Abstract
In the present study, the effects of δ-tocopherol (δ-T) on growth and apoptosis of human prostate cancer cells were determined and compared with that of α-tocopherol (α-T), a commonly used form of vitamin E. Treatment of human prostate cancer cells with δ-T resulted in strong growth inhibition and apoptosis stimulation, while the effects of α-T were modest. The strong effects of δ-T on the cells were associated with suppression of androgen receptor (AR) activity and decreased level of prostate specific antigen (PSA) that is a downstream target of the AR signaling. In the in vivo study, we found that δ-T had a more potent inhibitory effect on the formation and growth of prostate xenograft tumors than that of α-T. Moreover, δ-T inhibited proliferation and stimulated apoptosis in the tumors. The present study identified δ-T as a better form of vitamin E than α-T for future clinical studies of prostate cancer prevention.
Keywords: prostate cancer, vitamin E, tocopherol, apoptosis, AR signaling, xenograft tumor
Introduction
Vitamin E is present in many plant seeds and in our diet. It can be produced as a byproduct of the edible oil refining process. The vitamin E family consists of four tocopherols and four tocotrienols. Tocopherols have a saturated phytyl tail, whereas tocotrienols have an unsaturated isoprenoid side chain, which contains three double bonds.1,2 On the chromanol ring, the 4 variants (α-, β-, γ-, and δ-) are determined by the number and position of methyl groups.1,2 The predominant form of vitamin E in tissues is α-T, and its deficiency leads to ataxia in humans. The effects of vitamin E on the pathogenesis of human cancers have been investigated in many studies, and the results are generally inconsistent (reviewed in refs (3 and 4)). Studies on the correlation between tocopherols and cancer risk have shown that lower levels of tocopherols are associated with increased risk of lung, breast, and some other types of cancers.3−8 In an earlier clinical study, intake of α-T and β-carotene was found to be associated with a lower risk of prostate cancer.9 However, results from other studies including a large-scale human clinical trial do not support a protective role of α-T in cancer prevention.10−12 This discrepancy may be explained by the fact that other forms of vitamin E or a mixture of different tocopherols may be superior to α-T alone in preventing cancer development.
Since epidemiological studies indicate a protective role of vitamin E in cancer prevention, while clinical trials using α-T was not effective, it is important to determine the effect of the different tocopherols in the vitamin E family in cancer prevention. Previous studies have investigated the effects of α-T and γ-T in different types of cancers (reviewed in refs (3 and 4)), and recent studies from several laboratories demonstrated that a mixture of tocopherols that contains 13% α-T, 1.5% β-T, 57% γ-T, and 24% δ-T had potent preventive effects in a number of carcinogenesis models.13−17 However, studies on the anticancer effect and mechanisms of action for δ-T on prostate cancer are still lacking. The present study was therefore designed to explore the anticancer activity and mechanisms of action for δ-T in prostate cancer. In the present study, we determined the effects of δ-T on growth and apoptosis in different prostate cancer cells cultured in vitro. We also determined the in vivo effects of δ-T using a mouse subcutaneous xenograft tumor model. Our study provides the first evidence for the stronger activities of δ-T on growth inhibition and apoptosis stimulation in human prostate cancer cells as compared to α-T, γ-T, and a mixture of tocopherols. Our study also demonstrated that δ-T had a more potent inhibitory effect than α-T on the formation and growth of prostate LNCaP tumors in SCID mice.
Materials and Methods
Cells and Chemicals
Human prostate cancer LNCaP, VCaP, and CWR22Rv1cells were obtained from the ATCC (Rockville, MD, U.S.A.). α-T, γ-T and δ-T were from Sigma Co. (St. Louis, MO, U.S.A.). A mixture of tocopherol γ-TmT was purchased from the Cogns Corp. (Cincinnati, OH, U.S.A.). The extracellular matrix Matrigel was obtained from Corning Co. (Tewksbury, MA, U.S.A.). The various human prostate cancer cells were maintained in RPMI-1640 culture medium as described in our earlier study.17
Cell Viability
Cell viability was determined by the trypan blue exclusion assay. At the end of the experiments, the cells were trypsinized. Trypan blue solution (0.4%) was mixed with cell suspension in a ratio of 1:4, and the mixtures were kept at room temperature for 2 min for the cells to be stained with the dye. The number of viable cells was counted under a microscope (Nikon Optiphot, Tokyo, Japan). Cells absorbed the dye were counted as dead cells. The cells that did not absorb the dye were counted as live cells.18
Determination of Apoptosis
Apoptotic cells were identified in propidium iodide (PI) stained cells by morphology.19 At the end of the experiment, cells were spined to slides using a Shandon cytospin centrifuge (Thermo Scientific, Waltham, MA, U.S.A.) and fixed in a solution containing 50% acetone and 50% methanol. The cells were then stained with PI at a concentration of 1 μg/mL. A microscope (Nikon Optiphot, Tokyo, Japan) was used to identified apoptotic cells according to the morphological changes characteristic of apoptosis as described earlier.19
Western Blotting
After treatment, the cell lysates were prepared as described earlier.20 Proteins were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane. After blocking nonspecific binding sites with blocking buffer, the membrane was incubated overnight at 4 °C with PSA primary antibody (CBL-252, Millipore Co, Billerica, MA, U.S.A.). The β-actin was used as a loading control. Following removal of the primary antibody, the membrane was washed three times with TBS (PBS containing 0.05% tween 20) buffer at room temperature and then incubated with fluorochrome-conjugated secondary antibody (Santa Cruz Biotechnology Inc., CA, U.S.A.). The membrane was then washed with TBS three times. Final detection was done with an Odyseey infrared imaging system (Li-Cor Biotechnology, Lincoln, NE, U.S.A.).
AR Luciferase Reporter Assay
The CWR-22Rv1/AR cell line that stably expresses the AR-luciferase reporter gene was used in the present study.21 CWR-22Rv1/AR cells were treated with dihydrotestosterne (DHT), α-T and δ-T for 24 h. After treatment, the cells were harvested in a reporter lysis buffer and the luciferase activities were measured using luciferase assay kits from Promega (Madison, WI, U.S.A.), as described in our recent study.21
Subcutaneous LNCaP Tumors in SCID Mice
SCID mice originally obtained from Taconic Farms (Germantown, NY, U.S.A.) were bred in the animal facility in the Susan Lehman Cullman Laboratory for Cancer Research. Eight-week old SCID mice were housed in microisolator cages. Human prostate cancer LNCaP cells (2.0 × 106 cells/mouse) were suspended in culture medium and Matrigel (1:1) and injected subcutaneously into the back of the animals.22 The mice were randomly assigned to 3 experimental groups in the same day of the tumor cell injection. Group 1 was the control and the mice in this group were fed AIN 93 M diet. Mice in group 2 received AIN 93 M diet containing 0.3% α-T. Mice in group 3 were fed 0.3% δ-T in AIN 93 M diet. There were 10 animals in each experimental group. The experimental duration was 48 days. The mice were examined every day for the formation of subcutaneous tumors. When the subcutaneous tumor formed, tumor length and tumor width were measured once every third day and calculated as tumor size (length × width, cm2). The animal experiment was carried out under the Rutgers University Institutional Animal Care and Use Committee (IACUC)-approved protocol (RU02-001).
Tumor Cell Proliferation and Apoptosis
Proliferation of LNCaP tumor cell was measured by determining the number of mitotic cells, and apoptosis of the tumor cells was assessed by immunohistochemical staining for activated caspase-3. At the end of the experiment, tumors were excised from each mouse and weighted. Tumor tissues were fixed in buffered formalin for 24 h and then with ethanol for 48 h. Paraffin blocks of tumor tissues were prepared and paraffin sections of tumor tissues were processed for H&E staining. A microscope (Nikon Optiphot, Tokyo, Japan) was used to examine the tumor sections for mitotic cells. Activated caspase-3 was determined immunohistochemically using an anti activated caspase-3 antibody (#AF835, R&D Systems Ltd., Minneapolis, MN). Immunohistochemical staining was performed as described earlier.23 The number of cells with positive staining of activated caspase-3 was counted and expressed as % caspase-3+ cells.
Statistical Analyses
We used ANOVA (analysis of variance) and multiple comparison (Tukey-Kramer test) to perform statistical analyses in the present study. Differences in cell viability, apoptosis and AR activity in cells from different treatment groups were analyzed. Differences in tumor size and body weight between different treatment group in the in vivo study were also analyzed.
Results
Effects of Different Tocopherols on Growth and Apoptosis of Human Prostate Cancer Cells
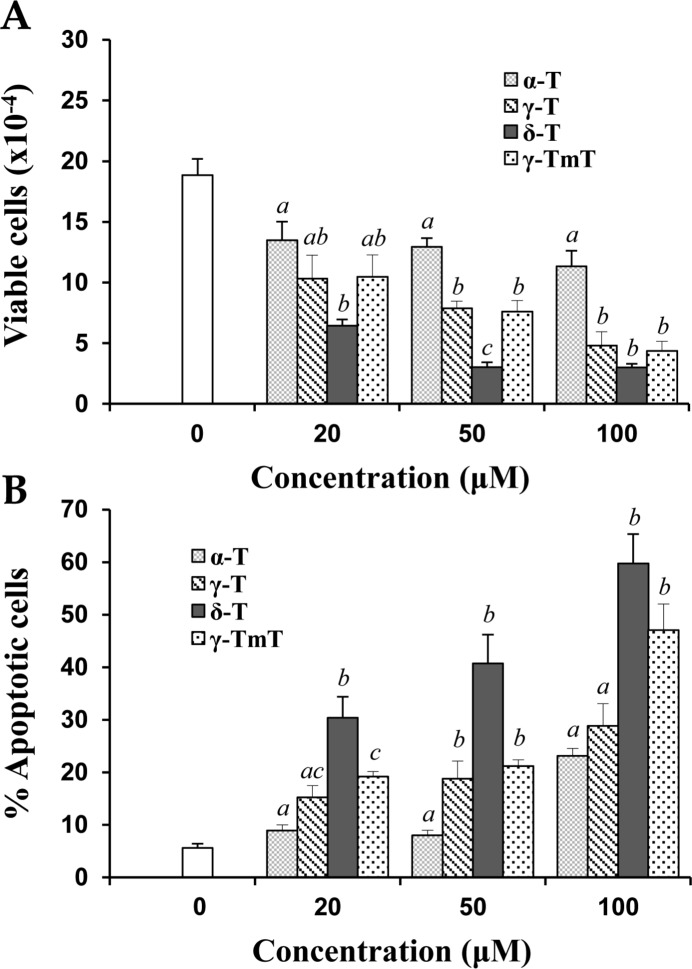
In initial studies, the effects of α-T, γ-T, δ-T, and γ-TmT on growth and apoptosis in human prostate cancer LNCaP cells were determined. As shown in Figure 1A, the different tocopherols dose-dependently inhibited the growth of cultured LNCaP cells. δ-T had a stronger inhibitory effect on the growth of LNCaP cells than the other tocopherols (Figure 1A). Additional experiments showed that treatment of LNCaP cells with different tocopherols resulted in a concentration-dependent increase in apoptosis. As shown in Figure 1B, δ-T had a more potent effect on inducing apoptosis in LNCaP cells than other tocopherols.
Figure 1.
Inhibition of growth and induction of apoptosis in LNCaP cells by α-T, γ-T, δ-T, and γ-TmT. Human prostate cancer LNCaP cells (0.5 × 105 cells/mL) were seeded in RPMI medium with 10% FBS and incubated 24 h to allow the cells attach to the dish. Then the cells were cultured in medium containing 1% FBS and treated with various concentrations of α-T, γ-T, δ-T, or γ-TmT. The treatment time was 96 h. The trypan blue exclusion assay was used to determine cell viability. Number of viable cells after treatment is shown in (A). Morphological assessment of PI-stained cells was used to determine apoptosis and the number of apoptotic cells is shown in (B). Different superscripts (a, b, and c) indicate statistical differences in the number of viable cells or percent apoptotic cells among different tocopherol isoforms (p < 0.05).
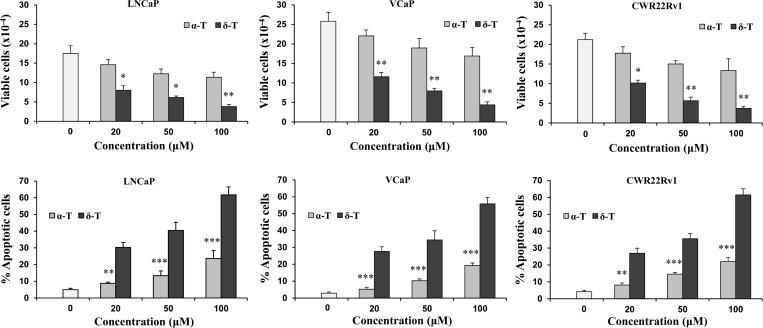
Since δ-T had stronger effects on growth inhibition and apoptosis induction than other tocopherols tested, we subsequently determined the effects of δ-T on different prostate cancer cells and compared its effects with α-T, the most common form of vitamin E. As shown in the upper panel of Figure 2, treatment with δ-T and α-T inhibited the growth of cultured LNCaP, VCaP, and CWR22Rv1 prostate cancer cells in a dose-dependent manner. δ-T had a stronger inhibitory effect on the growth of all three prostate cancer cell lines than α-T (upper panel of Figure 2). Treatment of different prostate cancer cells with α-T and δ-T also induced apoptosis in a dose-dependent manner. As shown in the lower panel of Figure 2, δ-T had a more potent effect on induction of apoptosis in different prostate cancer cells than α-T.
Figure 2.
Effects of α-T and δ-T on growth (upper panel) and apoptosis (lower panel) of cultured human prostate cancer cells. LNCaP, VCaP, and CWR22Rv1 cells (all at 0.5 × 105 cells/mL) were seeded in RPMI medium containing 10% FBS. After 24 h incubation, the cells were cultured in medium containing 1% FBS and treated with α-T or δ-T. The treatment time was 96 h. Graphes in the upper panel show the number of viable cells as determined by the trypan blue exclusion assay. Graphes in the lower panel show the number of apoptotic cells determined by morphological assessment. Differences for the number of viable or apoptotic cells between the α-T-treated group and the δ-T-treated group at different concentrations were statistically analyzed. *p < 0.05, **p < 0.01, and ***p < 0.001.
Effects of δ-T and α-T on AR Activity in CWR-22Rv1/AR Cells
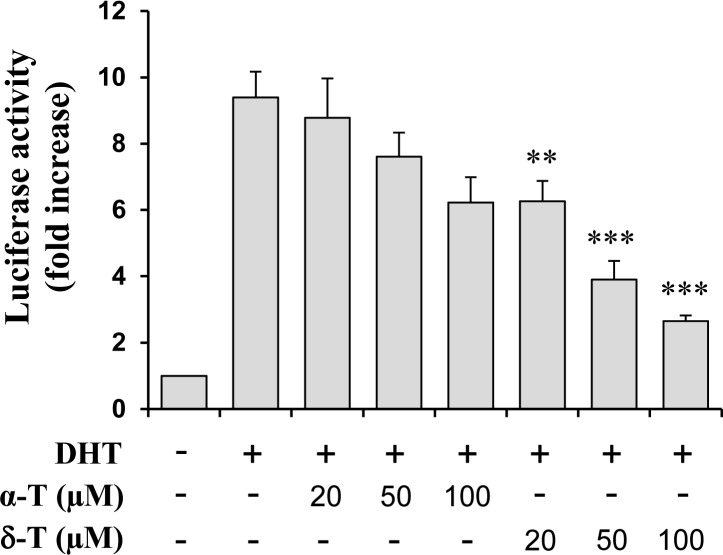
The effects of δ-T and α-T on AR activation was determined using the AR-luciferase reporter gene expression assay. AR activity in CWR-22Rv1/AR cells was stimulated by treatment with DHT and the inhibitory effect of δ-T and α-T on DHT-induced AR activation was determined. As shown in Figure 3, treatment with α-T and δ-T dose-dependently inhibited DHT-induced AR activation in CWR-22Rv1/AR cells. A stronger inhibitory effect of δ-T on AR activation than α-T was observed (Figure 3).
Figure 3.
Effect of α-T and δ-T on AR reporter activity in CWR-22Rv1/AR cells. CWR-22Rv1/AR cells were seeded at a density of 0.5 × 105 cells/mL of medium for 24 h. The medium was then changed to RPMI supplemented with 1% FBS, and the cells were treated with vehicle (control), with DHT (10 nM) alone or in combination with α-T or δ-T (20, 50, or 100 μM) for 24 h. The luciferase activity and protein concentration of the CWR-22Rv1/AR cells were measured. Each value represents the mean ± SE from three separate experiments. Differences for the relative luciferase activities between the α-T-treated group and the δ-T-treated group were analyzed by ANOVA with the Tukey-Kramer Multiple Comparison Test **p < 0.01, ***p < 0.001.
Effects of δ-T and α-T on the Level of PSA in CWR-22Rv1 and LNCaP Cells
To investigate whether decrease in AR activity by tocopherols leads to inhibition of AR signaling, we determined the level of PSA which is a down stream target of the AR signaling pathway. Treatment of CWR-22Rv1 and LNCaP cells with DHT caused an increase in the level of PSA (Figure 4). Treatment of the cells with δ-T strongly inhibited the DHT-induced increase in PSA while α-T only had small to moderate effects (Figure 4). The Western blots were analyzed by optical density measurement and normalized for actin. In the Western blot with LNCaP cells, the level of PSA relative to control (1.00) was 1.97 ± 0.12 in cells treated with DHT, 1.46 ± 0.09 in cells treated with DHT + α-T and 0.91 ± 0.08 in cells treated with DHT + δ-T. In the Western blot with CWR22Rv1 cells, the relative level of PSA was 1.00 in control, 2.01 ± 0.15 in cells treated with DHT, 1.02 ± 0.05 in cells treated with DHT + α-T and 0.48 ± 0.06 in cells treated with DHT + δ-T. Statistical analysis showed that the differences for density measurement between DHT + α-T and DHT + δ-T groups are statistically significant (p < 0.01) in both LNCaP and CWR22Rv1 cells. The result indicates that δ-T more potently inhibits activation of AR and its downstream target PSA as compared to α-T.
Figure 4.
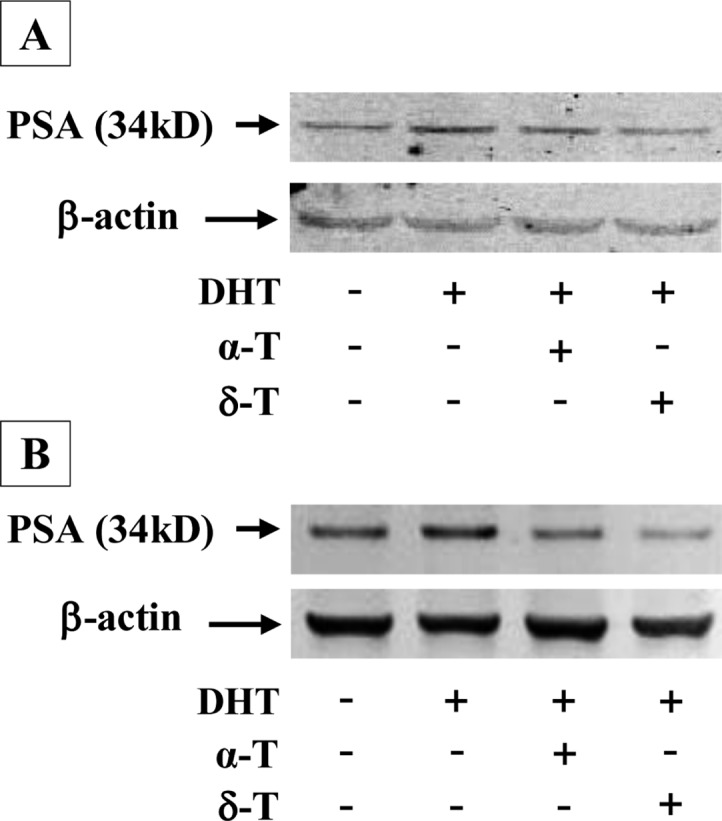
Effect of α-T and δ-T on the level of PSA in prostate cancer cells. CWR-22Rv1 and LNCaP cells were seeded at a density of 1 × 105 cells/mL of medium in 100 mm culture dishes (10 mL/dish) and incubated for 24 h. The medium was changed to RPMI supplemented with 1% FBS, and the cells were then treated with vehicle, with 10 nM DHT alone or together with α-T (50 μM) or δ-T (50 μM) for 48 h. PSA was determined by the Western blot analysis with anti-PSA antibody. The extent of protein loading was determined by blotting for β-actin. Representative blots for LNCaP (A) and CWR22Rv1 (B) cells are shown.
In Vivo Effects of δ-T and α-T
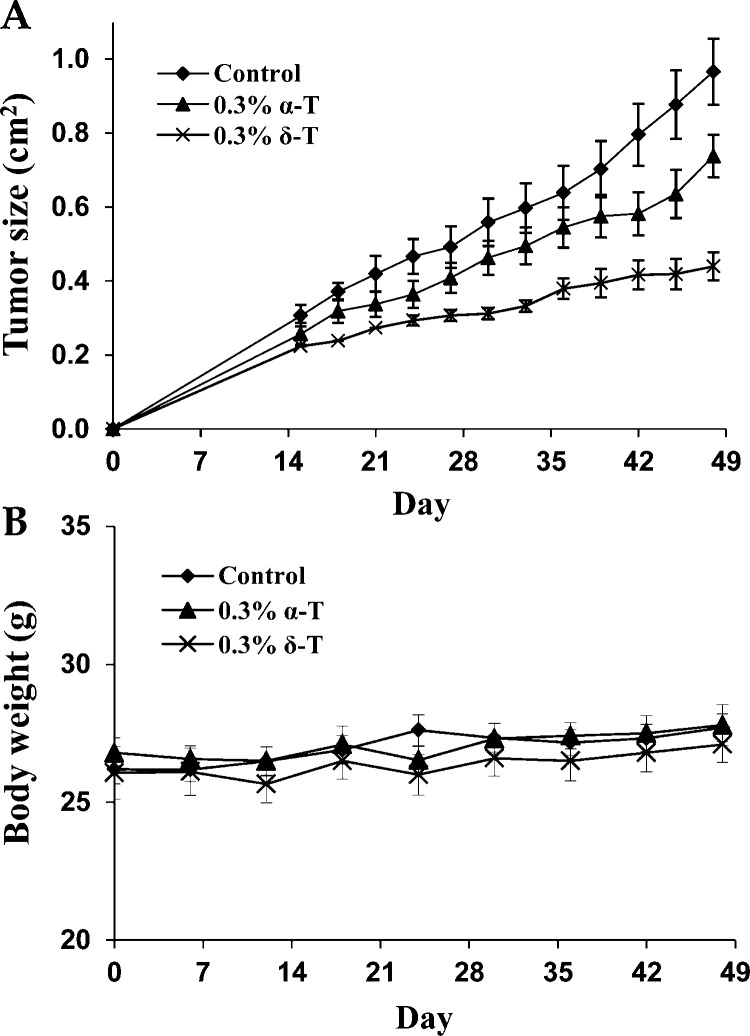
SCID mice were injected subcutaneously with LNCaP cells and received 0.3% α-T or 0.3% δ-T in diet. The tumor take rate was 100% in the control group, 70% in the α-T-treated group, and 50% in the δ-T-treated group. This result indicates that δ-T has a more potent inhibitory effect on the development of transplant prostate tumors than α-T. Treatment with δ-T also more potently inhibited the growth of LNCaP tumors than α-T (Figure 5). As shown in Table 1, the average tumor size at the end of the experiment (day 48) in the δ-T-treated group was significantly smaller than the α-T-treated group (p < 0.05). The tumor weight (g) was also measured at the end of the experiment (day 48). A statistically significant difference (p < 0.05) in the tumor weight between the δ-T-treated group and the α-T-treated group was found (Table 1). As shown in Figure 5B, mice in different treatment group had similar body weight during the experiment period. No statistically significant difference in body weight between any two groups was found at the end of the experiment (p > 0.05).
Figure 5.
In vivo effects of α-T and δ-T. Human prostate cancer LNCaP cells were injected subcutaneously into male SCID mice. The mice received AIN93 M diet (10 mice), AIN93 M diet containing 0.3% α-T (10 mice), or AIN93 M diet containing 0.3% δ-T (10 mice) for 48 days. (A) Tumor growth curve (measured as tumor size; cm2) in the control, 0.3% α-T-treated, and 0.3% δ-T-treated groups. (B) Body weight (g) of the mice in each group. Each value represents the mean ± SE.
Table 1. Effects of δ-T and α-T on Tumor Growth and Body Weight of SCID Micea.
| treatment group | tumor size (cm2) | tumor weight (g) | body weight (g) |
|---|---|---|---|
| control | 0.97 ± 0.08 | 0.62 ± 0.05 | 27.7 ± 0.50 |
| 0.3% α-T | 0.74 ± 0.05 | 0.54 ± 0.09 | 28.1 ± 0.74 |
| 0.3% δ-T | 0.44 ± 0.04b,d | 0.28 ± 0.06c,d | 27.5 ± 0.64 |
Human prostate cancer LNCaP cells were injected subcutaneously into SCID mice as indicated in the Materials and Methods. The mice were fed diet with 0.3% α-T or 0.3% δ-T. Tumor size (cm2) and tumor weight (g) were measured at the end of the experiment. Each value is the mean ± SE.
Indicates p < 0.001 compared to the control.
Indicates p < 0.01 compared to the control.
Indicates p < 0.05 compared to the 0.3% α-T-treated group.
Decreased Proliferation and Increased Apoptosis in LNCaP Tumors by α-T and δ-T
Tumor cell proliferation was measured by determining the number of mitotic cells in the xenograft tumors. We found that treatment of the mice with δ-T in diet resulted in a strong inhibition of tumor cell mitosis (Table 2). The differences in % mitotic cells between the δ-T-treated group and the control group, and between the δ-T-treated group and the α-T-treated group were statistically significant (p < 0.001). We found no statistically significant difference (p > 0.05) in % mitotic cells between the control group and the α-T-treated group (Table 2). As shown in Table 2 and Figure 6, treatment with δ-T also resulted in a strong increase in the number of caspase-3+ cells. The differences in the percentage of caspase-3+ between the δ-T-treated group and the control group, and between the δ-T-treated group and the α-T-treated group were statistically significant (p < 0.001). Treatment with α-T did not significantly increase the percentage of caspase-3+ cells (p > 0.05).
Table 2. Effects of δ-T and α-T on Tumor Cell Proliferation and Apoptosisa.
| treatment group | number of tumors | % mitotic cells | % caspase-3+ cells |
|---|---|---|---|
| control | 10 | 0.88 ± 0.03 | 0.57 ± 0.03 |
| 0.3% α-T | 7 | 0.78 ± 0.03 | 0.68 ± 0.05 |
| 0.3% δ-T | 5 | 0.50 ± 0.02b | 1.01 ± 0.05b |
The tumors from mice at the end of the experiment were analyzed for proliferation and apoptosis. Tumor cell proliferation was measured by determining mitotic cells. Apoptosis was measured by immunohistochemical staining with activated caspase-3. Each value is the mean ± SE.
Indicates p < 0.001 compared to the control or to the 0.3% α-T-treated group.
Figure 6.
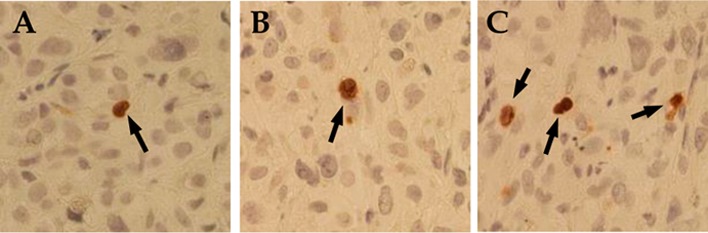
Effects of α-T and δ-T on expression of activated caspase-3 in prostate LNCaP xenograft tumors. Immunohistochemistry with an activated caspase-3 antibody was performed in paraffin sections of LNCaP tumors collected from the experiment described in Figure 5. Representative micrographes of caspase-3 immunostaining in LNCaP tumors from the control (A), α-T-treated (B), and δ-T-treated (C) mice are shown. Arrows indicate caspase-3 positive cells.
Discussion
In the present study, we demonstrated for the first time that δ-T had a potent inhibitory effect on the growth of prostate cancer cells cultured in vitro and grown as subcutaneous xenograft tumors in SCID mice. In initial studies, we determined the effects of α-T, γ-T, δ-T, and a mixture of tocopherols on cultured prostate cancer cells and found that δ-T had stronger effects on growth inhibition and apoptosis stimulation than α-T, γ-T, and the tocopherol mixture. In subsequent studies, we found that δ-T more potently inhibited the growth of LNCaP tumors in SCID mice than α-T. Most of the previous studies on vitamin E have focused on α-T. Studies on the effects of α-T on prostate cancer reveal inconsistent results.24−29 It has recently been shown by a number of laboratories that γ-T or a mixture of different tocopherols, γ-TmT, are more active than α-T for inhibiting prostate cancer.14,17,30−32 Accumulating evidence from experimental studies suggests that the α-T used in clinical trials may not be the correct form of vitamin E for cancer prevention. The strong inhibitory effect of δ-T on prostate cancer shown in our present studies indicates that δ-T may be a good candidate for future clinical trial of prostate cancer prevention.
The mechanisms by which different tocopherols inhibit the growth and induce apoptosis in prostate cancer cells are still largely unknown. Although many studies investigated the mechanisms of action for α-T and γ-T in cancer cells, there are very few studies for the mechanisms of anticancer activities of δ-T. A previous study showed that combination of γ-T with δ-T induced apoptosis in LNCaP cells by induction of cytochrome c release, activation of caspase-9 and caspase-3, and cleavage of poly-ADP-ribose polymerase.30 Recent studies by our collaboration team demonstrated that suppression of lung tumorigenesis by δ-T was associated with its ability to inhibit the formation of 8-OHdG, γ-H2AX, and nitrotyrosine, as well as to induce cell apoptosis.33 In another study, δ-T was found to induce PPAR-γ and PTEN, and reduce pAkt levels in breast cancer cells.34 The mechanisms for the effects of δ-T on prostate cancer are not clear. It is known that the AR signaling pathway is important for growth and survival of prostate cancer cells. Earlier studies have shown that analogues of α-T induced transcriptional repression of AR, inhibited AR activity and decreased the level of PSA.35,36 However, the effect of δ-T on AR signaling was not reported. In the present study, we demonstrated that δ-T had a more potent inhibitory effect than α-T on AR activation in prostate cancer cells as determined by the luciferase reporter gene expression assay. In addition, δ-T more potently decreased the level of PSA than α-T. Suppression of the AR signaling may be one of the mechanisms by which δ-T inhibits the growth and induces apoptosis in androgen sensitive prostate cancer cells. Preliminary results from our recent studies showed inhibitory effect of δ-T on the growth of PC-3 and Du145 cells (data not shown). This result indicates that δ-T also inhibited androgen-independent prostate cancer cells and that mechanisms other than AR signaling may be involved. Further studies are needed to explore the mechanisms of action for δ-T in androgen-independent prostate cancer cells.
Since δ-T showed strong effects on growth inhibition and apoptosis in cultured prostate cancer cells, we used the xenograft prostate tumor model to determine the in vivo effect of this tocopherol. We found in our earlier studies that 0.3% of different tocopherols in diet were effective.13−15 Therefore, 0.3% of α-T and δ-T in diet were used in our present in vivo study. Our study showed that treatment with 0.3% α-T in diet decreased the number of mice that formed LNCaP tumors. However, α-T administration did not significantly inhibit the growth of the tumors. Administration of 0.3% δ-T in the diet strongly inhibited the formation and growth of subcutaneous LNCaP tumors. In a recent pilot study, we found low plasma levels of δ-T (0.16–0.47 μM) in prostate cancer patients received 2 capsules of mix-tocopherol (containing 128 mg α-T, 200 mg γ-T, and 71 mg δ-T per capsule) for 7 or 14 days (data presented in the 2011 AACR annual meeting). The concentrations of α-T and δ-T to inhibit cultured prostate cancer cells are higher than the plasma levels of these compounds. It is possible that the in vivo effects of δ-T were cumulative. In addition, δ-T may interact with endogenous factors such as cytokines within tumor tissues leading to stronger anticancer effects. Mechanistic studies showed that treatment of mice with δ-T inhibited proliferation as reflected by decreased mitosis, and stimulated apoptosis as reflected by increased caspase-3 in LNCaP tumors. This result indicates that the strong inhibitory effect of δ-T on the growth of LNCaP tumors in SCID mice may be mediated by inhibition of proliferation and stimulation of apoptosis in the tumors. To our knowledge, this is the first report for an inhibitory effect of δ-T on the formation and growth of human prostate tumors in a xenograft model.
In summary, the present study showed that δ-T had more potent effects on inhibiting proliferation and inducing apoptosis in prostate cancer cells than α-T. The strong activities of δ-T were associated with suppression of AR activity and decreased level of PSA. Results from the in vivo study demonstrated a strong inhibitory effect of δ-T on the formation and growth of LNCaP tumors in SCID mice without apparent toxicity. Clinical studies for determining the potential preventive efficacy of δ-T on prostate cancer patients are warranted.
Acknowledgments
The authors dedicate this paper to Dr. Allan H. Conney, an outstanding and widely recognized cancer researcher who passed away on September 10, 2013. We thank Dr. Philip Fumanski for his valuable suggestions for the manuscript.
Glossary
Abbreviations Used
- α-T
(α-tocopherol)
- β-T
(β-tocopherol)
- γ-T
(γ-tocopherol)
- δ-T
(δ-tocopherol)
- AR
(androgen receptor)
- PSA
(prostate specific antigen)
- SCID
(severe combined immunodeficient)
- ANOVA
(analysis of variance)
Author Contributions
⊥ These two authors contributed equally to this work.
The present study was supported by the Guangdong Province Leadership Grant, China National Science Foundation Grants (Grant No. 81272452, 21102020, and 21272043), and the Rutgers Cancer Institute of New Jersey (CCSG P30-CA072720 RSD).
The authors declare no competing financial interest.
Author Status
# Deceased.
Funding Statement
National Institutes of Health, United States
References
- Constantinou C.; Papas A.; Constantinou A. I. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int. J. Cancer 2008, 15, 1234739–752. [DOI] [PubMed] [Google Scholar]
- Herrera E.; Barbas C. Vitamin E: action, metabolism and perspectives. J. Physiol. Biochem. 2001, 57143–56. [PubMed] [Google Scholar]
- Ju J.; Picinich S. C.; Yang Z.; Zhao Y.; Suh N.; Kong A. N.; Yang C. S. Cancer preventive activities of tocopherols and tocotrienols. Carcinogenesis 2010, 314533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Rad. Biol. Med. 2014, 72, 76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R.; Metcalfe C.; Fraser W. D.; Donovan J.; Hamdy F.; Neal D. E.; Lane J. A.; Martin R. M. Associations of circulating retinol, vitamin E, and 1,25-dihydroxyvitamin D with prostate cancer diagnosis, stage, and grade. Cancer Causes Control. 2012, 23111865–1873. [DOI] [PubMed] [Google Scholar]
- Yang C. S.; Suh N.; Kong A. N. Does vitamin E prevent or promote cancer?. Cancer Prev. Res. (Phila) 2012, 55701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banim P. J.; Luben R.; McTaggart A.; Welch A.; Wareham N.; Khaw K. T.; Hart A. R. Dietary antioxidants and the aetiology of pancreatic cancer: A cohort study using data from food diaries and biomarkers. Gut. 2013, 62101489–1496. [DOI] [PubMed] [Google Scholar]
- Epplein M.; Shvetsov Y. B.; Wilkens L. R.; Franke A. A.; Cooney R. V.; Le Marchand L.; Henderson B. E.; Kolonel L. N.; Goodman M. T. Plasma carotenoids, retinol, and tocopherols and postmenopausal breast cancer risk in the Multiethnic Cohort Study: A nested case-control study. Breast Cancer Res. 2009, 114R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Alpha-tocopherol, Beta-carotene Cancer Prevention Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N. Engl. J. Med. 1994, 330(15), 1029–1035. [DOI] [PubMed] [Google Scholar]
- Albanes D.; Till C.; Klein E. A.; Goodman P. J.; Mondul A. M.; Weinstein S. J.; Taylor P. R.; Parnes H. L.; Gaziano J. M.; Song X.; Fleshner N. E.; Brown P. H.; Meyskens F. L. Jr.; Thompson I. M. Plasma Tocopherols and Risk of Prostate Cancer in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Cancer Prev. Res. (Phila) 2014, 79886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I. M.; Cook N. R.; Gaziano J. M.; Gordon D.; Ridker P. M.; Manson J. E.; Hennekens C. H.; Buring J. E. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women’s Health Study: A randomized controlled trial. JAMA 2005, 294156–65. [DOI] [PubMed] [Google Scholar]
- Gaziano J. M.; Glynn R. J.; Christen W. G.; Kurth T.; Belanger C.; MacFadyen J.; Bubes V.; Manson J. E.; Sesso H. D.; Buring J. E. Vitamins E and C in the prevention of prostate and total cancer in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2009, 301152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolarek A. K.; So J. Y.; Thomas P. E.; Lee H. J.; Paul S.; Dombrowski A.; Wang C. X.; Saw C. L.; Khor T. O.; Kong A. N.; Reuhl K.; Lee M. J.; Yang C. S.; Suh N. Dietary tocopherols inhibit cell proliferation, regulate expression of ERα, PPARγ, and Nrf2, and decrease serum inflammatory markers during the development of mammary hyperplasia. Mol. Carcinog. 2013, 527514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Khor T. O.; Shu L.; Saw C. L.; Wu T. Y.; Suh N.; Yang C. S.; Kong A. N. A γ-tocopherol-rich mixture of tocopherols maintains Nrf2 expression in prostate tumors of TRAMP mice via epigenetic inhibition of CpG methylation. J. Nutr. 2012, 1425818–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G.; Xiao H.; Li G. X.; Picinich S. C.; Chen Y. K.; Liu A.; Lee M. J.; Loy S.; Yang C. S. A gamma-tocopherol-rich mixture of tocopherols inhibits chemically induced lung tumorigenesis in A/J mice and xenograft tumor growth. Carcinogenesis 2010, 314687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. S.; Suh N. Cancer prevention by different forms of tocopherols. Top. Curr. Chem. 2013, 329, 21–33. [DOI] [PubMed] [Google Scholar]
- Zheng X.; Cui X. X.; Khor T. O.; Huang Y.; Dipaola R. S.; Goodin S.; Lee M. J.; Yang C. S.; Kong A. N.; Conney A. Inhibitory Effect of a γ-Tocopherol-Rich Mixture of Tocopherols on the Formation and Growth of LNCaP Prostate Tumors in Immunodeficient Mice. Cancers (Basel) 2011, 343762–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altiok N.; Ersoz M.; Koyuturk M. Estradiol induces JNK-dependent apoptosis in glioblastoma cells. Oncol Lett. 2011, 261281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyl T.; Grzelkowska K.; Zimowska W.; Skierski J.; Wareski P.; Płoszaj T.; Trzeciak L. Expression of bcl-2 and bax in TGF-beta 1-induced apoptosis of L1210 leukemic cells. Eur. J. Cell Biol. 1998, 754367–374. [DOI] [PubMed] [Google Scholar]
- Ma L.; Li W. Emodin inhibits LOVO colorectal cancer cell proliferation via the regulation of the Bcl-2/Bax ratio and cytochrome c. Exp. Ther. Med. 2014, 841225–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. Y.; Ding N.; Du Z.; Cui X.; Wang H.; Wei X.; Conney A.; Zhang K.; Zheng X. Curcumin analogues with high activity for inhibiting human prostate cancer cell growth and androgen receptor activation. Mol. Med. Rep. 2014, 10, 1315–1322. [DOI] [PubMed] [Google Scholar]
- Hu G.; Li F.; Ouyang K.; Xie F.; Tang X.; Wang K.; Han S.; Jiang Z.; Zhu M.; Wen D.; Qin X.; Zhang L. Intrinsic gemcitabine resistance in a novel pancreatic cancer cell line is associated with cancer stem cell-like phenotype. Int. J. Oncol. 2012, 403798–806. [DOI] [PubMed] [Google Scholar]
- Qu J.; Lu W.; Li B.; Lu C.; Wan X. WWOX induces apoptosis and inhibits proliferation in cervical cancer and cell lines. Int. J. Mol. Med. 2013, 3151139–1147. [DOI] [PubMed] [Google Scholar]
- Venkateswaran V.; Fleshner N. E.; Klotz L. H. Synergistic effect of vitamin E and selenium in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2004, 7154–56. [DOI] [PubMed] [Google Scholar]
- Nakamura A.; Shirai T.; Takahashi S.; Ogawa K.; Hirose M.; Ito N. Lack of modification by naturally occurring antioxidants of 3,2′-dimethyl-4-aminobiphenyl-initiated rat prostate carcinogenesis. Cancer Lett. 1991, 583241–246. [DOI] [PubMed] [Google Scholar]
- McCormick D. L.; Rao K. V. Chemoprevention of hormone-dependent prostate cancer in the Wistar-Unilever rat. Eur. Urol. 1999, 355–6464–467. [DOI] [PubMed] [Google Scholar]
- Limpens J.; Schröder F. H.; de Ridder C. M.; Bolder C. A.; Wildhagen M. F.; Obermüller-Jevic U. C.; Krämer K.; van Weerden W. M. Combined lycopene and vitamin E treatment suppresses the growth of PC-346C human prostate cancer cells in nude mice. J. Nutr. 2006, 13651287–1293. [DOI] [PubMed] [Google Scholar]
- Fleshner N.; Fair W. R.; Huryk R.; Heston W. D. Vitamin E inhibits the high-fat diet promoted growth of established human prostate LNCaP tumors in nude mice. J. Urol. 1999, 16151651–1654. [PubMed] [Google Scholar]
- Basu A.; Grossie B.; Bennett M.; Mills N.; Imrhan V. Alpha-tocopheryl succinate (alpha-TOS) modulates human prostate LNCaP xenograft growth and gene expression in BALB/c nude mice fed two levels of dietary soybean oil. Eur. J. Nutr. 2007, 46134–43. [DOI] [PubMed] [Google Scholar]
- Jiang Q.; Wong J.; Ames B. N. Gamma-tocopherol induces apoptosis in androgen-responsive LNCaP prostate cancer cells via caspase-dependent and independent mechanisms. Ann. N.Y. Acad. Sci. 2004, 1031, 399–400. [DOI] [PubMed] [Google Scholar]
- Singh C. K.; Ndiaye M. A.; Siddiqui I. A.; Nihal M.; Havighurst T.; Kim K.; Zhong W.; Mukhtar H.; Ahmad N. Methaneseleninic acid and γ-Tocopherol combination inhibits prostate tumor growth in vivo in a xenograft mouse model. Oncotarget 2014, 5113651–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli P.; Caraglia M.; Abbruzzese A.; Beninati S. γ-Tocopherol inhibits human prostate cancer cell proliferation by up-regulation of transglutaminase 2 and down-regulation of cyclins. Amino Acids 2013, 44145–51. [DOI] [PubMed] [Google Scholar]
- Li G. X.; Lee M. J.; Liu A. B.; Yang Z.; Lin Y.; Shih W. J.; Yang C. S. δ-Tocopherol is more active than α - or γ -tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev. Res. (Phila) 2011, 43404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolarek A. K.; So J. Y.; Burgess B.; Kong A. N.; Reuhl K.; Lin Y.; Shih W. J.; Li G.; Lee M. J.; Chen Y. K.; Yang C. S.; Suh N. Dietary administration of δ- and γ-tocopherol inhibits tumorigenesis in the animal model of estrogen receptor-positive, but not HER-2 breast cancer. Cancer Prev. Res. (Phila) 2012, 5111310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Wu Y.; Malewicz B.; Lu J.; Li S.; Marshall J.; Ip C.; Dong Y. Augmented suppression of androgen receptor signaling by a combination of alpha-tocopheryl succinate and methylseleninic acid. Cancer 2006, 107122942–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P. H.; Wang D.; Chuang H. C.; Wei S.; Kulp S. K.; Chen C. S. alpha-Tocopheryl succinate and derivatives mediate the transcriptional repression of androgen receptor in prostate cancer cells by targeting the PP2A-JNK-Sp1-signaling axis. Carcinogenesis 2009, 3071125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]