ABSTRACT
Background: The perceived pain on injection site caused by subcutaneous (SC) self-injection may negatively affect acceptance and adherence to treatment in patients with multiple sclerosis (MS). Pain on injection may be caused by inaccurate injection technique, inadequate needle length adjustment, or repeated use of the same injection body area. However, information is lacking concerning the optimal needle depth to minimize the injection pain. Objective: The purpose of this program was to characterize the perceived injection-site pain associated with the use of various injection depths of the autoinjector of glatiramer acetate (GA) based on SC tissue thickness (SCT) of the injection site. Methods: This was a pilot program performed by MS-specialized nurses in patients with MS new to GA. Patients were trained by MS nurses on the preparation and administration of SC injection and on an eight-site rotation (left and right arms, thighs, abdomen, and upper quadrant of the buttock). The needle length setting was selected based on SCT measures as follows: 4 or 6 mm for SCT < 25 mm, 6 or 8 mm for SCT between 25 and 50 mm, and 8 or 10 mm for SCT > 50 mm. Injection pain was rated using a visual analog scale (VAS) at 5- and 40-minute postinjection and during two 24-day treatment periods. Results: Thirty-eight patients with MS were evaluated. The mean SCT ranged from 15.5 mm in the upper outer quadrant of the buttocks to 29.2 mm in the thighs. The mean perceived pain on injection was below 3 for all the injection sites, at both time points (5 and 40 minutes) and during both 24-day evaluation periods. The mean VAS scores were significantly greater after 5 minutes of injection compared with that reported 40-minute postinjection during both 24-day treatment periods and for all the injection areas. Mean VAS measures at 5- and 40-minute postinjection significantly decreased during the second 24-day treatment period with respect to that reported during the first 24 SC injections for all injection sites. Conclusions: Our findings suggest that the adjustment of injection depth of SC GA autoinjector according to SCT of body injection areas is suitable to maintain a low degree of postinjection pain. Moreover, our results also may indicate that the use of needle lengths of 6 mm or shorter is appropriate with regard to injection pain for adult patients with MS with SCT < 50 mm.
Keywords: glatiramer acetate, injection depth, multiple sclerosis, self-injection, subcutaneous thickness
Multiple sclerosis (MS) is a chronic, unpredictable demyelinating disease of the central nervous system, which leads to severe disability (Compston & Coles, 2008). Current first-line subcutaneous (SC) disease-modifying drugs for the treatment of relapsing-remitting multiple sclerosis (RRMS) include interferon beta-1b (IFNβ-1b; Betaferon and Extavia), IFNβ-1ba (Rebif), and glatiramer acetate (Copaxone), which are approved for the self-administration by SC injection in Europe. Despite the proven effectiveness of these therapies (Jacobs et al., 1996; Johnson et al., 1995; The IFNB Multiple Sclerosis Study Group, 1993; The PRISMS [Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis] Study Group, 1998), the requirement of frequent self-injection of MS therapy can be particularly troublesome as it may result in local injection-site reactions (LISRs). The SC IFNβ injections result in LISRs in 85%–92% of patients with MS during the early phase of treatment (Moses & Brandes, 2008), and 70% of patients receiving GA report an LISR (Copaxone, 2009). Erythema and pain are commonly reported LISRs associated with SC injections of IFNβ (Jacobs et al., 1996; The IFNB Multiple Sclerosis Study Group, 1993; The PRISMS [Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis] Study Group, 1998) and GA in patients with MS (Johnson et al., 1995). Although LISRs are rarely serious, they can lead to negative attitudes about injections, particularly in the early stages of treatment that may lead to poor adherence to DMT (Beer et al., 2011; Treadaway et al., 2009). Indeed, patient perception of pain caused by SC drug delivery may negatively affect acceptance and compliance with MS therapy (Treadaway et al., 2009). Certainly, nonadherence to SC DMT is a common problem in MS (Treadaway et al., 2009) with clinical consequences including reduced efficacy (Al-Sabbagh, Bennet, Kozma, Dickson, & Meletiche, 2013; Costello, Kennedy, & Scanzillo 2008; Rio et al., 2005).
In response to the difficulties self-injection entails, various autoinjection systems have been developed and introduced into the MS treatment armamentarium in an attempt to improve convenience and safety of self-injection and to reduce pain and anxiety (Lugaresi et al., 2008). Research efforts have specifically been focused on improving the needle design to reduce injection-site pain (Glenski & Conner, 2009; Jaber et al., 2008). The new autoinjection single-dose devices available for DMTs offer, among other helpful features, flexible depth adjustment and a hidden needle system that can help to overcome needle phobia. However, despite the technical advances in injecting devices, adherence to treatment in MS still remains suboptimal, probably because of incorrect injection technique, inadequate injection depth, and repeated use of the same injection site. Therefore, appropriate training on self-injection and improvement of injection comfort by adjusting the needle length may help patients to avoid missing doses and thus potentially improve adherence.
Pain on injection site has been reported as a reason for changing comfort settings in devices for frequent autoinjection in patients with MS (Devonshire et al., 2010). Hence, patients who usually experience pain may not have found their optimal injection depth. Indeed, nowadays, patients and their care providers have to try different injection depths in an attempt to find more comfortable and less painful injection settings. However, the optimal injection depth may vary among patients depending on their anatomical characteristics and the injection body area. Indeed, LISRs including injection-site pain are more likely to occur in the arms and thighs and less frequently in body areas with a higher proportion of SC adipose tissue (e.g., abdomen and buttocks). Therefore, as the thickness of SC adipose tissue (SCT) varies with position and with body mass index, individualization of depth setting should be considered for different groups of patients and injection body areas. As seen in diabetes, knowledge about the SCT would be essential in the selection of the appropriate injection depth and technique for SC drug delivery devices (Gibney, Arce, Byron, & Hirsch, 2010). However, there is no available information on the optimal needle length for SC injection of MS therapy to suit individual needs in patients with different anthropometric characteristics.
This project was conducted to characterize the perceived injection-site pain associated with the use of various needle lengths of the autoinjection device for daily SC administration of GA. For this purpose, an assistance program for needle depth adjustment according to the SCT was performed by MS-specialized nurses based on their own empirical experience in clinical practice.
Patients and Methods
Project Design and Patients
This project was conducted in nine Spanish Hospital Nursing Departments to evaluate an assistance improvement program of patients treated with SC GA injections. Men or women aged 18 years and older were eligible if they had a clinically definitive diagnosis of RRMS according to the revised 2005 or 2010 McDonald criteria (Polman et al., 2011; Polman, Wolinsky, & Reingold, 2005) and were new to SC injection therapy for MS. Patients were excluded if they were currently using and/or had used self-injecting therapies, including those indicated for other pathologies (i.e., diabetes). Patients included in the project had just initiated GA (Copaxone; TEVA Pharmaceutical Ltd., London, United Kingdom) from commercial sources according to routine clinical practice. The commercially available injections contain 20 mg of GA, supplied in prefilled syringes for SC delivery once daily. The GA injections were administered using the Autoject 2 for glass syringe. All patients participated voluntarily in the project after signing the informed consent.
In this project, 13 MS specialist nurses evaluated the degree of pain experienced from an assistance protocol of SC injection using different needle lengths selected according to the patients’ skinfold thickness in each body area. The measures were taken on the dominant side of the body. The needle length setting was selected in each patient based on SCT measures (in millimeters) as follows: 4 or 6 mm for SCT < 25 mm, 6 or 8 mm for SCT between 25 and 50 mm, and 8 or 10 mm for SCT > 50 mm. Of the two lengths tested for each SCT, the shortest was assigned to the dominant side considering that it generally has more muscle and less SCT. The development of this assistance program was based on the current GA self-injecting device (Autoject 2 for glass syringe) guidance for patients to find the right needle length depending on the depth of SC tissue (measured in inches) that a patient can pinch (Teva Neuroscience, 2013) and the depth settings available for this injecting device (4, 6, 8, and 10 mm).
The injection site was rotated as follows: left arm (day 1), right arm (day 2), left thigh (day 3), right thigh (day 4), left side of the abdomen (day 5), right side of the abdomen (day 6), left upper outer quadrant of the buttock (day 7), and right upper outer quadrant of the buttock (day 8). Patients had to perform three 8-site complete rotations for 24 days.
The project consisted of a baseline visit, defined as the first day of drug administration, and two or three (if needed) evaluation visits 24, 48, or 72 days after treatment initiation. The schedule of the project evaluation visits was made to coincide with the routine checkup appointments and the treatment dispensation at the center.
Project Conduct and Assessments
At the baseline visit, patients were instructed on the skin preparation for the injection, storage temperature, and preparation of the prefilled syringes and the self-injection technique. The project MS nurses administered the first injection with the autoinjector device to provide patients with appropriate training on injection procedure for home use. The injections could be self-injected, or patients could receive injections administered by a relative if trained about the use of the injecting device. Furthermore, MS nurses taught the patients not to exert excessive pressure on the skin with the autoinjector in order that adjusted injection depth did not vary. In addition, patients were instructed on the eight-site rotation program.
The SCT of patients was measured by the nurses using a plastic skinfold caliper. Seven different skinfolds were measured on the dominant side of the body: chest, axilla, triceps, subscapular, abdominal, suprailiac, and thigh. However, the skinfold thickness measurements used for assigning the needle length were obtained from the triceps, thighs, abdomen, and suprailiac. Patients were categorized in three subgroups according to their SCT: <25, 25–50, and >50 mm.
Fat mass index was calculated according to Jackson–Pollock equation (Jackson & Pollock, 1978, based on a sample aged 18–61 years) as follows: body density = 1.112 − (0.00043499 × sum of skinfolds) + (0.00000055 × square of the sum of skinfold sites) − (0.00028826 × age), where the skinfold sites (measured in millimeters) are the chest, axilla, triceps, subscapular, abdominal, suprailiac, and thigh.
Patients were given two paper-form diaries where the degree of injection-site pain experienced using each needle in the different injection sites had to be recorded. The patients had to rate the degree of pain from each injection using a visual analog scale (VAS) from 0 cm (no pain) to 10 cm (worst possible pain). The magnitude of pain at 5- and 40-minute postinjection in each body area had to be recorded in the patient diary during a 24-day treatment period. Because the perception of painful stimuli varies from person to person, patients participating in this project were used as their own controls. The patient diary included a body map to rotate injection areas (arms, thighs, abdomen, and upper outer quadrant of the buttock) where injection sites were specified. In addition, patients were instructed to observe for possible LISRs, which had also to be recorded in the patient diary. Patients were taught to identify swelling, redness (erythema), and itching as LISRs and advised not to treat LISRs (e.g., use of analgesic or topical corticosteroids, application of cold or heat in the injection site, etc.) to avoid masking the pain. However, if the patients experienced repeated and worrying LISRs, they could telephone their nurses to report these reactions to be properly treated if it was necessary.
After the first 24-day treatment, the participants returned to the center for a routine checkup by the nurses and gave back the patient diary, and the treatment for the second 24-day treatment period was dispensed. At this second visit, the VAS diary was checked by nurses, and VAS mean values of the three complete rotations by body site were calculated. When the median VAS score was ≥6, the needle length was reduced to the immediately lower length available. In the case of a patient with a mean VAS value ≥6 in the right and left sides of a body site, both needle lengths were reduced for the second treatment period. When the mean VAS value was ≥6 in one side of a body site and <6 in the other one, the needle length allocated to the side with a mean VAS value ≥6 was reduced whereas the injection depth of the other side was maintained. In the case that the mean VAS values were <6 in both body sides (right and left), the needle length associated to the greater VAS value was reduced to the immediately shorter length, which had not been used, whereas the length of the other body side was maintained for the following treatment period. After the second 24-day treatment period, when the patient returned for a checkup with the nurses, the needle length may be changed if necessary according to the aforementioned criteria for depth adjustment. When patients were not able to attend a regular checkup appointment with the nurse or they experienced very painful injections before the scheduled evaluation visit, nurses could decide to perform adjustments of depth setting at anytime, as long as the abovementioned criteria for changing the needle depth were met.
Statistical Considerations
Data obtained from patient diaries were analyzed using descriptive analyses. Quantitative variables were described using mean and standard errors, and qualitative variables were described using frequencies. Wilcoxon signed-rank test was used for comparing the mean VAS scores between 5- and 40-minute postinjection by body site and between the dominant and nondominant sides of the body at 5- and 40-minute postinjection. This nonparametric statistical test was also used to compare the perceived pain at 5 and 40 minutes between the first and second 24-day treatment periods by body site. Those patients who did not apply the project needle length recommendations (did not comply with the program) in neither of the injection sites were excluded from the pain analysis.
The statistical analysis was performed using the Statistical Package for the Social Sciences version 17.0 (SPSS, Inc., Chicago, IL).
Results
Demographic, Clinical, and Anthropometric Characteristics
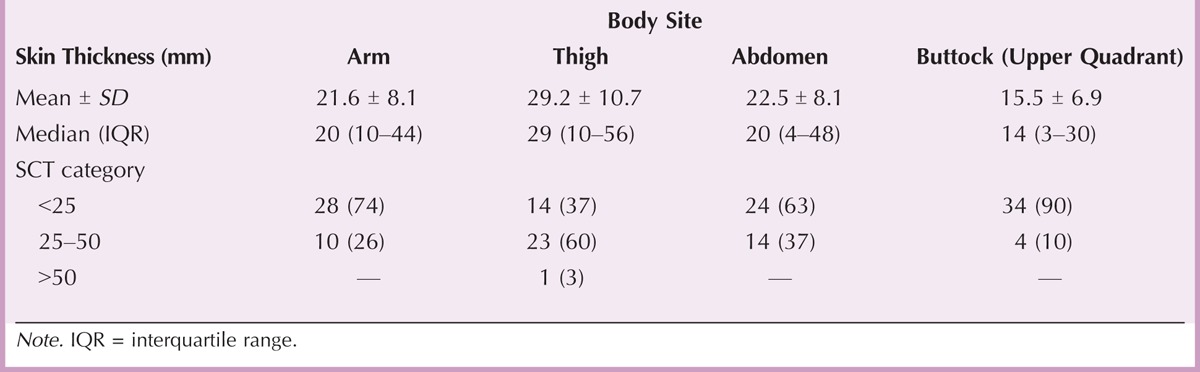
From June 2012 to December 2012, 47 patients from nine Spanish centers were enrolled in the project. Of the 47 patients included, nine patients were not evaluable: three failed to complete the VAS diary, and six did not use the needle length assigned by the MS nurse according to the project program. Therefore, 38 patients with MS were evaluated. The demographic, clinical, and anthropometric characteristics of the patients are displayed in Table 1. The median age of the patients was 39 years, and nearly 70% were women. Most patients (82%) showed right-side dominance. Mean fat mass index was 1.1 kg/m2. The mean SCT ranged from 15.5 mm in the upper outer quadrant of the buttocks to 29.2 mm in the thighs. Most patients had SCT < 25 mm in the arms (74%), abdomen (63%), and upper quadrant of the buttocks (89.5%). However, SCT between 25 and 50 mm was the most frequent in the thighs (60.5%). The skin thickness categories (<25, 25–50, and >50 mm) by body site are displayed in Table 2.
TABLE 1.
Patients’ Demographic, Clinical, and Anthropometric Characteristics
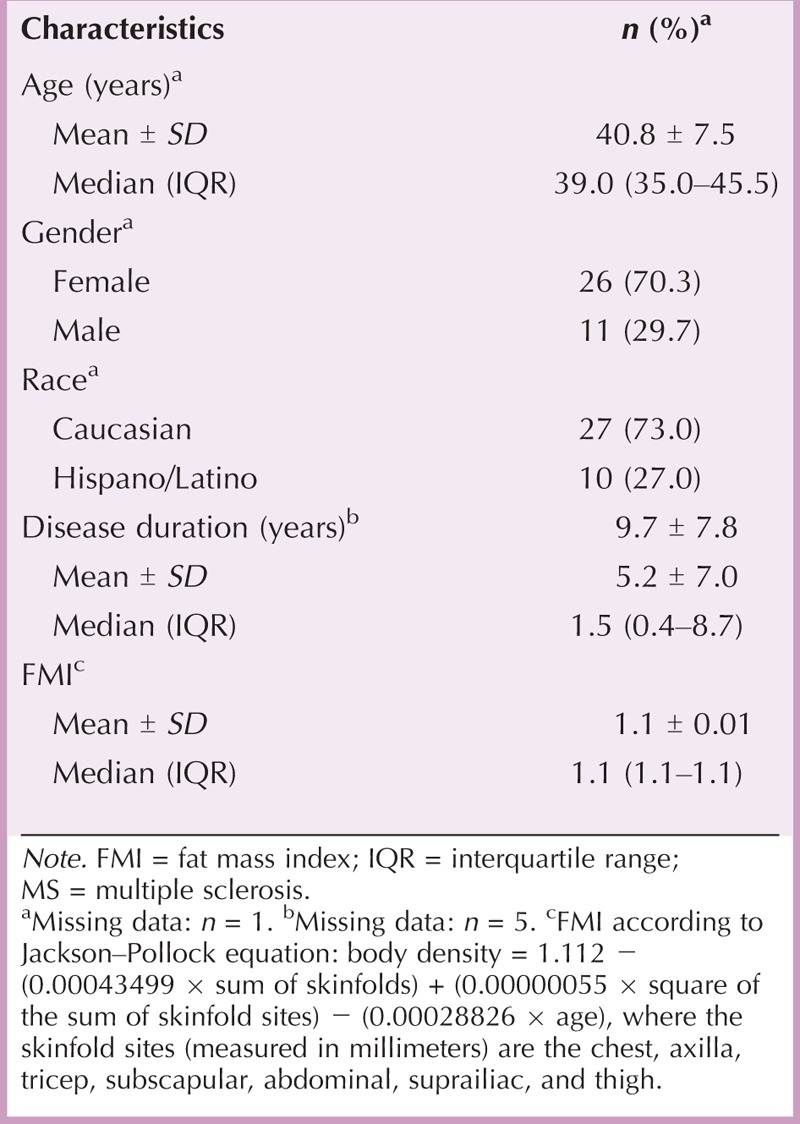
TABLE 2.
Skin Thickness (Millimeters) by Body Site
Analysis of Pain
Thirty-eight patients were analyzed for injection-related pain. Moreover, the VAS scores from an injection applied with a different needle depth than that recommended by MS nurses were not included in the analysis of pain.
The mean pain on injection, as assessed by using the VAS diary, was below 3 for all the injection sites, both time points (5 and 40 minutes) and across both 24-day evaluation periods. The VAS score was significantly greater after 5 minutes of injection compared with that reported 40 minutes after injection in all the body areas designed for injection and during the first and second 24-day treatment periods (p < .0001 for all comparisons). The mean VAS scores between the dominant and nondominant sides of the body (regardless of right or left body side dominance) were similar for all the injection sites and for 5- and 40-minute postinjection after the first and second treatment periods (Figure 1).
FIGURE 1.
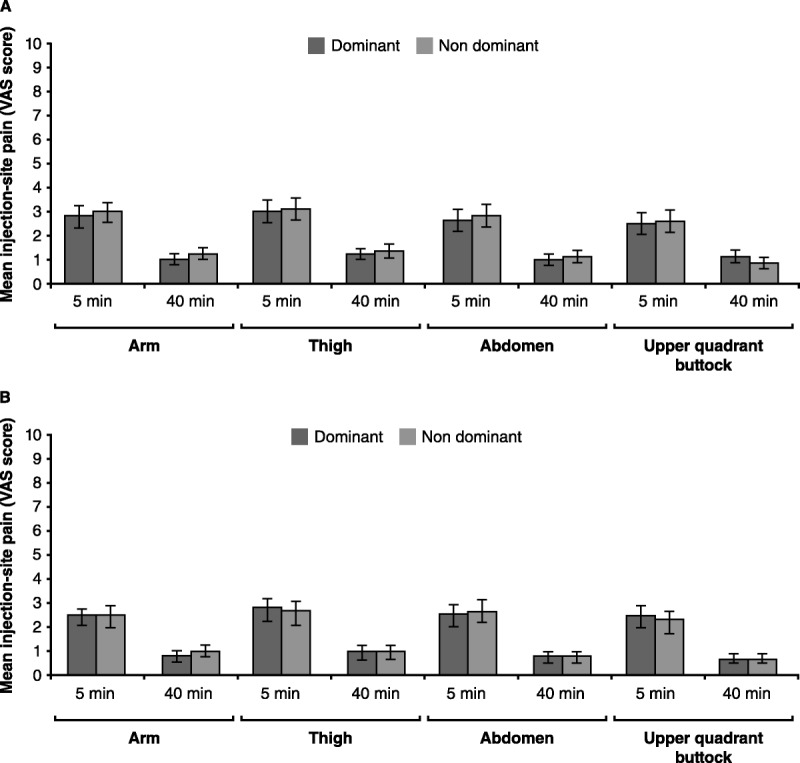
Median Pain (and Standard Error), Assessed by VAS, at 5 and 40 Minutes Postinjection During the First (A) and Second (B) 24-Day Treatment Periods in the Dominant and Nondominant Sides of the Injection Body Areas (Arm, Thigh, Abdomen, and Upper Quadrant of the Buttock)
The number of patients requiring needle length reduction after the first 24-day treatment by body site was as follows: seven (20%) in the nondominant side of thigh and two (13.2%) in both thighs, nine (25.7%) in the nondominant arm, seven (20%) and one (2.8%) in the nondominant and dominant sides of the abdomen, and eight (22.8%) in the nondominant side of the upper quadrant of the buttock. Mean VAS measures at 5 and 40 minutes significantly decreased during the second 24-day treatment period as compared with the mean perceived pain during the first 24 SC injections for all injection sites (p < .05 for all comparisons). The mean VAS score during the second 24-day evaluation period was below 1 after 5-and 40-minute postinjection (Figure 2). The analyses regarding the third 24-day treatment period initially considered could not be performed because of the low sample available to obtain reliable data, given that most patients maintained minimal pain scores by the second 24-day evaluation period.
FIGURE 2.
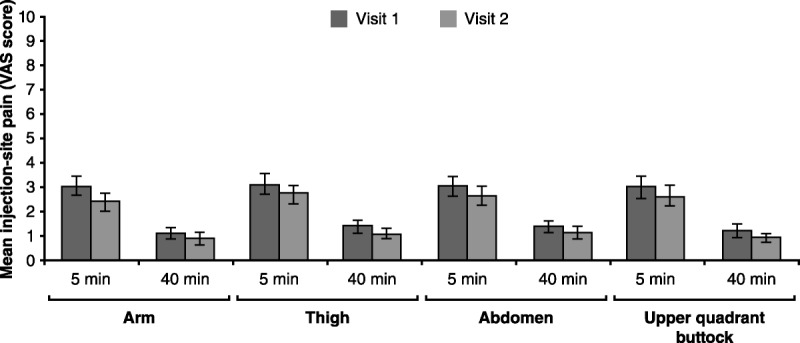
Median VAS Scores (and Standard Error) During the First and Second 24-Day Treatment Periods at 5 and 40 Minutes Postinjection in the Arm, Thigh, Abdomen, and Upper Quadrant of the Buttock
The proportion of pain-free patients during the first 24 days of treatment ranged from 5% in the arms to 14% in the abdomen after 5 minutes of injection and between 32% in the thigh to 40% in the abdomen 40 minutes after injection. In addition, the percentage of patients showing no pain after 5-and 40-minute postinjection during the second treatment period was between 10% and 19% and ranged from 35% to 48% in the thigh and in the upper quadrant of the buttock, respectively. The perceived pain during the first 24-day period was <5 in over 80% of patients at 5-minute postinjection in all body sites and in 97%–100% after 40 minutes of injection. The proportion of patients reporting VAS scores >7 ranged from 6% and 9% after 5 minutes of injection at both 24-day treatment periods. None of the patients reported injection pain >7 after 40-minute postinjection during the course of treatment periods evaluated.
Regarding the analysis of the perceived pain in relation to the injection depths assessed (4, 6, and 8 mm), we found that it was below 4 and 1.5 at 5-and 40-minute postinjection, respectively, for all the injection sites and across both 24-day treatment periods evaluated (Figure 3). In addition, there were no significant differences in the pain on injection between the different injection depths applied at any of the time points evaluated and during the treatment periods assessed for each body area (data not shown).
FIGURE 3.
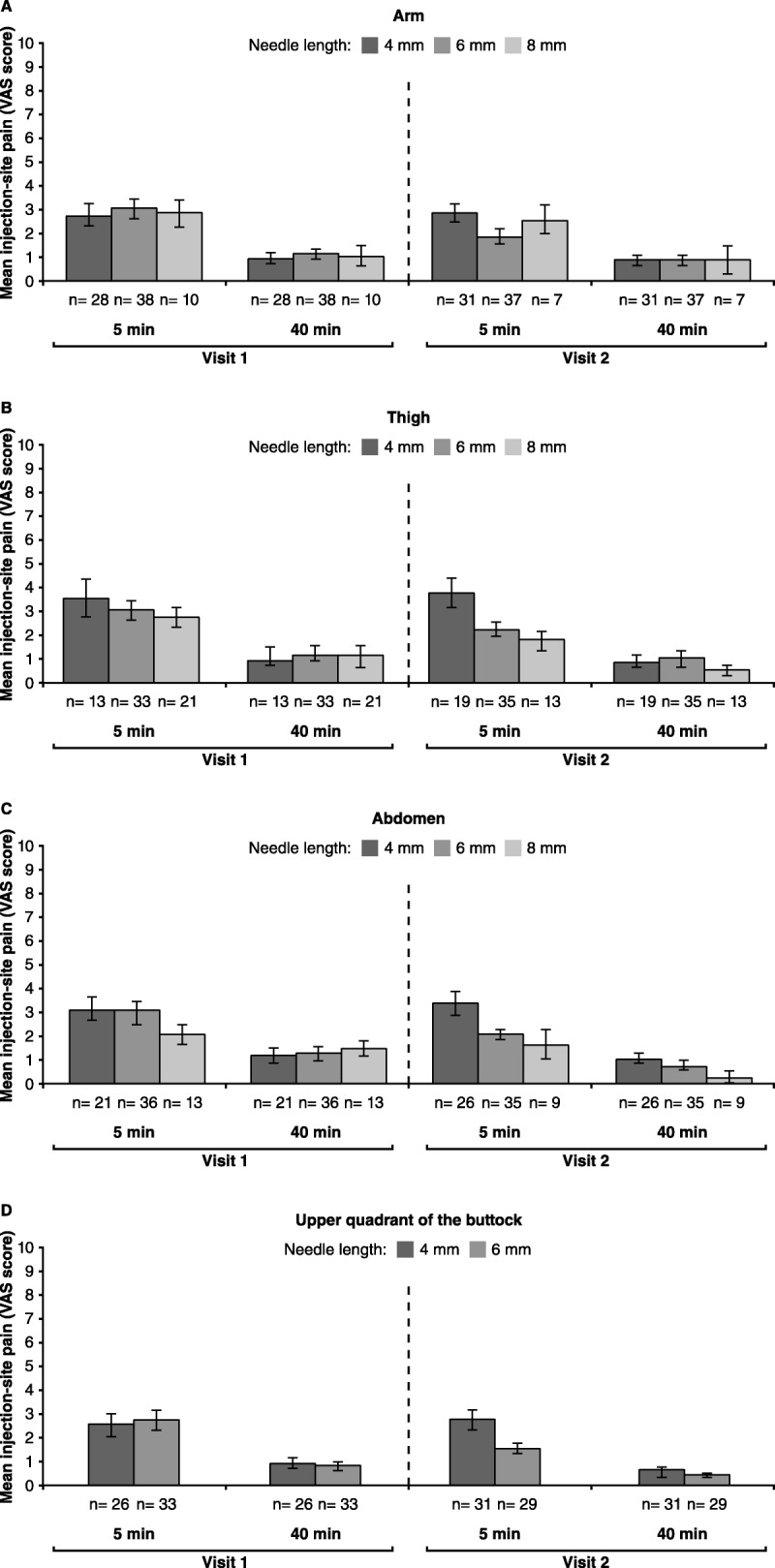
Mean VAS Scores (and Standard Error) Reported with the 4-, 6-, and 8-mm Needles at 5 and 40 Minutes Postinjection and During the First and Second Treatment Periods (Visits 1 and 2) in the Arm (A), Thigh (B), Abdomen (C), and Upper Quadrant of the Buttock (D)
Note. There was only 1 patient who used the 10-mm needle in the study because of a SCT > 50 mm in the thigh (B). In addition, D shows the VAS scores for the 4- and 6-mm needle given that only 3 patients at visit 1 and 2 patients at visit 2 used the 8-mm needle in the upper quadrant of the buttock and data would not be comparable. (B) There were 35 evaluable patients for pain at the thigh: 33 patients applied injections according to the needle depth adjustment protocol in both thighs, and 2 patients used the suitable depth in only one thigh (6- and 8-mm needle, respectively). (C) There were 37 evaluable patients for pain at the abdomen: 33 patients applied the recommended needle depth in both sides of the abdomen, and 4 patients used the adequate needle depth in only one side (3 patients: 6-mm needle; 1 patient: 8-mm needle). (D) There were 33 evaluable patients for pain at the upper quadrant of the buttock: 29 patients used the recommended needle depth for both sides, and 4 patients applied the adequate needle depth in only one side (6-mm needle).
Analysis of Other LISRs
The proportion of patients who reported any occurrence of LISRs during the two consecutive 24-day evaluation periods was 76.3% (n = 29). The LISRs after self-injection, recorded in the patient diary, occurred in 27 (71.1%) during the first 24-day treatment period. The percentage of patients experiencing LISRs was reduced to 55.3% throughout the second 24-day period. The most common LISRs reported during the first 24 days of treatment were swelling (63.2%), itching (47.4%), and redness (44.7%). These LISRs were also the most frequent LISRs reported for the second treatment period but in a lower proportion: swelling (44.7%), redness (42.1%), and itching (34.2%).
Discussion
This pilot project is the first to evaluate the effect on pain of certain needle lengths for SC injection of GA selected on the basis of individual anthropometric characteristics of patients with MS. One of the main findings of our project is that the mean pain on injection, as assessed using a VAS diary, was below 3 after 5 and 40 minutes of SC GA injection, across both 24-day evaluation periods and for all the injection sites. These findings may therefore suggest that the self-injection program assessed in this pilot experience may be suitable to minimize pain on the injection site in naive patients with MS that initiate GA therapy. Furthermore, we have shown here that there were no significant differences in the pain between the dominant and nondominant sides of the body, where a deeper depth was assigned, in none of the injection sites or time points evaluated. Overall, these findings could indicate that the recommendation regarding the use of a shorter needle length in the dominant body side would be suitable for SC self-injection in all anatomical areas commonly used for injection.
Moreover, it should be noted that the pain reported at 5-minute postinjection was significantly superior to that seen after 40 minutes of injection, where the median pain was below 1 for all body sites. The needle depths selected in this program therefore enable a significant reduction of pain in a very short time after the injection is applied. Furthermore, pain on injection diminished over the course of the treatment, and it was even lower during the second 24-day treatment period. These results could be related to the optimization of the injection technique by patients and the continuous support provided by MS nurses, which play a key role in the management of LISRs during MS therapy, particularly with the widespread introduction of injecting devices for SC DMT delivery. Indeed, although detailed information on injection preparation and administration was not formally collected, the low degree of pain reported suggests that patients may have received proper training on injection preparation and administration at treatment initiation.
In addition, although MS nurses could decide to reduce the initially assigned needle length if mean VAS scores was ≥6 after the first 24-day treatment period, it is interesting to note that only a small proportion of patients required needle length reductions after the first 24 injections. Hence, these results may suggest that the protocol of injection depth adjustment recommended in this project may be adequate from the start of SC GA therapy.
Of note, there were a few isolated cases that recorded nearly the maximum of pain in the VAS diary in all injection sites and regardless of the time after injection (5 and 40 minutes) and the time on treatment. A possible explanation for this finding could be that these patients may have experienced anxiety about the use of needles that could have increased the perceived pain at the time of injection. Indeed, it has been suggested that anxiety may increase the extent to which patients are aware of side effects and also their perception of the magnitude of such event (Lugaresi et al., 2012). Furthermore, to interpret these isolated data, it should be born in mind that pain is a subjective issue and, whenever these patients felt any pain, they scored the maximum. These patients would therefore not be representative of the general population considering the notably lower VAS scores reported by most patients.
Overall, the recommended program evaluated appears to be adequate for DMT-naive patients to maintain minimal pain on injections during the course of SC GA therapy. Moreover, the fact that there were no differences between the 4-, 6-, and 8-mm needle with respect to injection pain could suggest the appropriateness of each needle length for the SCT where it was used. Of note, the 6-mm needle was the only one evaluated in nearly all patients given that all but one patient had SCT < 50 mm and considering that it was also used in those patients with SCT of <25 or 25–50 mm according to the injection depth adjustment program. However, the lower proportion of patients using the 8-mm needle, particularly in the arms and upper quadrant of the buttock, does not allow us to draw firm conclusions regarding its suitability in all injection areas. Therefore, the findings of this pilot experience may suggest that the 6-mm depth could be a suitable standard depth to be used in all injection areas to maintain a low degree of pain in case that the skinfold measure cannot be performed. However, we cannot support that the 6-mm needle may offer the same benefit regarding pain in obese patients as this population was not represented in our project. Further studies would be required to better define the patient groups that might derive less pain from certain needle lengths of SC GA injection.
Finally, safety does not appear to be compromised by the recommended protocol for self-injection. Indeed, the incidence of LISRs in our project (76%) is similar to that reported for GA injections (70%; Copaxone, 2009). Although the wheals seemed to be superior in size at treatment initiation, they were normally reduced during the course of treatment. However, a long-term evaluation will be needed to adequately assess the safety related to the use of different needle lengths for the SC injection of GA.
The findings of this program must be considered in the context of their limitations. The main limitations of our project arise from the small population analyzed and the design as a pilot experience. Moreover, a further potential limitation of this project is the lack of control group in which the project program of needle depth adjustment based on SCT was not applied. However, the lack of control may be at least in part overcome by the fact that the patients are their own controls in this project.
Despite the abovementioned limitations, to our knowledge, this project is the first to examine the effect of different needle lengths selected according to the SCT on the incidence of injection site pain in patients naive to SC MS therapy who initiate self-injection of daily DMT. Hence, although modest, our findings might offer a welcome addition considering the lack of information on strategies to minimize pain to SC injections of MS therapy. Indeed, considering that the perceived pain caused by SC drug delivery may negatively affect compliance and acceptance of MS therapy, this project may represent the initial steps toward addressing an important issue for patients with MS who are trying to incorporate the SC self-injection process into their daily lives. Moreover, although this assistential program has been evaluated with GA, further studies may assess self-injection programs based on anthropometric characteristics of patients with other injecting devices used for the frequent SC delivery of DMT. Furthermore, this project may offer a basis for further clinical investigation of needle length selection according to SCT and other particular characteristics of patients to suit individual needs of patients with MS in an attempt to increase adherence to DMT.
In conclusion, this project suggests that the adjustment of injection depth of SC GA device according to SCT of body injection areas is suitable to maintain a low degree of injection pain and a manageable profile of LISRs in patients with MS who are new to DMT. Moreover, our findings may also indicate that the use of the needle lengths of 6 mm or shorter could be adequate with respect to injection pain in adult patients with MS with SCT < 50. Larger studies will be needed to assess the optimal depth according to the SCT of each injection area to create a body map of depth requirements depending on anthropometric characteristics of patients that may lead to the individualization of SC GA therapy.
Acknowledgment
The authors would like to acknowledge TEVA Pharma S.L.U. for supporting the study.
Footnotes
The authors declare that, during their professional career, they have acted as a board member and/or have received honoraria as a consultant and/or speakers’ fees and support for traveling to meetings for the study or other purposes from one or more of the following companies: Novartis, Biogen, Merck, Teva, Glaxo, and Genzyme. P. Francoli and R. Sánchez-De la Rosa belong to the Medical & HEOR Department of TEVA Pharma Ltd. This study was funded by TEVA Pharma S.L.U. Otherwise, the authors declare no conflicts of interest.
References
- Al-Sabbagh A., Bennet R., Kozma C., Dickson M., Meletiche D. (2013). Medication gaps in disease-modifying therapy for multiple sclerosis are associated with an increased risk of relapse. Journal of Neurology, 255, S79. [Google Scholar]
- Beer K., Muller M., Hew-Winzeler A. M., Bont A., Maire P., You X., Curtius D. (2011). The prevalence of injection-site reactions with disease-modifying therapies and their effect on adherence in patients with multiple sclerosis: An observational study. BMC Neurology, 11, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A., Coles A. (2008). Multiple sclerosis. Lancet, 372, 1502– 1517. [DOI] [PubMed] [Google Scholar]
- Copaxone. (2009). Summary of products characteristics. Retrieved from http://www.aemps.gob.es/cima/especialidad.do?metodo=verFichaWordPdf&codigo=64205&formato=pdf&formulario=FICHAS [Google Scholar]
- Costello K., Kennedy P., Scanzillo J. (2008). Recognizing nonadherence in patients with multiple sclerosis and maintaining treatment adherence in the long term. Medscape Journal of Medicine, 10, 225. [PMC free article] [PubMed] [Google Scholar]
- Devonshire V., Arbizu T., Borre B., Lang M., Lugaresi A., Singer B., Cornelisse P. (2010). Patient-rated suitability of a novel electronic device for self-injection of subcutaneous interferon beta-1a in relapsing multiple sclerosis: An international, single-arm, multicentre, Phase IIIb study. BMC Neurology, 10, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney M. A., Arce C. H., Byron K. J., Hirsch L. J. (2010). Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: Implications for needle length recommendations. Current Medicine Research Opinion, 26, 1519– 1530. [DOI] [PubMed] [Google Scholar]
- Glenski S., Conner J. (2009). 29 gauge needles improve patient satisfaction over 27 gauge needles for daily glatiramer acetate injections. Drug Healthcare Patient Safety, 1, 81– 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber A., Bozzato G. B., Vedrine L., Prais W. A., Berube J., Laurent P. E. (2008). A novel needle for subcutaneous injection of interferon beta-1a: Effect on pain in volunteers and satisfaction in patients with multiple sclerosis. BMC Neurology, 8, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. S., Pollock M. L. (1978). Generalized equations for predicting body density. British Journal of Nutrition, 40, 497– 504. [DOI] [PubMed] [Google Scholar]
- Jacobs L. D., Cookfair D. L., Rudick R. A., Herndon R. M., Richert J. R., Salazar A. M., Whitham R. H. (1996). Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Annals of Neurology, 39, 285– 294. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Brooks B. R., Cohen J. A., Ford C. C., Goldstein J., Lisak R. P., Schiffer R. B. (1995). Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: Results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology, 45, 1268– 1276. [DOI] [PubMed] [Google Scholar]
- Lugaresi A., Durastanti V., Gasperini C., Lai M., Pozzilli C., Orefice G., Millefiorini E. (2008). Safety and tolerability in relapsing-remitting multiple sclerosis patients treated with high-dose subcutaneous interferon-beta by Rebiject autoinjection over a 1-year period: The CoSa study. Clinical Neuropharmacology, 31, 167– 172. [DOI] [PubMed] [Google Scholar]
- Lugaresi A., Florio C., Brescia-Morra V., Cottone S., Bellantonio P., Clerico M., Paolillo A. (2012). Patient adherence to and tolerability of self-administered interferon beta-1a using an electronic autoinjection device: A multicentre, open-label, phase IV study. BMC Neurology, 12, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses H., Jr., Brandes D. W. (2008). Managing adverse effects of disease-modifying agents used for treatment of multiple sclerosis. Current Medicine Research Opinion, 24, 2679– 2690. [DOI] [PubMed] [Google Scholar]
- Polman C. H., Reingold S. C., Banwell B., Clanet M., Cohen J. A., Filippi M., Wolinsky J. S. (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology, 69, 292– 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman C. H., Wolinsky J. S., Reingold S. C. (2005). Multiple sclerosis diagnostic criteria: Three years later. Multiple Sclerosis, 11, 5– 12. [DOI] [PubMed] [Google Scholar]
- Rio J., Porcel J., Tellez N., Sanchez-Betancourt A., Tintore M., Arevalo M. J., Montalban X. (2005). Factors related with treatment adherence to interferon beta and glatiramer acetate therapy in multiple sclerosis. Multiple Sclerosis, 11, 306– 309. [DOI] [PubMed] [Google Scholar]
- Teva Neuroscience. (2013). Your Copaxone injection guide . Retrieved from http://copaxone.com/pdfs/Injecting_Guide.aspx [Google Scholar]
- The IFNB Multiple Sclerosis Study Group. (1993). Interferon beta-1b is effective in relapsing-remitting multiple sclerosis: I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology, 43, 655– 661. [DOI] [PubMed] [Google Scholar]
- The PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. (1998). Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet, 352 (9139), 1498– 1504. [PubMed] [Google Scholar]
- Treadaway K., Cutter G., Salter A., Lynch S., Simsarian J., Corboy J., Frohman E. M. (2009). Factors that influence adherence with disease-modifying therapy in MS. Journal of Neurology, 256, 568– 576. [DOI] [PubMed] [Google Scholar]


