Abstract
Eight new cardiac glycosides/aglycones (antiaritoxiosides A–G, 1–7, and antiarotoxinin B, 8), two new coumarins (anticarins A–B, 41–42), and two new flavanones (antiarones L–K, 43–44) were isolated from trunk bark of Antiaris toxicaria together with 53 known compounds. The new structures were established by extensive analysis of spectroscopic data. Compound 1 (10-carboxy and 3α-hydroxy) and compounds 3–6 (10-hydroxy) contain unique substituents that are rarely found in cardiac glycosides. The cytotoxic effects of isolated compounds against ten human cancer cell lines, KB, KB-VIN, A549, MCF-7, U-87-MG, PC-3, 1A9, CAKI-1, HCT-9 and S-KMEL-2, were tested using the sulforhodamine B assay. Five compounds (12, 16, 20, 22, and 31) showed significant cytotoxicity against all ten cancer cell lines, with notable potency at the ng/mL level against some cell lines, which merits further development as clinical trial candidates.
Keywords: Antiaris toxicaria, Cardiac glycosides, Coumarins, Cytotoxicity
1. Introduction
Cardiac glycosides are naturally occurring compounds with a steroidal framework and are used for the treatment of congestive heart failure and as anti-arrhythmic agents. They also have been used as arrow poisons, abortifacients, emetics, diuretics, and heart tonics.1 Mechanistically, their anti-arrhythmic activity results from inhibition of Na+/K+-ATPase, and leads to an increased intracellular calcium concentration. Recent research indicated that binding of cardiac glycosides to Na+/K+-ATPase can activate multiple downstream signal transduction pathways that regulate many important physiological and pathological states.2 Less well known are the emerging role of this compounds category in the prevention and/or treatment of proliferative diseases, such as cancer. Because cardenolide-like compounds are often quite toxic, however, the potential use of such compounds for the treatment of cancer was abandoned 40 years ago. Even so, studies have revealed that some cardiac glycosides can selectively inhibit human tumor cell growth, but not normal cellular proliferation through complex cell signal transduction mechanisms.1 Specifically, cardiac glycosides can inhibit growth of cancer cells, induce apoptosis via activation of caspase-3, induce cytochrome C release from mitochondria, and generate reactive oxygen species. In addition, clinical trials of cardiac glycosides and extracts containing them have been initiated to assess the anticancer potential.2 Therefore, the use of certain cardiac glycosides may represent a worthwhile approach for control of cancer, despite their narrow therapeutic index.
In a previous investigation of bioactive compounds with potential therapeutic value, we reported cardiotonic principles from Antiaris toxicaria (Pers.) Lesch.3 As a continuation of our anticancer research program based on natural products, an ethanolic extract of A. toxicaria (Moraceae) was found to show significant cytotoxicity against KB cells. A. toxicaria, also called ‘upas tree’, is widely distributed in tropical rain forests of Southeast Asia. Phytochemical studies have shown that this plant contains cardiac glycosides,3,4 prenylaurones,5 chalcones,6 flavanones,6 and dihydrochalcones.7 Prior investigations also indicated that this species exhibited a broad spectrum of biological activities, such as anti-tumor,8–12 anti-arrhythmic,3 and 5-lipoxygenase inhibition effects.12 Therefore, an intensive investigation of this cytotoxic extract was carried out and led to the isolation of 12 new compounds, together with 53 known compounds. Herein, we describe the isolation, structural elucidation, and antitumor activity of these isolates against ten human cancer cell lines. Preliminary structure–activity relationship (SAR) correlations are also described. In addition, based on the structures of 10-hydroxy-19-nor-cardenolides and 19-nor-cardenolides identified from this plant, a biogenetic pathway of cardiac glycoside has been proposed.
2. Results and discussion
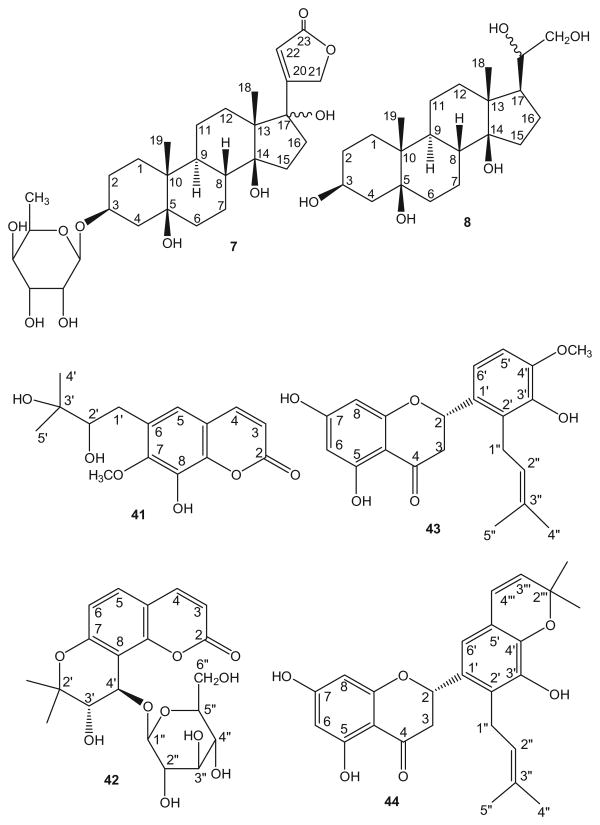
Twelve new compounds, including eight cardiac glycosides/ aglycones (1–8), two coumarins (41–42) and two flavanones (43–44) (Fig. 1), together with 53 known compounds, were isolated from an EtOH extract of fresh trunk bark of A toxicaria through column chromatography on silica gel, Diaion HP-20, Sephadex H-20 and semipreparative HPLC. The subfractions were examined by H2SO4 solution spray on TLC for cardiac glycosides, which show as green spots, and subjected to a series of column chromatographic steps in order to obtain cardenolides/aglycones. The structures of new compounds were elucidated on the basis of spectroscopic methods, including 2D NMR techniques.
Figure 1.
Chemical structures for new compounds.
The HRFAB mass spectrum of 1 showed a molecular ion-related [M+H]+ peak at m/z 567.2808, corresponding to the molecular formula C29H42O11. The NMR signals were due to one methyl, eleven methylene, eight methine and six quaternary carbons as determined by DEPT 135 spectroscopy (Table 1). Compound 1 showed a UV absorption maximum at 216 nm and IR absorption at 1734 cm −1 (γ-lactone carbonyl), which were indicative of a butenolactone system.13 The 1H NMR spectrum of 1 (Table 2) showed characteristic signals for a butenolactone ring at δH 4.98 and 5.27 (each 1H, d, J = 18.1 Hz, H-21a, and b) and 6.08 (s, H-22), as well as a methyl singlet at δH 0.98 (s, H-18), suggesting 1 to be a cardenolide with a C-18 methyl group. A strong carbonyl absorption in the IR spectrum at 1734 cm−1 and a carbon signal at δC 176.7 in the 13C NMR spectrum suggested that a carboxylic acid was present at C-19. Comparison of the 1H and 13C NMR spectra of 1 with those of antiaroside R (9),4 which was isolated from the n-BuOH soluble fraction, showed that the two structures were very similar, except for the differences in glycosidic signals and orientation at C-3. An anomeric proton signal at δH 6.08 (s) in the 1H NMR spectrum and signals at δC 99.8, 72.7, 72.8, 74.3, 69.9, and 18.6 in the 13C NMR spectrum indicated the presence of α-rhamnose. The α-orientation of C-3 was deduced from the coupling constant values of H-3 (dddd, J = 11.0, 11.0, 5.4, 5.4 Hz). This assignment was supported by downfield shifts of H-2a, H-3, and H-4a from δH 1.43 (m), 4.37 (br s, W1/2 = 15.7 Hz), and 1.67 (d, J =12.7 Hz) in 9 to δH 2.19 (m), 4.81 (dddd, J = 11.0, 11.0, 5.4, 5.4 Hz), and 2.19 (m) in 1. HMBC correlation of H-1′ with C-3 [δC 72.9/δH 6.08 (s)] inferred that the rhamnose unit was linked to C-3. Hence, the structure shown was established for 1 (antiaritoxioside A).
Table 1. 13C NMR data for antiaritoxioside A-G (1–7) and antiarotoxinin B (8).
| Carbon | 1‡ | 2‡ | 3‡ | 4‡ | 5 | 6 | 7‡ | 8 |
|---|---|---|---|---|---|---|---|---|
| 1 | 36.3 | 26.9 | 25.3 | 26.9 | 26.7 | 22.0 | 23.6 | 25.8 |
| 2 | 22.4 | 28.7 | 27.4 | 28.6 | 27.9 | 26.4 | 26.8 | 28.6 |
| 3 | 72.9 | 73.9 | 73.8 | 73.8 | 73.2 | 72.3 | 74.1 | 67.8 |
| 4 | 32.1 | 35.6 | 34.8 | 35.6 | 35.1 | 30.0 | 36.1 | 36.1 |
| 5 | 72.9 | 74.7 | 73.1 | 74.2 | 74.3 | 30.4 | 73.4 | 74.5 |
| 6 | 43.3 | 35.8 | 35.1 | 35.9 | 36.5 | 33.2 | 40.8 | 37.8 |
| 7 | 28.4 | 24.3 | 24.5 | 24.8 | 24.7 | 26.0 | 21.3 | 24.4 |
| 8 | 42.3 | 40.7 | 41.3 | 40.4 | 40.4 | 48.2 | 41.2 | 39.0 |
| 9 | 41.0 | 40.2 | 39.4 | 37.5 | 37.7 | 42.0 | 37.3 | 40.0 |
| 10 | 55.7 | 74.4 | 74.4 | 74.7 | 74.4 | 34.0 | 39.2 | 41.3 |
| 11 | 23.5 | 21.6 | 22.8 | 30.8 | 30.7 | 22.1 | 26.1 | 22.0 |
| 12 | 39.9 | 40.0 | 40.4 | 74.7 | 74.7 | 39.7 | 31.4 | 40.3 |
| 13 | 50.2 | 50.1 | 50.3 | 56.7 | 56.9 | 50.1 | 51.9 | 47.8 |
| 14 | 84.8 | 84.6 | 85.0 | 85.0 | 85.1 | 83.8 | 86.7 | 83.8 |
| 15 | 33.4 | 33.0 | 33.0 | 33.4 | 33.4 | 32.2 | 33.7 | 33.1 |
| 16 | 27.2 | 27.5 | 27.2 | 27.9 | 29.2 | 22.1 | 34.3 | 19.0 |
| 17 | 51.5 | 51.3 | 51.3 | 46.4 | 46.2 | 51.4 | 87.8 | 51.5 |
| 18 | 16.3 | 16.3 | 16.3 | 10.3 | 10.3 | 16.4 | 13.1 | 15.5 |
| 19 | 176.7 | 17.0 | 17.4 | |||||
| 20 | 176.1 | 176.3 | 176.4 | 176.7 | 176.8 | 176.0 | 174.0 | 71.1 |
| 21 | 73.8 | 73.8 | 73.8 | 74.0 | 74.1 | 73.6 | 73.8 | 65.8 |
| 22 | 117.6 | 117.7 | 117.6 | 117.5 | 117.4 | 117.6 | 116.6 | |
| 23 | 174.6 | 174.8 | 174.6 | 174.7 | 174.8 | 174.5 | 173.2 | |
| 1′ | 99.8 | 100.8 | 99.3 | 100.7 | 99.4 | 99.8 | 99.8 | |
| 2′ | 72.7 | 72.6 | 72.2 | 72.6 | 69.8 | 72.9 | 69.6 | |
| 3′ | 72.8 | 73.0 | 73.1 | 72.9 | 73.6 | 72.9 | 73.8 | |
| 4′ | 74.3 | 73.9 | 73.8 | 73.7 | 73.7 | 74.1 | 73.3 | |
| 5′ | 69.9 | 70.7 | 70.8 | 70.7 | 70.0 | 70.0 | 70.0 | |
| 6′ | 18.6 | 18.6 | 18.8 | 18.6 | 17.0 | 18.6 | 17.3 |
δ Value in pyridine-d5 (100 MHz).
δ Value in pyridine-d5 (75 MHz).
Table 2. 1H NMR data for antiaritoxioside A-G (1–7) and antiarotoxinin B (8).
| Proton | 1 | 2‡ | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1a/b | 1.76 (br d, 13.6)/3.29 (ddd, 13.6, 13.6, 5.7) | 1.90 (m)/2.19 (m) | 2.04 (m)/2.44 (m) | 1.95 (m)/2.27 (br dd, 8.4, 2.3) | 1.92 (m)/2.46 (br s dd, 14.5, 2.9) | 1.23 (m)/2.07 (m) | 1.32 (ddd, 13.5, 13.5, 3.8)/2.21 (m) | 1.45 (t, 11.7)/2.21 (td, 12.5, 4.4) |
| 2a/b | 2.19 (m)/2.27 (m) | 1.67 (m)/2.04 (m) | 1.81 (m)/2.14 (m) | 1.69 (br d, 14.8)/2.00 (m) | 1.81 (br d, 13.1)/2.17 (m) | 1.52 (m) | 1.92 (m)/2.07 (m) | 1.81 (m) |
| 3 | 4.81 (dddd, 11.0, 11.0, 5.4, 5.4) | 4.29 (br s, 6.6)† | 4.40 (br s, 15.7)† | 4.27 (br s, 7.9)† | 4.48 (br s, 7.7)† | 4.18 (br s, 9.9)† | 4.52 (br s, 9.8)† | 4.44 (br s, 11.3) |
| 4a/b | 2.19 (m)/2.45 (br d, 12.8) | 1.90 (m)/2.11 (m) | 1.81 (m)/2.04 (m) | 1.97 (m)/2.15 (m) | 1.92 (m)/2.09 (br d, 9.7) | 1.23 (m)/1.48 (br t, 4.0) | 1.56 (m)/1.97 (m) | 1.78 (m)/2.32 (dd, 14.9, 2.5) |
| 5 | 2.07 (m) | |||||||
| 6a/b | 2.19 (m)/2.27 (m) | 1.55 (m)/2.25 (m) | 1.65 (m)/2.14 (m) | 1.56 (dd, 11.2, 6.3)/1.97 (m) | 1.63 (br d, 13.0)/2.28 (m) | 1.78 (m)/2.02 (m) | 1.49 (m)/2.07 (m) | 1.62 (br d, 13.0)/2.03 (m) |
| 7a/b | 2.27 (m)/2.68 (br d, 11.8) | 1.23 (m)/2.33 (br. d, 9.8) | 1.65 (m)/2.15 (m) | 1.32 (br d, 13.2)/ 2.29 (br s dd, 9.9, 3.3) | 2.12 (m)/2.32 (m) | 1.55 (m)/1.78 (m) | 1.30 (m)/1.64 (br t, 13.5) | 1.40 (m)/2.36 (td, 13.3, 2.0) |
| 8 | 2.27 (m) | 2.36 (d, 12.3) | 2.15 (m) | 2.48 (br t, 11.8) | 2.48 (br dd, 12.8, 2.9) | 1.29 (m) | 1.99 (m) | 1.76 (dt, 12.2, 11.8) |
| 9 | 2.27 (m) | 1.63 (m) | 1.65 (m) | 1.78 (ddd, 8.7, 8.7, 3.0) | 1.81 (br d, 13.1) | 1.24 (m) | 2.39 (m) | 2.03 (m) |
| 10 | 1.43 (m) | |||||||
| 11a/b | 1.88 (m) | 1.55 (m)/1.90 (m) | 1.38 (m)/2.26 (m) | 2.15 (m)/2.24 (m) | 2.12 (m)/2.25 (m) | 1.66 (br d, 2.3)/1.84 (m) | 1.84 (br t, 13.9)/2.25 (m) | 1.40 (m) |
| 12a/b | 1.46 (m) | 1.40 (m) | 1.38 (m) | −/3.74 (br t, 7.8) | −/3.76 (br t, 9.7) | 1.31 (m) | 2.17 (m)/ 2.39 (m) | 1.42 (t, 11.9) |
| 15a/b | 1.93 (m)/2.19 (m) | 1.90 (m)/2.04 (m) | 1.81 (m)/2.04 (m) | 1.73 (m)/1.97 (m) | 1.92 (m) | 1.35 (m) | 1.19 (m)/1.49 (m) | 1.89 (m)/2.09 (dt, 12.6, 9.8) |
| 16a/b | 1.93 (m)/2.03 (dd, 20.0, 10.8) | 2.04 (m) | 1.81 (m)/2.04 (m) | 2.15 (m) | 2.12 (m) | 1.94 (m)/2.07 (m) | 1.97 (m)/2.21 (m) | 1.92 (m)/2.26 (m) |
| 17 | 2.76 (dd, 9.1, 3.2) | 2.78 (br d, 7.7) | 2.79 (br t,2.5) | 3.76 (br t, 7.4) | 3.76 (br t, 9.7) | 2.76 (br d, 8.8) | 2.21 (m) | |
| 18 | 0.98 (s) | 1.05 (s) | 1.15 (s) | 1.28 (s) | 1.29 (s) | 0.99 (s) | 1.26 (s) | 1.31 (s) |
| 19 | 1.17 (s) | 1.19 (s) | ||||||
| 20 | 4.36 (t, 6.5) | |||||||
| 21a/b | 4.98 (d, 18.1)/5.27 (d, 18.1) | 5.13 (d, 18.2)/5.30 (d, 18.2) | 5.01 (d, 18.0)/5.29 (d, 18.0) | 5.09 (d, 17.9)/5.23 (d, 17.9) | 5.03 (d, 18.1)/5.33 (d, 18.1) | 5.02 (d, 17.8)/5.31 (br d, 17.8) | 5.07 (dd, 18.2, 1.4)/5.21 (dd, 18.2, 1.4) | 3.89 (dd, 10.5, 6.1)/4.08 (dd, 10.5, 7.2) |
| 22 | 6.08 (s) | 6.12 (s) | 6.12 (s) | 6.22 (s) | 6.23 (s) | 6.12 (s) | 6.23 (d, 1.4) | |
| 1′ | 5.44 (br s) | 5.49 (br s) | 5.40 (d, 5.8) | 5.33 (br s) | 5.43 (d, 8.1) | 5.41 (br s) | 5.39 d, 8.1) | |
| 2′ | 4.27 (br s) | 4.54 (br s) | 3.96 (d, 4.7) | 4.48 (br s) | 4.50 (dd, 8.1, 3.1) | 4.54 (m) | 4.46 (br d, 8.1) | |
| 3′ | 4.44 (dd, 8.9, 3.4) | 4.44 (dd, 9.0, 3.2) | 4.67 (m) | 4.38 (dd, 8.6, 2.8) | 4.77 (t, 3.1) | 4.54 (m) | 4.70 (m) | |
| 4′ | 4.19 (dd, 8.9, 8.9) | 4.31 (m) | 3.66 (d, 6.2) | 4.21 (br t, 8.6) | 4.13 (d, 3.1) | 4.29 (m) | 4.11 (br s) | |
| 5′ | 4.32 (dq, 8.9, 6.1) | 4.22 (m) | 4.34 (dq, 6.2, 4.6) | 4.18 (dq, 8.6, 5.9) | 4.61 (q, 6.6) | 4.29 (m) | 4.59 (q, 6.4) | |
| 6′a/b | 1.59 (d, 6.1) | 1.63 (d, 6.0) | 1.59 (d, 4.6) | 1.61 (d, 5.9) | 1.53 (d, 6.6) | 1.67 (d, 4.7) | 1.56 (d, 6.4) |
δ Value in pyridine-d5 (400 MHz), coupling constants in Hz are given in parentheses.
W1/2 (Hz): width of 1/2 peak high.
δ Value in pyridine-d5 (300 MHz), coupling constants in Hz are given in parentheses.
A molecular formula of C28H42O10 was deduced for compound 2, 12 mass units less than that of periplorhamnoside (11).14 The 1H and 13C NMR data of the two compounds were similar except for the C-19 and C-10 signals. The carbon signal at δC 17.2 (C-19) in 11 was absent in 2. Furthermore, the carbon signal at δC 41.2 (C-10) in 11 was present at δC 74.4 (C-10) in 2. Thus, both the NMR and MS data were indicative of a hydroxy rather than methyl group at C-10 in 2. The β-orientation of the C-3 monosaccharide unit was deduced from the W1/2 constant of H-3 (br s, W1/2 = 6.6 Hz). Therefore, the structure of 2 was determined as demethyl-10-hydroxy-periplorhamnoside and 2 was named antiaritoxioside B.
Compound 3 showed a pseudo molecular ion peak at m/z 539.2855 in its HRFABMS and had the same molecular formula, C28H42O10, as toxicarioside M (10),15 which was also isolated from the n-BuOH extract of this plant. The UV, IR and NMR data of 3 strongly resembled those of 10, consistent with a general structure containing a central cardenolide moiety dioxygenated at C-3, and C-5 and a hydroxy group in the 10-position. The sole significant differences were in certain glycosidic signals (Tables 1 and 2). An anomeric proton signal at δH 5.40 (d, J = 5.8 Hz) in the 1H NMR spectrum and signals at δC 99.3, 72.2, 73.1, 73.8, 70.8, and 18.8 in the 13C NMR spectrum indicated the presence of β-allomethylose. An NOE effect from H-1′ to H-3 suggested that the allomethylose unit was attached to C-3. These data agreed with the replacement of the antiarosyl unit in 10 by an allomethylosyl unit in 3. Thus, the structure of 3 was established for antiaritoxioside C.
A molecular formula of C28H43O11 was deduced for compound 4, 12 mass units less than that of β-antiarin (34),16 isolated from the CHCl3 soluble fraction. The 1H and 13C NMR data of the two compounds were similar except for the absence of a C-19 aldehyde group and a downfield shift of C-10 from δC 55.0 in 34 to δC 74.7 in 4. These data suggested that the aldehyde group in 34 was replaced by a hydroxy group in 4. This conclusion was supported by 3JCH HMBC correlations from H-1b and H-11b to C-10. The β-orientations of the C-3 and -12 OH groups were deduced from the coupling constants of H-3 (δH 4.27, br. s, W1/2 = 7.9 Hz) and H-12 (δH 3.74, br t, J = 7.8 Hz). Thus, 4 was determined as 10-hydroxy-19-nor-β-antiarin and named antiaritoxioside D.
Compound 5 had the same molecular formula, C28H43O11, as 4. The UV, IR and NMR data also strongly resembled those of 4, consistent with a structure containing a 19-nor-cardenolide moiety tetraoxygenated at C-3, C-5, C-10 and C-12. The sole significant differences occurred in glycosidic signals of the molecules (Tables 1 and 2). The sugar proton signals of 5 indicated the presence of a β-antiarosyl moiety. These data were consistent with the replacement of the rhamnosyl unit in 5 by an antiarosyl unit in 5. The β-orientations of C-3 and C-12 were deduced by the W1/2 of H-3 (br s, 7.7 Hz) and H-12 coupling constant (br t, J = 9.7 Hz). Thus, the structure of 5 was assigned as shown, and 5 was named antiaritoxioside E.
The HRFAB mass spectrum of 6 showed a molecular ion-related [M+Na]+ peak at m/z 529.2774, corresponding to the molecular formula C28H42O8. The 1H and 13C NMR data (Tables 1 and 2) indicated that 6 was a 19-nor-cardenolide monosaccharide with a hexose sugar unit. Analyses of COSY, HMQC and HMBC spectra indicated that the sugar was α-rhamnose. Comparison of the 1H and 13C NMR spectra of 6 with those of malayoside (29),3,16 showed that the two structures were very similar, except for that 6 did not contain the C-19 aldehyde group found in 29 based on the presence of a methine (−CH) proton at δH 1.43 (m), upfield shift of C-10 from δC 51.2 to δC 34.0, and absence of CHO signals. Compound 6 was thus assigned to be 19-nor-malayoside and named antiaritoxioside F.
Compound 7 (C29H45O10) was 16 mass units larger than 11.14 The 1H/13C NMR spectra of 7 and 11 were very similar, except for the sugar moiety signals and one oxygenated carbon in 7. The spectra of 11 showed a proton signal at δH 2.81 (dd, J = 8.9, 5.1 Hz) and a carbon signal at δC 51.4 (C-17), while in 7, the former signal was absent and the latter signal was replaced by an oxygenated carbon signal at δC 87.8 (C-17). Thus, an OH group was attached at C-17 in 7, and this postulate was supported by a downfield shift of C-16 from δC 27.35 in 11 to δC 34.3 in 7, together with HMBC correlations of H-18 to C-17. An anomeric proton signal at δH 5.39 (d, J = 8.1 Hz) and carbon signals at δC 99.8, 69.6, 73.8, 73.7, 70.0, and 17.3 indicated the presence of an antiarosyl moiety. An NOE effect between H-3 and H-1′ placed the antiarosyl unit on C-3, and a W1/2 coupling constant of 9.8 Hz for H-3 indicated α-orientation. Therefore, the structure 7 was established for antiaritoxioside G.
Compound 8 (C21H36O5) had 21 signals in the 13C NMR spectrum corresponding to two methyl, ten methylene, five methine, and four quaternary carbon atoms. The 1H and 13C NMR spectra of 8 displayed signals characteristic of the steroid skeleton of a cardenolide. However, the absence of typical signals for the olefinic group and carbonyl carbon of the butenolactone ring, suggested that the five-membered lactone in 8 was absent. This hypothesis was confirmed by the presence of a methylene group at δC 65.8/ δH 3.89 (1H, dd, J = 10.5, 6.1 Hz) and 4.08 (1H, dd, J = 10.5, 7.2 Hz, H-21) and one methine at δC 71.1/δH 4.36 (1H, dd, J = 7.2, 6.1 Hz, H-20), as well as the absence of any significant UV absorption. In addition to three OH groups on the steroid skeleton (C-3, C-5 and C-14), two OH groups were assigned at C-20 and C-21, based on carbon signals at δC 71.1 and 65.8, respectively. Orientation of the C-3 OH was also determined by the W1/2 of H-3 (br s, W1/2 = 11.3 Hz). The above analysis established the structure of 8 as shown. This compound was reported as an intermediate in the synthesis of periplogenin by Peoghenghi et al.17 It has been isolated from a natural source for the first time and named antiarotoxinin B.
Compound 41 was obtained as colorless syrup, and its molecular formula was determined to be C15H18O6 on the basis of HREIMS ([M]+, m/z 294.1100). The IR spectrum showed absorption bands at 3400 and 1711 cm−1 for a hydroxyl group and α, β-unsaturated lactone, respectively. UV absorptions at 316 and 258 nm,18 and two AB type system protons at δH 7.94 and 6.32 (each 1H, d, J = 9.6 Hz, H-4 & -3) suggested that 41 was a coumarin derivative.19 The 1H NMR spectrum of 41 also showed an aromatic proton at δH 7.06 (1H, s), three hydroxy protons at δH 9.60, 4.39 and 4.23 (each 1H, D2O exchangeable), one methoxy group at δH 3.79 (3H, s) and one 2,3-dihydroxyisopentyl group at δH 1.12 and 1.10 (each 3H, s), 3.36 (1H, m), 2.95 (1H, d, J = 13.2 Hz) and 2.39 (1H, dd, J = 13.2, 10.4 Hz). The 13C NMR spectrum of 41 revealed the presence of 15 carbons resonances including two methyl, one methoxy, one methylene, four methine and seven quaternary carbons. Comparison of the 1H and 13C NMR spectra of 41 with those of skimminan, which was isolated from Skimmia anquetelia by Sharma et al.,20 showed that the two structures were very similar, except for the absence of signals for a sugar and one additional methoxy group (δH 3.79, δC 60.5) in the former. The HMBC spectrum showed correlations of the methoxy group at δH 3.79 with C-7 (δC 149.9) and of H-1′ (δH 2.95, and 2.39) with C-5 (δC 119.7), C-6 (δC 131.0) and C-7 (δC 149.9). Therefore, the methoxy group was attached at C-7 and the 2, 3-dihydroxyisopentyl moiety was located at C-6. This assignment was further confirmed by NOE effects between H-4 (δH 7.94) and H-5 (δH 7.06), as well as H-5 and H-1′ for 41. From the above data, new compound 41 was elucidated as 8-hydroxy-7-methoxy-6-[2′, 3′-dihydroxyisopentyl]coumarin, and has been designated as anticarin A.
Compound 42, +38.2° (c 0.25, MeOH), was isolated as colorless needles. The HRFABMS exhibited a pseudo-molecular ion at m/z 425.1367 for [M+H]+, consistent with the molecular formula C20H24O10. UV absorptions at 326, 302 (sh), 259, 247 and 220 (sh) nm,18 and IR band at 1709 (OC=O) cm−1, as well as two AB type protons at δH 7.96 (1H, d, J = 9.2 Hz, H-4) and 6.26 (1H, d, J = 9.2 Hz, H-3) suggested that 42 was a coumarin derivative.19 In the 1H NMR spectrum, the presence of two additional AB type protons at δH 7.54 and 6.76 (each 1H, d, J = 8.8 Hz), two vicinal methine protons at δH 4.84 (1H, d, J = 2.4 Hz) and 3.94 (1H, dd, J = 4.4, 2.4 Hz), and two methyl groups at δH 1.27 (6H, s), indicated that 42 was a khellactone glycoside.21 The signal at δH 4.84 was assigned as H-4′ based on its correlations to C-7 (δC 156.6), C-9 (δC 155.0), C-8 (δC 108.6), and C-1″ (δC 103.8) in the HMBC spectrum, whereas the signal at δH 3.94 was assigned as H-3′ based on its correlation to C-3′ in the HMQC spectrum, as well as long range correlations of two methyl groups (δH 1.27) to C-3′ (δC 70.6). An anomeric proton signal at δH 4.71 (1H, d, J = 8.0 Hz, H-1″) in the 1H NMR spectrum and signals at δC 103.8, 74.0, 76.8, 70.3, 77.3 and 61.5 in the 13C NMR spectrum suggested the presence of β-glucose. The coupling constant of the anomeric doublet (δH 4.71, 8.0 Hz) suggested a β-glucosidic linkage for the khellactone. The FAB-MS spectrum showed an ion fragment at m/z 263[M+-162], also indicating the presence of a hexose moiety. The β-glucose unit was placed at C-4′ on the basis of HMBC correlation between H-1″ of the glucose unit and C-4′ of the aglycone moiety, together with NOE correlation between H-1″ and H-4′. Accordingly, the structure of 42 was established as 4′-β-glucosyl-khellactone, and 42 has been named anticarin B.
Compound 43 was obtained as colorless obtained as colorless needles. The molecular formula formula was established as C21H22O6 from m/z 370.1412 [M]+ in HREIMS. The IR spectrum of 43 showed absorption bands at 3456 and 3175 cm−1 for one or more hydroxy group(s) and 1638 cm−1 for a conjugated carbonyl functionality. The presence of a flavanone skeleton was evident from UV [226 (sh), 288, and 328 (sh) nm],18 and 1H [δH 2.66 (1H, dd, J =17.2, 2.8 Hz, H-3α), 3.16 (1H, dd, J = 17.2, 13.2 Hz, H-3β) and 5.62 (1H, dd, J = 13.2, 2.8 Hz, H-2)] and 13C [δC 79.9 (C-2), 43.1 (C-3) and 197.4 (C-4)] NMR spectra. The 1H NMR spectrum further showed three hydroxy groups [δH 12. 17, 9.69 and 7.55 (each 1H, s)], two singlet aromatic protons [δH 5.96 (2H, s)], AB system aromatic protons [δH 6.90 (1H, d, J =8.4 Hz) and 7.06 (1H, d, J = 8.4 Hz)], a methoxy moiety [δH 3.86 (3H, s)], and an isoprenyl group [δH 1.67 (3H, s), 1.63 (3H, s), 3.52 (2H, d, J = 6.4 Hz) and 5.13 (1H, t, J = 6.4 Hz)]. The locations of these substituents were determined by HMQC and HMBC experiments. Based on 3J-correlations between the proton at δH 5.96 with C-5 (δC 165.1), C-7(δC 167.2), and C-10 (δC 103.0) in the HMBC experiment and with C-6 in a HMQC spectrum, the singlet aromatic protons were assigned to H-6 and H-8, respectively, of ring-A HMBC correlations of the proton at δH 6.90 to C-1′ (δC 130.5) and C-3′ (δC 144.8) and at δH 7.06 to C-2 (δC 79.9) and C-2′ (δC 126.9) suggested that the AB system aromatic protons belonged H-5′ and H-6′, respectively. Combined with HMBC correlations of H-1″ (δH 3.52) to C-1′ (δC 130.5), C-2′ (δC 126.9), C-3′ (δC 144.8), and C-2″ (δC 124.0), and methoxy protons (δH 3.86) to C-4′ (δC 148.0) implied that the isoprenyl group was attached to C-2′, while the methoxy group was located at C-4′. These assignments were further confirmed by an NOE effect between the methoxy group and H-5′ (δH 6.90). Finally, a hydroxy group was attached to C-3′. The S-configuration of C-2 was determined by a positive Cotton effect observed at 312 nm and a negative one at 286 nm in the circular dichroism (CD) spectrum of 43.22 Based on these observations, 43 was established as (2S)-3′,5,8-trihydroxy-4′-methoxy-6,7-methylenedioxy-2′-prenylflavanone, and named antiarone L.
The molecular formula of 44 was determined as C25H26O6 from a molecular ion peak at m/z 423.1733 [M+H]+ in its HRESIMS. The UV (324, 286, and 231 nm),18 1H, and 13C NMR spectra indicated that 44 was also a flavanone. The 1H and 13C NMR spectra of 44 closely resembled those of 43 except that signals for a 2,2-dimethylpyran ring unit were present at δH 6.40 (1H, d, J = 9.8 Hz, H-4″′), 5.73 (1H, d, J = 9.8 Hz, H-3″′), 1.20 and 1.18 (6H, s, 2″′-CH3) and δC 122.7 (C-4″′), 131.3 (C-3″′), 77.5 (C-2″′), 29.1 & 32.4 (2″′-CH3) in 44, and the methoxy group found 43 was absent. Correlations of H-1″ [δH 3.48 (2H, d, J = 6.8 Hz)] to C-1′ (δC 130.1), C-2′ (δC 124.0) and C-3′ (δC 143.8) in the HMBC spectrum indicated an isoprenyl group was attached to C-2′, whereas long range correlations of H-4″′ (δH 6.40) to C-4′ (δC 140.5), C-5′ (δC 133.1) and C-6′ (δC 120.0) and of H-3″′ (δH 5.73) to C-5′ suggested that the 2,2-dimethylpyran ring was fused to C-5′ and 4′. The absolute stereochemistry of 44 was deduced to be 2S because of a positive Cotton effect at 314 nm and a negative one at 290 nm in the circular dichroism (CD) spectrum of 44.22 Thus, the structure of compound 44 was assigned as (2S)-2″′,2″′-dimethylpyrano-3′,5,8-trihydroxy-2′-prenylflavanone, and named antiarone K.
Antiaroside R (9),4 toxicarioside M (10),15 periplorhamnoside (11),14 antiaroside A (12),3 peripalloside (13),14 cheiranthoside (14),23 strophanthidol (15),24 convallatoxal (16),16 cannogenol (17),25 antiaroside B (18),3 strophanthidin (19),14 convallatoxin (20),16 strophathojavoside (21),26 desglucocheirotoxin (22),24 strophalloside (23),14 convalloside (24),27 glucostrophalloside (25),28 cheirotoxin (26),29 antiaroside C (27),3 antiaroside D (28),3 malayoside (29),3,16 antiarigenin (30),14 α-antiarin (31),16 antialloside (32),26 toxicarioside B (33),30 β-antiarin (34),16 antiaroside E (35),3 antiaroside F (36),3 antiaroside G (37),3 antiaroside H (38),3 antiaroside I (39),3 antiarotoxinin A (40),3 (+)-marmesin (45),31, decursinol (46),21 (R)-peucedanol (47),32 oxypeucedanin hydrate (48),33 (2S)-pinocembrin (49),34 antiarones F (50),6 I (51),6 vanillic acid (52),35 p-hydroxybenzoic acid (53),36, vanillin (54),35 (3S,4R)-hydroxymellein (55),37 2, 4,6-trihydroxy-5-methoxyactophenone (56),38 6-acetoxy-2,4-dihydroxyacetophenone (57),39 syringic aicd (58),40 koaburaside (59),41 vanilloyl β-d-glucose (60),42 uridine (61),43 xanthine (62),44 adenosine (63),36 3-hydroxy-5-methoxybenzalacetophenone (64),45 and β-sitosterol (65)35 were also isolated and identified by comparison of their physical and spectroscopic properties with those reported in the literature.
Cardenolides 11–40 and coumarins 41, 42, and 45 were assayed for anti-proliferative effects against 10 human cancer cell lines, KB, KB-VIN, A549, MCF-7, U-87-MG, PC-3, 1A9, CAKI-1, HCT-9, and S-KMEL-2.46 The cytotoxic data are shown in Table 3. Among the tested isolates, compounds 11, 12, 16, 17, 19–25, 29, 31–35, and 42 exhibited potent effects against all cancer cell lines. Among them, compounds 12, 16, 20, 22, and 31 showed ng/mL potency against one or more cancer cell lines. Compound 12 exhibited an ED50 of 0.0097 μg/mL against KB cancer cell lines. Compound 16 demonstrated ED50 values of 0.0040, 0.0076, and 0.0061 μg/mL against A549, 1A9 and CAKI-1 cells, respectively. Compound 20 showed ED50 values of 0.0027, 0.0065, 0.0031, 0.0014 and 0.0013 μg/mL against A549, MCF-7, CAKI-1, HCT-9 and s-KMEL-2 cells, respectively. Compound 22 exhibited an ED50 of 0.0092 μg/mL against A549 cells. Finally, compound 31 showed an ED50 of 0.0044 μg/mL against PC-3 cells. Common structural features in all five compounds included a 5β-OH, 13β-Me, 14β-OH, and 17β-α, β-unsaturated-γ-butyrolactone. Compounds 20, 22, and 31 contained a C-18 β-CHO, while 12 and 16 contained β-CH3 and −CH2OH, respectively. Compound 31 contained a C-12 β-OH, while compounds 12, 16, 20, and 22 were unsubstituted. All five compounds contained a 3β-sugar moiety, 3β-O-β-d-antiarose in 12, 22, and 31, 3β-O-α-l-rhamnose in 16 and 20.
Table 3. Cytotoxicity (ED50 μg/mL) of isolated compounds from A. toxicariaa.
| Compound | KB* | KB-VIN | A549 | MCF-7 | U-87-MG | PC-3 | 1A9 | CAKI-1 | HCT-9 | S-KMEL-2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 0.054 | 0.020 | 0.012 | 0.018 | 0.082 | 0.021 | 0.012 | 0.014 | 0.044 | 0.017 |
| 12 | 0.0097 | 0.068 | 0.044 | 0.063 | 0.15 | 0.078 | 0.020 | 0.018 | 0.090 | 0.073 |
| 14 | 1.5 | 1.7 | 0.12 | 0.58 | 1.9 | 0.60 | 0.53 | 0.51 | 0.94 | 0.16 |
| 15 | 2.7 | 3.0 | 0.14 | 0.69 | 2.9 | 0.81 | 0.66 | 0.67 | 6.4 | 0.57 |
| 16 | 0.025 | 0.014 | 0.0040 | 0.012 | 0.036 | 0.013 | 0.0076 | 0.0061 | 0.020 | 0.014 |
| 17 | 0.45 | 0.50 | 0.050 | 0.21 | 0.50 | 0.15 | 012 | 0.11 | 0.46 | 0.70 |
| 19 | 0.61 | 0.69 | 0.11 | 0.38 | 0.72 | 0.17 | 0.15 | 0.14 | 0.51 | 0.13 |
| 20 | 0.014 | 0.013 | 0.0027 | 0.0065 | 0.014 | 0.094 | 0.030 | 0.0031 | 0.0014 | 0.0013 |
| 21 | 0.066 | 0.051 | 0.016 | 0.042 | 0.078 | 0.052 | 0.013 | 0.014 | 0.067 | 0.064 |
| 22 | 0.044 | 0.018 | 0.0092 | 0.015 | 0.054 | 0.018 | 0.012 | 0.014 | 0.044 | 0.015 |
| 23 | 0.081 | 0.050 | 0.015 | 0.024 | 0.043 | 0.063 | 0.013 | 0.014 | 0.085 | 0.063 |
| 24 | 0.2 | 0.18 | 0.070 | 0.15 | 0.40 | 0.095 | 0.072 | 0.071 | 0.20 | 0.42 |
| 25 | 0.12 | 0.083 | 0.038 | 0.096 | 0.15 | 0.084 | 0.057 | 0.065 | 0.13 | 0.12 |
| 27 | 1.5 | 1.6 | 0.48 | 1.4 | 1.7 | 0.42 | 0.39 | 0.32 | 1.5 | 0.26 |
| 28 | 1.7 | 1.9 | 0.38 | 1.0 | 4.2 | 0.53 | 0.74 | 0.93 | 1.9 | 0.31 |
| 29 | 0.30 | 0.32 | 0.080 | 0.19 | 0.37 | 0.11 | 0.076 | 0.073 | 0.18 | 0.62 |
| 30 | 5.3 | 5.1 | 0.59 | 1.3 | 4.6 | 1.2 | 0.12 | 1.3 | 4.5 | 0.41 |
| 31 | 0.14 | 0.15 | 0.059 | 0.10 | 0.017 | 0.0044 | 0.058 | 0.072 | 0.15 | 0.13 |
| 32 | 0.065 | 0.075 | 0.046 | 0.12 | 0.15 | 0.085 | 0.062 | 0.068 | 0.11 | 0.12 |
| 33 | 0.13 | 0.14 | 0.058 | 0.092 | 0.13 | 0.091 | 0.063 | 0.070 | 0.11 | 0.12 |
| 34 | 0.067 | 0.061 | 0.013 | 0.018 | 0.071 | 0.050 | 0.014 | 0.015 | 0.066 | 0.065 |
| 35 | 0.49 | 0.57 | 0.070 | 0.42 | 0.73 | 0.16 | 0.13 | 0.13 | 0.35 | 0.12 |
| 36 | 1.8 | 2.2 | 0.54 | 1.3 | 4.5 | 0.89 | 0.97 | 1.2 | 3.4 | 0.32 |
| 37 | 5.3 | 17.5 | ||||||||
| 38 | 5.9 | 7.1 | 0.76 | 3.1 | 7.3 | 1.8 | 1.4 | 1.5 | 2.5 | 1.3 |
| 39 | 2.0 | 2.3 | 0.36 | 1.3 | 3.6 | 0.90 | 0.83 | 1.1 | 2.9 | 0.30 |
| 40 | 4.3 | 15.0 | ||||||||
| 41 | 10.8 | >20 | 1.5 | 1.5 | 1.5 | 6.7 | 1.3 | |||
| 42 | 5.8 | 4.1 | 1.2 | 3.1 | 7.4 | |||||
| 45 | >20 | >20 |
Compounds 1–9 were not tested due to insufficient quantities remaining.
Cell line, ED50 in μg/mL.
The following specific structure-activity correlations were noted in these studies. Changing the orientation of the glycoside from 3β in potent cardenolide 20 to 3α in 27 significantly decreased the cytotoxicity, for example, from 0.0027 to 0.48 μg/mL against A549, from 0.0065 to 1.4 against MCF-7, from 0.0031 to 0.31 μg/mL against CAKI-1, from 0.0014 to 1.5 μg/mL against HCT-9, and from 0.0013 to 0.26 μg/mL against s-KMEL-2. Compound 28 with a 3α-diglycoside was also one of the least potent cardenolides. Activity decreased dramatically when the 3β-O-α-l-rhamnose of 16 (ED50 values 0.0.0040–0.036 μg/mL) was changed to 3β-OH in 15 (ED50 values 0.14–6.5 μg/mL). Replacement of the C-10 CH3 of 12 with COOH in 38 together with addition of a 12β-OH decreased cytotoxicity from 0.0097 to 5.9 μg/mL against KB cells. Similarly, changing the C-10 CH2OH of 16 to H in 39 decreased cytotoxicity from 0.0040 to 0.36 μg/mL against A549 cells. Compounds showing the lowest potency against the cancer cell lines included 14, 15, 27, and 28 with ED50 values ranging from 0.12 to 4.2 μg/mL, as well as 30 and 36–40 with ED50 values of 0.36–17.5 μg/mL. Notably, a C-10 COOH, COO-glucose, or H was found in 36–39 rather than CHO, CH3, or CH2OH as found in the more potent compounds. While the cytotoxic effects of new compounds 1–6 were not examined in this report,46 the cytotoxic effects of structurally similar cardiac glycosides have been reported.4,10 Our observations were generally consistent with the prior findings (for example, compound 9 with COO-glucose at C-10 and compounds with α-oriented substituents at C-3 exhibited lower cytotoxicity).4 In summary, from our investigation, the β-orientation of C-3, C-3 monosaccharide, and C-10 substituents (CHO, CH3, or CH2OH) are crucial factors in the cytotoxicity effects of cardiac glycosides. Prior reports also indicated that compounds with C-10 OH may exhibit significant cytotoxicity.10
Among the three tested coumarins, compound 41 and the structurally related linear dihydrofuranocoumarin 45 showed no cytotoxic activity, whereas the angular dihydropyranocoumarin 42 exhibited broad and significant cytotoxicity against KB-VIN, A549, MCF-7, PC-3, 1A9, CAKI-1 and S-KMEL-2 cell lines with ED50 values ranging from 1.2 to 4.1 μg/mL.
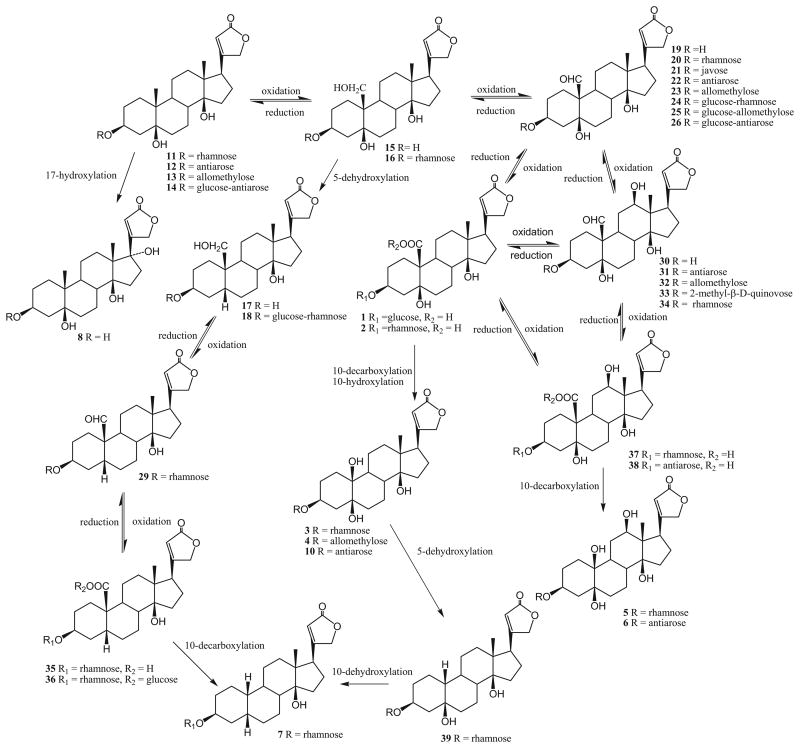
It is a definite possibility that the structurally similar cardiac glycoside metabolites were derived biogenetically from a general catabolic process. A possible biogenetic transformation pathway is presented in Scheme 1 for the first time on the basis of evidence from natural sources. The proposed biosynthesis could explain the catabolic progression of structurally interesting cardiac glycosides.
Scheme 1.
Biosynthetic pathway of cardiac glycosides.
3. Conclusions
In summary, this paper described the isolation and structure elucidation of 12 new compounds, including eight cardiac glycosides/aglycones, antiaritoxiosides A-G (1–7) and antiarotoxinin B (8), two coumarins, anticarin A–B, and two flavanones, antiarones L–K. The cytotoxic activity of cardiac glycosides and coumarins against ten cancer cell lines also reported. Compounds 12, 16, 20, 22, and 31 showed significant cytotoxicity against all 10 cancer cell lines, with notable potency at the ng/mL level against some cell lines. SAR correlations were also identified. While many cardiac glycosides are reported in the literature, compounds with 10-carboxy (e.g., 1, 9) and 3α-hydroxy (e.g., 1) substituents are rare. Furthermore, the C-10 substituents, including COOH, CHO, CH2OH, CH3, OH and H, of cardiac glycosides can help to explain the catabolic progression of structurally interesting cardiac glycosides.
4. Experimental
4.1. General procedures
Optical rotations were measured with a JASCO DIP-370 polarimeter. IR spectra were determined as KBr discs on a Shimazu FTIR-8501 spectrophotometer. UV spectra were acquired on a Hitachi UV-3210 spectrophotometer. The 1H and 13C NMR spectra were recorded on Bruker Avance 300 (1H at 300 MHz) and AMX 400 (1H at 400 MHz) spectrometers. The 1H and 13C chemical shifts (ppm) were referenced to the solvent peaks for pyridine-d5 at δH 8.71 and δC 149.9, DMSO-d6 at δH 2.49 and δC 39.5, acetone-d6 at δH 2.05 and δC 29.9, 206.7, and methanol-d4 at δH 3.31 and δC 49.0. ESIMS, FABMS and HRMS were taken with a VG 70–250 S spectrometer by direct inlet system. Merck silica gel 60 (Merck 70–230, 230–400 mesh) was used for column chromatography. Glass sheets of silica gel 60 F254 (Merck 0.2 mm thick) were used for TLC Melting points were measured on a Yanagimoto MP-S3 micromelting point apparatus and are uncorrected.
4.2. Plant material
Trunk bark of A toxicaria was collected from Yunnan, China, and authenticated by C.S. Kuoh (Department of Life Sciences, National Cheng Kung University, Tainan, Taiwan). A voucher specimen (NCKUWu 92012) has been deposited in the Herbarium of National Cheng Kung University, Tainan, Taiwan, R.O.C.
4.3. Extraction and isolation
Trunk bark of A toxicaria (6.0 kg) was cut into small pieces and extracted with 95% EtOH (20 L × 3). Evaporation of the solvent under reduced pressure provided 239.0 g of crude extract, which was partitioned between CHCl3−H2O, and n-BuOH−H2O, successively, to yield CHCl3 (AT-C, 65.1 g), n-BuOH (AT-B, 100.2 g), and H2O (AT-W, 73.7 g) fractions. The CHCl3 fraction was subjected to silica gel CC using increasing polarity mixtures of n-hexanes-acetone as eluant to give 14 fractions (AT-C-1 ∼ 14). AT-C-2 was recrystallized to obtain 65 (42.5 mg). AT-C-3 was chromatographed on silica gel using n-hexane–diisopropyl ether (3:1) to obtain 56 (1.2 mg), 57 (1.7 mg), and 64 (11.5 mg). AT-C-4 was chromatographed on silica gel using n-hexane–diisopropyl ether (3:1) to obtain 43 (12.4 mg), 49 (3.1 mg), 50 (10.0 mg), 51 (175.4 mg), 55 (1.2 mg), and 64 (2.4 mg). AT-C-5 was chromatographed on silica gel eluted with n-hexane–diisopropyl ether (1:1) to give 43 (18.0 mg), 44 (1.0 mg), 49 (3.7 mg), 50 (17.1 mg), and 55 (2.4 mg). AT-C-6 was chromatographed on silica gel using diisopropyl ether−MeOH (40:1) to obtain 7 (8.7 mg), 19 (103.2 mg), 46 (3.1 mg), 52 (8.7 mg), 54 (12.3 mg), and 65 (12.5 mg). AT-C-7 was chromatographed on silica gel and eluted with n-hexane–diisopropyl ether (1:1) to give 15 (6.6 mg) and 21 (7.0 mg). AT-C-8 was chromatographed on silica gel using EtOAc−MeOH (20:1) to obtain 6 (2.1 mg), 8 (19.6 mg), 17 (3.2 mg), 29 (8.8 mg), 35 (37.6 mg), and 39 (7.5 mg). AT-C-9 and 10 were chromatographed on silica gel using EtOAc−MeOH (20:1) to obtain 29 (21.4 mg), and 20 (117.2 mg), respectively.
The n-BuOH fraction was subjected to Diaion HP-20 CC eluting with a H2O−MeOH gradient system to give 12 fractions (AT-B-1 ∼ 12). AT-B-4 and 5 were chromatographed on silica gel using CHCl3−MeOH (9:1) to obtain 4 (1.3 mg), 5 (1.5 mg), 30 (58.9 mg), 31 (11.0 mg), 32 (4.3 mg), 34 (7.3 mg), 37 (34.7 mg), 38 (147.4 mg), 52 (10.0 mg), 53 (10.2 mg), and 54 (5.5 mg), successively. AT-B-6 was chromatographed on silica gel using CHCl3−MeOH (9:1) to obtain 31 (118.4 mg). AT-B-7 and 8 were chromatographed on silica gel using CHCl3−MeOH−H2O (9:1:005) to obtain 1 (3.0 mg), 2 (3.8 mg), 10 (0.5 mg), 14 (9.1 mg), 16 (6.1 mg), 18 (12.3 mg), 20 (431.2 mg), 22 (4.3 mg), 23 (5.9 mg), 24 (91.2 mg), 25 (18.3 mg), 26 (0.5 mg), 27 (112.3 mg), 28 (12.2 mg), 36 (5.9 mg), 41 (25.3 mg), 42 (5.8 mg), 47 (6.7 mg), and 58 (2.7 mg), successively. AT-B-9 and 10 were chromatographed on silica gel using CHCl3−MeOH−H2O (9:1:0.05) to obtain 3 (0.3 mg), 11 (10.9 mg), 12 (9.8 mg), 13 (1.9 mg), 20 (12.5 mg), 22 (29.9 mg), 23 (17.8 mg), 33 (13.5 mg), 40 (0.7 mg), 45 (1.2 mg), 48 (0.9 mg), and 53 (2.8 mg), successfully.
The H2O fraction was subjected to Diaion HP-20 CC eluting with a H2O−MeOH gradient system to give 10 fractions (AT-W-1 ∼ 10). AT-W-2 and 3 were chromatographed on silica gel using EtOAC−MeOH−H2O (4:1:0.5) to obtain 61 (2.3 mg), and 62 (10.7 mg). AT-W-4 was chromatographed on silica gel using EtOAC−MeOH−H2O (4:1:0.5) to obtain 59 (22.4 mg), 60 (29.0 mg), and 63 (1.2 mg). AT-W-5 was chromatographed on silica gel using CHCl3−MeOH−H2O (4:1:0.005) to obtain 59 (12.6 mg). AT-W-6 and 7 was chromatographed on silica gel using EtOAC−MeOH−H2O (4:1:0.5) to obtain 58 (17.3 mg). AT-W-8 and 9 were chromatographed on Sephadex LH-20 using a H2O−MeOH gradient system to obtain 9 (0.7 mg), 20 (43.2 mg), 24 (26.8 mg), 28 (26.4 mg), 31 (20.2 mg) and 32 (1.3 mg), successfully.
4.4. Antiaritoxioside A (1)
Colorless syrup; +189.13° (c 0.01 in MeOH); UV λmax (MeOH)/nm (log ε) 216 (4.21); IR νmax/cm−1 (KBr) 3400, 2930, 1734, 1651, 1506, 1456, 1394, 1229, 1026; 1H NMR data (pyridine-d5) see Table 2; 13C NMR data (pyridine-d5) see Table 1; FABMS m/z 567 (13%) [M+H]+; HRFABMS m/z 567.2808 (C29H43O11 [M+H]+ requires 567.2805).
4.5. Antiaritoxioside B (2)
Colorless syrup; −9.52° (c 0.04 in MeOH); UV λmax(MeOH)/nm (log ε) 215 (4.31); IR νmax/cm−1 (KBr) 3440, 2937, 1744, 1647, 1634, 1454, 1032; 1H NMR data (pyridine-d5) see Table 2; 13C NMR data (pyridine-d5) see Table 1; FABMS m/z 539 (8%) [M+H]+; HRFABMS m/z 539.2855 (C28H43O10 [M+H]+ requires 539.285).
4.6. Antiaritoxioside C (3)
Colorless syrup; −242.2° (c 0.003 in MeOH); UV λmax (MeOH)/nm (log ε) 213 (4.14); IR νmax/cm−1 (KBr) 3410, 2928, 1734, 1647, 1636, 1541, 1456, 1076, 1036; 1H NMR data (pyridine-d5) see Table 2; 13C NMR data (pyridine-d5) see Table 1; FABMS m/z 539 (6%) [M+H]+; HRFABMS m/z 539.2855 (C28H43O10 [M+H]+ requires 539.2856).
4.7. Antiaritoxioside D (4)
Colorless syrup; −45.88° (c 0.01 in MeOH); UV λmax (MeOH)/nm (log ε) 214 (4.17); IR νmax/cm−1 (KBr) 3410, 2932, 1742, 1649, 1454, 1377, 1029; 1H NMR data (pyridine-d5) see Table 2; 13C NMR data (pyridine-d5) see Table 1; FABMS m/z 555 (3%) [M+H]+; HRFABMS m/z 555.2805 (C28H43O11 [M+H]+ requires 555.2805).
4.8. Antiaritoxioside E (5)
Colorless syrup; −69.72° (c 0.02 in MeOH); UV λmax (MeOH)/nm (log ε) 271 (3.63), 218 (4.36); IR νmax/cm−1 (KBr) 3410, 2945, 1732, 1640, 1630, 1452, 1385, 1171, 1109, 1078,1028, 997; 1H NMR data (pyridine-d5) see Table 2; 13C NMR data (pyridine-d5) see Table 1; FABMS m/z 555 (8%) [M+H]+; HRFABMS m/z 555.2802 (C28H43O11 [M+H]+ requires 555.2805).
4.9. Antiaritoxioside F (6)
Colorless syrup; −15.28° (c 0.02 in MeOH); UV λmax (MeOH)/nm (log ε) 216 (4.32); IR νmax/cm−1 (KBr) 3410, 2926, 1742, 1645, 14474, 1377, 1049; 1H NMR data (pyridine-d5) see Table 2; 13C NMR data (pyridine-d5) see Table 1; FABMS m/z 529 (7%) [M+Na]+, 507 (5%) [M+H]+; HRFABMS m/z 529.2774 (C28H42-NaO8 [M+Na]+ requires 529.2777).
4.10. Antiaritoxioside G (7)
Colorless powder (CHCl3−MeOH); mp 227–228 °C; −57.69° (c 0.01 in MeOH); UV λmax (MeOH)/nm (log ε) 215 (4.62); IR νmax/cm−1 (KBr) 3350, 2949, 1753, 1383, 1286, 1175, 1101, 1022, 991; 1H NMR data (pyridine-d5) see Table 2; 13C NMR data (pyridine-d5) see Table 1; FABMS m/z 553 (2%) [M+H]+; HRFABMS m/z 553.3014 (C29H45O10 [M+H]+ requires 553.3012).
4.11. Antiarotoxinin B (8)
Colorless powder (CHCl3−MeOH); mp >280 °C; −31.34° (c 0.02 in MeOH); IR νmax/cm−1 (KBr) 3261, 2936, 2882, 1470, 1448, 1383, 1088, 1047; 1H NMR data (pyridine-d5) see Table 2; 13C NMR data (pyridine-d5) see Table 1; EIMS m/z 368 (8%) [M]+, 350 (28%), 319 (39%), 301 (41%), 296 (100%), 290 (15%), 283 (20%), 272 (14%), 147 (12%), 121 (15%), 107 (28%), 97 (39%); HREIMS m/z 368.2560 (C21H36O5 [M]+ requires 368.2563).
4.12. Anticarin A (41)
Colorless syrup; +16.3° (c 0.058, MeOH); UV λmax (MeOH)/nm (log ε) 316 (3.60), 258 (3.48); IR νmax/cm−1 (KBr) 3400, 2926, 1711, 1607, 1572, 1450, 1279; 1H NMR (400 MHz, DMSO-d6) δH 9.60 (1H, br s, D2O exchangeable, OH), 7.94 (1H, d, J = 9.6 Hz, H-4), 7.06 (1H, s, H-5), 6.32 (1H, d, J = 9.6 Hz, H-3), 4.39 (1H, d, J = 6.0 Hz, D2O exchangeable, 2′-OH), 4.23 (1H, br s, D2O exchangeable, 3′-OH), 3.79 (3H, s, OCH3), 3.36 (1H, m, H-2′), 2.95 (1H, d, J = 13.2 Hz, H-1′), 2.39 (1H, dd, J = 13.2, 10.4 Hz, H-1′), 1.12 (3H, s, H-4′), 1.10 (3H, s, H-5′); 13C NMR (100 MHz, DMSO-d6) δC 160.3 (C-2), 149.9 (C-7), 145.0 (C-4), 142.3 (C-9), 137.2 (C-8), 131.0 (C-6), 119.7 (C-5), 115.0 (C-10), 114.0 (C-3), 77.8 (C-2′), 71.9 (C-3′), 60.5 (OCH3), 31.4 (C-1′), 26.6 (C-5′), 24.8 (C-4′); EIMS m/z 294 (28%) [M]+, 261 (7%), 236 (79%), 235 (37%), 221 (23%), 205 (100%), 193 (56%), 177 (20%), 163 (25%), 147 (22%), 59 (84%); HREIMS m/z 294.1100 (C15H18O6 [M]+ requires 294.1103).
4.13. Anticarin B (42)
Colorless needles (MeOH); mp. 265–267 °C; +38.2° (c 0.2523, MeOH); UV λmax (MeOH)/nm (log ε) 326 (4.18), 302 (3.92, sh), 259 (3.46), 247 (3.50), 220(4.13, sh), 204 (4.67); IR νmax/cm−1 (KBr) 3300, 1709, 1605, 1592, 1541, 1306, 1126, 1076, 1036; 1H NMR (400 MHz, DMSO-d6) δH 7.96 (1H, d, J = 9.2 Hz, H-4), 7.54 (1H, d, J = 8.8 Hz, H-5), 6.76 (1H, d, J = 8.8 Hz, H-6), 6.26 (1H, d, J = 9.2 Hz, H-3), 5.29 (1H, d, J = 4.4 Hz, D2O exchangeable, 3′-OH), 4.93 (2H, m, D2O exchangeable, 3″, 4″-OH), 4.84 (1H, br. d, J = 2.4 Hz, H-4′), 4.71 (1H, d, J = 8.0 Hz, H-1″), 4.52 (1H, br s, D2O exchangeable, 4′-OH), 4.42 (1H, t, J = 6.0 Hz, D2O exchangeable, 6″-OH), 3.94 (1H, dd, J = 4.4, 2.4 Hz, H-3′), 3.76 (1H, br. dd, J = 12.0, 6.0 Hz, H-6″), 3.50 (1H, br s dd, J = 12.0, 6.0 Hz, H-6″), 3.25 (1H, m, H-5″), 3.22 (1H, t, J = 8.4 Hz, H-3″), 3.05 (1H, m, H-4″), 2.90 (1H, t, J = 8.4 Hz, H-2″), 1.27 (6H, s, 20-CH3 × 2); 13C NMR (100 MHz, DMSO-d6) δC 160.1 (C-2), 156.6 (C-7), 155.0 (C-9), 144.8 (C-4), 129.1 (C-5), 114.1 (C-6), 112.0 (C-3, 10), 108.6 (C-8), 103.8 (C-1″), 78.5 (C-2′), 77.3 (C-5″), 76.8 (C-3″), 74.0 (C-2″), 71.4 (C-4′), 70.6 (C-3′), 70.3 (C-4″), 61.5 (C-6″), 24.9 & 24.3 (2′-CH3 × 2); FABMS m/z 425 (23%) [M+H]+, 371 (8%), 307 (10%), 263 (75%), 245 (31%), 219 (74%), 154 (100%), 136 (86%); HRFABMS m/z 425.1367 (C20H25O10 [M+H]+ requires 425.1369).
4.14. Antiarone L (43)
Colorless needles (acetone); mp. 131–133°C; −3.0° (c 0.53, MeOH); UV λmax (MeOH)/nm (log ε) 328 (3.63, sh), 288 (4.31), 226 (4.44, sh), 206 (4.86); IR νmax/cm−1 (KBr) 3456, 3175, 2964, 2924, 1638, 1605, 1497, 1460, 1375, 1277, 1163, 1082; CD (c 5.4 × 10−5, MeOH) [θ]208 −9580, [θ]224 +38450, [θ]286 −22450, [θ]312 +52330; 1H NMR (400 MHz, acetone-d6) δH 12.17 (1H, br s, D2O exchangeable, 5-OH), 9.69 (1H, br s, D2O exchangeable, 8-OH), 7.55 (1H, br s, D2O exchangeable, 3′-OH), 7.06 (1H, d, J =8.4 Hz, H-60), 6.90 (1H, d, J = 8.4 Hz, H-5′), 5.96 (2H, s, H-6&8), 5.62 (1H, dd, J = 13.2, 2.8 Hz, H-2), 5.13 (1H, t, J = 6.4 Hz, H-2″), 3.86 (3H, s, OCH3), 3.52 (2H, d, J = 6.4Hz, H-1″), 3.16 (1H, dd, J = 17.2, 13.2 Hz, H-3α), 2.66 (1H, dd, J = 17.2, 2.8 Hz, H-3β), 1.67&1.63 (each 3H, s, H-4″&5″); 13C NMR (acetone-d6, 100 MHz) δC 197.4 (C-4), 167.2 (C-7), 165.1 (C-5), 164.4 (C-9), 148.0 (C-4′), 144.8 (C-3′), 131.4 (C-3″), 130.5 (C-1′), 126.9 (C-2′), 124.0 (C-2″), 118.2 (C-6′), 109.5 (C-5′), 103.0 (C-10), 96.7 (C-8), 95.7 (C-6), 79.9 (C-2), 56.2 (OCH3), 43.1 (C-3), 25.7 & 17.9 (C-4″ & 5″), 25.0 (C-1″); EIMS m/z 370 (100%) [M]+, 352 (10%), 219 (12%), 153 (26%), 125 (18%); HREIMS m/z 370.1412 (C21H22O6 [M]+ requires 370.1416).
4.15. Antiarone K (44)
Colorless powder (acetone); mp 171–173 °C; −44.7° (c 0.001, MeOH); UV λmax (MeOH)/nm (log ε) 324 (3.41, sh), 286 (4.06), 231 (4.35, sh), 213 (4.25); IR νmax/cm−1 (KBr) 3600–3000, 2963, 2926, 2855, 1639, 1522, 1466, 1456, 1340, 1271, 1161, 1085; CD (c 5.0 × 10−5, MeOH) [θ]210 −10580, [θ]229 +39460, [θ]290 −25380, [θ]314 +52190; 1H NMR (acetone-d6, 400 MHz) δH 12.18 (1H, br s, D2O exchangeable, 5-OH), 7.70 (1H, br s, D2O exchangeable, 3′-OH), 6.56 (1H, s, H-6′), 6.40 (1H, d, J = 9.8 Hz, H-1″′), 5.95 (2H, s, H-6 & 8), 5.73 (1H, d, J = 9.8 Hz, H-2″′), 5.60 (1H, dd, J = 13.4, 2.6 Hz, H-2), 5.13 (1H, t, J = 6.8 Hz, H-2″), 3.48 (2H, d, J = 6.8 Hz, H-1″), 3.17 (1H, dd, J = 17.0, 13.4Hz, H-3α), 2.64 (1H, dd, J = 17.0, 2.6 Hz, H-3β), 1.63&1.67 (each 3H, s, H-4″&5″), 1.18 & 1.20 (each 3H, s, 2″′-CH3 × 2); 13C NMR (acetone-d6, 100 MHz) δC 197.4 (C-4), 167.2 (C-7), 165.2 (C-5), 164.5 (C-9), 143.8 (C-3′), 140.5 (C-4′), 133.1 (C-5′), 131.3 (C-3″ & 3″′), 120.1 (C-1′), 127.1 (C-2′), 124.0 (C-2″), 122.7 (C-4″′), 120.0 (C-6′), 103.0 (C-10), 96.7 (C-8), 95.7 (C-6), 77.5 (C-2″′), 77.0 (C-2), 43.2 (C-3), 32.4 & 29.1 (C-2″′-CH3 × 2), 25.7&17.9 (C-4″& 5″), 25.2 (C-1″); EIMS m/z 436 (100%) [M]+; 418 (10%), 286 (25%), 255 (18%); 153 (26%); HREIMS m/z 436.1889 (C26H28O6 [M]+ requires 436.1886).
4.16. In vitro cytotoxicity assay
The sulforhodamine B assay was used according to the procedures developed and validated at NCI.47 Doxorubicin was used as the positive control antitumor drug. The in vitro anticancer activities are expressed as ED50 values, which is the test compound concentration (μg/mL) that reduced the cell number by 50% after 72 h of continuous treatment. The values were interpolated from dose-response data. Each test was performed in triplicate with variation less than 5%. The ED50 values determined in each of independent tests varied less than 10%. Compound stock solutions were prepared in DMSO with the final solvent concentration ≤ 1% DMSO (v/v), a concentration without effect on cell replication. The cells were cultured at 37 °C in RPMI-1640 supplemented with 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 2% (w/v) sodium bicarbonate, 10% (v/v) fetal bovine serum, and 100 μg/mL kanamycin in a humidified atmosphere containing 5% CO2.
Acknowledgments
The authors acknowledge financial support from the National Science Council, Taiwan, Republic of China awarded to T.S. Wu. Partial support was received from NIH Grant No. CA-177584-01 awarded to K.H. Lee.
References and notes
- 1.Newman RA, Yang P, Pawlus AD, Block KI. Mol Interventions. 2008;8:36. doi: 10.1124/mi.8.1.8. and references therein. [DOI] [PubMed] [Google Scholar]
- 2.Prassas I, Diamandis EP. Nat Rev Drug Disc. 2008;7:926. doi: 10.1038/nrd2682. [DOI] [PubMed] [Google Scholar]
- 3.Shi LS, Liao YRM, Su J, Lee AS, Kuo PC, Damu AG, Kuo SC, Sun HD, Lee KH, Wu TS. J Nat Prod. 2010;73:1214. doi: 10.1021/np9005212. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Q, Tang JS, Hu MJ, Liu J, Chen HF, Gao H, Wang GH, Li SL, Hao XJ, Zhang XK, Yao XS. J Nat Prod. 2013;76:1771. doi: 10.1021/np4005147. [DOI] [PubMed] [Google Scholar]
- 5.Hano Y, Mitsui P, Nomura T. Heterocycles. 1990;30:1023. [Google Scholar]
- 6.Hano Y, Mitsui P, Nomura T. Heterocycles. 1990;31:1315. [Google Scholar]
- 7.Hano Y, Mitsui P, Nomura T, Kawai T, Yoshida Y. J Nat Prod. 1991;54:1049. [Google Scholar]
- 8.Dong WH, Mei WL, Zhao YX, Zeng YB, Zuo WJ, Wang H, Li XN, Dai HF. Planta Med. 2011;77:1730. doi: 10.1055/s-0030-1271045. [DOI] [PubMed] [Google Scholar]
- 9.Dai HF, Gan YJ, Que DM, Wu J, Wen ZC, Mei WL. J Asian Nat Prod Res. 2009;11:832. doi: 10.1080/10286020903164285. [DOI] [PubMed] [Google Scholar]
- 10.Dai HF, Gan YJ, Que DM, Wu J, Wen ZC, Mei ML. Molecules. 2009;14:3694. doi: 10.3390/molecules14093694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang KC. The Pharmacology of Chinese Herbs. CRC Press; Louisville: 1993. pp. 60–61. [Google Scholar]
- 12.Uno Y, Mitsui P, Nomura T. Jpn Kokai Tokkyo Koho JP04,169, 548 [92, 169, 548] (cl Co 7(49/84), 17, Jun 1992, Appl 90/295, 298, 02 Nov 1990, 599. [CA 117: P239819v (1992)]. [Google Scholar]
- 13.Scott AI. Interpretation of the Ultraviolet Spectra of Natural Product. Pergamon Press; New York: 1964. p. 235. [Google Scholar]
- 14.Muehlradt P, Weiss Ek, Reichstein T. Helv Chem Acta. 1964;47:2164. [Google Scholar]
- 15.Levrier C, Kiremire B, Guéritte F, Litaudon M. Fitoterapia. 2012;83:660. doi: 10.1016/j.fitote.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Wehrli W, Schindler O, Reichstein T. Helv Chem Acta. 1962;45:1183. [Google Scholar]
- 17.Peoghenghi R, Philipp A, Gaudry R. Tetrahedron Lett. 1963;29:2045. [Google Scholar]
- 18.Scott AI. Interpretation of the Ultraviolet Spectra of Natural Product. Pergamon Press; New York: 1964. p. 142. [Google Scholar]
- 19.Chen IS, Chang CT, Sheen WS, Teng CM, Tsai IL, Duh CH, Ko FN. Phytochemistry. 1996;41:525. doi: 10.1016/0031-9422(95)00625-7. [DOI] [PubMed] [Google Scholar]
- 20.Sharma RK, Negi DS, Gibbons S, Otsuka H. Planta Med. 2008;74:175. doi: 10.1055/s-2008-1034281. [DOI] [PubMed] [Google Scholar]
- 21.Sano K, Yosioka I, Kitagawa I. Chem Pharm Bull. 1975;23:20. [Google Scholar]
- 22.Antus S, Baitz-Gacs E, Kajtar J, Snatzke G, Tokes AL. Liebigs Ann Chem. 1994;5:497. [Google Scholar]
- 23.Lei ZH, Nakayama H, Kuniyasu A, Tai BS, Nohara T. Chem Pharm Bull. 2002;50:861. doi: 10.1248/cpb.50.861. [DOI] [PubMed] [Google Scholar]
- 24.Juslen C. Soc Sci Fennica, Commentationes Phys. 1962;2:61. [CA 57:15482i (1963)] [Google Scholar]
- 25.Wehrli W. Helv Chem Acta. 1962;45:1206. [Google Scholar]
- 26.Muehlradt P, Weiss Ek, Reichstein T. Ann Chem. 1965;685:253. [CA 63: 13389a (1964)] [Google Scholar]
- 27.Brandt R, Kaufmann H, Reichstein T. Helv Chem Acta. 1966;49:2469. [Google Scholar]
- 28.Makarevich IF, Klimenko OI, Kolesnikov DG. Chem Nat Compd. 1974;10:619–622. [Google Scholar]
- 29.Makarevich IF, Kolesnikov DG, Belokon VF. Chem Nat Compd. 1974;10:616. [Google Scholar]
- 30.Carter CA, Gray EA, Schneider TL, Lovett CMJr, Scott L, Messer AC, Richardson DP. Tetrahedron. 1997;53:16957. [Google Scholar]
- 31.Jimenez B, Grande MC, Anaya J, Torres P, Grande M. Phytochemistry. 2000;53:1025. doi: 10.1016/s0031-9422(99)00524-5. [DOI] [PubMed] [Google Scholar]
- 32.Ikeshiro Y, Mase I, Tomita Y. Phytochemistry. 1994;35:1339. [PubMed] [Google Scholar]
- 33.Harkar S, Razoan TK, Waight ES. Phytochemistry. 1984;23:419. [Google Scholar]
- 34.Fukui H, Goto K, Tabata M. Chem Pharm Bull. 1988;36:4174. doi: 10.1248/cpb.36.4174. [DOI] [PubMed] [Google Scholar]
- 35.Wu TS, Shi LS, Kuo SC. Phytochemistry. 1999;50:1411. [Google Scholar]
- 36.Wu TS, Yeh JH, Wu PL. Phytochemistry. 1995;40:121. [Google Scholar]
- 37.Evidente A, Andolfi A, Fiore M, Spanu E, Maddau L, Franceschini A, Marras F, Motta A. J Nat Prod. 2006;69:671. doi: 10.1021/np050393l. [DOI] [PubMed] [Google Scholar]
- 38.Phadke PS, Rao AVR, Venkataraman K. Indian J Chem. 1967;5:131. [Google Scholar]
- 39.Apsimon JW, Haymes NB, Sim KY, Whelley WB. J Chem Soc. 1963:3780. [Google Scholar]
- 40.Chen CY, Chang FR, Teng CM, Wu YC. J Chin Chem Soc. 1999;46:77. [Google Scholar]
- 41.Kazuya K, Katsuyoshi M, Kazuo K, Taichi O. Chem Pharm Bull. 1994;42:1669. [Google Scholar]
- 42.Yadava VS, Misra K. Indian J Chem Sect B. 1989;28:875. [Google Scholar]
- 43.Jacques R. J Am Chem Soc. 1987;109:316. [Google Scholar]
- 44.Masao O, Masami T, Toru Y, Masami S, Yasumi U, Shunrou K. J Agric Biol Chem. 1989;53:1469. [Google Scholar]
- 45.Rao KV. J Heterocycl Chem. 1975;12:725. [Google Scholar]
- 46.The new cardiac glycosides (1-8) were obtained in minute quantity from A. toxicaria. Samples were sent for anti-inflammatory testing and insufficient quantities remained for cytotoxicity evaluation.
- 47.Rao KV, Kapicak LS. J Heterocycl Chem. 1976;13:1073. [Google Scholar]