Abstract
Background
Chemotherapy-induced peripheral neuropathy (CIPN) is a common toxicity secondary to chemotherapy. Genetic factors may be important in predisposing patients to this adverse effect.
Patients and Methods
We studied 950 primary lung cancer patients, who received platinum or platinum-combination drug chemotherapy and who had DNA available for study. We analyzed epidemiological risk factors in 279 CIPN patients and 456 non-CIPN patients and genetic risk factors in 141 CIPN patients and 259 non-CIPN patients. The risk factors studied included demographic, diagnostic, and treatment data, as well as 174 tag SNPs (single nucleotide polymorphisms) across 43 candidate genes in the glutathione, cell cycle, DNA repair, cell signaling, and apoptosis pathways.
Results
Patients who had diabetes mellitus were more likely to have CIPN (p=0.0002). Other epidemiologic risk factors associated with CIPN included number of cycles (p=0.0004) and type of concurrent chemotherapy (p<0.001) . SNPs most associated with CIPN were in glutathione peroxidase 7 (GPX7) gene (p values 0.0015 and 0.0028, unadjusted and adjusted) and in ATP-binding cassette sub-family C member 4 (ABCC4) gene (p values 0.037 and 0.006, unadjusted and adjusted). We also found other suggestive associations in methyl-o-guanine-methyl-transferase (MGMT) and glutathione-S-transferase (GST) isoforms.
Conclusions
Epidemiological and genetic risk factors associated with CIPN in this cohort, included the type of chemotherapy drug, intensity of chemotherapy treatment, and genes known to be associated with chemotherapy resistance. These findings suggest that differentiating between cytotoxic and neurotoxic mechanisms of chemotherapy drugs is challenging but represents an important step toward individualized therapy and improving quality of life for patients.
Keywords: Chemotherapy induced peripheral neuropathy, genetic, single nucleotide polymorphism, platinum drugs, paclitaxel, diabetes
Introduction
Combination chemotherapy with a platinum drug or a platinum drug combined with a taxane is used for many types of cancer including ovarian, testicular, lung, breast, and colorectal [1, 2]. Chemotherapy-induced peripheral neuropathy (CIPN) is a common toxicity associated with this treatment [3]. It is typically a sensory neuropathy that may necessitate dose reduction and lead to impaired quality of life [3-5]. Treatment and preventive strategies have had limited success [6-10]. CIPN is the major dose limiting toxicity for this common chemotherapy regime. The mechanism underlying neurotoxicity is not understood. Apoptosis of primary sensory dorsal root ganglion (DRG) neurons due to formation of platinum adducts in nuclear and mitochondrial DNA is a central mechanism in rodent models [11-19]. This results in mitochondrial dysfunction and disruption in cell cycle [14, 15, 19]. Thus the mechanisms of neurotoxicity and cancer cytotoxicity have many similarities and separating the beneficial cytotoxic effect from the neurotoxic effect may be difficult.
A different approach to preventing neurotoxicity may result from understanding why some patients develop CIPN, while others do not. This can be approached from an epidemiological and genetic perspective. A large study using Medicare insurance records identified that the number of cycles of chemotherapy, patient age and cumulative drug dose, were associated with the risk of developing CIPN [2, 20-22]. Genetic risk factors have been associated with polymorphisms in glutathione-S-transferase (GST) gene family isoforms [2, 20-22], especially GSTP-1 [2, 20-22] signaling pathways, metal transporters, growth factors, and DNA repair genes [2] [11, 23-27].
We now report both an epidemiological and multi-gene association study in a cohort of 950 primary lung cancer patients who received platinum and platinum-taxane chemotherapy. Medical records were available for identifying demographic and epidemiologic data for all of these patients. DNA samples were available for the genetic study. We used a candidate pathway-based approach because it is a hypothesis driven approach to the use of single nucleotide polymorphism data. This contrasts with genome-wide association studies (GWAS) that may identify polymorphisms in genes not mechanistically known to be associated with metabolism of the specific drug but also identify many more false-positive associations. The genes and pathways chosen were based on their known involvement with metabolism that focuses on glutathione, cell cycle, cell signaling, apoptosis, and DNA repair pathways, all of which have been implicated in animal model studies of CIPN [2, 11, 23-27].
Methods and Materials
Patient Cohort and Inclusion Criteria
Patients included in this study were chosen from a previously described, larger cohort of primary lung cancer patients [28-32]. Patients had a diagnosis of primary lung cancer in the electronic medical record system and were seen at Mayo Clinic between 1997 and 2006. Patients who were treated with at least one dose of platinum chemotherapy were included. A Mayo Clinic chest pathologist verified lung cancer diagnoses. All patients consented to participate in the study and to have their medical record information reviewed by our study team. 950 patients met the inclusion criteria for the study (Table 1).
Table 1.
Inclusion and Exclusion Criteria for Nested Case Control Study
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1. Diagnosis of primary lung cancer 2. Seen at Mayo Clinic for primary lung cancer between 1997 and 2006 3. Primary lung cancer pathology confirmed by Mayo Clinic pathologist 4. Received at least one dose of platinum (Cisplatin, Carboplatin) for primary lung cancer 5.Genotyped for SNPs on peripheral whole blood 6.Consented for use of medical records and study participation |
1. Toxicity data not available throughout and after all platinum chemotherapy 2.CIPN developed greater than 6 months after last dose of platinum chemotherapy 3.Unclear or uncommon CIPN Symptoms noted (i.e. not primarily sensory neuropathy, significant motor deficit, not primarily stocking and glove distribution, etc) and excluded by the neurologists on our study team |
Data Abstraction
Trained study personnel abstracted the data. Demographic and comorbidity data were abstracted from medical records, the social security death index, and annual follow-up questionnaires. Diagnostic data, treatment data, and CIPN status were abstracted from Mayo Clinic medical records and/or outside medical records. Demographic data included age, gender, ethnicity, and performance score status. Treatment data included type of platinum drug, type of other chemotherapy drugs, number of cycles, and response to treatment. Diagnostic data included type, stage, and grade of lung cancer. All data were entered and maintained in our secure SAS-DMS database (SAS Institute Inc., Cary, NC)
Identification of peripheral neuropathy
For this study CIPN was defined as distal symmetrical sensory symptoms or signs in a stocking or glove and stocking distribution. Patients were determined to have CIPN if their medical record mentioned paresthesia and/or peripheral neuropathy as a toxicity using the U.S. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) [33]. This defines CIPN as a disorder characterized by functional disturbances of sensory neurons resulting in abnormal cutaneous sensations of tingling, numbness, pressure, cold, and warmth that are experienced in the absence of a stimulus. CIPN must have been noted by a medical professional (M.D., R.N., or P.A.) in direct care of the patient. Neuropathy symptoms must not have been noted before starting chemotherapy nor have been first noted later than six months after the last dose of chemotherapy. If neurological symptoms were not typical for a primary sensory neuropathy the study neurologists re-reviewed the patient’s medical record and determined eligibility. All CIPN negative patients had to have toxicity data available throughout their chemotherapy and at least 6 months follow-up. Patients with unknown CIPN status were defined as those who had neuropathic symptoms prior to chemotherapy, those who had unusual symptoms and/or distribution of symptoms or had neuropathy first noted more than six months after the last dose of platinum chemotherapy. Other neurologic diseases included the presence of CNS metastases or any pre-existing neurologic disorder such as stroke.
Patients with diabetes, with or without neuropathy were excluded from the genetic association study but were included in the epidemiological analysis to determine whether diabetes was a risk factor for development of neuropathy.
Epidemiological Study and Statistical Analyses
Patients were stratified into four comorbidity groups: patients with central nervous system (CNS) metastases, patients with diabetes mellitus (DM), patients with any other neurological disease (e.g. stroke) and patients who had none of these comorbidities. The number and percentage of patients with and without CIPN in each group were noted, and odds ratios, 95% confidence intervals, and p values were calculated for each of these groups after comparison with the no comorbidities group (reference group).
Patients with CIPN and with no comorbidities (N=157) were then compared with patients without CIPN and with no comorbidities (N=292) for common demographic, diagnostic, and treatment risk factors. We performed a secondary analysis on the larger group of patients (N=279 with CIPN and N=456 without CIPN) which included patients with comorbidities but excluded those with unknown CIPN status (figure 1). These demographic, diagnostic, and treatment data were analyzed as appropriate using Pearson's chi squared or Fisher's exact tests for categorical variables, and unpaired t tests for quantitative variables. The magnitudes of differences between study groups were summarized using odds ratios and 95% confidence intervals. All analyses were performed on JMP 9.0 and SAS 9.3 (SAS Institute Inc., Cary, N.C., U.S.A.).
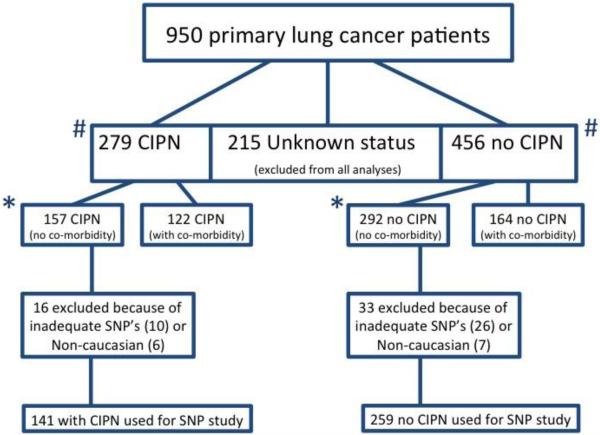
Figure 1.
Flowchart of patient cohort, nested case control study. 157 patients with CIPN and no co-morbidities * and 292 patients without CIPN and no co-morbidities* were used in the primary analysis in the epidemiological study. The total group of 279 CIPN and 456 no CIPN # were used in a secondary, confirmatory analysis. For the genetic study only the patients who either definitely had (n=141) or did not have (n=259) CIPN and who had usable SNP data were used.
Genetic Pathway Based Association Study
For the genetic association study, only CIPN positive (N=141) and CIPN negative (N=259) patients who had no major comorbidities (i.e. diabetes mellitus, any neurological disease, CNS metastases) and who were of Caucasian/European ancestry were included to limit confounding prognostic factors and population stratification (figure 1.).
Patients were genotyped by the Mayo Clinic Genotyping Core facility using an Illumina GoldenGate SNP array on peripheral blood DNA. A total of 174 SNPs were analyzed after passing quality control. The 174 SNPs were within 43 genes in 5 major pathways: glutathione, DNA repair, cell cycle, apoptosis, and cell signaling/metabolic. See Supplementary Table A for the SNPs genotyped and analyzed. Both univariate and multivariate analyses were performed within Plink 1.07 (Harvard University, Cambridge, MA, U.S.A.) and SAS 9.3 (SAS Institute Inc., Cary, NC, U.S.A.) [34]. In univariate analyses, additive models were utilized for the primary assessment of each SNP using Fisher's exact tests. Odds ratio (OR) and 95% confidence interval (CI) were also calculated via allelic tests. To correct for multiple comparisons, Q values were calculated with the false discovery rate (FDR) set at 20%. In multivariate analyses, logistic regression models were utilized with a weighted propensity score method to control for confounding effects of known clinical outcome predictors. The patient-specific variables used to construct the propensity scores include age at platinum chemotherapy, gender, and smoking status; cell type, grade, and stage of lung cancer; dose and type of platinum drug and surgical resection. Additional covariates adjusted in the models include having radiation therapy or not, total number of cycles of platinum chemotherapy, and whether receiving a taxane or not. As a step of verification, candidate genes with significant SNPs were further analyzed by haplotype.
Results
Epidemiological Study
Of the 950 patients included in this cohort, a total of 279 patients had CIPN, 456 patients did not have CIPN, and 215 patients had unknown CIPN status (figure 1). Table 2 shows the p values and odds ratios of CIPN in each group compared to the reference group with no comorbidities. Patients who had diabetes mellitus were more than twice as likely to have CIPN [p= 0.0002, OR=2.41 (1.51-3.86)]. Neither the CNS metastases nor neurological disease groups were significantly associated with the likelihood of developing CIPN. Similar results were found in the secondary analysis when the patients with comorbidities were compared (data not shown).
Table 2.
Number (percent) of patients in each comorbidity category with regards to CIPN status.
| Category | CIPN N=279 | No CIPN N=456 | Unknown CIPN N=215 | P value [Odds Ratio (95% Confidence Interval)] |
|---|---|---|---|---|
| No Comorbidities | 157 (56.3) | 292 (64.0) | 142 (66.1) | Reference Group |
| Diabetes Only | 48 (17.2) | 37 (8.1) | 17 (7.9) | 0.0002 [2.41 (1.51-3.86)] |
| CNS Mets Only | 29 (10.4) | 47 (10.3) | 19 (8.8) | 0.59 [1.15 (0.69-1.90)] |
| Neuro Disease Only | 36 (12.9) | 46 (10.1) | 25 (11.6) | 0.12 [1.46 (0.90-2.35)] |
| Multiple Categories | 9 (3.2) | 34 (7.5) | 12 (5.6) | Not done |
Patients who had diabetes mellitus were more likely to have CIPN than not have CIPN (p<0.002). DM=diabetes mellitus as comorbidity. Neuro=any neurological disease as comorbidity. CNS=had central nervous system metastases. Total patients in the whole cohort=950.
Primary and secondary analyses of the demographic information demonstrated no significant differences in age at cancer diagnosis, age at chemotherapy or gender associated with the development of CIPN in the no comorbidities group or in the whole cohort (Supplement table E). There were also no significant differences in the risk of developing CIPN in either the primary (with no co-morbidities) or secondary (with co-morbidities) analyses related to tumor type, stage, grade or response to chemotherapy (Supplement table F).
Treatment risk factors associated with CIPN included whether or not the patient received a taxane drug with platinum [p<0.001, OR=2.71 (1.58-4.64)]. Thus patients treated with a platinum drug and a taxane were more than twice as likely to develop CIPN as those treated with only a platinum drug. The number of cycles of chemotherapy was also associated with increased risk for CIPN. In the primary analysis, patients with CIPN had received on average 5.7 (SD 2.8) cycles compared to 5.0 (SD 3.1) cycles for those without CIPN. When the patients with comorbidities were added in (secondary analysis) this effect became greater. Patients with CIPN received 5.6 (SD 2.8) cycles compared to 4.7 (SD 2.9) cycles for those without CIPN (p=.0004). Patients receiving concurrent radiation therapy were less likely to develop CIPN [p<0.001, OR=0.33 (0.20-0.53)]. These risk factors were apparent both in the stratified as well as the whole cohort. No other treatment factors were found to be associated with CIPN in this cohort.
Genetic Association Study: SNP Analyses
Sixteen CIPN and 33 no CIPN patients were excluded from the genetic analyses due to technically inadequate SNP data or non-Caucasian/European ancestry (n=13). A total of 141 patients with CIPN and 259 without CIPN were analyzed for the genetic association study (figure 1). Several SNPs showed associations with the development of CIPN during platinum or platinum-taxane chemotherapy.. The most significant SNPs from this study (i.e. SNPs with either adjusted or unadjusted p values <0.10) are summarized in Table 3, and a summary of all SNPs analyzed are shown in Supplementary Tables B and C. The most significant SNP, rs3753753, was within the GPX7 gene [univariate p value of 0.0015 and multivariate p value of 0.0028, adjusted OR=1.70 (1.20-2.40)]. This SNP was study-wide significant (q=0.155) during univariate analysis. SNP, rs1729786, within the ABCC4 gene also showed association with CIPN status [p values: 0.038 unadjusted and 0.006 adjusted, adjusted OR: 0.630 (0.45-0.87)]. Other genes that showed modest association with CIPN included GSTA4 (glutathione-S-transferase alpha 4), ABCC2 (ATP-Binding Cassette Sub-Family C member 2), MGMT, XPC (Xeroderma pigmentosum, complementation group C), MSH3 (MutS Homolog 3), RAD51, and RRMI (Ribonucleosidediphosphate reductase large subunit).. For haplotype analyses, a total of 39 haplotypes were analyzed. The most associated haplotype with CIPN was in GPX7, providing further evidence of association of GPX7 with CIPN (Supplementary Table D).
Table 3.
Most significant SNPs in association study by univariate and multivariate analyses (p<0.10).
| SNP | Gene | Pathway | P Value Additive | OR Unadjusted (95% CI) | P Value Adjusted | OR Adjusted (95% CI) |
|---|---|---|---|---|---|---|
| rs3753753 | GPX7 | Glutathione | 0.0015* | 1.59 (1.15-2.19) | 0.0028 | 1.70 (1.20-2.40) |
| rs1729786 | ABCC4 | Glutathione | 0.0377 | 0.68 (0.50-0.92) | 0.0058 | 0.63 (0.45-0.87) |
| rs11016884 | MGMT | DNA repair | 0.0556 | 0.67 (0.49-0.93) | 0.0104 | 0.64 (0.46-0.90) |
| rs2733537 | XPC | Glutathione | 0.0534 | 1.43 (1.06-1.93) | 0.0701 | 1.34 (0.98-1.85) |
| rs10764901 | MGMT | DNA repair | 0.0697 | 1.45 (1.05-2.00) | 0.0582 | 1.39 (0.99-1.94) |
| rs3092981 | RAD51 | DNA repair | 0.0726 | 8.67 (0.88-85.12) | 0.0445 | 0.59 (0.35-0.99) |
| rs2268166 | RRM1 | DNA repair | 0.0333 | 0.44 (0.20-0.98) | 0.0539 | 0.44 (0.19-1.01) |
| rs26279 | MSH3 | DNA repair | 0.1457 | 1.39 (1.01-1.91) | 0.0467 | 1.39 (1.01-1.93) |
| rs3756980 | GSTA4 | Glutathione | 0.0568 | 0.68 (0.45-1.01) | 0.1069 | 0.69 (0.44-1.08) |
| rs12243174 | MSH2 | DNA repair | 0.1696 | 1.37 (0.98-1.92) | 0.0726 | 1.37 (0.97-1.93) |
| rs4715352 | GSTA5 | Glutathione | 0.0937 | 1.37 (0.98-1.93) | 0.0846 | 1.38 (0.96-1.99) |
| rs1047635 | GPX7 | Glutathione | 0.1869 | 1.31 (0.98-1.75) | 0.0629 | 1.35 (0.98-1.84) |
| rs6492763 | ABCC4 | Glutathione | 0.1599 | 1.30 (0.96-1.75) | 0.0406 | 1.42 (1.02-1.98) |
| rs17189561 | ABCC4 | Glutathione | 0.2852 | 0.70 (0.46-1.07) | 0.0505 | 0.65 (0.42-1.00) |
| rs3136326 | MSH6 | DNA repair | 0.2695 | 1.43 (0.93-2.18) | 0.0933 | 1.46 (0.94-2.28) |
=study-wide significant (q=0.1556) after false discovery rate set at <20%.
Discussion
Peripheral neuropathy is the most common dose-limiting toxicity for many chemotherapy agents. It occurs in 30-40% of patients treated with platinum and taxane drugs [3]. Using data from IMS Health (Danbury, Connecticut) it has been calculated that between 390,470 and 465,441 patients develop CIPN each year with an annual cost of $2.39 – 2.73 billion dollars. Multiple treatment approaches to protect against CIPN have failed [10]. An alternative approach is to examine risk factors for an individual patient to develop neuropathy and then incorporate that into their treatment planning. In this study we used epidemiological and genetic factors to explore the feasibility of this approach
Patients who had diabetes mellitus were more likely to develop CIPN. It is important to note that these patients did not have symptomatic neuropathy before treatment began. This association has been previously suggested but not demonstrated. The association could be due to existing asymptomatic diabetic neuropathy since up to 50% of diabetic patients may have asymptomatic neuropathy [35]. From the practical perspective, oncologists and patients with diabetes should be informed that they are more likely to develop symptomatic CIPN.
We confirmed the previously reported association between the number of chemotherapy cycles [2] and risk of developing neuropathy. This apparent dose-response relationship suggests that CIPN is not due to a single patient related factor but rather to an aggregation of host factors that are common in the population. The observation that patients taking two neurotoxic drugs, a platinum and a taxane drug, are at 2.7 times greater risk of developing CIPN than patients on one drug further supports the proposal that all patients are at risk, although it is not understood why the 40% who develop neuropathy are susceptible. The observation that exposure to radiation appeared to be protective against CIPN is probably artifactual, due to a confounding effect that patients receiving radiation are more likely to get treated with etoposide (a rarely neurotoxic agent) instead of the highly neurotoxic taxanes.
We also report several SNPs and genes associated with the development of CIPN. SNPs with the strongest association with CIPN in this study were in GPX7 and ABCC4; MGMT and GST family were less strongly associated. These genes have previously shown to be associated with platinum resistance in cancer cells [21, 23, 30, 32, 36-41]. Therefore, genetic factors playing a role in a cancer's response to platinum are also likely to influence the development of CIPN. Although we did not find associations with previously reported SNP's that have been associated with neuropathy, [42, 43] the ones identified here are in closely related pathways. The GPX gene family is known to have an impact on mitochondrial function and reactive oxygen species production and scavenging [41] an important potential mechanism for development of CIPN. Further, MGMT is a gene that repairs guanine adducts in DNA after exposure to alkylating agents such as cisplatin [37]. Therefore, the repair capacity of DNA adducts is likely at least a contributing factor in the development of CIPN.
Limitations of this study include sample size and limited number of genes and SNPs studied (174 tagSNPs), presence of unknown confounders and biases, and the limited generalizability due to the lack of non-Caucasian ethnic populations. In the future, studies assessing other genetic risk factors for neuropathy (e.g. the more than 60 genes identified as being associated with inherited neuropathies) may provide additional data for individualizing treatment.
In conclusion, several epidemiological risk factors associated with the development of CIPN were identified. The most important was diabetes mellitus. The identification of specific polymorphisms associated with increased risk of developing CIPN should further encourage genomic studies accompanied by careful phenotyping of patients. This approach has the potential for developing individualized treatment regimens based on genomic testing.
Supplementary Material
Highlights.
Cohort of 950 primary lung cancer patients treated with a platinum drug.
DNA and clinical data available for all patients.
40% developed peripheral neuropathy.
Diabetes and drug dose increased risk of neuropathy.
SNPs associated with glutathione metabolism and DNA repair associated with neuropathy.
Acknowledgements
We thank Mayo Clinic CCaTS (Center for Clinical and Translational Science) for support for this manuscript. We also thank Susan Ernst and Jane Meyer for their help in preparation and submission of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gershenson DM, Wharton JT, Herson J, Edwards CL, Rutledge FN. Single-agent cis-platinum therapy for advanced ovarian cancer. Obstet Gynecol. 1981;58(4):487–96. [PubMed] [Google Scholar]
- 2.Nurgalieva Z, Xia R, Liu CC, Burau K, Hardy D, Du XL. Risk of chemotherapy-induced peripheral neuropathy in large population-based cohorts of elderly patients with breast, ovarian, and lung cancer. Am J Ther. 2010;17(2):148–58. doi: 10.1097/MJT.0b013e3181a3e50b. [DOI] [PubMed] [Google Scholar]
- 3.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 4.Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15(29):3081–94. doi: 10.2174/092986708786848569. [DOI] [PubMed] [Google Scholar]
- 5.Cavaletti G. Peripheral neurotoxicity of platinum-based chemotherapy. Nat Rev Cancer. 2008;8(1):1p following 71. doi: 10.1038/nrc2167-c1. author reply 1p following. [DOI] [PubMed] [Google Scholar]
- 6.Pachman DR, Barton DL, Watson JC, Loprinzi CL. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther. 2011;90(3):377–87. doi: 10.1038/clpt.2011.115. [DOI] [PubMed] [Google Scholar]
- 7.Cavaletti G, Minoia C, Schieppati M, Tredici G. Protective effects of glutathione on cisplatin neurotoxicity in rats. Int J Radiat Oncol Biol Phys. 1994;29(4):771–6. doi: 10.1016/0360-3016(94)90565-7. [DOI] [PubMed] [Google Scholar]
- 8.Pace A, Giannarelli D, Galie E, Savarese A, Carpano S, Della Giulia M, et al. Vitamin E neuroprotection for cisplatin neuropathy: a randomized, placebo-controlled trial. Neurology. 2010;74(9):762–6. doi: 10.1212/WNL.0b013e3181d5279e. [DOI] [PubMed] [Google Scholar]
- 9.Pisano C, Pratesi G, Laccabue D, Zunino F, Lo Giudice P, Bellucci A, et al. Paclitaxel and Cisplatin-induced neurotoxicity: a protective role of acetyl-L-carnitine. Clin Cancer Res. 2003;9(15):5756–67. [PubMed] [Google Scholar]
- 10.Albers JW, Chaudhry V, Cavaletti G, Donehower RC. Interventions for preventing neuropathy caused by cisplatin and related compounds. The Cochrane database of systematic reviews. 2011;(2):CD005228. doi: 10.1002/14651858.CD005228.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dzagnidze A, Katsarava Z, Makhalova J, Liedert B, Yoon MS, Kaube H, et al. Repair capacity for platinum-DNA adducts determines the severity of cisplatin-induced peripheral neuropathy. J Neurosci. 2007;27(35):9451–7. doi: 10.1523/JNEUROSCI.0523-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ta LE, Espeset L, Podratz J, Windebank AJ. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology. 2006;27(6):992–1002. doi: 10.1016/j.neuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Alaedini A, Xiang Z, Kim H, Sung YJ, Latov N. Up-regulation of apoptosis and regeneration genes in the dorsal root ganglia during cisplatin treatment. Exp Neurol. 2008;210(2):368–74. doi: 10.1016/j.expneurol.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Bottone MG, Soldani C, Veneroni P, Avella D, Pisu M, Bernocchi G. Cell proliferation, apoptosis and mitochondrial damage in rat B50 neuronal cells after cisplatin treatment. Cell Prolif. 2008;41(3):506–20. doi: 10.1111/j.1365-2184.2008.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer SJ, McDonald ES, Gross L, Windebank AJ. Alterations in cell cycle regulation underlie cisplatin induced apoptosis of dorsal root ganglion neurons in vivo. Neurobiol Dis. 2001;8(6):1027–35. doi: 10.1006/nbdi.2001.0426. [DOI] [PubMed] [Google Scholar]
- 16.Gill JS, Windebank AJ. Cisplatin-induced apoptosis in rat dorsal root ganglion neurons is associated with attempted entry into the cell cycle. J Clin Invest. 1998;101(12):2842–50. doi: 10.1172/JCI1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: a potential mechanism for neurotoxicity. Neurobiol Dis. 2005;18(2):305–13. doi: 10.1016/j.nbd.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 18.McDonald ES, Windebank AJ. Cisplatin-induced apoptosis of DRG neurons involves bax redistribution and cytochrome c release but not fas receptor signaling. Neurobiol Dis. 2002;9(2):220–33. doi: 10.1006/nbdi.2001.0468. [DOI] [PubMed] [Google Scholar]
- 19.Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K, et al. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis. 2011;41(3):661–8. doi: 10.1016/j.nbd.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barahmani N, Carpentieri S, Li XN, Wang T, Cao Y, Howe L, et al. Glutathione S-transferase M1 and T1 polymorphisms may predict adverse effects after therapy in children with medulloblastoma. Neuro Oncol. 2009;11(3):292–300. doi: 10.1215/15228517-2008-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YC, Tzeng CH, Chen PM, Lin JK, Lin TC, Chen WS, et al. Influence of GSTP1 I105V polymorphism on cumulative neuropathy and outcome of FOLFOX-4 treatment in Asian patients with colorectal carcinoma. Cancer Sci. 2010;101(2):530–5. doi: 10.1111/j.1349-7006.2009.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009;8(1):10–6. doi: 10.1158/1535-7163.MCT-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14(5):1291–5. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 24.Scuteri A, Galimberti A, Maggioni D, Ravasi M, Pasini S, Nicolini G, et al. Role of MAPKs in platinum-induced neuronal apoptosis. Neurotoxicology. 2009;30(2):312–9. doi: 10.1016/j.neuro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Liu JJ, Jamieson SM, Subramaniam J, Ip V, Jong NN, Mercer JF, et al. Neuronal expression of copper transporter 1 in rat dorsal root ganglia: association with platinum neurotoxicity. Cancer Chemother Pharmacol. 2009;64(4):847–56. doi: 10.1007/s00280-009-1017-6. [DOI] [PubMed] [Google Scholar]
- 26.Fischer SJ, Podratz JL, Windebank AJ. Nerve growth factor rescue of cisplatin neurotoxicity is mediated through the high affinity receptor: studies in PC12 cells and p75 null mouse dorsal root ganglia. Neurosci Lett. 2001;308(1):1–4. doi: 10.1016/s0304-3940(01)01956-5. [DOI] [PubMed] [Google Scholar]
- 27.Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient Receptor Potential Vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol Pain. 2010;6:15. doi: 10.1186/1744-8069-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Z, Aubry MC, Deschamps C, Marks RS, Okuno SH, Williams BA, et al. Histologic grade is an independent prognostic factor for survival in non-small cell lung cancer: an analysis of 5018 hospital- and 712 population-based cases. J Thorac Cardiovasc Surg. 2006;131(5):1014–20. doi: 10.1016/j.jtcvs.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 29.Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128(1):452–62. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 30.Pankratz VS, Sun Z, Aakre J, Li Y, Johnson C, Garces YI, et al. Systematic evaluation of genetic variants in three biological pathways on patient survival in low-stage non-small cell lung cancer. J Thorac Oncol. 2011;6(9):1488–95. doi: 10.1097/JTO.0b013e318223bf05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang P, Ebbert JO, Sun Z, Weinshilboum RM. Role of the glutathione metabolic pathway in lung cancer treatment and prognosis: a review. J Clin Oncol. 2006;24(11):1761–9. doi: 10.1200/JCO.2005.02.7110. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Sun Z, Cunningham JM, Aubry MC, Wampfler JA, Croghan GA, et al. Genetic variations in multiple drug action pathways and survival in advanced stage non-small cell lung cancer treated with chemotherapy. Clin Cancer Res. 2011;17(11):3830–40. doi: 10.1158/1078-0432.CCR-10-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Department of Health and Human Services NIoH National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. 2010 [Google Scholar]
- 34.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817–24. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- 36.Moyer AM, Sun Z, Batzler AJ, Li L, Schaid DJ, Yang P, et al. Glutathione pathway genetic polymorphisms and lung cancer survival after platinum-based chemotherapy. Cancer Epidemiol Biomarkers Prev. 2010;19(3):811–21. doi: 10.1158/1055-9965.EPI-09-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maki Y, Murakami J, Asaumi J, Tsujigiwa H, Nagatsuka H, Kokeguchi S, et al. Role of O6-methylguanine-DNA methyltransferase and effect of O6-benzylguanine on the anti-tumor activity of cis-diaminedichloroplatinum(II) in oral cancer cell lines. Oral Oncol. 2005;41(10):984–93. doi: 10.1016/j.oraloncology.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Yan XD, Pan LY, Yuan Y, Lang JH, Mao N. Identification of platinum-resistance associated proteins through proteomic analysis of human ovarian cancer cells and their platinum-resistant sublines. J Proteome Res. 2007;6(2):772–80. doi: 10.1021/pr060402r. [DOI] [PubMed] [Google Scholar]
- 39.Funke S, Timofeeva M, Risch A, Hoffmeister M, Stegmaier C, Seiler CM, et al. Genetic polymorphisms in GST genes and survival of colorectal cancer patients treated with chemotherapy. Pharmacogenomics. 2010;11(1):33–41. doi: 10.2217/pgs.09.132. [DOI] [PubMed] [Google Scholar]
- 40.Scuteri A, Galimberti A, Ravasi M, Pasini S, Donzelli E, Cavaletti G, et al. NGF protects dorsal root ganglion neurons from oxaliplatin by modulating JNK/Sapk and ERK1/2. Neurosci Lett. 2010;486(3):141–5. doi: 10.1016/j.neulet.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 41.Schulz R, Emmrich T, Lemmerhirt H, Leffler U, Sydow K, Hirt C, et al. Identification of a glutathione peroxidase inhibitor that reverses resistance to anticancer drugs in human B-cell lymphoma cell lines. Bioorg Med Chem Lett. 2012;22(21):6712–5. doi: 10.1016/j.bmcl.2012.08.091. [DOI] [PubMed] [Google Scholar]
- 42.Khrunin AV, Moisseev A, Gorbunova V, Limborska S. Genetic polymorphisms and the efficacy and toxicity of cisplatin-based chemotherapy in ovarian cancer patients. Pharmacogenomics J. 2010;10(1):54–61. doi: 10.1038/tpj.2009.45. [DOI] [PubMed] [Google Scholar]
- 43.Oldenburg J, Kraggerud SM, Grov EK, Dahl AA, Lothe RA, Fossa SD. Platinum, polymorphisms, and personality: Impact on long-term neurotoxicity. J Clin Oncol. 2008;26(15S):5036. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.