Abstract
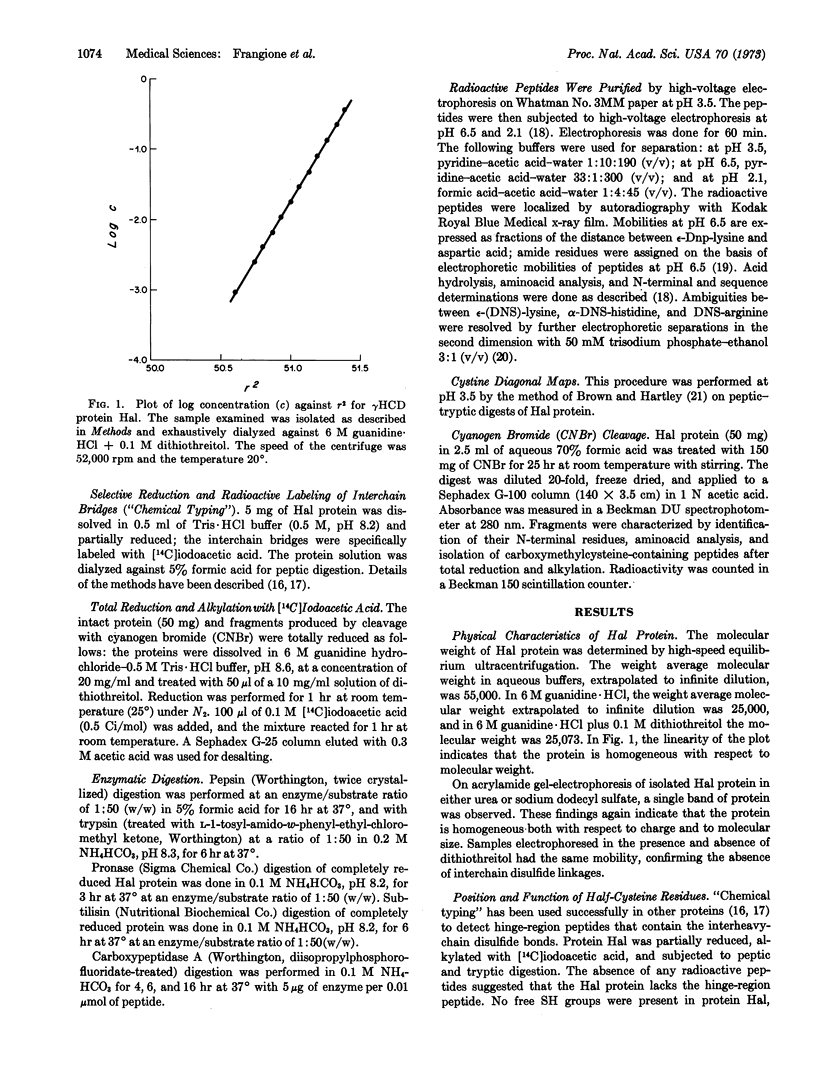
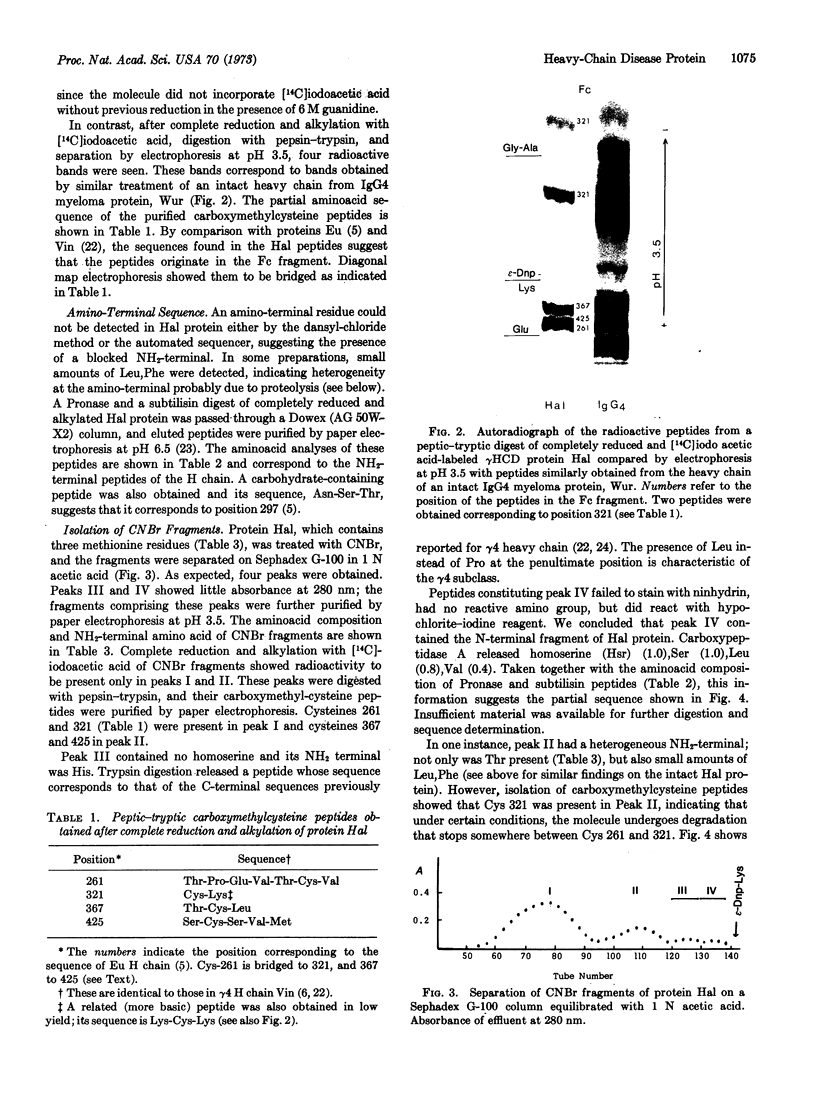
Protein Hal is a human γ4 heavy-chain disease protein whose molecular weight is reduced from 55,000 to 25,000 in 6 M guanidine due to the lack of disulfide bonds between heavy chains. Studies of aminoacid sequence indicate that it contains a gap of about 240 residues, starting 10 residues from the N-terminal end and including the rest of the Fd fragment, as well as the hinge region. Normal sequence apparently resumes at a methionine residue (position 252) in the second constant domain (CH2) and seems normal from there to the carboxyl end of the molecule. These results imply that reinitiation of translation at an internal AUG codon occurs in protein Hal.
Keywords: heavy-chain disease, aminoacid sequence, internal deletion, methionine 252
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capecchi M. R. Initiation of E. coli proteins. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1517–1524. doi: 10.1073/pnas.55.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. M., Franklin E. C., Frangione B. Molecular defect in a gamma-2 heavy chain. Science. 1972 Apr 14;176(4031):187–189. doi: 10.1126/science.176.4031.187. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN E. C. STRUCTURAL STUDIES OF HUMAN 7S GAMMA-GLOBULIN (G IMMUNOGLOBULIN). FURTHER OBSERVATIONS OF A NATURALLY OCCURRING PROTEIN RELATED TO THE CRYSTALLIZABLE (FAST) FRAGMENT. J Exp Med. 1964 Nov 1;120:691–709. doi: 10.1084/jem.120.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett J. W., Deutsch H. F., Smithies O. Hinge-region deletion localized in the IgG-globulin Mcg. Immunochemistry. 1973 Feb;10(2):115–118. doi: 10.1016/0019-2791(73)90238-3. [DOI] [PubMed] [Google Scholar]
- Frangione B., Franklin E. C. Chemical typing of the immunoglobulins IgM, IgA1 and IgA2. FEBS Lett. 1972 Feb 15;20(3):321–323. doi: 10.1016/0014-5793(72)80096-6. [DOI] [PubMed] [Google Scholar]
- Frangione B., Franklin E. C. Heavy chain diseases: clinical features and molecular significance of the disordered immunoglobulin structure. Semin Hematol. 1973 Jan;10(1):53–64. [PubMed] [Google Scholar]
- Frangione B., Milstein C., Franklin E. C. Chemical typing of immunoglobulins. Nature. 1969 Jan 11;221(5176):149–151. doi: 10.1038/221149a0. [DOI] [PubMed] [Google Scholar]
- Frangione B., Milstein C. Partial deletion in the heavy chain disease protein ZUC. Nature. 1969 Nov 8;224(5219):597–599. doi: 10.1038/224597a0. [DOI] [PubMed] [Google Scholar]
- Frangione B., Milstein C., Pink J. R. Structural studies of immunoglobulin G. Nature. 1969 Jan 11;221(5176):145–148. doi: 10.1038/221145a0. [DOI] [PubMed] [Google Scholar]
- Frangione B., Milstein C. Variations in the S-S bridges of immunoglobins G: interchain disulfide bridges of gamma G3 myeloma proteins. J Mol Biol. 1968 May 14;33(3):893–906. doi: 10.1016/0022-2836(68)90326-4. [DOI] [PubMed] [Google Scholar]
- Frangione B., Prelli F., Mihaesco C., Wolfenstein C., Mihaesco E., Franklin E. C. Structural studies of immunoglobulin G, M and A heavy chains. Ann N Y Acad Sci. 1971 Dec 31;190:71–82. doi: 10.1111/j.1749-6632.1971.tb13524.x. [DOI] [PubMed] [Google Scholar]
- Frangione B., Wolfenstein-Todel C. Partial duplication in the "hinge" region of IgA 1 myeloma proteins. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3673–3676. doi: 10.1073/pnas.69.12.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin E. C., Frangione B. The molecular defect in a protein (CRA) found in gamma-1 heavy chain disease, and its genetic implications. Proc Natl Acad Sci U S A. 1971 Jan;68(1):187–191. doi: 10.1073/pnas.68.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448. doi: 10.1146/annurev.bi.40.070171.002205. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Peterson P. A., Cunningham B. A., Berggård I., Edelman G. M. 2 -Microglobulin--a free immunoglobulin domain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1697–1701. doi: 10.1073/pnas.69.7.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink J. R., Buttery S. H., De Vries G. M., Milstein C. Human immunoglobulin subclasses. Partial amino acid sequence of the constant region of a gamma 4 chain. Biochem J. 1970 Mar;117(1):33–47. doi: 10.1042/bj1170033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T., Weber K., Ganem D., Miller J. H. Translational restarts: AUG reinitiation of a lac repressor fragment. Proc Natl Acad Sci U S A. 1972 Apr;69(4):897–901. doi: 10.1073/pnas.69.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahl J. W. The C-terminal sequences of the heavy chains of human immunoglobulin G myeloma proteins of differing isotopes and allotypes. Biochem J. 1967 Dec;105(3):1019–1028. doi: 10.1042/bj1051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabhai A., Brenner S. A mutant which reinitiates the polypeptide chain after chain termination. J Mol Biol. 1967 Jul 14;27(1):145–162. doi: 10.1016/0022-2836(67)90357-9. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D. M., Fanning E. M., Percy M. E., Parr D. M., Connell G. E. Deletions in immunoglobulin polypeptide chains as evidence for breakage and repair in DNA. Science. 1971 May 7;172(3983):574–577. doi: 10.1126/science.172.3983.574. [DOI] [PubMed] [Google Scholar]
- Smithies O., Poulik M. D. Initiation of protein synthesis at an unusual position in an immunoglobulin gene? Science. 1972 Jan 14;175(4018):187–189. doi: 10.1126/science.175.4018.187. [DOI] [PubMed] [Google Scholar]
- Terry W. D., Ein D. Structural studies of gamma heavy chain disease proteins. Ann N Y Acad Sci. 1971 Dec 31;190:467–471. doi: 10.1111/j.1749-6632.1971.tb13556.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilkinson J. M., Press E. M., Porter R. R. The N-terminal sequence of the heavy chain of rabbit immunoglobulin IgG. Biochem J. 1966 Aug;100(2):303–308. doi: 10.1042/bj1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R., Blumenschein G. R., Terry W. D. A new type of human gamma heavy chain disease protein: immunochemical and physical characteristics. Immunochemistry. 1970 Apr;7(4):373–381. doi: 10.1016/0019-2791(70)90240-5. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]