Abstract
Berries are gaining increasing importance lately for their chemopreventive and therapeutic potential against several cancers. In earlier studies, a blueberry-supplemented diet has shown protection against 17β-estradiol (E2)-mediated mammary tumorigenesis. This study tested both preventive and therapeutic activities of diet supplemented with whole blueberry powder (50:50 blend of Tifblue and Rubel). Animals received 5% blueberry diet, either 2 weeks prior to or 12 weeks after E2 treatment in preventive and therapeutic groups, respectively. Both interventions delayed the tumor latency for palpable mammary tumors by 28 and 37 days, respectively. Tumor volume and multiplicity were also reduced significantly in both modes. The effect on mammary tumorigenesis was largely due to down-regulation of CYP 1A1 and ER-α gene expression and also favorable modulation of microRNA (miR-18a and miR-34c) levels. These data suggest that the blueberry blend tested is effective in inhibiting E2-mediated mammary tumorigenesis in both preventive and therapeutic modes.
Keywords: blueberry, estrogen, breast cancer, chemoprevention, chemotherapy, ACI rats, miRNA modulation, blood chemistry, hematopoietic parameters
Introduction
Breast cancer is the most common cancer among American women and the second leading cause of cancer deaths. It is estimated that 232,340 women will be diagnosed with breast cancer and 39,620 will die in 2013.1 Accumulating data from epidemiological and experimental studies have shown that reproductive hormones, particularly E2, play a significant role in breast cancer etiology. Breast carcinogenesis is highly interlinked with the high levels of E2 and its metabolism to catechols mediated by cytochrome P450 enzymes.2 Other phase I and phase II enzymes involved in the metabolic activation and deactivation include estrone sulfatase, sulfotransferases, catechol-O-methyltransferase, and uridine-5′-diphosphate glucuronosyltransferase.3 The biological effects of E2 and its metabolites are mediated by two distinct E2 receptors (ER-α and ER-β) by binding with varying affinities.4
MicroRNAs (miRNAs) are small noncoding RNAs, and their expressions have been correlated with specific breast cancer pathological features such as E2 and progesterone receptor expression, proliferation index, and tumor stage.5 In an independent study, the expression of 667 miRNAs in 29 breast tumor and 21 adjacent normal tissues demonstrate 130 miRNAs to have significant differential expressions in breast tumors when compared to the normal adjacent tissues.6 miRNA expression is suppressed or stimulated by E2 and other E2 receptor ligands in human breast cancer cells, rat mammary gland, and other E2 responsive organs such as the endometrium and uterus.7
There is growing evidence from in vitro and in vivo studies that consumption of fruits and vegetables is associated with reduced risk of developing cancer.8 Berries are rich in anthocyanins, which are considered to be a good candidate for preventing the development of cancer by protecting cells from the damage caused by reactive oxygen species.9 There are over 500 anthocyanins characterized, but a great majority of them contain a core structure of six anthocyanidins, namely, cyanidin, delphinidin, malvidin, pelargonidin, peonidin, and petunidin.10 Anthocyanins have mono-, di-, and tricyclic sugars attached to the anthocyanidin core structure and possess high antioxidant properties.11
Berries have received much attention lately due to their cancer chemopreventive potential. Blueberry (BB) is among the few fruits that contain five of the major anthocyanidins (cyanidin, delphinidin, malvidin, peonidin, and petunidin).12 Anthocyanins activate phase II enzymes and induce apoptosis as well as demonstrate antiproliferative, anti-inflammatory, and antiangiogenesis properties.13 In our recent study, an equimolar mixture of five major anthocyanidins was found to synergistically inhibit proliferation of two non-small-cell lung cancer cell lines both in cell culture and in vivo.14 In another study, we have demonstrated that dietary blueberry (Berkley) when provided in chemoprevention mode (5%, w/w) inhibited E2-mediated mammary tumorigenesis in ACI rats.15
In this study, we report on both chemopreventive and therapeutic potential of highbush blueberry powder (50:50 blend of Tifblue and Rubel). We also report the possible mechanism by which BB may inhibit mammary tumorigenesis.
Materials and Methods
Diet
Freeze-dried highbush whole blueberry powder (50:50 blend of Tifblue and Rubel) was received from the U.S. Highbush Blueberry Council (Folsom, CA, USA). This blend contains 38 mg/g total phenolics and 21 mg/g total anthocyanins. AIN-93 M diet and AIN-93 M diet supplemented with 2.5 and 5% BB were prepared in pellet form by Harlan-Teklad (Madison, WI, USA) and stored at 4 °C in vacuum-sealed bags until use. BB diet was customized to have the same calorific value as AIN-93 M.
Animal Study
Animal experiments were executed in agreement with an approved protocol from the Institutional Animal Care and Use Committee at the University of Louisville. Female ACI rats (5–6 weeks old) were purchased from Harlan Sprague–Dawley, Inc. (Indianapolis, IN, USA). After a week of acclimation, animals were randomized into seven groups (Table 1). Groups 1, 4, and 7 were provided with AIN-93 M diet, and groups 2 and 5 were fed a diet supplemented with BB (2.5% w/w). Groups 3 and 6 received diet supplemented with 5% BB. After 2 weeks of experimental diet intervention, groups 4–7 were implanted subcutaneously with a 1.2 cm silastic implant containing 9 mg of 17β-estradiol (E2) as described.16 Group 7 received control AIN-93 M diet for 14 weeks and then was changed to BB diet (5% w/w) after the appearance of first palpable tumor in control group. As the BB diet in group 7 started after the appearance of tumors, this was considered as therapeutic intervention. Body weight and diet consumption were recorded weekly until euthanasia. Starting from 12 weeks of E2 treatment, animals were palpated weekly for tumor incidence and multiplicity. When the palpable tumors reached >90% in group 4, animals in all groups were euthanized by CO2 asphyxiation. Blood was collected by cardiac puncture to isolate serum and plasma. Lung, liver, brain, mammary tissue, and pituitary gland were collected, weighed, and snap frozen for further analysis. Mammary tumor size was measured by a Vernier caliper. Small pieces of mammary tissue and pituitary gland were fixed in 10% formalin for histopathological analysis.
Table 1. Experimental Design.
| group | treatment | no. of animals |
|---|---|---|
| 1 | untreated | 8 |
| 2 | 2.5% BB diet | 5 |
| 3 | 5% BB diet | 5 |
| 4 | E2 + control (AIN-93 M diet) | 24 |
| 5 | E2 + 2.5% BB diet | 20 |
| 6 | E2 + 5% BB diet (preventive) | 20 |
| 7 | E2 + 5% BB diet (therapeutic) | 20 |
Histopathology
To assess the presence of microscopic tumors and pathological changes in the mammary glands of rats treated with E2 for 12 weeks, 5 μm mammary tissue sections (n = 21) from multiple previous studies were stained with hematoxylin and eosin and analyzed by a board-certified veterinary pathologist.
Immunohistochemistry
Tissue sections from five randomly selected animals from each group were stained for proliferating cell nuclear antigen (PCNA) using a PCNA staining kit (Life Technologies, Grand Island, NY, USA) following the manufacturer’s protocol. Five to six different sites in each slide were scored for deeply stained epithelial nuclei by two cytopathologists and represented as percent deeply stained cells.
Plasma Prolactin
Plasma prolactin levels were quantified using a Rat Prolactin ELISA Immuno-assay kit (Alpco, Salem, NH, USA) following the manufacturer’s instructions. Briefly, plasma samples (n = 5) from E2-treated groups were diluted 100- or 200-fold, and plasma from groups that did not receive E2 was diluted 2-fold prior to the analysis. The samples were added to a 96-well plate incubated with rat prolactin sample buffer followed by enzyme-labeled anti-rat prolactin antibody. Then samples were incubated with 3,3′,5,5′-tetramethylbenzidine (TMB) and substrate solution (provided in the kit) followed by measurement of the optical density at 450 nm immediately after the addition of stop solution. About 25 μL of diluted plasma samples was measured against various concentrations of calibrator samples ranging from 5 to 80 ng/mL of prolactin levels.
Western Blot Analysis
Western blot analysis was done as described elsewhere.14 Briefly, whole-cell lysates were prepared from mammary tissues using radioimmunoprecipitation assay (RIPA) lysis buffer (Santa Cruz Biotechnology Inc., Dallas, TX, USA), and protein concentrations were measured by using the bicinchoninic acid (BCA) method. Proteins were separated on MES-SDS-Bis-Tris 4–12% gradient gel (Invitrogen, Carlsbad, CA, USA). The proteins were transferred to PVDF membranes and probed for cyclin D1, PCNA, estrogen receptor-alpha (ER-α), c-myc, and progesterone receptor (PR). Equal loading of the protein was confirmed by β-actin.
Total RNA Isolation and qRT-PCR Analysis
Total RNA from frozen mammary tissues was isolated using Trizol reagent (Invitrogen). RNA concentrations were measured by NanoDrop (N2000c, NanoDrop Corp., Wilmington, DE, USA). qRT-PCR was performed with 100 ng of RNA using a qScript One-Step SYBR Green qRT-PCR Kit (Quanta Biosciences, Gaithersburg, MD, USA) with 500 pmol of forward and reverse primers for each gene. The genes and primer sequences are as follows: CYP1A1 (forward, 5′ GGGATATAGAAGCCATTCAGACTTG 3′; reverse, 5′ TGGAGACCTTCCGACATTCAT 3′); ER-α (forward, 5′ GGCACATGAGTAACAAAGGCA 3′; reverse, 5′ GGCATGAAGACGATGAGCAT 3′); 18S (forward, 5′ GGGAGGTAGTGACGAAAAATAACAAT 3′; reverse, 5′ TTGCCCTCCAATGGATCCT 3′). Quantitative PCR was carried out on a 7500 Fast-Real Time PCR instrument (Applied Biosystems, Green Island, NY, USA), following the manufacturer’s guideline. The gene expressions were calculated by relative quantification method (2ΔΔCt) comparing with control and normalizing to 18S RNA.
Small RNA Isolation/miRNA Analysis
Small RNAs (<200 bp) were isolated from mammary tissues using a mirVana microRNA isolation kit (Invitrogen) following the manufacturer’s instruction. The concentration of the small RNA was quantified by Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). qPCR analysis of miR-18a, miR20a, miR25, and miR-34c was performed using a TaqMan miRNA Reverse Transcription Kit and TaqMan gene-specific miRNA assays (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Relative changes in miRNA expressions were quantified by comparison with the control and normalization to 5S RNA.
Toxicity Analysis
Whole blood was collected at the time of euthanasia to analyze serum chemistry and hematological parameters, as described previously.17 Liver functions were analyzed by measuring the levels of aspartate transaminase, alanine aminotransferase, alkaline phosphatase, γ-glutamyl transpeptidase, and amylase in serum. Kidney functions were analyzed by measuring blood urea nitrogen, creatinine, and various electrolytes in serum. Various hematological parameters such as white blood cells (WBCs), red blood cells, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and platelet count were analyzed from whole blood. Five random samples from each group were analyzed.
Statistical Analysis
All statistical comparisons between controls and E2-treated groups were made using an unpaired one-tailed Student’s t test. Tumor latency was analyzed using a nonparametric log-rank test. Diet consumption was analyzed using an unpaired two-tailed Student’s t test, and body weight was analyzed by two-way ANOVA. All statistical analyses were done in GraphPad prism software, version 4.3 (San Diego, CA, USA). A p value of ≤0.05 was considered significant.
Results
Food Consumption and Body Weight
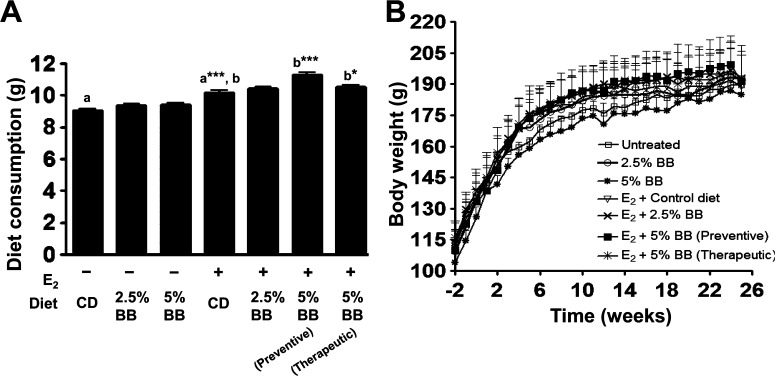
There was no significant difference in diet consumption among groups receiving either control diet or AIN-93 M diet supplemented with 2.5 and 5% BB, in the absence of E2 treatment. The diet consumption, however, increased in E2-treated animals fed control diet or BB-supplemented diets (Figure 1A). The increase was significant in animals receiving the 5% BB diet in both preventive and therapeutic mode; the increase was insignificant in the 2.5% BB diet group. At the end of the study (25 weeks), one-way ANOVA analysis showed no significant difference in body weights among the control and E2-treated animals (Figure 1B).
Figure 1.
Average diet consumption and body weight:. (A) Average diet consumption by ACI rat per day fed with AIN-93 M diet and diet supplemented with 2.5 and 5% BB in the presence and absence of E2. Values denote mean ± SD of diet consumption over a period of 25 weeks. Asterisk (a) indicates significant difference of E2 control from untreated control (p = 0.0001). Asterisk (b) indicates significant difference of BB diets from E2 control (p = 0.001 and 0.044). (B) Average body weight of rats receiving either AIN-93 M or BB diet. One-way ANOVA analysis showed that there was no significant difference in body weight after 25 weeks of E2 treatment.
Tumor Incidence, Multiplicity, and Volume
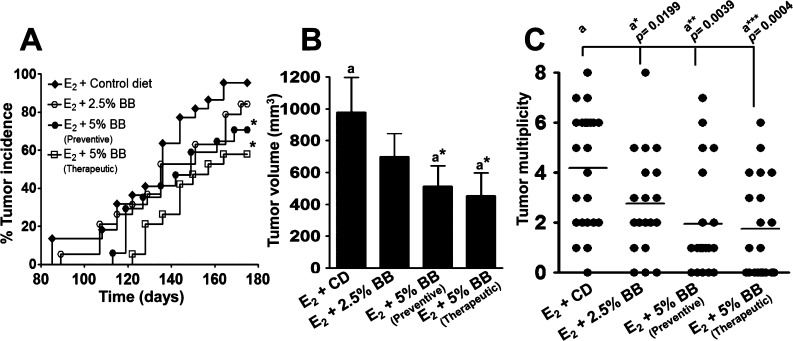
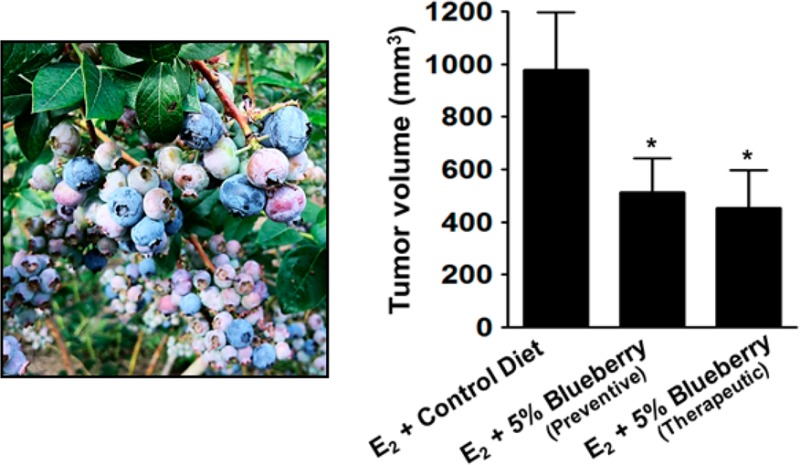
BB diet was highly effective in favorably modulating the various tumor indices (latency, incidence, multiplicity, and burden). BB diet dose dependently delayed the tumor latency. The groups receiving 5% BB diet in both the chemopreventive and therapeutic modes had a significant delay with the first tumor appearance in the control group by 28 and 37 days, respectively, as compared to the age-matched control animals (Figure 2A). The group that received 2.5% BB diet also had delayed tumor latency but only slightly, about 7 days. At the end of the study, the control group developed palpable mammary tumors in 96% animals, whereas animals provided with the 5% BB diet in chemopreventive and therapeutic groups had palpable tumors only in 60% (p = 0.0109) and 55% (p = 0.0021) of animals, respectively; the 2.5% BB diet also showed some (11%) but insignificant reduction in tumor incidence compared to the control group. Tumor volume and multiplicity were measured after the animals had been euthanized. Animals receiving control diet had an average tumor volume of 979 ± 218 mm3, whereas animals on the 5% BB diet had significant reductions, 512 ± 130 mm3 (p = 0.0398) and 452 ± 144 mm3 (p = 0.0106), for chemopreventive and therapeutic mode, respectively (Figure 2B). Animals on the 2.5% BB diet showed a modest but insignificant (0.1435) reduction with an average tumor volume of 698 ± 145 mm3. Tumor multiplicity in control animals was 4.17 ± 0.48 per animal, and it was reduced to 2.17 ± 0.52 (p = 0.0039) and 1.75 ± 0.45 (p = 0.0004) by the 5% BB diet given in chemopreventive and therapeutic mode, respectively (Figure 2C); the 2.5% BB diet also showed a significant reduction in tumor multiplicity, 2.75 ± 0.45 (p = 0.0199).
Figure 2.
Effect of blueberry diet on (A) tumor incidence, (B) tumor volume, and (C) tumor multiplicity. (A) Tumor incidence was calculated from the weekly palpation report and analyzed using a nonparametric log-rank test. Asterisk indicates significant difference of 5% BB diet [preventive (p = 0.0109) and therapeutic mode (p = 0.0021)] from E2-treated control. (B) Tumor volume and multiplicity were calculated at the time of euthanasia and analyzed using unpaired one-tailed Student’s t test. Asterisk indicates that the tumor volume of both 5% BB diets (p = 0.0398 and p = 0.0106) was significantly different from E2-treated control.
Liver, Mammary, and Pituitary Weights
At the end of the study, the liver, mammary, and pituitary tissue weights were not significantly different among the groups without E2 treatment. However, the differences were significant with E2 treatment. Mammary tissue weight with 5% BB diet in chemopreventive mode was modestly offset (p = 0.0486) from E2-treated control. There was a 4–5-fold increase in the pituitary weight in E2-treated animals; however, this was significantly offset with the 5% BB diet in preventive (33.9 ± 5.6 mg vs 42.2 ± 10.7 mg; p = 0.0031) and therapeutic (34.4 ± 5.3 mg; p = 0.0031) modes (Table 2). Pituitary weights in animals receiving the 2.5% BB diet also showed significant decrease (34.4 ± 8.0 mg; p = 0.0070).
Table 2. Comparison of Body Weight and Other Organ Weightsa between ACI Rats Fed Control Diet or Diet Supplemented with Blueberry.
| group | body weight (g) | liver (g) | mammary (g) | pituitary (mg) |
|---|---|---|---|---|
| untreated (n = 8) | 189.4 ± 6.8 | 4.9 ± 0.4 | 4.6 ± 0.9 | 11.2 ± 1.3 |
| 2.5% BB diet (n = 5) | 192.6 ± 15.9 | 5.2 ± 1.0 | 4.4 ± 0.9 | 12.2 ± 1.0 |
| 5% BB diet (n = 5) | 185.0 ± 6.6 | 4.8 ± 0.4 | 4.6 ± 0.4 | 9.8 ± 1.4 |
| E2 + control AIN 93 M diet (n = 24) | 189.0 ± 14.2 | 6.3b ± 0.4 | 6.4b ± 1.5 | 42.2b ± 10.7 |
| p value | 0.0001 | |||
| E2 + 2.5% BB diet (n = 18) | 192.3 ± 12.0 | 6.5 ± 0.6 | 5.7 ± 1.5 | 34.4c ± 8.0 |
| p value | 0.007 | |||
| E2 + 5% BB diet (preventive) (n = 18) | 191.2 ± 17.5 | 6.7 ± 0.8 | 5.6 ± 2.0 | 33.9c ± 5.6 |
| p value | 0.0486 | 0.0031 | ||
| E2 + 5% BB diet (therapeutic) (n = 18) | 192.4 ± 12.0 | 6.5 ± 0.5 | 5.9 ± 1.2 | 34.4c ± 5.3 |
| p value | 0.0031 |
Body weight and organ weight values are expressed as mean ± SD.
Values that are significantly higher than untreated control.
Values that are significantly higher than E2-treated animals. Average body weight of animals is measured at the time of euthanasia.
Histopathology
In all animals that were treated with E2 for 12 weeks (n = 21), there was expansion of mammary tissue with proliferation of terminal buds that differentiated into acini. The acinar epithelial cells were frequently vacuolated, and the acini contained amphophilic inspissated secretory material filling the lumina (Figure 3A). Concomitantly, there was proliferation of ductules and ducts with variable grades of epithelial dysplasia (n = 13). The ductular epithelial cells were cuboidal with distinct cell borders and moderate amounts of eosinophilic cytoplasm. Often (n = 4), the nuclei showed karyomegaly and were hyperchromatic, containing one to three distinct nucleoli (Figure 3B). Mitotic figures were not present in the sections examined. In >50% of cases (n = 11), the ducts were ectatic and lined by hyperplastic epithelium that formed two or more layers (Figure 3A). Ductular adenomas (n = 3) were characterized by the development of a 100–250 μm long papillary projection from the ductular walls that narrowed the lumina (Figure 3A, inset). The neoplastic cells contained scant cytoplasm and round nuclei with one to three distinct nucleoli. In all cases, desquamated epithelial cells were present within the ductular lumina. Prominent periductular fibrosis was a feature of all adenomas. Multifocal perivascular mast cell infiltration correlated with the severity of the lesion in all cases. In addition, the ductular epithelial dysplasia correlated with the size of expansion of the terminal bud.
Figure 3.
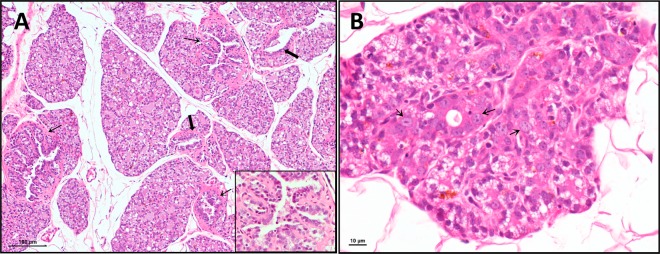
Presence of epithelial dysplasia and ductular papillomas from mammary tissue of rats treated with E2 implant for 12 weeks. (A) Image of mammary section at 40× magnification showing long papillary projections from ductular walls (thin arrows) and ducts lined with hyperplastic epithelium with two or more layers (bold arrows). Inset shows 400× magnification of papillary projections. (B) Mammary section at 600× magnification with nuclei showing karyomegaly, which are hyperchromatic containing one to three distinct nucleoli (thin arrows).
Cell Proliferation Index
Cell proliferation was analyzed by staining tumor and adjacent mammary tissue for PCNA protein markers using antibody-based assay. After 25 weeks, there was no significant difference in cell proliferation among animals receiving either control or 2.5 and 5% BB diets. However, the E2 treatment increased the cell proliferation to nearly 6-fold (p = 0.0095) (Figure 4). This increase in the cell proliferation was significantly offset by 34 and 35% with 5% BB diet administered in chemopreventive and therapeutic mode, respectively (Figure 4); the effect was insignificant with the 2.5% BB diet.
Figure 4.
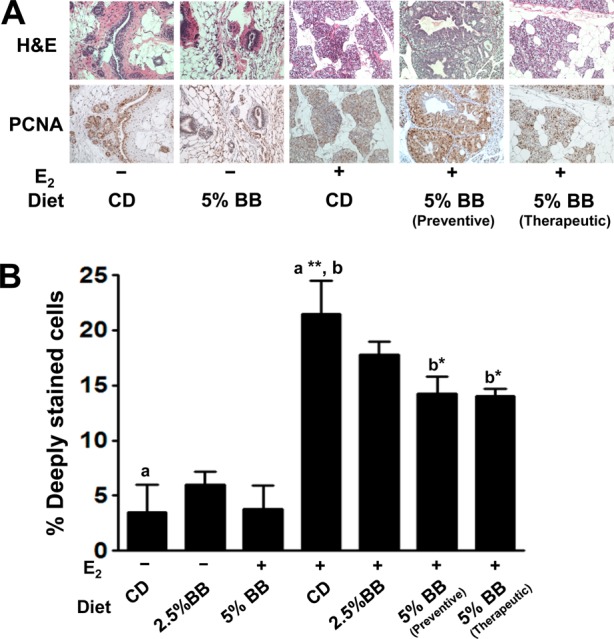
Proliferative index and effect of blueberry diet with or without estrogen (E2) treatment. (A) Immunohistochemical staining for proliferating cell nuclear antigen (PCNA). Representative photomicrographs are 20× magnification of normal and hyperplastic mammary tissue. (B) Graph denotes the percentage of deeply stained cells for PCNA in mammary tissues (n = 5). a Statistically significant from untreated control (p = 0.0095). b Statistically significant from E2-treated control (p = 0.0373 and p = 0.0251).
Plasma Prolactin
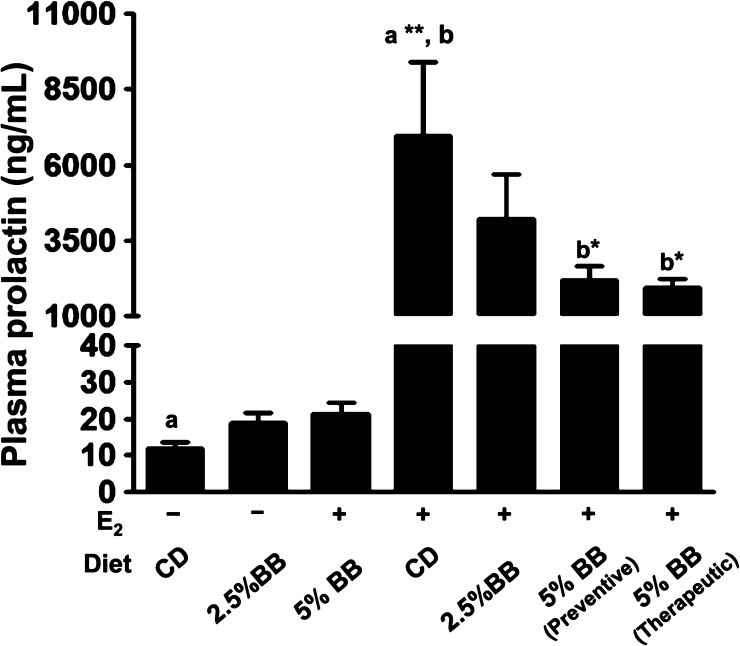
No significant difference was observed in the levels of plasma prolactin between untreated groups and BB diet groups. On the other hand, there was a substantial increase (p = 0.0035) in prolactin levels with E2 treatment (6973 ± 2443 ng/mL) compared to untreated control (11.8 ± 1.8 ng/mL). After 25 weeks, animals provided with 5% BB diet in chemopreventive (2205 ± 438 ng/mL; p = 0.05) and therapeutic mode (1950 ± 285 ng/mL; p = 0.01) demonstrated significant reduction in the elevated levels of plasma prolactin (Figure 5). A modest but insignificant reduction in prolactin levels (4193 ± 1509 ng/mL) was also observed with the 2.5% BB diet.
Figure 5.
Circulating plasma prolactin levels among rats receiving either control diet or diet supplemented with 2.5 and 5% BB. Values represent the average of six samples ± SE done in duplicates. a Statistically significant from untreated control (p = 0.0035). b Statistically significant from E2-treated control (∗, p = 0.05; ∗∗, p = 0.01).
Modulation of Estrogen-Specific Genes
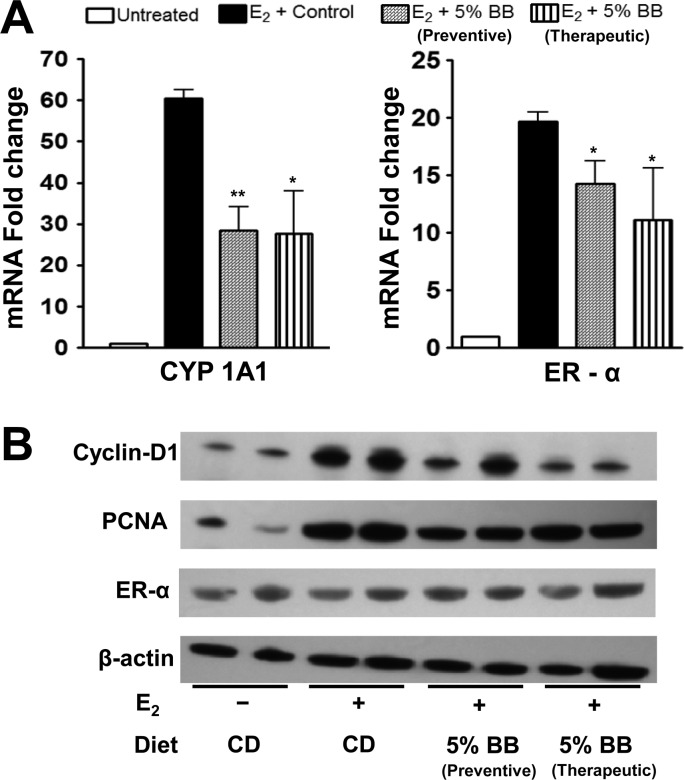
There was a pronounced effect of E2 on the expressions of cytochrome CYP 1A1 and ER-α at the mRNA levels. All groups were compared against the untreated control, and the fold change was expressed as mean ± standard error. E2 treatment up-regulated the CYP 1A1 mRNA levels by 60-fold. The 5% BB intervention provided in chemopreventive and chemotherapeutic modes both significantly offset the E2-associated increase in CYP1A1 to 28.3 ± 6-fold (p = 0.0011) and 27.6 ± 10.3-fold (p = 0.0102), respectively. Similarly, the mRNA expression level of ER-α in E2-treated animals was up-regulated by 19.7 ± 2-fold compared to untreated control, and this increase was also significantly offset by the 5% BB diet, chemopreventive mode (14.2 ± 2-fold; p = 0.0201) and therapeutic mode (11.1 ± 4-fold; p = 0.05) (Figure 6A). Western blot analysis showed that E2 treatment up-regulated both cyclin D1 and PCNA. Intervention of 5% BB diet in both preventive and therapeutic modes down-regulated cyclin D1 and PCNA at protein levels. However, ER-α protein level was not modulated with either E2 treatment or administration of BB diet.
Figure 6.
(A) mRNA expression of CYP1A1 and ER-α in mammary tissues. The total RNA isolated from the mammary tissue was analyzed by qPCR. Data represent the average of four animals ± SE. Asterisk represents significant difference from E2-treated control (p = 0.0011 and 0.01). (B) Protein expression of cyclin D1, PCNA, and ER-α in mammary tissue. The total cellular lysate isolated from the mammary tissues was analyzed by Western blot.
Modulation of Estradiol-Specific miRNAs
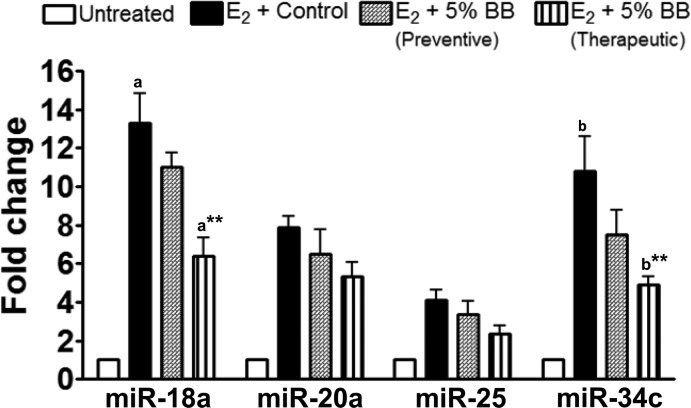
We analyzed four E2-specific miRNAs (miR-18a, -20a, -25, and -34c) on the basis of our previous study in this animal model18 to determine the effect of BB treatment on their modulation. All four miRNAs were significantly up-regulated under the influence of E2 intervention in agreement with our previous findings. miR-18a and miR-34c were up-regulated about 13- and 11-fold, respectively, compared to untreated control. The effect of E2 on miR-18a and miR-34c was reduced to 6.4-fold (p = 0.0013) and 4.9-fold (p = 0.0033), respectively, with the 5% BB diet in therapeutic mode (Figure 7). Berry diet given in chemopreventive mode also offset these two elevated miRNAs, but the effect was not as pronounced. There was no significant effect of BB treatment on the up-regulated miR-20a and miR-25 expression levels.
Figure 7.
Expression of miRNAs 18a and 34c in mammary tissues. The small RNA was isolated by mirVana microRNA kit and quantified by Bioanalyzer. qPCR analysis was performed using a TaqMan microRNA Reverse Triscription Kit and TaqMan gene-specific MicroRNA assays. Graph represents the average of four rats ± SE done in duplicates. Asterisk indicates significant difference from E2-treated control (p = 0.0013 and 0.0033).
Effect of Dietary Blueberry on Serum Chemistry and Hematological Parameters
Analysis of serum for liver enzymes such as aspartate transaminase, alanine aminotransferase, alkaline phosphatase, γ-glutamyl transpeptidase, amylase, and lipase indicated that they were within the normal range, and no difference was found with E2 or BB diet or in combination compared to control, suggesting that berry diets had no toxic effects (Table 3). Serum was analyzed for plasma proteins (albumin and globulin), glucose, cholesterol, and triglycerides (Table 3). Analysis of blood urea nitrogen, creatinine, and various electrolytes showed no significant changes, indicating normal kidney functions with BB treatment (Table 3). Whole blood from all of the groups was analyzed for hematological parameters such as white blood cells, red blood cells, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and platelets. No difference in any of the hematological parameters was found in any group compared to aged-matched control animals (Table 3). The levels of neutrophils and lymphocytes measured in whole blood showed no significant difference among all groups (Table 3).
Table 3. Serum and Hematological Parameters for Assessment of Toxicity and Tolerance of Blueberry Diet.
| E2 + 5% BB diet |
|||||
|---|---|---|---|---|---|
| untreated | 5% BB diet | E2 + control AIN-93 M diet | preventive | therapeutic | |
| serum enzymes | |||||
| AST (SGOT) | 342 ± 130 | 276 ± 80 | 236 ± 31 | 217 ± 51 | 263 ± 49 |
| ALT (SGPT) | 56 ± 9 | 38 ± 5 | 45 ± 10 | 49 ± 7 | 48 ± 11 |
| alk phosphatase | 31 ± 9 | 41 ± 8 | 33 ± 17 | 32 ± 9 | 20 ± 6 |
| GGT | 8 ± 2 | 3 ± 0 | 5 ± 2 | 4 ± 2 | 4 ± 1 |
| amylase | 552 ± 91 | 585 ± 40 | 548 ± 72 | 585 ± 130 | 630 ± 36 |
| lipase | 25 ± 0 | 50 ± 25 | 25 ± 0 | 26 ± 1 | 25 ± 0 |
| plasma proteins | |||||
| albumin (g/dL) | 4 ± 0.1 | 4 ± 0.3 | 4 ± 0.1 | 4 ± 0.4 | 5 ± 0.1 |
| globulin (g/dL) | 3 ± 0.5 | 3 ± 0.3 | 4 ± 0.3 | 4 ± 0.2 | 4 ± 0.3 |
| glucose (mg/dL) | 122 ± 36 | 139 ± 46 | 90 ± 30 | 118 ± 33 | NDa |
| cholesterol (mg/dL) | 118 ± 14 | 98 ± 10 | 131 ± 16 | 124 ± 20 | 151 ± 13 |
| triglycerides (mg/dL) | 103 ± 27 | 108 ± 20 | 91 ± 23 | 99 ± 28 | 119 ± 56 |
| kidney function | |||||
| BUN (mg/dL) | 24 ± 10 | 21 ± 3 | 21 ± 4 | 20 ± 4 | 24 ± 2 |
| creatinine (mg/dL) | 0.4 ± 0.1 | 0.5 ± 0 | 0.44 ± 0.1 | 0.44 ± 0.1 | 0.48 ± 0.1 |
| phosphorus (mg/dL) | 16 ± 6 | 19 ± 2 | 13 ± 4 | 13 ± 2 | 17 ± 4 |
| calcium (mg/dL) | 11 ± 1 | 12 ± 1 | 12 ± 0.3 | 12 ± 0.3 | 12 ± 0.4 |
| magnesium (mequiv/dL) | 4 ± 0 | 4 ± 0.3 | 3 ± 0.3 | 3 ± 0.3 | 4 ± 0.2 |
| sodium (mequiv/dL) | 146 ± 5 | 147 ± 4 | 144 ± 1 | 141 ± 2 | 141 ± 2 |
| potassium (mequiv/dL) | 8 ± 4 | 9 ± 1 | 8 ± 0.9 | 7 ± 0.3 | 12 ± 2 |
| chloride (mequiv/dL) | 81 ± 42 | 100 ± 2 | 98 ± 3 | 97 ± 2 | 96 ± 2 |
| hematological parameters | |||||
| WBC (103/μL) | 5 ± 2 | 4 ± 0.3 | 5 ± 2 | 6 ± 3 | 4 ± 2 |
| RBC (106/μL) | 8 ± 0.1 | 8 ± 0.5 | 6 ± 1 | 6 ± 2 | 7 ± 0.4 |
| HGB (g/dL) | 13 ± 0.3 | 13 ± 1 | 9 ± 4 | 10 ± 2 | 11 ± 1 |
| HCT (%) | 44 ± 3 | 46 ± 2 | 35 ± 3 | 33 ± 7 | 38 ± 4 |
| MCV (fL) | 55 ± 3 | 55 ± 3 | 58 ± 4 | 55 ± 3 | 55 ± 3 |
| MCH (pg) | 17 ± 0.3 | 16 ± 0.3 | 17 ± 1 | 16 ± 1 | 20 ± 8 |
| MCHC (g/dL) | 30 ± 2 | 29 ± 2 | 29 ± 1 | 30 ± 1 | 30 ± 2 |
| platelet count (103/μL) | 854 ± 100 | 807 ± 334 | 872 ± 140 | 648 ± 216 | 863 ± 61 |
| neutrophils | 634 ± 287 | 684 ± 203 | 2106 ± 1608 | 2229 ± 1394 | 1398 ± 581 |
| lymphocytes | 4222 ± 1882 | 3475 ± 666 | 2347 ± 675 | 3102 ± 1628 | 2458 ± 1078 |
ND, not determined.
Discussion
Cancer chemopreventive agents are either natural products or their synthetic analogues, which inhibit the transformation of normal cells to premalignant cells or the progression of premalignant cells to malignant cells.19 It is believed that chemopreventive agents can inhibit oncogenic pathways, inhibit growth, and induce apoptosis in cancer cells.20 Conventional chemopreventive agents are administered long-term and believed to block or delay the progression of transformed cells by modulating cell proliferation or differentiation.21 They also prevent activation of carcinogen, trap carcinogen before reaching the active site, and enhance detoxification systems.21 On the other hand, standard therapeutic drugs are toxic to both cancer and normal cells, which results in therapeutic activity and side effects to normal cells. Cytotoxicity to normal cells may lead to secondary tumors.20
This study was conducted to test both the chemopreventive and therapeutic potential of Tifblue and Rubel blueberry blend against E2-mediated mammary tumorigenesis. We had previously reported on the chemopreventive effect of Berkley blueberry on mammary tumorigenesis.15,22 This is the first study of its kind in which the therapeutic potential of blueberry has been shown. We also confirmed the microscopic tumors and precancerous conditions by histological evaluation of mammary gland sections from rats treated and necropsied at 12 weeks of E2 treatment. Variable ductular epithelial changes including dysplasia, hyperplasia, and adenoma formation were found, suggesting various stages of carcinogenesis. In addition, periductular fibroplasia as well as mast cell infiltration positively correlated with degree of dysplasia.
Similar to our earlier studies15,22 this Tifblue–Rubel blueberry blend at 2.5 and 5% given in the chemopreventive mode was highly effective in increasing the tumor latency by 7 and 28 days, respectively. The administration of 2.5% BB diet showed significant reduction in tumor multiplicity and pituitary weight. In our earlier studies, 2.5% BB diet made from the mixture of three blueberries was not effective against E2 treatment; this could be because the E2 implant size was 3 cm long containing 27 mg of E2.22 In another study with a similar 9 mg E2 implant, a 2.5% diet showed protective effects that were in the same vicinity as reported by us previously.23 In both studies, the 2.5% BB diet had only modest effect and did not significantly reduce the tumor volume, tumor latency, and molecular markers investigated. The higher dose of BB (5%) administered in chemopreventive mode was highly protective in reducing tumor volume and tumor multiplicity and increased tumor latency to 28 days, in agreement with our previous findings.15 However, the protective effect of blueberry was slackened after 20 weeks of E2 treatment, likely due to the exponential growth rate of the cells to reach the malignant stage. For the first time BB diet was administered after 12 weeks of E2 treatment to determine the therapeutic effect against precancerous and microscopic adenomas. When compared with animals that received 5% BB diet in chemopreventive mode, the therapeutic group showed a significantly enhanced effect by reducing tumor volume and multiplicity and increasing the tumor latency to 37 days, that is, 9 days more than the preventive groups. Even when the BB diet was initiated after 14 weeks of E2 treatment, the therapeutic group elicited protective effects. The therapeutic potential of BB anthocyanidins has been shown by others against human prostate cancer24 and by us against lung cancer xenograft.14 However, there is no study for direct comparison in E2-induced mammary tumorigenesis. Interestingly, the present data also suggest that BB diet can be started in the postinitiation stage of tumorigenesis and still elicit a response similar to that of chemopreventive mode.
Bioavailability of anthocyanins in vivo is crucial for their biological effects. Anthocyanins are absorbed intact in the glycosidic form.25,26 They are rapidly absorbed from the stomach and jejunum.27,28 Yet >50% of the ingested anthocyanins are found in the cecum,29 implying poor absorption. Anthocyanin structure exists as a flavilium cation in acidic conditions, which is stable, but converts to unstable quinoidal or carbinol structure at neutral or alkaline conditions.30 However, it has also been shown that anthocyanins are stabilized by flavanols and ascorbic acid present in the food matrix.31,32 This may explain the higher bioavailability of anthocyanins when given as a whole fruit powder relative to individual anthocyanins,33 indicating strong synergism occurs in absorption between coexisting molecules in fruit. Furthermore, in a study with healthy subjects with ileostomy, nearly 40% of anthocyanins were recovered in the ileal fluid, indicating that despite the difference in pH, the anthocyanins were stable and large amounts will pass from the small intestine to the large intestine.34 Yet, all of the bioavailability studies were done with bolus doses of pure anthocyanins or fruit powder/extracts.35 Anthocyanins were not detected in the plasma of animals fed a diet mixed with sources rich in anthocyanins or the pure compound.29 However, lack of detection of anthocyanins in the plasma does not indicate lack of tissue bioavailability. When anthocyanins were delivered intravenously, a biphasic decay of plasma levels was observed, indicating tissue distribution.33 In a parallel publication in this issue, we have discussed in detail the bioavailability of anthocyanins and its levels in lung tissue.12 We have demonstrated the presence of all five anthocyanidins, delphinidin, cyanidin, peonidin, petunidin, and malvidin, in lung tissues. Thus, anthocyanins are bioavailable and bioaccumulate in non-gastrointestinal tissues.
In agreement with our previous studies, the effect of E2 was reflected in the body, liver, mammary, and pituitary weights of the animals.15 The transitory increase in body weight and organ weight is majorly due to the E2 and may not be due to the diet consumption. Moreover, control AIN-93 M diet and diets supplemented with 2.5 and 5% BB were isocaloric. Although there was no effect of BB diet on body weight gain and organ weights, it was found to significantly offset E2-associated elevation in the weight of pituitary tumors. The pituitary weight was affected due to the increase in plasma prolactin levels induced by E2, resulting in pituitary prolactinomas.36 However, the E2-mediated mammary tumor model was well studied and standardized to reduce the side effect to the pituitary gland.16 Earlier studies have shown that prolonged supply of E2 increases the production of prolactin in E2-sensitive rat strains.37,38 High circulatory levels of prolactin in serum are one of the major reasons for animals to develop E2-induced mammary tumors.39 Berry diet at 2.5 and 5% significantly offset the prolactin levels elevated by E2.
E2 also induces the production of phase I enzyme, CYP1A1. It catalyzes the conversion of E2 into its metabolites (2E2 and 4E2), which further undergo redox cycle leading to the formation of reactive oxygen species.40 In this study, we showed that BB diet significantly reduced the expression of CYP1A1, suggesting its protective role against mammary carcinogenesis. Studies have shown that cyclin D1 and E2 receptors have important roles in regulating proliferation of breast epithelial cells.41,42 It is well established that E2 via ER-α elicits rapid signals driving cancer cells to proliferation. This biological effect of E2-mediated by ER-α was reduced significantly in 5% BB diet provided in therapeutic mode. Regulation of cyclin D1 gene expression has been associated with changes in the proliferation rate of breast cancer cells.43 Induction of cyclin D1 expression occurs rapidly and is a critical feature of the mitogenic action of E2. The expression of cyclin D1 was down-regulated by 2–2.6-fold with 5% BB diet in chemopreventive and therapeutic modes, respectively. Investigation of PCNA in nuclear proteins revealed that dietary BB reduced the expression levels significantly. PCNA was shown to interact with ER-α both in the absence and in the presence of DNA, to enhance the interaction of ER-α with ERE-containing DNA, and associate with endogenous E2-responsive genes.44 These findings clearly indicate that BB diet could modulate major E2-associated proliferation markers that lead to protective effects against mammary tumorigenesis in animals treated with E2.
Another interesting finding in this study is from the analysis of miRNAs related to the ER-α family. Aberrant miRNA expression is implicated in E2-related breast, uterine, and ovarian cancer development and progression.7 Hormones play a crucial role in regulating miRNAs by both genomic (transcriptional) and nongenomic mechanisms.45 Lorio et al. identified miRNAs having expression correlated with specific breast cancer biopathologic features, such as E2 and progesterone receptor expression, tumor stage, vascular invasion, or proliferation index.5 Targeting specific miRNAs for inhibiting specific stages of a cancer46 is being explored. Several miRNAs that regulate ER are involved in synchronized feedback mechanisms as a component of ER activation. Many of these ER-regulating miRNAs therefore are either E2-inducible (miR-18a and let-7) or subject to E2-mediated repression (miR-206, miR-221/222, and miR-145).47 This laboratory has recently identified miRNAs that modulated under the influence of E2 in this animal model and report aberrant changes in miRNA expression profile as early as 3 weeks of E2 treatment.48 We chose four miRNAs, miR-18a, -20a, -25, and -34c, on the basis of our previous study where these miRNAs were up-regulated with E2 to understand the influence of BB diet on their modulation. Increases in miR-18a and miR-20a mature forms were observed after E2 stimulation.18 In this study, BB diet administered in therapeutic mode significantly reduced miR-18a and miR-34c, whereas chemopreventive mode showed modest but significant reduction. These findings provide initial insights of BB diet in decreasing the E2-induced miRNA levels. However, the BB diet failed to modulate miR-20a and -25 expression levels, which remain the subject of further investigation.
In the chemopreventive mode, the rats are fed BB diet as young as 6–7 weeks old. The relative enhanced effect observed with the therapeutic approach could be due to the adaptive response of the rats to the BB phytochemicals in the chemopreventive mode. For example, the CYP1A1 mRNA level in mammary tissue was highest at 3 weeks after E2 implant and gradually decreased with time.15,23 The effect of the BB phytochemicals was more pronounced in the inhibition of conversion of normal cells to precancerous or precancerous frank tumors, but were not as effective once the tumors reached the exponential growth rate. Thus, by the time the mammary cells started exhibiting precancerous conditions, the efficacy was better when BB phytochemicals were introduced into the system than when they were already in the circulation. Nevertheless, both approaches were effective in delaying the onset and growth of palpable tumors.
In summary, this is the first demonstration indicating that BB diet is highly protective even in preinitiated E2-mediated mammary tumors in ACI rats through its effects by modulating cell proliferation and molecular targets. Thus, the consumption of BB can be used as an effective strategy for the treatment of E2-associated breast cancer and possible prevention of its relapse and metastasis. Our study demonstrates the chemopreventive and therapeutic potential of blueberry and advances our understanding of the working mechanisms in search of potential future drugs.
Acknowledgments
We thankfully acknowledge Dr. Anil Poudel for imaging histopathological slides.
This work was supported by the Highbush Blueberry Council, California, USPHS Grants CA-118114 and CA-125152, and the Agnes Brown Duggan Endowment. R.C.G. holds the Agnes Brown Duggan Chair in Oncological Research.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Howlader N.; Noone A. M. Krapcho M.; Garshell J.; Neyman N.; Altekruse S. F.; Kosary C. L.; Yu M.; Ruhl J.; Tatalovich Z.; Cho H.; Mariotto A.; Lewis D. R.; Chen H. S.; Feuer E. J.; Cronin K. A.. SEER Cancer Statistics Review, 1975–2010; National Cancer Institute: Bethesda, MD, 2013. [Google Scholar]
- Yager J. D.; Davidson N. E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006, 354, 270–282. [DOI] [PubMed] [Google Scholar]
- Lakhani N. J.; Venitz J.; Figg W. D.; Sparreboom A. Pharmacogenetics of estrogen metabolism and transport in relation to cancer. Curr. Drug Metab. 2003, 4, 505–513. [DOI] [PubMed] [Google Scholar]
- Bhavnani B. R.; Tam S. P.; Lu X. Structure activity relationships and differential interactions and functional activity of various equine estrogens mediated via estrogen receptors (ERs) ERalpha and ERbeta. Endocrinology 2008, 149, 4857–4870. [DOI] [PubMed] [Google Scholar]
- Iorio M. V.; Ferracin M.; Liu C. G.; Veronese A.; Spizzo R.; Sabbioni S.; Magri E.; Pedriali M.; Fabbri M.; Campiglio M.; Menard S.; Palazzo J. P.; Rosenberg A.; Musiani P.; Volinia S.; Nenci I.; Calin G. A.; Querzoli P.; Negrini M.; Croce C. M. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005, 65, 7065–7070. [DOI] [PubMed] [Google Scholar]
- Romero-Cordoba S.; Rodriguez-Cuevas S.; Rebollar-Vega R.; Quintanar-Jurado V.; Maffuz-Aziz A.; Jimenez-Sanchez G.; Bautista-Pina V.; Arellano-Llamas R.; Hidalgo-Miranda A. Identification and pathway analysis of microRNAs with no previous involvement in breast cancer. PLoS One 2012, 7, e31904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge C. M. miRNAs and estrogen action. Trends Endocrinol. Metab. 2012, 23, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke D.; Steward W. P.; Gescher A. J.; Marczylo T. Anthocyans from fruits and vegetables – does bright colour signal cancer chemopreventive activity?. Eur. J. Cancer 2005, 41, 1931–1940. [DOI] [PubMed] [Google Scholar]
- Devasagayam T. P.; Tilak J. C.; Boloor K. K.; Sane K. S.; Ghaskadbi S. S.; Lele R. D. Free radicals and antioxidants in human health: current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [PubMed] [Google Scholar]
- Mazza G.; Cacace J. E.; Kay C. D. Methods of analysis for anthocyanins in plants and biological fluids. J. AOAC Int. 2004, 87, 129–145. [PubMed] [Google Scholar]
- He J.; Giusti M. M. Anthocyanins: natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [DOI] [PubMed] [Google Scholar]
- Aqil F.; Vadhanam M. V.; Jeyabalan J.; Cai J.; Singh I. P.; Gupta R. C.. Detection of anthocyanins/anthocyanidins in animal tissues. J. Agric. Food Chem. 2013, DOI: 10.1021/jf500467b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. S.; Stoner G. D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausar H.; Jeyabalan J.; Aqil F.; Chabba D.; Sidana J.; Singh I. P.; Gupta R. C. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett. 2012, 325, 54–62. [DOI] [PubMed] [Google Scholar]
- Ravoori S.; Vadhanam M. V.; Aqil F.; Gupta R. C. Inhibition of estrogen-mediated mammary tumorigenesis by blueberry and black raspberry. J. Agric. Food Chem. 2012, 60, 5547–5555. [DOI] [PubMed] [Google Scholar]
- Ravoori S.; Vadhanam M. V.; Sahoo S.; Srinivasan C.; Gupta R. C. Mammary tumor induction in ACI rats exposed to low levels of 17beta-estradiol. Int. J. Oncol. 2007, 31, 113–120. [PubMed] [Google Scholar]
- Bansal S. S.; Vadhanam M. V.; Gupta R. C. Development and in vitro-in vivo evaluation of polymeric implants for continuous systemic delivery of curcumin. Pharm. Res. 2011, 28, 1121–1130. [DOI] [PubMed] [Google Scholar]
- Castellano L.; Giamas G.; Jacob J.; Coombes R. C.; Lucchesi W.; Thiruchelvam P.; Barton G.; Jiao L. R.; Wait R.; Waxman J.; Hannon G. J.; Stebbing J. The estrogen receptor-alpha-induced microRNA signature regulates itself and its transcriptional response. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 15732–15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. Y.; Hail N. Jr.; Lotan R. Apoptosis as a novel target for cancer chemoprevention. J. Natl. Cancer Inst. 2004, 96, 662–672. [DOI] [PubMed] [Google Scholar]
- Blagosklonny M. V. Carcinogenesis, cancer therapy and chemoprevention. Cell Death Differ. 2005, 12, 592–602. [DOI] [PubMed] [Google Scholar]
- Wattenberg L. W. What are the critical attributes for cancer chemopreventive agents?. Ann. N.Y. Acad. Sci. 1995, 768, 73–81. [DOI] [PubMed] [Google Scholar]
- Aiyer H. S.; Srinivasan C.; Gupta R. C. Dietary berries and ellagic acid diminish estrogen-mediated mammary tumorigenesis in ACI rats. Nutr. Cancer 2008, 60, 227–234. [DOI] [PubMed] [Google Scholar]
- Aiyer H. S.; Gupta R. C. Berries and ellagic acid prevent estrogen-induced mammary tumorigenesis by modulating enzymes of estrogen metabolism. Cancer Prev. Res. 2010, 3, 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeez B. B.; Siddiqui I. A.; Asim M.; Malik A.; Afaq F.; Adhami V. M.; Saleem M.; Din M.; Mukhtar H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-kappaB signaling. Cancer Res. 2008, 68, 8564–8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H.; Inaba H.; Kishi M.; Tominaga S.; Hirayama M.; Tsuda T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J. Agric. Food Chem. 2001, 49, 1546–1551. [DOI] [PubMed] [Google Scholar]
- Miyazawa T.; Nakagawa K.; Kudo M.; Muraishi K.; Someya K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3,5-diglucoside, into rats and humans. J. Agric. Food Chem. 1999, 47, 1083–1091. [DOI] [PubMed] [Google Scholar]
- Matuschek M. C.; Hendriks W. H.; McGhie T. K.; Reynolds G. W. The jejunum is the main site of absorption for anthocyanins in mice. J. Nutr. Biochem. 2006, 17, 31–36. [DOI] [PubMed] [Google Scholar]
- Talavera S.; Felgines C.; Texier O.; Besson C.; Lamaison J. L.; Remesy C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J. Nutr. 2003, 133, 4178–4182. [DOI] [PubMed] [Google Scholar]
- Talavera S.; Felgines C.; Texier O.; Besson C.; Mazur A.; Lamaison J. L.; Remesy C. Bioavailability of a bilberry anthocyanin extract and its impact on plasma antioxidant capacity in rats. J. Sci. Food Agric. 2006, 86, 90–97. [Google Scholar]
- Brouillard R.Chemical structure of anthocyanins. In Anthocyanins as Food Colours; Markakis P., Ed.; Academic Press: New York, 1982; pp 1–40. [Google Scholar]
- Ozkan M. Degradation of anthocyanins in sour cherry and pomegranate juices by hydrogen peroxide in the presence of added ascorbic acid. Food Chem. 2002, 78, 499–504. [Google Scholar]
- Rossetto M.; Vanzani P.; Mattivi F.; Lunelli M.; Scarpa M.; Rigo A. Synergistic antioxidant effect of catechin and malvidin 3-glucoside on free radical-initiated peroxidation of linoleic acid in micelles. Arch. Biochem. Biophys. 2002, 408, 239–245. [DOI] [PubMed] [Google Scholar]
- Ichiyanagi T.; Shida Y.; Rahman M. M.; Hatano Y.; Konishi T. Bioavailability and tissue distribution of anthocyanins in bilberry (Vaccinium myrtillus L.) extract in rats. J. Agric. Food Chem. 2006, 54, 6578–6587. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Barrio R.; Borges G.; Mullen W.; Crozier A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J. Agric. Food Chem. 2010, 58, 3933–3939. [DOI] [PubMed] [Google Scholar]
- McGhie T. K.; Walton M. C. The bioavailability and absorption of anthocyanins: towards a better understanding. Mol. Nutr. Food Res. 2007, 51, 702–713. [DOI] [PubMed] [Google Scholar]
- Stone J. P.; Holtzman S.; Shellabarger C. J. Neoplastic responses and correlated plasma prolactin levels in diethylstilbestrol-treated ACI and Sprague-Dawley rats. Cancer Res. 1979, 39, 773–778. [PubMed] [Google Scholar]
- Wiklund J.; Rutledge J.; Gorski J. A genetic model for the inheritance of pituitary tumor susceptibility in F344 rats. Endocrinology 1981, 109, 1708–1714. [DOI] [PubMed] [Google Scholar]
- Wiklund J.; Wertz N.; Gorski J. A comparison of estrogen effects on uterine and pituitary growth and prolactin synthesis in F344 and Holtzman rats. Endocrinology 1981, 109, 1700–1707. [DOI] [PubMed] [Google Scholar]
- Blankenstein M. A.; Broerse J. J.; van Zwieten M. J.; van der Molen H. J. Prolactin concentration in plasma and susceptibility to mammary tumors in female rats from different strains treated chronically with estradiol-17 beta. Breast Cancer Res. Treat. 1984, 4, 137–141. [DOI] [PubMed] [Google Scholar]
- Liehr J. G. Is estradiol a genotoxic mutagenic carcinogen?. Endocr. Rev. 2000, 21, 40–54. [DOI] [PubMed] [Google Scholar]
- Zwijsen R. M.; Wientjens E.; Klompmaker R.; van der Sman J.; Bernards R.; Michalides R. J. CDK-independent activation of estrogen receptor by cyclin D1. Cell 1997, 88, 405–415. [DOI] [PubMed] [Google Scholar]
- Yu Q.; Geng Y.; Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature 2001, 411, 1017–1021. [DOI] [PubMed] [Google Scholar]
- Castro-Rivera E.; Samudio I.; Safe S. Estrogen regulation of cyclin D1 gene expression in ZR-75 breast cancer cells involves multiple enhancer elements. J. Biol. Chem. 2001, 276, 30853–30861. [DOI] [PubMed] [Google Scholar]
- Schultz-Norton J. R.; Gabisi V. A.; Ziegler Y. S.; McLeod I. X.; Yates J. R.; Nardulli A. M. Interaction of estrogen receptor alpha with proliferating cell nuclear antigen. Nucleic Acids Res. 2007, 35, 5028–5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge C. M. Estrogen regulation of microRNA expression. Curr. Genomics 2009, 10, 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassen S.; Miska E. A.; Caldas C. MicroRNA: implications for cancer. Virchows Arch. 2008, 452, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q.; Eades G. MicroRNA regulatory networks provide feedback mechanisms for steroid receptor signaling. J. Steroids Hormon. Sci. 2012, 3, e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munagala R.; Aqil F.; Vadhanam M. V.; Gupta R. C. MicroRNA ‘signature’ during estrogen-mediated mammary carcinogenesis and its reversal by ellagic acid intervention. Cancer Lett. 2013, 339, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]