Transperineal in-bore MR imaging–guided biopsy allows for a fully controlled needle placement and MR imaging confirmation of the deployment in the target and detects a large number of relevant cancers, many of which are located anteriorly.
Abstract
Purpose
To determine the detection rate, clinical relevance, Gleason grade, and location of prostate cancer (PCa) diagnosed with and the safety of an in-bore transperineal 3-T magnetic resonance (MR) imaging–guided prostate biopsy in a clinically heterogeneous patient population.
Materials and Methods
This prospective retrospectively analyzed study was HIPAA compliant and institutional review board approved, and informed consent was obtained. Eighty-seven men (mean age, 66.2 years ± 6.9) underwent multiparametric endorectal prostate MR imaging at 3 T and transperineal MR imaging–guided biopsy. Three subgroups of patients with at least one lesion suspicious for cancer were included: men with no prior PCa diagnosis, men with PCa who were undergoing active surveillance, and men with treated PCa and suspected recurrence. Exclusion criteria were prior prostatectomy and/or contraindication to 3-T MR imaging. The transperineal MR imaging–guided biopsy was performed in a 70-cm wide-bore 3-T device. Overall patient biopsy outcomes, cancer detection rates, Gleason grade, and location for each subgroup were evaluated and statistically compared by using χ2 and one-way analysis of variance followed by Tukey honestly significant difference post hoc comparisons.
Results
Ninety biopsy procedures were performed with no serious adverse events, with a mean of 3.7 targets sampled per gland. Cancer was detected in 51 (56.7%) men: 48.1% (25 of 52) with no prior PCa, 61.5% (eight of 13) under active surveillance, and 72.0% (18 of 25) in whom recurrence was suspected. Gleason pattern 4 or higher was diagnosed in 78.1% (25 of 32) in the no prior PCa and active surveillance groups. Gleason scores were not assigned in the suspected recurrence group. MR targets located in the anterior prostate had the highest cancer yield (40 of 64, 62.5%) compared with those for the other parts of the prostate (P < .001).
Conclusion
In-bore 3-T transperineal MR imaging–guided biopsy, with a mean of 3.7 targets per gland, allowed detection of many clinically relevant cancers, many of which were located anteriorly.
© RSNA, 2014
Introduction
Prostate cancer (PCa) is one of the most frequently diagnosed cancers in men, but only a minority of these cancers will cause relevant morbidity and mortality (1,2). Prostate biopsy is typically performed by using transrectal ultrasonographically (US) guided sampling in a nontargeted systematic pattern, with 12–15 cores taken bilaterally at the apex, base, and midgland regions of the prostate. Transperineal prostate mapping biopsies for diagnosis or treatment planning can be performed without imaging or by using transrectal US guidance with a transperineal template–guided approach in the operating room (3,4). Transperineal biopsy results have shown improved cancer detection rates, improved anteroapical sampling, reduced false-negative results, and reduced risk of underestimating disease volume and grade (5).
Imaging-guided and targeted needle biopsy is a mainstay of cancer diagnosis in many diseases, such as breast and lung cancers. A critical feature of this approach is the ability to obtain image confirmation of the biopsy needle in the lesion or target immediately before deployment.
The introduction of multiparametric magnetic resonance (MR) imaging of the prostate, with T2-weighted, diffusion-weighted, and dynamic contrast agent–enhanced imaging, has resulted in rapid expansion of the role of MR imaging in the detection and localization of PCa (6–10) and of MR imaging–guided or –targeted prostate interventional procedures (11,12). There are now several MR imaging–guided biopsy approaches that use multiparametric MR imaging for prebiopsy target identification and localization of the suspected lesion.
There are important differences in how multiparametric MR imaging–targeted biopsies can be performed. These have been reviewed in several recent meta-analyses (13–16). In essence, there are six configurations from which to choose: The navigation or guidance method can be transrectal US or MR imaging, samples can be taken either in the bore or outside the magnet, and the access route can be either transrectal or transperineal.
Transperineal in-bore MR imaging–guided biopsy at 0.5 T was first reported in 2001 (17), and it was shown to allow clear needle visualization and to be safe, feasible, and accurate. Since that time, with the exception of a case report, a small (n = 10) study at 3 T, and a report of registration methods (18–20), there have been no prospective clinical studies of transperineal MR imaging–guided biopsy. The feasibility of using transperineal MR imaging–guided biopsy in potential patient populations, such as men with no prior PCa diagnosis, men with PCa who are undergoing active surveillance (AS), or men with biochemical failure after definitive treatment, has not been ascertained. This study was conducted to determine the detection rate, clinical relevance, Gleason grade, and location of PCa lesions diagnosed with and the safety of an in-bore transperineal 3-T MR imaging–guided prostate biopsy in a clinically heterogeneous patient population. We hypothesized that transperineal MR imaging–guided biopsy of a low number of targets would yield a high number of clinically relevant cancers.
Materials and Methods
Patient Population
The institutional review board of the hospital (Brigham and Women’s Hospital, Boston, Mass) approved this Health Insurance Portability and Accountability Act–compliant prospective study. All men who underwent transperineal MR imaging–guided biopsy between January 2011 and August 2013 were enrolled after providing informed consent. All authors had full control of and access to all data pertaining to the study. Inclusion criteria were at least one suspected lesion at typical 3-T multiparametric MR imaging and at least one of the following: (a) elevated prostate-specific antigen (PSA), (b) prior negative transrectal US-guided biopsy, or (c) inability to undergo transrectal biopsy owing to rectal surgery. Exclusion criterion was prior prostatectomy or contraindication to 3-T MR imaging. All patients were divided into one of the three following subgroups, depending on clinical presentation: (a) men with no prior PCa diagnosis (hereafter, no PCa group), (b) men with PCa who were considering or undergoing AS (hereafter, AS group), and (c) men with biochemical failure after prior therapy for PCa who had a suspected recurrence (SR) (hereafter, SR group).
Multiparametric MR Imaging Protocol
Prebiopsy multiparametric MR imaging was performed by using a 3-T MR imager (Signa HDxt 3.0 T; GE Healthcare, Milwaukee, Wis) with both endorectal (Medrad, Warrendale, Pa) and pelvic phased-array coils. Bowel peristalsis was suppressed by administering 1 mg of glucagon intramuscularly. The imaging sequences used were T2 weighted, diffusion weighted (b = 500 and 1400 sec/mm2), and dynamic contrast enhanced, after intravenous injection of gadopentetate dimeglumine (Magnevist; Bayer Healthcare Pharmaceuticals, Montville, NJ) at 0.1 mmol per kilogram of body weight (Table 1) (6). Apparent diffusion coefficient maps were generated from diffusion-weighted images for b values of both 500 and 1400 sec/mm2. In addition, semiquantitative (ie, maximum slope, time to peak, and area under the plasma concentration time curve in the first 90 seconds) and quantitative (ie, forward volume transfer constant and fractional volume of extracellular space per unit volume of tissue) pharmacokinetic maps of the dynamic contrast-enhanced data were derived by using research software (OncoQuant; GE Global Research, Niskayuna, NY) (21).
Table 1.
Typical Pre- and Intraprocedural Prostate 3-T MR Imaging Protocol
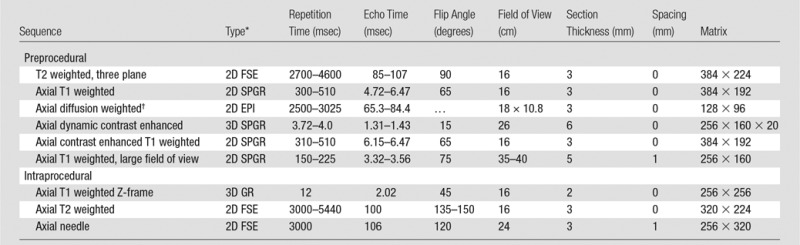
Source.—Reference 6.
EPI = single-shot echo-planar imaging, FSE = fast spin echo, GR = gradient-recalled echo, SPGR = spoiled gradient recalled, 3D = three-dimensional, 2D = two-dimensional.
b values = 0, 500, and 1400 sec/mm2.
Prebiopsy Image Analysis
To prospectively identify all suspected targets for biopsy, three radiologists (K.T., F.M.F., and C.M.C.T., with more than 10 years of experience each in prostate MR imaging) independently reviewed all MR sequences and images. Clinical data, including PSA value and prior biopsy reports (when applicable), were available at the time of analysis. All images were displayed and interpreted by using a picture archiving and communication system and a research workstation by running visualization and medical image computing software (22) (3D Slicer, www.slicer.org) (Fig 1). After a review of all available images for all pulse sequences was performed by using previously published criteria (6), all lesions suspicious for cancer were identified and rated as follows: 1, definitely not malignant; 2, probably not malignant; 3, indeterminate; 4, probably malignant; or 5, definitely malignant (23).
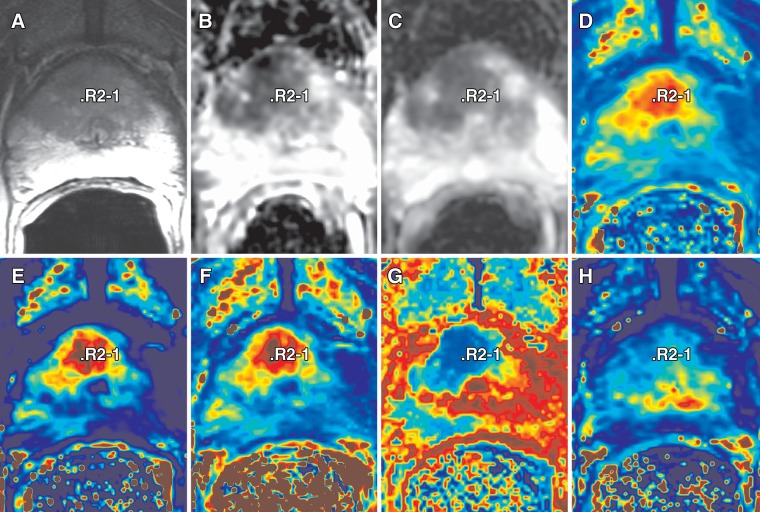
Figure 1:
Multiparametric MR imaging review for a 68-year-old man with elevated PSA (25.95 ng/mL [25.95 μg/L]) and a history of two previous negative transrectal US-guided biopsies. Transperineal MR imaging–guided biopsy revealed PCa with Gleason score of 4+4 in anterior central gland (R2-1), leading to subsequent radical prostatectomy. Image review and target selection were performed by using 3D Slicer. Setup enables concurrent visualization of different multiparametric sequences reformatted to acquisition plane of T2-weighted series. A, T2-weighted; B, apparent diffusion coefficient (b = 500 sec/mm2); C, apparent diffusion coefficient (b = 1400 sec/mm2); D, area under the curve; E, forward volume transfer constant; F, maximum slope; G, time to peak; and, H, fractional volume of extracellular space images.
Transperineal MR Imaging–guided Biopsy Procedure
All men attended prebiopsy clinic visits during which the benefits and risks of the procedure were explained and informed consent for both the biopsy and administration of sedation was obtained. At this visit, blood testing for coagulation status and serum PSA was performed. The biopsy was performed in either a 3-T wide-bore system (Siemens Verio 3T; Siemens Healthcare, Erlangen, Germany) or a ceiling-mounted 3-T wide-bore MR imager (IMRIS/Siemens Verio; IMRIS, Minnetonka, Minn).
The interventional radiologist (K.T.) reviewed all biopsy targets on the day the biopsy was performed and ranked them in descending order of suspicion for malignancy on the basis of the readers’ scores. If two reviewers selected the same lesion for biopsy, only one target was included in the biopsy plan. In case of discrepancies between the radiologists, all targets underwent biopsy.
The patient was placed in the supine position in the imager gantry, an intravenous line was sited, and his legs were elevated by using an in-house–designed table-top device to allow for transperineal access (19) (Fig 2). The skin of the perineum was prepared and draped in a sterile manner, and the needle guidance template was positioned. All patients received intravenous sedation consisting of a benzodiazepine (2–5 mg of midazolam hydrochloride) and an opioid (50–300 μg of fentanyl citrate). Baseline MR imaging was performed (see Table 1), including T1-weighted imaging, to register the template to the patient’s imaging space (19). Then, by using pre- and intraprocedural axial T2-weighted images and nonrigid registration (20,24), all target and lesion locations were reidentified by using the 3D Slicer software (Fig 3).
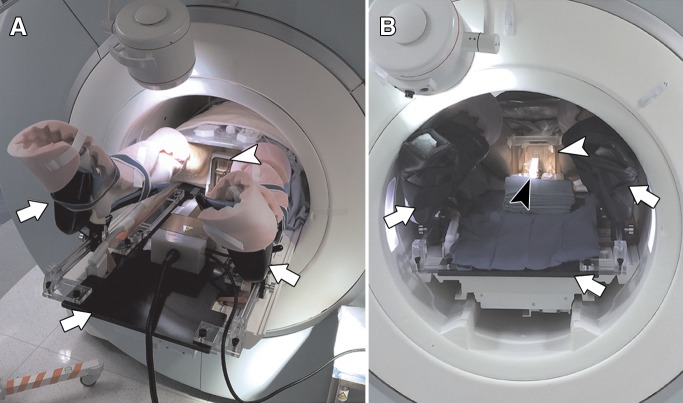
Figure 2:
Photographs show transperineal MR imaging–guided biopsy procedure setup. A, Patient is placed onto custom-made MR imaging–compatible table top with leg supports (arrows) with legs in lithotomy position, and template is placed against perineum (arrowhead, Smart Template). B, In-bore view with patient on table top (arrows), template in place (white arrowhead, poly[methyl methacrylate] manual template), and biopsy needle in place (black arrowhead).
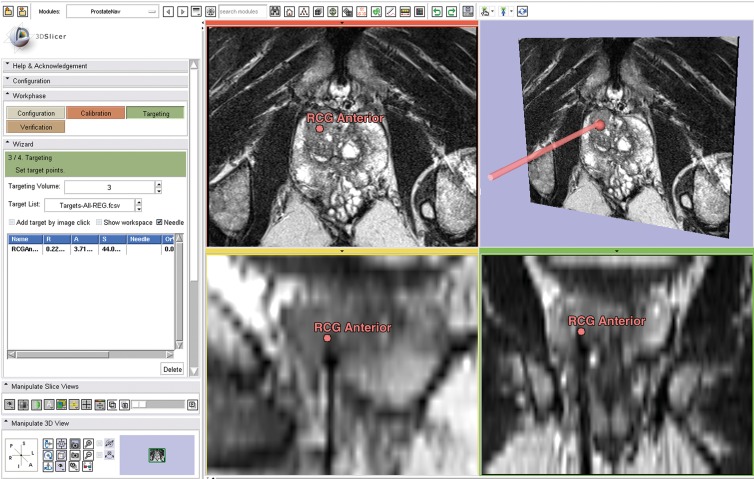
Figure 3:
Screenshot shows intraprocedural visualization of imaging for needle position confirmation for transperineal prostate biopsy in a 71-year-old man with no rectal access and increasing PSA (31.09 ng/dL [31.09 μg/L]). Targeted biopsy yielded Gleason 4+3 PCa in right anterior central gland. Image shows 3D Slicer targeting module during biopsy with needle confirmation image loaded. Left: control panel. Top middle: axial T2-weighted MR image. Top right: three-dimensional view, including calculated needle path (arrow). Bottom middle: sagittal T2-weighted MR image shows target and needle tract. Bottom right: coronal T2-weighted MR image, with target and needle track. RGC = right central gland.
All biopsy samples were obtained after local anesthesia was administered (2% sodium bicarbonate–buffered lidocaine, 10–20 mL per session) by using an 18-gauge side-cutting MR-compatible core biopsy needle (MRI Bio Gun, E-Z-EM, Westbury, NY; Single Action Biopsy Device, US Biopsy, Franklin, Ind; Semi-Automatic Biopsy Gun, Invivo, Schwerin, Germany; Fully Automatic Biopsy Gun, Invivo). Depending on the sample quality, one to five samples were taken. Needle insertion was guided by either a standard poly(methyl methacrylate) template with a fixed 5-mm grid or an MR imaging–compatible robotic Smart Template, both described elsewhere (19,25). For each target, the needle depth and skin entry location were derived from the MR images. All needles were manually advanced, and locations were confirmed by using T2-weighted images. For each target, the localization within the prostate was determined by one of two radiologists (K.T. or T.P.) in three dimensions: side (left or right), height (base, midgland, or apex), and sagittal localization (anterior, central, or posterior).
Procedure time.—Typical imaging duration was derived on the basis of imaging protocols and Digital Imaging and Communications in Medicine time stamps for the imaging, needle placements, and registration parts of the procedure.
Pain and adverse events.—Patient pain was assessed intra- and postprocedurally by using the visual analog scale, recording pain on a 0–10 scale, as per institutional guidelines. Procedure complications were assessed by observing changes during intraprocedural imaging (eg, periprostatic hematoma) or with standard follow-up consulting of the patients (eg, urinary retention, infection, hematospermia, hematuria) and recorded for analysis.
Pathologic findings.—Tissue samples were collected, fixed in formaldehyde, and labeled according to the location and target number. This critical step allowed for site-specific MR-pathology correlation. All specimens were handled in the routine clinical manner. Diagnosis was recorded for each positive core, and Gleason patterns were also recorded, except in men who had undergone prior treatment. Biopsy specimens with at least one component of Gleason 4 or positive biopsy specimens of patients with recurrent cancer were deemed clinically relevant.
Statistical Analyses
Continuous quantities are reported as means ± standard deviations and were compared between groups by using one-way analysis of variance followed by Tukey honestly significant difference post hoc comparisons. Discrete quantities were compared between groups and localizations by using χ2 tests. For all tests, a P value of .05 was considered to indicate a significant difference. All statistical calculations were performed by using the R statistics package (version 3.0.1) (26) and Medcalc (Medcalc Software, Ostend, Belgium).
Results
Ninety consecutive transperineal MR imaging–guided biopsy sessions were performed in 87 men. Three men had second or repeat transperineal MR–guided biopsies, with an average repeat biopsy interval of 16.2 months. Three additional transperineal biopsy procedures were performed in the study time frame and were excluded, two were postprostatectomy procedures for evaluation of a recurrent PCa, and one was performed with a different imager at 1.5 T because of a 3.0-T–incompatible implant. Prebiopsy multiparametric MR imaging was used to identify a mean of 3.7 targets ± 1.7 per gland, targeting a total of 332 suspected foci. The mean patient age was 66.2 years ± 6.9; mean PSA value, 12.4 ng/mL ± 11.5 (12.4 µg/L ± 11.5); mean prostate volume, 54.8 mL ± 30.0; and mean PSA density, 0.26 ng/mL2 ± 0.22 (0.26 µg/L2 ± 0.22) (Table 2). Patients in the SR group were older than those in the no prior PCa group (P = .0303) and had a lower mean PSA value (P < .001) and a smaller prostate (P = .029), but no significant difference in PSA density could be found (P = .411). No significant differences in the number of targets or the number of positive targets per session could be found (Table 2). Fifty-five of 90 (61.1%) procedures were performed by using the manual in-bore template, and 35 of 90 (38.9%) with the in-bore Smart Template (25).
Table 2.
Characteristics of the Study Population
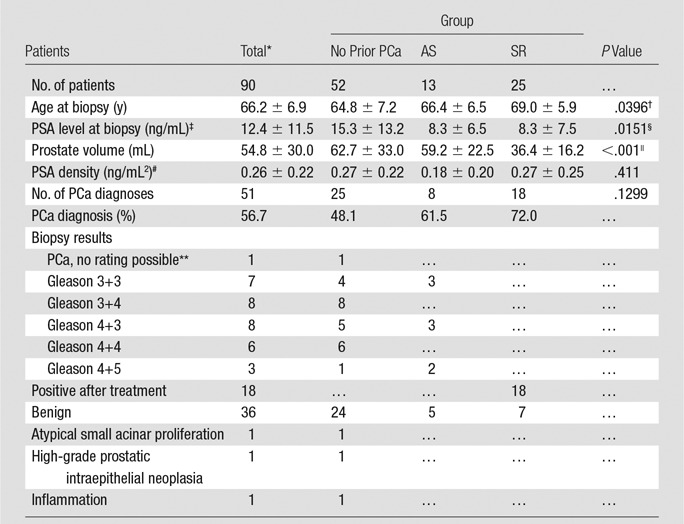
Note.—Where applicable, values are means ± standard deviations.
Three repeat cases with a minimum repeat interval of 11.6 months were treated as separate entities.
SR vs no prior PCa group: P = .0303, AS vs no prior PCa group: P = .716, AS vs SR group: P = .497.
To convert to Système International (SI) units in micrograms per liter, multiply by 1.0
SR vs AS group: P = .00319, SR vs no prior PCa group: P < .001, no prior PCa vs AS: P > .99.
SR vs no prior PCa: P = .0290, SR vs AS: P > .99, no prior PCa vs AS: (P = .0109).
To convert to SI units in micrograms per liter squared, multiply by 1.
Sample quality was inadequate for Gleason grading.
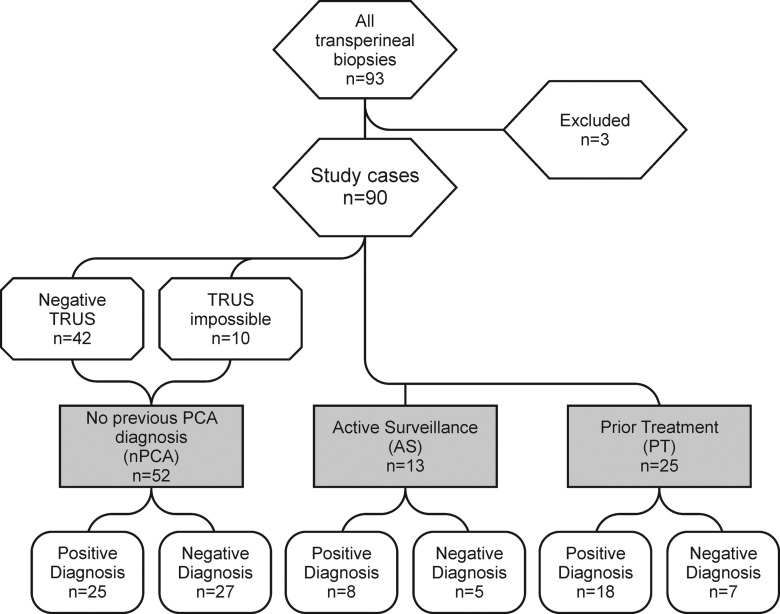
The overall cancer detection rate was 56.7% (51 of 90 men), with 29.2% positive targets (97 of 332) (Tables 2, 3). The patient subgroups and biopsy results are summarized in the study workflow diagram (Fig 4).
Table 3.
Biopsy Results
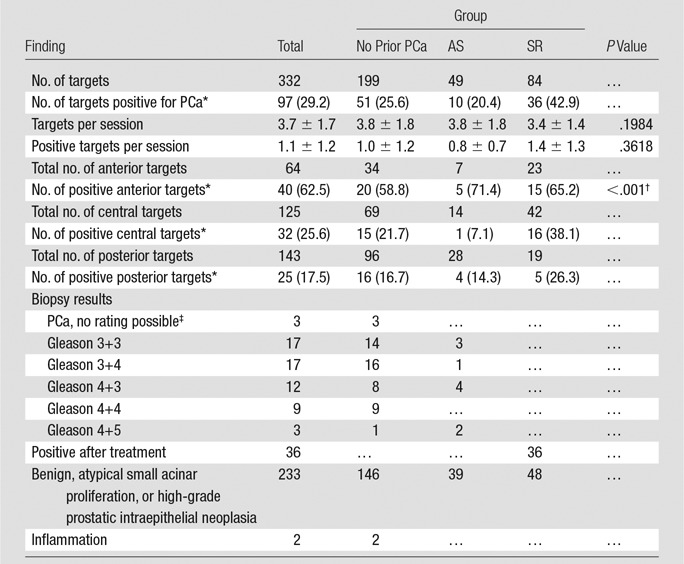
Data in parentheses are percentages.
Overall comparison of the positivity rate in the anterior part against the central and posterior parts.
The quality of the sample was inadequate for Gleason grading.
Figure 4:
Study flow diagram. Patients were separated into three groups: patients with no previous PCa (nPCA) diagnosis, patients undergoing or considering AS, and patients with SR after prior treatment for PCa. TRUS = transrectal US.
No Prior PCa Diagnosis Group
Of the 52 men who underwent transperineal MR imaging–guided biopsies 25 (48.1%) received results that were positive for PCa, and 20 of 25 were Gleason 3+4 or higher. One hundred ninety-nine targets were identified (3.8 ± 1.8 per biopsy procedure), of which 51 (25.6%) were positive for cancer. Previously performed transrectal US-guided biopsies did not result in a positive PCa diagnosis in 41 (78.8%) of these 52 men. The remainder lacked rectal access, owing to surgery or stenosis, rendering transrectal US impossible.
AS Group
Eight (61.5%) of 13 men in the AS group had positive transperineal MR imaging–guided biopsy findings in 10 (20.4%) of 49 targets. Five (62.5%) of the eight men had Gleason 4+3 or higher grade cancers.
SR Group
In the SR group of 25 patients, 19 patients had undergone prior brachytherapy alone, four had undergone external beam radiation and androgen deprivation therapy, two had undergone external beam radiation therapy alone, and one patient had undergone external beam radiation therapy and brachytherapy before the procedure. Eighteen (72.0%) of the 25 patients had positive transperineal MR imaging–guided biopsy findings. Eighty-four targets were identified, of which 36 (42.9%) were positive at biopsy. Owing to the effects of previous radiation, the pathologists did not assign Gleason scores or patterns in this group.
Procedure Time
The preparation imaging included a localizer (43 seconds), a T1-sequence for template registration (2 minutes and 24 seconds), and a T2-weighted sequence for target registration (5 minutes and 57 seconds), accounting for 9 minutes and 4 seconds of imaging time. The biopsy procedure time, as determined from first needle confirmation image to the last needle confirmation image, lasted a mean of 67.8 minutes (median, 63.8 minutes), with an mean of 21.4 minutes per predefined target (median, 18.1 minutes).
Postprocedure Complications
The procedure was well tolerated by all men. Median intraprocedural pain, as measured, per hospital policy, by using a visual analog scale (0 = no pain, 10 = severe pain), was 1 (interquartile range, 1). The median postprocedural pain, as measured on the same scale, was zero (interquartile range, zero). Twenty (22.2%) of the 90 patients developed a periprostatic hematoma apparent at imaging, all of which were asymptomatic and resolved without any further intervention. Five (5.5%) of the 90 patients required temporary catheterization for postprocedural urinary retention, all of which resolved after 6 days ± 4.6. Transient hematuria and/or hematospermia occurred in 11 (12.2%) of the 90 patients. No cases of prostatitis or infection were observed or reported.
PCa Gleason Grade and Location
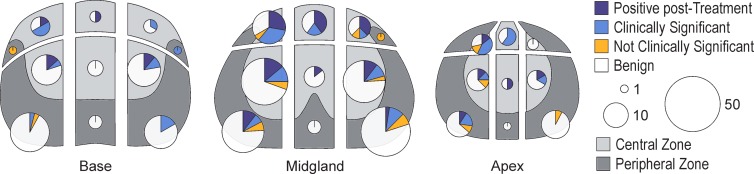
In the study population as a whole, 43 (84.3%) of the 51 PCas, as revealed at transperineal MR imaging–guided biopsy, were either Gleason pattern 3+4 or higher or recurrent PCa (Table 2). In the group with pathologically assigned Gleason scores (excluding the men with prior radiation therapy), 25 (78.1%) of the 32 had a Gleason 4 or higher pattern, and the remaining 21.9% (seven of 32) were Gleason pattern 3+3. For the no prior PCa and AS patient groups, the percentages of men with Gleason pattern 4 or higher were 80.0% (20 of 25) and 62.5% (five of eight), respectively. On a per target basis, 77 (79.4%) of 97 targets had Gleason pattern 4 or higher or were recurrent cancer, with rates of 66.7% (34 of 51), 70.0% (seven of 10), and 100% (36 of 36) for the no prior PCa diagnosis, AS, and SR groups, respectively (Table 3, Fig 5).
Figure 5:
Figure shows location and histopathologic outcome of biopsies in prostate, as divided into anterior, posterior, and central zones. Pie chart size is proportional to number of biopsies performed. Clinically significant = Gleason grade of 3+4 or higher, not clinically significant = Gleason grade of 6 or lower. Although more biopsies were performed in central and posterior parts of prostate, a higher proportion of positive biopsies was observed in anterior portion of prostate.
The majority of the suspected targets in all groups (143 of 332) were located posteriorly, with 125 targets in the center and 64 in the anterior section (Table 3, Fig 4). The cancer rates by location within the gland were 62.5% in the anterior region (40 of 64), 25.6% in the central region (32 of 125), and 17.5% in the posterior region (25 of 143), with a higher positive biopsy rate in the anterior gland (P < .001). The percentage of positive targets was consistently higher in the anterior gland in all three patient groups, at 58.8% in the no prior PCa diagnosis group (20 of 34), 71.4% in the AS group (five of seven), and 65.2% in the SR group (15 of 23), as compared with the central gland (21.7% [15 of 69], 7.1% [one of 14], and 38.1% [16 of 42], respectively) or the posterior part of the prostate (16.7% [16 of 96], 14.3% [four of 28], and 26.3% [five of 19], respectively).
Discussion
In this study of 90 transperineal wide-bore 3-T multiparametric MR imaging–guided prostate biopsy procedures, we found a large number (56.7% [51 of 90]) of cancers. Our patients experienced minimal pain and only minor adverse events. Our results showed that a substantial percentage of clinically relevant (Gleason pattern 4 or greater or disease recurrence) cancers were detected in all three patient groups. In the specimens assigned a Gleason score (excluding men with prior radiation therapy), 78.1% (25 of 32) were shown to be clinically relevant cancer (Gleason pattern 4 or higher). In the first subgroup of men with no prior cancer diagnosis, 48.1% (25 of 52) had cancer detected. Transrectal US did not allow a positive PCa diagnosis in 78.8% (41 of 52) of these men. Even more importantly, 80% (20 of 25) of these cancers were clinically relevant, with a Gleason pattern of 3+4 or higher. MR imaging and MR imaging–guided biopsy should therefore be considered in all men for whom there is a high clinical suspicion for PCa in whom a diagnosis has not been achieved. The AS group in our study also had a high rate (61.5% [eight of 13]) of cancers detected, and 62.5% (five of eight) of these had a Gleason pattern of 4 or higher and, thus, may not be suitable for AS.
The patient subgroups showed an expected difference in prostate volume for the patients previously treated and, paired with a lower PSA in SR patients, there was no difference in PSA density. Also, the older age of SR patients compared with the other groups was not surprising. Many of the comparisons of patient characteristics involving the AS group were not significant, possibly related to the small patient numbers.
Prostate multiparametric MR imaging has matured to a point where it has become valuable in a variety of diagnostic and therapeutic interventions. There are now several options available, beyond transrectal US guidance, to biopsy the prostate in conjunction with prebiopsy multiparametric MR imaging results. It is important to understand the approaches used in all MR imaging–guided biopsy reports, and correct terminology should be used to avoid confusion. The terms MR imaging guidance and MR imaging targeted have both been used interchangeably when MR images define the target, but neither term should be taken to infer that intraprocedural in-bore MR imaging guidance was actually used, as it was in our study. In a review by Nelson et al (16), 20 MR imaging–guided biopsy studies were analyzed; 18 obtained the biopsies through a transrectal route, one through a transperineal route, and one through a transgluteal route. The one transperineal MR imaging–guided prostate biopsy study reviewed by Nelson et al was reported by Hadaschik et al (27) and used prebiopsy MR imaging to identify biopsy targets, but needle guidance and deployment in real time used transrectal US rather than intraprocedural MR imaging guidance. Kasivisvanathan et al (28) also reported on an “MR targeted transperineal biopsy” in which the needle deployment was based on the operator’s cognitive knowledge of the prebiopsy 1.5-T MR imaging findings. The sampling was performed with the patient under general anesthesia by using a grid template in the operating room with US guidance.
The most common approach to MR imaging–guided biopsy uses preacquired MR imaging and real-time transrectal US out of bore. This requires an image registration method to allow display of the earlier MR images with the real-time transrectal US images on the day of the biopsy (29–31). These all use different rigid or nonrigid algorithms to help reidentify the biopsy targets in transrectal US. Another out-of-bore approach uses the MR imaging in a cognitive manner, with the operator interpreting the previously acquired MR imaging information either as reported or by direct visualization and then performing a transrectal US sampling directed to the cognitively recalled locations (28). The transrectal MR imaging–guided biopsy studies (32–40) report cancer positivity rates of 8%–59%. Puech et al (41) compared cognitive and fusion techniques, showing no significant differences in biopsy yield. MR imaging allowed identification of and was used to obtain biopsies from 1.39 (37) to 4.3 (33) targets, with per-target cancer yields of between 3% and 33%. Previous reports have compared systematic 12-core transrectal US biopsy with MR imaging–targeted biopsy and found that the latter revealed 16% more grade 4 or 5 cancers (42). Siddiqui et al (43) recently compared the Gleason grades from MR imaging-US fusion biopsy with those obtained with 12-core transrectal US, and the MR imaging-US fusion targeted biopsy allowed detection of 67% more cancers with a Gleason grade of 4+3 or greater than did the 12-core US biopsy alone. The cancer detection rate and target numbers in our study are concordant (56.7% [51 of 90], mean of 3.7 targets), albeit at the high end of these results.
There are important differences in adverse events in the comparison of transrectal and transperineal approaches. In-bore or out-of-bore transrectal MR imaging–guided biopsy shares the same urosepsis risk as transrectal US, and whole-gland access can be limited, because the clinically available transrectal devices do not allow free manipulation of the needle in the bore or image-guided repositioning in real time, and the needle and target locations may not be confirmed (44).
In comparison, the transperineal route allows complete freedom of needle manipulation. It can be placed anywhere on the perineum and avoids transgression of the rectal wall, and thus, it is a sterile biopsy with a substantially reduced risk of infection (45). The lack of postbiopsy infection or sepsis is important in contrast to the 3%–11% infection rates previously reported for transrectal US, especially in light of rising antibiotic-resistant infections after transrectal biopsies (46–48). It can be performed in men with difficult or impossible rectal access, such as patients who have undergone proctocolectomy or those with rectal stenosis from prior radiation treatment (49).
Another major advantage of the transperineal approach is the easy access to any aspect of the gland, especially the anterior region of the prostate (50). Our results show a significantly higher (P < .001) rate (62.5% [40 of 64]) of positive cancer findings in the anterior part of the prostate compared with that in the rest of the gland (central [25.6%, 32 of 125] and posterior [17.5%, 25 of 143]), although the influence of a patient selection bias cannot be ruled out. In any case, this is an especially challenging area for transrectal US-guided biopsy.
The following limitations must be considered when interpreting the results of our data. The study was conducted at a single center. A multicenter approach would be desirable for evaluating the influence of different magnets, setups, equipment, and postprocessing settings. The pathologic results reported are all based only on biopsy cores. We did not have whole-mount or routine radical prostatectomy results, and thus, we cannot report the false-negative biopsy results. Owing to the very nature of the MR imaging–targeted biopsy technique, only patients with MR imaging–visible lesions were included, introducing a potential selection bias. Of the three subgroups, the AS group was small, which limits our conclusions in this important group, although we do believe the high rate of positive results from anteriorly located lesions is an important issue in these patients who are often enrolled into AS protocols on the basis of transrectal US sampling. There is heterogeneity among the men who had undergone treatment, because some had undergone radiation alone and others underwent it combined with androgen suppression. When cancer is found in this population, no Gleason pattern is assigned by pathologists.
In general, all MR imaging biopsy approaches have reported a higher cancer yield than do those with transrectal US, with more clinically relevant disease being detected with MR imaging approaches (13,16). There are important considerations with all these MR imaging–guided biopsies related to resource use, length of this procedure, and overall cost-benefit analysis that are beyond the scope of our study but will be the focus of future research.
In summary, transperineal in-bore 3-T MR imaging–guided biopsy provides a direct approach to sample all MR imaging–defined targets and was successful in all three patient subgroups. It resulted in high cancer detection rates, with high Gleason scores, which were highest in men with SR and in the anterior gland. The procedure and setup, with image registration, allows access to the prostate with relative ease without moving the patient during the procedure and enables fully controlled needle placement and MR imaging–confirmed deployment in the target.
Advances in Knowledge
■ Transperineal MR imaging–guided prostate biopsy had a high rate of cancer detection and a high rate of clinically relevant cancers (Gleason pattern 4 and abnormal prostate-specific antigen in treatment-naive or previously treated men) diagnosed, with a low number of targets (mean, 3.7 per gland).
■ Transperineal MR imaging–guided prostate biopsy can be performed with a low rate of complications (periprostatic hematoma, 22.2% [20 of 90]; hematuria or hematospermia, 12.2% [11 of 90]; urinary retention, 5.5% [five of 90]; no known infections) and is tolerated well by the patients.
Implication for Patient Care
■ In-bore MR imaging–guided transperineal prostate biopsy can be regarded as a safe and well-tolerated option for sampling of any location within the prostate gland.
Acknowledgments
Acknowledgments
Pharmacokinetic analysis was performed by using custom research software courtesy of Sandeep N. Gupta, PhD (GE Global Research, Niskayuna, NY). The authors thank Louise Greenberg, MEd, for her indispensable help with the institutional review board and project management, and all our nurses and technologists, especially Angela Roddy Kanan and Janice Fairhurst, for their dedication and excellent patient care.
Received January 28, 2014; revision requested March 18; revision received April 18; accepted June 9; final version accepted June 24.
T.P. supported by RWTH Aachen University Hospital.
Funding: This research was supported by the National Institutes of Health (grants R01CA111288, P41RR019703, P41EB015898, P01CA067165, U01CA151261, and U54EB005149).
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Disclosures of Conflicts of Interest: T.P. Activities related to the present article: grant from RWTH Aachen University. Activities not related to the present article: grant and nonfinancial support from Siemens Healthcare, nonfinancial support from Philips Healthcare and RSNA. Other relationships: disclosed no relevant relationships. K.T. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: grant from Cannon. Other relationships: disclosed no relevant relationships. A.F. disclosed no relevant relationships. S.S. disclosed no relevant relationships. J.T. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: grant from Siemens Medical Solutions USA. Other relationships: disclosed no relevant relationships. F.M.F. disclosed no relevant relationships. M.G.V. disclosed no relevant relationships. A.S.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: personal fees as a member of the ad board for Myrian, Dendreon, and Sanofi Aventis. Other relationships: disclosed no relevant relationships. R.V.M. disclosed no relevant relationships. W.M.W. disclosed no relevant relationships. N.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: Director of AZE Technology. Other relationships: disclosed no relevant relationships. C.M.C.T. disclosed no relevant relationships.
Abbreviations:
- AS
- active surveillance
- PCa
- prostate cancer
- PSA
- prostate-specific antigen
- SR
- suspected recurrence
References
- 1.Prostate cancer key statistics . American Cancer Society Web site. http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics. Published August 26, 2013. Updated March 12, 2014. Accessed July 5, 2013.
- 2.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol 2012;13(8):790–801. [DOI] [PubMed] [Google Scholar]
- 3.Onik G, Miessau M, Bostwick DG. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J Clin Oncol 2009;27(26):4321–4326. [DOI] [PubMed] [Google Scholar]
- 4.Arumainayagam N, Ahmed HU, Moore CM, et al. Multiparametric MR imaging for detection of clinically significant prostate cancer: a validation cohort study with transperineal template prostate mapping as the reference standard. Radiology 2013;268(3):761–769. [DOI] [PubMed] [Google Scholar]
- 5.Chang DT, Challacombe B, Lawrentschuk N. Transperineal biopsy of the prostate: is this the future? Nat Rev Urol 2013;10(12):690–702. [DOI] [PubMed] [Google Scholar]
- 6.Hegde JV, Mulkern RV, Panych LP, et al. Multiparametric MRI of prostate cancer: an update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J Magn Reson Imaging 2013;37(5):1035–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurhanewicz J, Vigneron D, Carroll P, Coakley F. Multiparametric magnetic resonance imaging in prostate cancer: present and future. Curr Opin Urol 2008;18(1):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shukla-Dave A, Hricak H, Kattan MW, et al. The utility of magnetic resonance imaging and spectroscopy for predicting insignificant prostate cancer: an initial analysis. BJU Int 2007;99(4):786–793. [DOI] [PubMed] [Google Scholar]
- 9.Villers A, Puech P, Mouton D, Leroy X, Ballereau C, Lemaitre L. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol 2006;176(6 Pt 1):2432–2437. [DOI] [PubMed] [Google Scholar]
- 10.Fütterer JJ, Heijmink SW, Scheenen TW, et al. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology 2006;241(2):449–458. [DOI] [PubMed] [Google Scholar]
- 11.Haker SJ, Mulkern RV, Roebuck JR, et al. Magnetic resonance-guided prostate interventions. Top Magn Reson Imaging 2005;16(5):355–368. [DOI] [PubMed] [Google Scholar]
- 12.D’amico AV, Tempany CM, Schultz D, et al. Comparing PSA outcome after radical prostatectomy or magnetic resonance imaging-guided partial prostatic irradiation in select patients with clinically localized adenocarcinoma of the prostate. Urology 2003;62(6):1063–1067. [DOI] [PubMed] [Google Scholar]
- 13.Moore CM, Robertson NL, Arsanious N, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol 2013;63(1):125–140. [DOI] [PubMed] [Google Scholar]
- 14.Robertson NL, Emberton M, Moore CM. MRI-targeted prostate biopsy: a review of technique and results. Nat Rev Urol 2013;10(10):589–597. [DOI] [PubMed] [Google Scholar]
- 15.Overduin CG, Fütterer JJ, Barentsz JO. MRI-guided biopsy for prostate cancer detection: a systematic review of current clinical results. Curr Urol Rep 2013;14(3):209–213. [DOI] [PubMed] [Google Scholar]
- 16.Nelson AW, Harvey RC, Parker RA, Kastner C, Doble A, Gnanapragasam VJ. Repeat prostate biopsy strategies after initial negative biopsy: meta-regression comparing cancer detection of transperineal, transrectal saturation and MRI guided biopsy. PLoS ONE 2013;8(2):e57480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hata N, Jinzaki M, Kacher D, et al. MR imaging-guided prostate biopsy with surgical navigation software: device validation and feasibility. Radiology 2001;220(1):263–268. [DOI] [PubMed] [Google Scholar]
- 18.D’Amico AV, Cormack RA, Tempany CM. MRI-guided diagnosis and treatment of prostate cancer. N Engl J Med 2001;344(10):776–777. [DOI] [PubMed] [Google Scholar]
- 19.Tokuda J, Tuncali K, Iordachita I, et al. In-bore setup and software for 3T MRI-guided transperineal prostate biopsy. Phys Med Biol 2012;57(18):5823–5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fedorov A, Tuncali K, Fennessy FM, et al. Image registration for targeted MRI-guided transperineal prostate biopsy. J Magn Reson Imaging 2012;36(4):987–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 1999;10(3):223–232. [DOI] [PubMed] [Google Scholar]
- 22.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenkrantz AB, Kim S, Lim RP, et al. Prostate cancer localization using multiparametric MR imaging: comparison of Prostate Imaging Reporting and Data System (PI-RADS) and Likert scales. Radiology 2013;269(2):482–492. [DOI] [PubMed] [Google Scholar]
- 24.Oguro S, Tokuda J, Elhawary H, et al. MRI signal intensity based B-spline nonrigid registration for pre- and intraoperative imaging during prostate brachytherapy. J Magn Reson Imaging 2009;30(5):1052–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song SE, Tokuda J, Tuncali K, Tempany CM, Zhang E, Hata N. Development and preliminary evaluation of a motorized needle guide template for MRI-guided targeted prostate biopsy. IEEE Trans Biomed Eng 2013;60(11):3019–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. [Google Scholar]
- 27.Hadaschik BA, Kuru TH, Tulea C, et al. A novel stereotactic prostate biopsy system integrating pre-interventional magnetic resonance imaging and live ultrasound fusion. J Urol 2011;186(6):2214–2220. [DOI] [PubMed] [Google Scholar]
- 28.Kasivisvanathan V, Dufour R, Moore CM, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol 2013;189(3):860–866. [DOI] [PubMed] [Google Scholar]
- 29.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol 2011;186(4):1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marks L, Young S, Natarajan S. MRI-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol 2013;23(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Natarajan S, Marks LS, Margolis DJ, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol 2011;29(3):334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beyersdorff D, Winkel A, Hamm B, Lenk S, Loening SA, Taupitz M. MR imaging-guided prostate biopsy with a closed MR unit at 1.5 T: initial results. Radiology 2005;234(2):576–581. [DOI] [PubMed] [Google Scholar]
- 33.Engehausen DG, Engelhard K, Schwab SA, et al. Magnetic resonance image-guided biopsies with a high detection rate of prostate cancer. ScientificWorldJournal 2012;2012:975971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franiel T, Stephan C, Erbersdobler A, et al. Areas suspicious for prostate cancer: MR-guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding—multiparametric MR imaging for detection and biopsy planning. Radiology 2011;259(1):162–172. [DOI] [PubMed] [Google Scholar]
- 35.Hambrock T, Fütterer JJ, Huisman HJ, et al. Thirty-two-channel coil 3T magnetic resonance-guided biopsies of prostate tumor suspicious regions identified on multimodality 3T magnetic resonance imaging: technique and feasibility. Invest Radiol 2008;43(10):686–694. [DOI] [PubMed] [Google Scholar]
- 36.Hambrock T, Somford DM, Hoeks C, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol 2010;183(2):520–527. [DOI] [PubMed] [Google Scholar]
- 37.Hoeks CM, Schouten MG, Bomers JG, et al. Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol 2012;62(5):902–909. [DOI] [PubMed] [Google Scholar]
- 38.Roethke M, Anastasiadis AG, Lichy M, et al. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol 2012;30(2):213–218. [DOI] [PubMed] [Google Scholar]
- 39.Schwab SA, Kuefner MA, Adamietz B, et al. MRI-guided core biopsy of the prostate in the supine position: introduction of a simplified technique using large-bore magnet systems. Eur Radiol 2013;23(5):1415–1419. [DOI] [PubMed] [Google Scholar]
- 40.Singh AK, Krieger A, Lattouf JB, et al. Patient selection determines the prostate cancer yield of dynamic contrast-enhanced magnetic resonance imaging-guided transrectal biopsies in a closed 3-Tesla scanner. BJU Int 2008;101(2):181–185. [DOI] [PubMed] [Google Scholar]
- 41.Puech P, Rouvière O, Renard-Penna R, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy—prospective multicenter study. Radiology 2013;268(2):461–469. [DOI] [PubMed] [Google Scholar]
- 42.Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int 2011;108(8 Pt 2):E171–E178. [DOI] [PubMed] [Google Scholar]
- 43.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 2013;64(5):713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krieger A, Iordachita II, Guion P, et al. An MRI-compatible robotic system with hybrid tracking for MRI-guided prostate intervention. IEEE Trans Biomed Eng 2011;58(11):3049–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lange D, Zappavigna C, Hamidizadeh R, Goldenberg SL, Paterson RF, Chew BH. Bacterial sepsis after prostate biopsy: a new perspective. Urology 2009;74(6):1200–1205. [DOI] [PubMed] [Google Scholar]
- 46.Zaytoun OM, Vargo EH, Rajan R, Berglund R, Gordon S, Jones JS. Emergence of fluoroquinolone-resistant Escherichia coli as cause of postprostate biopsy infection: implications for prophylaxis and treatment. Urology 2011;77(5):1035–1041. [DOI] [PubMed] [Google Scholar]
- 47.Djavan B, Waldert M, Zlotta A, et al. Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: results of a prospective European prostate cancer detection study. J Urol 2001;166(3):856–860. [PubMed] [Google Scholar]
- 48.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol 2011;186(5):1830–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Symons JL, Huo A, Yuen CL, et al. Outcomes of transperineal template-guided prostate biopsy in 409 patients. BJU Int 2013;112(5):585–593. [DOI] [PubMed] [Google Scholar]
- 50.Satoh T, Matsumoto K, Fujita T, et al. Cancer core distribution in patients diagnosed by extended transperineal prostate biopsy. Urology 2005;66(1):114–118. [DOI] [PubMed] [Google Scholar]