Abstract
Melanoma differentiation-associated gene-7/interleukin-24 (mda-7/IL-24) displays a broad range of antitumor properties including cancer-specific induction of apoptosis, inhibition of tumor angiogenesis and modulation of antitumor immune responses. In our study, we elucidated the role of MDA-7/IL-24 in inhibiting growth of breast cancer-initiating/stem cells. Ad.mda-7 infection decreased proliferation of breast cancer-initiating/stem cells without affecting normal breast stem cells. Ad.mda-7 induced apoptosis and endoplasmic reticulum stress in breast cancer-initiating/stem cells similar to unsorted breast cancer cells and inhibited the self-renewal property of breast cancer-initiating/stem cells by suppressing Wnt/β-catenin signaling. Prevention of inhibition of Wnt signaling by LiCl increased cell survival upon Ad.mda-7 treatment, suggesting that Wnt signaling inhibition might play a key role in MDA-7/IL-24-mediated death of breast cancer-initiating/stem cells. In a nude mouse subcutaneous xenograft model, Ad.mda-7 injection profoundly inhibited growth of tumors generated from breast cancer-initiating/stem cells and also exerted a potent “bystander” activity inhibiting growth of distant uninjected tumors. Further studies revealed that tumor growth inhibition by Ad.mda-7 was associated with a decrease in proliferation and angiogenesis, two intrinsic features of MDA-7/IL-24, and a reduction in vivo in the percentage of breast cancer-initiating/stem cells. Our findings demonstrate that MDA-7/IL-24 is not only nontoxic to normal cells and normal stem cells but also can kill both unsorted cancer cells and enriched populations of cancer-initiating/stem cells, providing further documentation that MDA-7/IL-24 might be a safe and effective way to eradicate cancers and also potentially establish disease-free survival.
Keywords: MDA-7/IL-24, apoptosis, Wnt signaling, cancer-initiating/stem cells, breast cancer
Breast cancer continues to be a leading cause of cancer-related deaths in women worldwide despite significant advances in screening techniques that lead to early disease detection. This disease evades most conventional therapies frequently leading to relapse although the primary lesion is eradicated.1 Over the last years, evidence has accumulated supporting the notion that tumors are organized in a hierarchy of heterogeneous cell populations with different biological properties and that the ability to sustain tumor formation and growth exclusively resides in a small proportion of tumor cells, termed cancer stem cells or tumor-initiating cells.2,3 In breast cancer, a population of CD24−/low/CD44+ cells has been isolated that is highly enriched for cancer-initiating/stem cells. This population is 1,000 times more tumorigenic than cell populations that are depleted of CD24−/low/CD44+ cells, and injection of as few as 200 cells leads to tumor formation in Severe combined immunodeficiency (SCID) mice.4 Breast cancer-initiating/stem cells can be established from patients’ surgical specimens or breast cancer cell lines and encompass undifferentiated cells capable of self-renewal, extensive proliferation as clonal nonadherent spherical clusters and differentiation along different mammary epithelial lineages.4–6
Tumor-initiating/stem cells have the important feature of self-renewal, which is an intrinsic property of stem cells.7 Wnt/β-catenin signaling is one of the key pathways that promote self-renewal of breast cancer-initiating/stem cells.5 Activation of Wnt target genes is mediated by β-catenin, which translocates into the nucleus and binds to the transcription factor T-cell factor/lymphoid enhancer factor (TCF/LEF). The level of intracellular β-catenin is modulated by a multiprotein complex consisting of glycogen synthase kinase 3β (GSK3β), adenomatous polyposis coli, casein kinase 1α and axin. GSK3β promotes the ubiquitin-proteasome degradation of β-catenin by phosphorylating three specific amino acids, Ser33/Ser37/Thr41, on β-catenin.8,9 Similarly, Akt (protein kinase B) is a central regulator in the Wnt and PI3K signaling pathways.10 Apoptosis resistance, a characteristic feature of cancer cells, has been demonstrated prominently in cancer-initiating/stem cells.11 Breast cancer-initiating/stem cells display high levels of expression of apoptotic regulating proteins including Bcl-2, NF-κB and Akt.2,3,11 A comparative gene expression profile study illustrated that a side population of cells isolated from MCF-7 when compared to the non-side population of MCF-7 cells displayed alterations in several different pathways documenting deregulation of a number of apoptotic genes including BCL-2, BCL-xL, IAPs, PI3K and FADD.12 Consequently, the effective targeting of breast cancer-initiating/stem cells with novel antitumor agents has the potential to significantly improve outcome for women with both early and advanced stages of breast cancer.
Using subtraction hybridization combined with induction of cancer cell terminal differentiation, our laboratory cloned melanoma differentiation-associated gene-7/interleukin-24, a novel member of the IL-10-related cytokine gene family.13 Subsequent experiments document that mda-7/IL-24 has nearly ubiquitous antitumor properties in vitro and in vivo,14–21 which culminated in a successful entry into the clinic where safety and clinical efficacy when administered by adenovirus (Ad.mda-7; INGN 241) were shown in a phase 1 clinical trial in patients with advanced cancers.18 Forced mda-7/IL-24 expression in cancer cells in vitro and in vivo inhibits angiogenesis22; stimulates an antitumor immune response23; sensitizes cancer cells to radiation-, chemotherapy- and antibody-induced killing14,16,24,25 and elicits potent “antitumor bystander activity.”26,27 After forced expression of mda-7/IL-24 by an adenovirus, the MDA-7/IL-24 protein interacts with the endoplasmic reticulum (ER) chaperone protein BiP/GRP78 and initiates a cascade of “unfolded protein response” (UPR) events in tumor cells that culminate in apoptosis.28 We have shown that mda-7/IL-24 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice.29 Moreover, chemotherapeutic agent- and antibody-mediated killing is enhanced in the presence of mda-7/IL-24 providing a synergistic effect thereby expanding treatment options for breast cancer patients.30–32 We have also found that a conditionally replication competent adenovirus expressing mda-7/IL-24, a cancer terminator virus (CTV), displays dual cancer-specific activity resulting in eradication of both primary and distant breast carcinomas in nude mice.33 It has been reported that Ad.mda-7 downregulates both the PI3K and the Wnt/β-catenin signaling pathways in breast, lung and pancreatic cancer cells and remedies aberrant signaling in tumor cells, resulting in restoration of apoptosis induction.34,35
In our study, we investigated the role of mda-7/IL-24 on breast cancer-initiating/stem cells. Our findings show that Ad.mda-7 is able to inhibit the growth of different breast cancer-initiating/stem cells via induction of ER stress and apoptosis without exerting any deleterious effect in normal stem cells. We also document that Ad.mda-7 inhibits self-renewal of breast cancer-initiating/stem cells by inhibiting the Wnt signaling pathway and Ad.mda-7 effectively inhibits in vivo growth of tumors originating from breast cancer-initiating/stem cells. Accordingly, Ad.mda-7 might provide an effective means of safely eradicating both unsorted cancer cells and cancer-initiating/stem cells, thereby establishing an enduring therapeutic response in patients with breast cancer.
Material and Methods
Cell culture
MCF-7, MDA-MB-231, T47D human breast carcinoma cell lines and MCF-10A, a normal breast cell line, were obtained from the American Type Culture Collection and were cultured as recommended. Human mammary epithelial cells (HMEpC) were obtained from Promocell Promocell, Boston, MA, USA and cultured as recommended. Cells were infected with 100 plaque-forming units (pfu)/cell of Ad.mda-7 or Ad.vec and analyzed as described.28 Cell viability by MTT assay was performed as described.29,33
Flow cytometry and sorting
Breast cancer cells were detached with trypsin, washed once in Fluorescence activated cell sorter (FACS) buffer (phosphate buffered saline (PBS) containing 1–2% bovine serum albumin (BSA) and 5 mM EDTA), stained with anti-CD24-FITC and anti-CD44-PE using 10 μl of antibody per 106 cells and incubated at 4°C for 15 min. After incubation, cells were washed once with FACS buffer, suspended in FACS buffer at 2 × 106 cells/ml and cancer-initiating/stem cells were sorted on an Aria cell sorter (BD Biosciences, San Jose, CA). Live cells were gated on the basis of forward and side scatters, and single cells were gated on the basis of forward scatter and pulse width. Gates were determined by analysis of unstained cells and isotype-specific stains.4
Mammosphere cultures and inhibition assays
The sorted breast cancer cells were plated and cultured in ultra-low attachment plates (Corning Life Sciences, Corning, NY) in a serum-free mammary epithelial growth medium supplemented with B27 (Life Technologies, NY), 20 ng/ml EGF (Epidermal growth factor) and 20 ng/ml bFGF (basic fibroblast growth factor) (BD Biosciences) as described.5,6 To quantify the effect of Ad.mda-7 on mammospheres, different concentrations of Ad.mda-7 (pfu) were used to infect primary mammosphere cultures, whereas the second passage cultures were grown in the absence of Ad.mda-7. After 7 days of culture, the number of mammospheres was counted under a microscope.36
Annexin V and propidium iodide staining
The breast cancer-initiating/stem cells were seeded in six-well plates (2 × 105 per well) and were infected with Ad.mda-7 or Ad.vec for 48 hr and processed and evaluated for Annexin V binding as described.33
Western blotting analysis
Preparation of whole-cell lysates and Western blotting for MDA-7/IL-24 (1:100, mouse monoclonal, GenHunter Corp., Nashville, TN), BiP/GRP78 (1:1,000, rabbit polyclonal, Santa Cruz, Dallas, TX), GRP94 (1:1,000, rabbit polyclonal, Santa Cruz), GADD133 (1:1,000, rabbit polyclonal, Santa Cruz), PARP, Bcl2, Bcl-xL, phospho-Akt, phospho-β-catenin, phospho-GSK3β, phospho-eIF2α, Akt, β-catenin and GSK3β (1:1,000, rabbit monoclonal, Cell Signaling, Boston, MA) protein levels were as described.28
Caspase assays
The breast cancer-initiating/stem cells were seeded in 96-well (10,000 cells per well) ultralow attachment plates and were infected with Ad.mda-7 or Ad.vec for 48 hr. After treatment caspase 3/7 activities were measured using CellPlayer™ 96-well kinetic Caspase 3/7 reagent kits following the manufacturer’s protocol (Essen Bioscience Corp. Ann Arbor, MI).
Reporter assays
Breast cancer-initiating/stem cells were suspended in 24-well ultralow attachment plates and were transduced with lentiviruses expressing TCF/LEF Reporter (TCF/LEF-Luc) at an optimized multiplicity of infection (MOI) of 5 for 48 hr in a final volume of 1.0 ml serum-free medium. The cells were infected with Ad.mda-7 for 12 hr and cell lysates were prepared and assayed for luciferase activity using Luciferase Assay System (Promega, Madison, WI) according to the manufacturer’s instructions.36
Human breast cancer xenografts in athymic nude mice and immunohistochemistry
T47D-initiating/stem cells (1 × 104) were injected s.c. in 100 μl of PBS in both flanks of female athymic nude mice (NCRnu/nu; 4-week old; ≈20 g of body weight) and xenograft studies were done as described previously.29 Formalin-fixed and paraffin-embedded specimens were sectioned, 3- to 4-μm thick, and immunohistochemistry was done as described previously.25
Statistical analysis
Data are represented as the mean ± standard error of mean and analyzed for statistical significance using one-way analysis of variance followed by Newman–Keuls test as a post hoc test. A p value of <0.05 was considered significant.
Results
Ad.mda-7 infection results in expression of MDA-7/IL-24 in breast cancer-initiating/stem cells
To determine if Ad.mda-7 is effective in selectively inhibiting growth and inducing apoptosis in breast cancer-initiating/stem cells versus normal stem cells and to define potential therapeutic applications, we sorted initiating/stem cells (CD24−/low/CD44+), which were ~1–5% of the total unsorted population, from different breast cancer cells, including MCF-7, MDA-MB-231 and T47D, as well as MCF-10A37 and HMEpC normal breast epithelial cells, and cultured them in serum-free media. Initially, we confirmed the expression of MDA-7/IL-24 in different cell types 48 hr after Ad.mda-7 infection. Ad.mda-7 infection resulted in a dose-dependent expression of MDA-7/IL-24 protein in all types of initiating/stem cells, irrespective of their cancer or normal origin (Fig. 1 and Supporting Information Fig. S1A).
Figure 1.
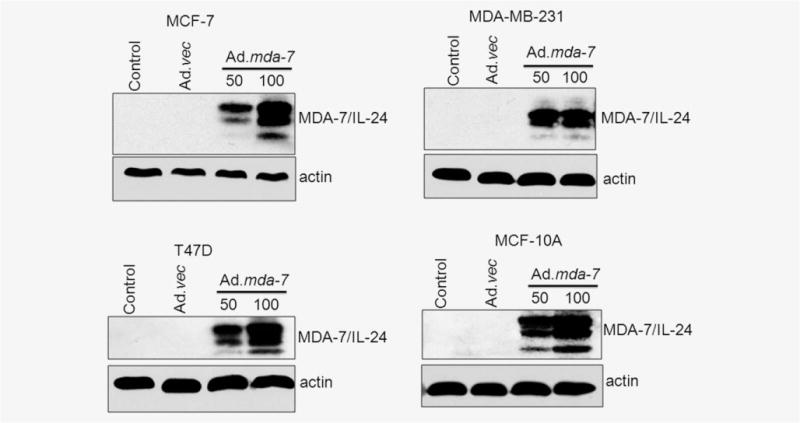
Ad.mda-7 infection results in expression of MDA-7/IL-24 in breast cancer-initiating/stem cells. MCF-7-, T47D-, MDA-MB-231- and MCF-10A-derived initiating/stem cells were sorted from their respective cell lines by flow cytometry and infected with either Ad.mda-7 (50 or 100 pfu) or Ad.vec (100 pfu) for 48 hr and total proteins were isolated. The expressions of MDA-7/IL-24 and actin (as a loading control) proteins were analyzed by Western blotting.
Ad.mda-7 inhibits proliferation and induces ER stress and apoptosis in breast cancer-initiating/stem cells
We demonstrated previously that infection of unsorted populations of breast cancer cells with Ad.mda-7 inhibits their proliferation and induces apoptosis.29–33 To investigate the effect of Ad.mda-7 on breast cancer-initiating/stem cells, we sorted cancer stem cells and non-stem cell populations from MCF-7 cells by flow cytometry using CD24 and CD44 as markers (Fig. 2a). Both CD24- and CD44-negative non-stem cells, CD24-positive cells, CD44-positive cells and both CD24- and CD44-positive cells were infected with Ad.mda-7 at different doses. Ad.mda-7 effectively reduced cell viability in all of these cell populations in a dose-dependent manner as measured by standard MTT assays (Fig. 2b). The finding that Ad.mda-7 inhibited growth of non-stem cell populations and stem cell populations to a similar extent indicates that mda-7/IL-24 might be an effective cancer therapeutic for chemotherapy-resistant breast cancer cells, which are enriched with cancer-initiating/stem cells.7,11,12 Similarly, Ad.mda-7 dose-dependently inhibited viability of cancer-initiating/stem cells sorted from T47D and MDA-MB-231 cells without affecting the normal stem cells derived from MCF-10A or HMEpC cells, thereby further confirming the cancer cell-specific nature of mda-7/IL-24 (Fig. 2c and Supporting Information Fig. S1B). Annexin V staining performed 48 hr post-Ad.mda-7 infection demonstrated a strong dose-dependent induction of apoptosis in cancer-initiating/stem cells, but not in normal stem cells (Fig. 2d). Dose-dependent apoptosis induction by Ad.mda-7 was further confirmed after Ad.mda-7 infection by increased cleavage of PARP and caspases, downregulation of the anti-apoptotic protein Bcl-2 and upregulation of the proapoptotic protein Bax in cancer-initiating/stem cells sorted from MCF-7 and T47D cells (Fig. 2e and Supporting Information Fig. S2A). Moreover, Western blotting and caspase assays by CellPlayer assay showed that both caspase 3 and 7 were activated in T47D-sorted cells and only caspase 7 was activated in MCF-7-sorted cells as these cells are deficient in caspase 3 (Figs. 2e and 2f).
Figure 2.
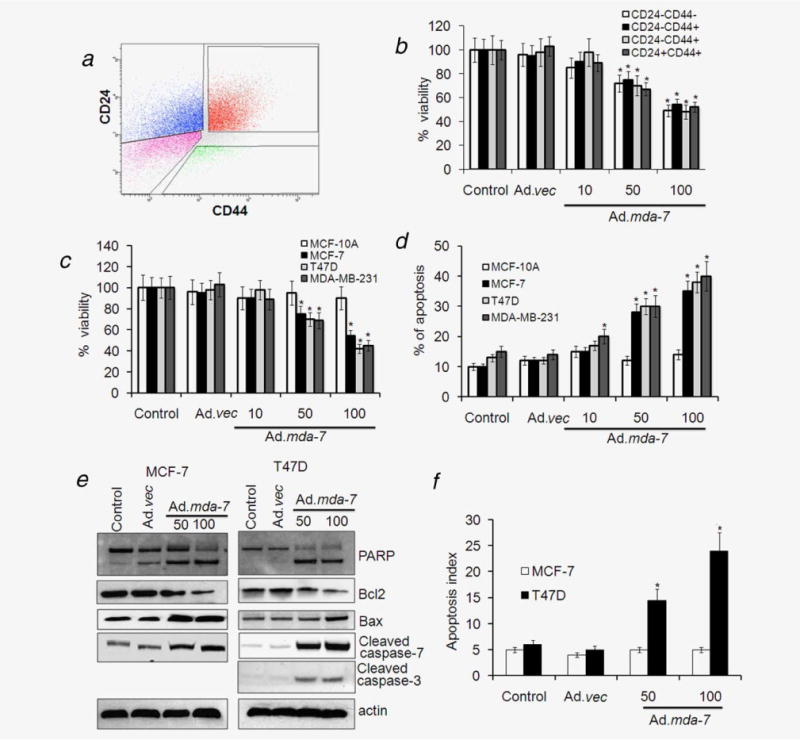
Ad.mda-7 infection induces apoptosis in breast cancer-initiating/stem cells. (a) MCF-7 cells were analyzed by flow cytometry for CD44 and CD24 expression. The gates shown were used for sorting of cells with the indicated phenotypes. (b) The sorted populations, based on surface markers, were infected with Ad.mda-7 (10, 50 and 100 pfu/cell) or Ad.vec (100 pfu/cell) and after 48 hr cell proliferation was measured by MTT assay. (c and d) Cancer-initiating/stem cells from MCF-7, T47D, MDA-MB-231 and MCF-10A were infected with Ad.mda-7 (50 or 100 pfu/cell) or Ad.vec (100 pfu/cell) and after 48 hr cell proliferation was measured by MTT assay (c) and Annexin V staining assay using flow cytometry (d) measured apoptosis. MCF-7- and T47D-initiating cells were infected with Ad.mda-7 (50 or 100 pfu/cell) or Ad.vec (100 pfu/cell) and cell lysates were prepared after 48 hr. (e) MCF-7- and T47D-initiating cells were infected with Ad.mda-7 (50 or 100 pfu/cell) or Ad.vec (100 pfu/cell) and cell lysates were prepared after 48 hr. Activation of poly(ADP-ribose) polymerase-1 (PARP), caspases 3 and 7 and expression of Bcl-2 and Bax were detected by Western blotting analysis. (f) After 48-hr infection with Ad.mda-7 or Ad.vec the apoptotic index as indicative of caspase 3/7 activity in MCF-7- and T47D-initiating cells was determined using a fluorescence microscope. The apoptotic index was calculated by dividing the number of caspase 3/7 fluorescent cells by the total number of DNA-containing cells after staining with Vybrant DyeCycle Green. Data represent mean ± SD (n = 3). Asterisk (*) represents significant difference (p < 0.5) with corresponding control.
We next analyzed the modulation of mda-7/IL-24 downstream genes and signaling pathways that contribute to its tumor-suppressing properties in the context of breast cancer-initiating/stem cells. MDA-7/IL-24 induces an ER stress response (UPR)28; therefore, we determined the expression levels of ER stress markers. The levels of BiP/GRP78, GRP94 and GADD153, and activation of p-eIF2α were significantly higher upon infection with Ad.mda-7 when compared to control and Ad.vec-infected breast cancer-initiating/stem cells (Fig. 3a). In addition, ER stress response element (ERSE) reporter assays confirmed that Ad.mda-7 increased ER stress in a dose-dependent manner (Fig. 3b). These findings further confirm that Ad.mda-7 also maintains its bona fide downstream effects in breast cancer-initiating/stem cells.
Figure 3.
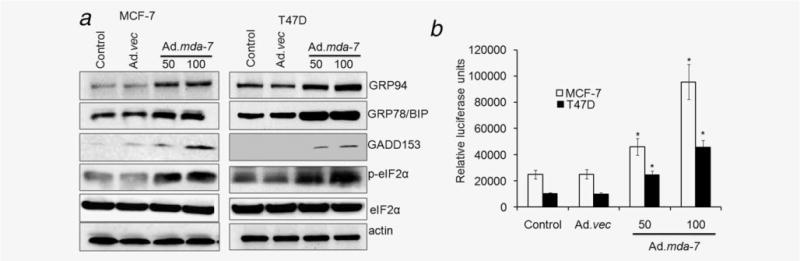
Ad.mda-7 induces ER stress in breast cancer-initiating/stem cells. MCF-7- and T47D-initiating/stem cells were infected with Ad.mda-7 (50 or 100 pfu/cell) or Ad.vec (100 pfu/cell) and cell lysates were prepared after 48 hr. (a) Changes in BiP/GRP78, GRP94 GADD153 and activation of phospho-eukaryotic initiation factor 2 (p-eIF2α) proteins were evaluated by Western blotting. (b) MCF-7- and T47D-initiating/stem cells were transfected with ERSE reporter followed by infection with Ad.mda-7 or Ad.vec for 24 hr and dual Luciferase assays were performed and promoter activity values are expressed as arbitrary units using a Renilla reporter for internal normalization. Data represent mean ± SD (n = 3). Asterisk (*) represents significant difference (p < 0.5) with corresponding control.
Ad.mda-7 inhibits mammosphere formation and Wnt signaling in breast cancer stem cells
Breast cancer-initiating/stem cells are enriched in nonadherent spherical clusters of cells, termed mammospheres, and these cells are capable of yielding secondary spheres and differentiating along multiple lineages.5,6 To evaluate whether Ad.mda-7 could suppress the formation of mammospheres in vitro, we infected breast cancer-initiating/stem cells, obtained from MCF-7 and T47D cells, with Ad.mda-7 for 48 hr and then cultured these cells in nonadherent conditions to determine mammosphere formation in the absence of Ad.mda-7. Compared to control and Ad.vec infection, Ad.mda-7 inhibited in a dose-dependent manner not only the number of mammospheres (Fig. 4a) but also the size of the spheres (Fig. 4b), indicating a reduced self-renewal capacity of these initiating/stem cells. It should be noted that Ad.mda-7 inhibited mammosphere formation in both MCF-7 and T47D cancer-initiating/stem cells even at 10 pfu, a dose at which no cytotoxic effect was observed in cell viability assays.
Figure 4.
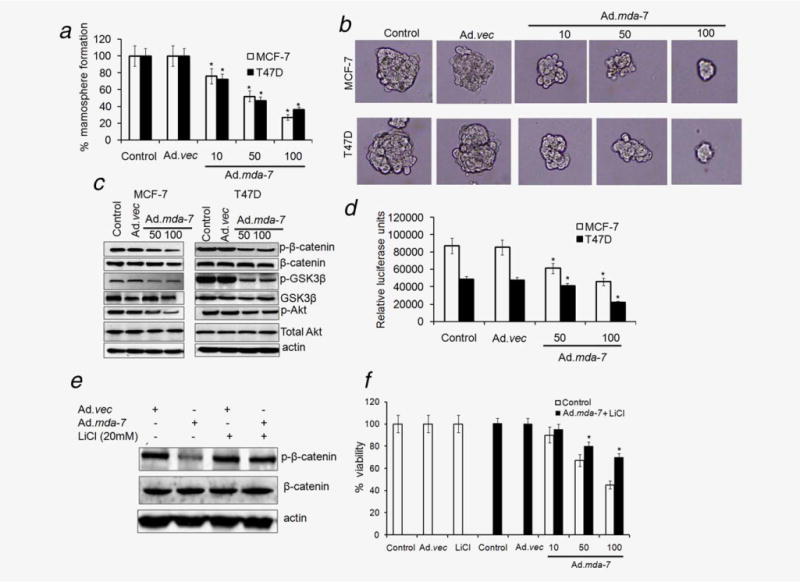
Ad.mda-7 inhibits mammosphere formation and Wnt signaling in breast cancer-initiating/stem cells. (a) Cancer-initiating/stem cells from MCF-7 and T47D were infected with Ad.mda-7 (50 or 100 pfu/cell) or Ad.vec (100 pfu/cell) for 48 hr and the infected cells were seeded for formation of secondary mammospheres as described in “Material and Methods” section and quantified by counting the mammospheres by microscope. Data represent mean ± SD (n = 3). (b) Photomicrograph of primary mammospheres following the indicated treatments (magnification, ×100). (c) MCF-7- and T47D-initiating/stem cells were infected with Ad.mda-7 (50 or 100 pfu/cell) or Ad.vec (100 pfu/cell) and cell lysates were prepared after 48 hr and changes in phospho- and total β-catenin, GSK3β and Akt expression were evaluated by Western blotting. (d) MCF-7- and T47D-initiating/stem cells were infected with a TCF/LEF TOP-luc lentiviral reporter system for 48 hr followed by infection with Ad.mda-7 (50 or 100 pfu/cell) or Ad.vec (100 pfu) for 12 hr and relative luciferase activity was measured as described in the “Material and Methods” section. (e and f) T47D-initiating/stem cells were infected with 100 pfu/cell of Ad.mda-7 or Ad.vec in the presence of LiCl (20 mM) for 48 hr and expression of phospho- and total β-catenin was analyzed (e) and cell proliferation was measured by MTT assay (f). Asterisk (*) represents significant difference (p < 0.5) when compared to Ad.mda-7-infected cells without LiCl.
There is considerable evidence that the Wnt pathway regulates self-renewal in breast cancer-initiating/stem cells.8,9 In addition, previous reports have shown that Ad.mda-7 inhibits the Wnt/β-catenin pathway in lung and breast cancer cells.34 Therefore, we determined the effect of Ad.mda-7 on Wnt signaling in breast cancer-initiating/stem cells by analyzing the levels of protein expression in this signaling pathway. Ad.mda-7 transduction of MCF-7- and T47D-derived breast cancer-initiating/stem cells resulted in a significant dose-dependent downregulation of p-β-catenin and p-GSK3β (Fig. 4c and Supporting Information Figs. S2B and S2C). Ad.mda-7 also decreased the expression of p-AKT, without affecting total Akt expression (Fig. 4c and Supporting Information Fig. S2D), which regulates initiating/stem cell self-renewal downstream of Wnt signaling.10 Transduction with Ad.vec did not alter Wnt or PI3K pathway proteins, thereby confirming the specificity of the MDA-7/IL-24 effect. To confirm that downregulation of β-catenin protein level decreases its transcriptional activity, we used a TCF/LEF TOP-luc lentiviral reporter system. Infection with Ad.mda-7 resulted in significant downregulation of β-catenin/LEF-dependent luciferase activity in MCF-7- and T47D-derived cancer-initiating/stem cells, further confirming the role of MDA-7/IL-24 in the inhibition of the Wnt signaling pathway (Fig. 4d). LiCl inactivates GSK3β through Ser9 phosphorylation, which in turn reduces the phosphorylation of β-catenin at Ser33/Ser37/Thr41 and its degradation.38 Ad.mda-7-mediated inhibition of β-catenin phosphorylation was reversed upon treatment of breast cancer-initiating/stem cells with LiCl as indicated by the lack of change in p-β-catenin levels upon Ad.mda-7 infection when performed in the presence of LiCl when compared to infection with only Ad.mda-7 (Fig. 4e). More importantly, pretreatment of breast cancer-initiating/stem cells with LiCl significantly prevented cell death induced by Ad.mda-7 as demonstrated by MTT assays (Fig. 4f). Taken together, these data suggest that downregulation of the Wnt/β-catenin self-renewal pathway might contribute to the inhibitory effects of MDA-7/IL-24 on breast cancer-initiating/stem cells.
Ad.mda-7 eradicates primary and inhibits distant tumors generated by breast cancer-initiating/stem cells in nude mice
To determine whether the in vitro growth inhibitory activity of Ad.mda-7 in breast cancer-initiating/stem cell translated into the in vivo state, T47D-initiating/stem cells (1 × 104) were subcutaneously injected into the left and right flanks of athymic nude mice. After palpable tumors of ~100 mm3 developed on both flanks, in ~ 30–40 days, the animals received seven intratumoral injections over a 4-week period with 1 × 108 pfu of Ad.vec or Ad.mda-7 in tumors on the left flank. The tumors on the right flank were not injected with virus. T47D-initiating/stem cells formed large, aggressive and actively proliferating tumors in control-untreated and in Ad.vec-injected animals. In contrast, the Ad.mda-7-treated group showed profound growth inhibition of the left flank-injected tumors, which was evident by 2 weeks after initiating the therapeutic treatment protocol (Figs. 5a and 5b). Furthermore, a significant inhibition of growth of the right-sided uninjected tumors was also observed upon treatment with Ad.mda-7 in the left flank tumor, highlighting the potent “bystander” antitumor effect of MDA-7/IL-24 (Figs. 5a and 5b). These results were anticipated, because direct injection of the right-side tumor with a nonreplicating adenovirus expressing mda-7/IL-24 (Ad.mda-7) results in high levels of MDA-7/IL-24 protein in the injected tumor, whereas secretion of this cytokine “bystander effect” will generate less MDA-7/IL-24 in the circulation and in the distant tumor and the inhibitory effect will therefore be less profound. These findings provide definitive evidence for therapeutic efficacy of Ad.mda-7 in breast cancer-initiating cells in vivo.
Figure 5.
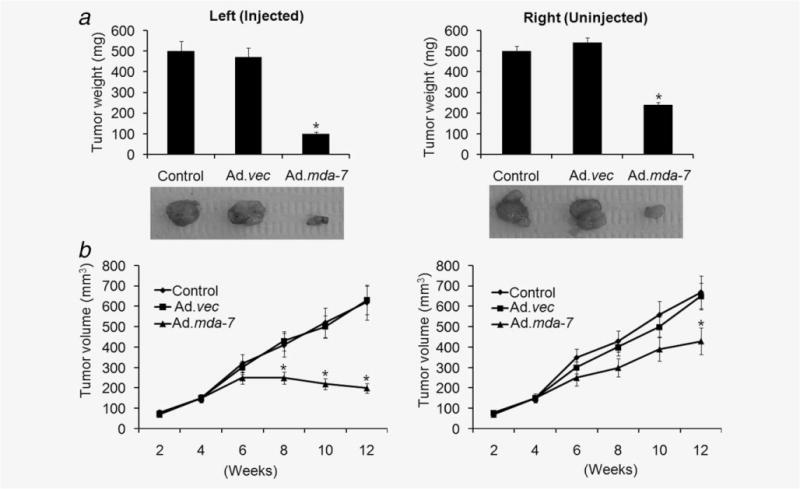
Ad.mda-7 eradicates primary tumors and inhibits distant tumors generated by breast cancer-initiating/stem cells in nude mice. Tumor xenografts from T47D-initiating/stem cells were established in athymic nude mice in the left and right flanks and only tumors on the left side were injected with PBS (control) or with the indicated Ad for 3 weeks (total of seven injections). (a) Measurement of tumor weight at the end of the study. Lower panel, picture of isolated tumors from the left-treated and right-untreated regions of animals. (b) Measurement of tumor volume at the end of the study. The data represent the mean ± SD with a minimum of five mice per group. Asterisk (*) represents significant difference (p < 0.5) with corresponding control.
We next determined the efficacy of Ad.mda-7 in in vivo transgene delivery by staining for MDA-7/IL-24 protein, CD-31 (which monitors angiogenesis and microvessel formation) and Ki-67 (which monitors cell proliferation). Tumors were harvested from the animals after three injections and tumor sections were interrogated by immunohistochemistry. Strong MDA-7/IL-24 staining was observed in left-sided tumors only upon Ad.mda-7 treatment (Fig. 6a and Supporting Information Fig. 3S). No significant MDA-7/IL-24 staining was observed in right-sided untreated tumors after three Ad.mda-7 injections of the left tumor, possibly reflecting the need for more injections to produce sufficient quantities of MDA-7/IL-24 in the circulation to induce expression in the untreated tumor. However, compared to Ad.vec treatment, Ad.mda-7 treatment resulted in a significant reduction in staining for CD-31 and Ki-67 in both left- and right-sided tumors (Fig. 6a and Supporting Information Fig. 3S). Analysis of cells isolated from in vivo tumor tissue by flow cytometry indicated that Ad.mda-7 administration decreased breast cancer-initiating/stem cells when compared to control unsorted tumor cells (Fig. 6b). These results may indicate that angiogenesis and cell proliferation in untreated tumors are sensitive to lower levels of circulating MDA-7/IL-24, which would need to be confirmed by further experiments. In total, these findings indicate that in breast cancer-initiating/stem cells Ad.mda-7 can efficiently generate MDA-7/IL-24 protein that exerts a potent “bystander” effect in distant tumors by inhibiting cell proliferation and angiogenesis (Figs. 5 and 6).
Figure 6.
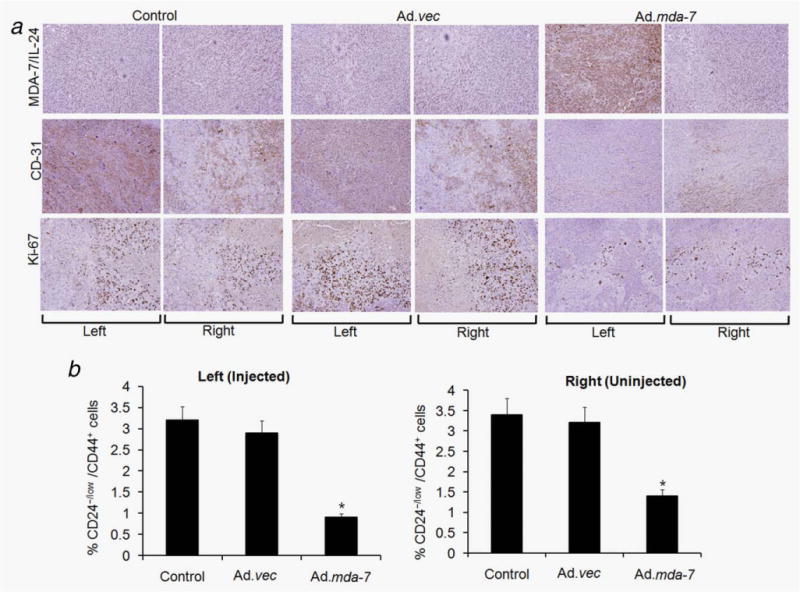
Immunohistochemical analysis of tumor xenografts induced by T47D-initiating/stem cells infected with Ad.vec or Ad.mda-7 or controls injected with PBS. Tumor tissues were harvested and formalin-fixed and paraffin-embedded sections were immunostained for MDA-7/IL-24, CD-31 and Ki-67. The left and right tumors were from animals injected three times (left tumor) with PBS, Ad.vec or Ad.mda-7. Experimental details are given in “Material and Methods” section. (b) Single-cell suspensions were generated from the tumors of all groups and were fixed and stained with CD24 and CD44 antibodies to quantify the percentage of CD24−/low/CD44+ cells by fluorescence-activated cell sorting. The data represent the mean ± SD with a minimum of five mice per group. Asterisk (*) represents significant difference (p < 0.5) vs. corresponding control.
Discussion
It is hypothesized that a small proportion of cancer-initiating/stem cells found within a tumor may be the prime movers of cancer regrowth after therapy, thereby having profound clinical implications for cancer therapeutics and prevention.2,39 Cancer-initiating/stem cells display resistance to conventional therapies and are associated with cancer relapse/recurrence after therapy.40,41 Moreover, the lack of efficacy of current therapies in advanced and metastatic disease requires novel approaches that can specifically or selectively target cancer-initiating/stem cell populations. In these contexts, therapies that are directed against both differentiated cancer cells and cancer-initiating/stem cells might provide advantages to treat cancer. We and others have established that mda-7/IL-24 has significant potential as an anticancer therapeutic because of its multiplicity of antitumor properties, its nontoxic effects to normal cells and tissues and its safety and efficacy as observed in clinical trials.14,16–21 Accordingly, based on the profound antitumor activity of MDA-7/IL-24 and to examine its potential effects in cancer-initiating/stem cells, we used both in vitro and in vivo systems to determine whether Ad.mda-7 is biologically active against purified populations of breast cancer-initiating/stem cells.
Several techniques have been developed to isolate and characterize breast cancer-initiating/stem cells in vitro. We sorted CD44+/CD24−/low cells as breast cancer-initiating/stem cells and observed that Ad.mda-7 effectively and equally inhibited proliferation of the initiating/stem as well as the non-stem breast cancer cell populations. In fact, the cancer-initiating/stem cells did not demonstrate any resistance toward MDA-7/IL-24 as has been observed for chemotherapeutic reagents and radiation.42,43 It is noteworthy that the doses of Ad.mda-7 (pfu) used in our study are comparable to those previously shown to induce cell death in unselected tumor cell populations.28,29 Moreover, Ad.mda-7 also selectively inhibited cancer-initiating/stem cells, while exerting minimal toxicity to normal stem cells derived from immortal MCF-10A and primary HMEpC cells. These observations are consistent with previous reports demonstrating that MDA-7/IL-24 preferentially targets transformed versus normal cells.29,33 We now demonstrate that MDA-7/IL-24 induces apoptosis and ER stress in breast cancer-initiating/stem cells making this therapeutic cytokine an attractive and beneficial option for targeting cancer-initiating/stem cells.44 Similar to epithelial tumor cells14,28 and leukemia cells,45 Ad.mda-7 triggers a response classically associated with ER stress induction, including upregulation of BiP/GRP78, GRP94 and GADD153, as well as eIF2α phosphorylation, in various breast cancer-initiating/stem cells. These findings indicate that the same genetic/epigenetic changes that elicit tumor cell sensitivity to mda-7/IL-24 are maintained in the entire tumor population (both breast cancer non-stem and stem cells), which is important for developing effective therapies for breast cancer.
The Wnt/β-catenin signaling pathway is implicated in a wide range of developmental processes including maintenance of stem cell compartments in adult tissue.8 As a novel target for cancer stem cell therapy, previous studies showed that anti-Wnt-2 monoclonal antibody induced apoptosis in malignant melanoma cells and inhibited tumor growth.46 Several phytochemicals, such as selenium, EGCG (Epigallo-catechin gallate) and vitamin D, have recently been shown to inhibit Wnt signaling in cancers and could potentially be excellent candidates for targeting cancer-initiating/stem cells.47 MDA7/IL-24 delivered by adipose-derived stromal/mesenchymal stem cells48 has potent proapoptotic activity toward different cancer cell lines and reduced tumor growth in the TRAMP-C2-Ras (TC2Ras) prostate cancer model.48 MDA-7/IL-24 expressed in neural stem cells in combination with neural stem cells expressing TRAIL display potent antitumor activity against glioblastoma multiforme brain tumors.49 Here, we show that MDA-7/IL-24 downregulates the Wnt/β-catenin self-renewal pathway in breast cancer-initiating/stem cells, through activation of GSK3β and Akt inhibition resulting in a decrease of β-catenin phosphorylation (Ser33/Ser37/Thr41) and proteasome degradation. Similar findings were also observed in lung and breast cancer cells further confirming that Ad.mda-7 might provide an effective means of eradicating both cancer non-stem and cancer-initiating/stem cells through overlapping mechanisms.30 The in vitro efficacy of MDA-7/IL-24 was also confirmed in vivo demonstrating that MDA-7/IL-24 is able to profoundly reduce tumor burden in treated tumors developed from breast cancer-initiating/stem cells and also has potent “bystander” antitumor effects in a distant untreated population of breast cancer-initiating/stem cells. This finding is consistent with our previous observations showing that secreted MDA-7/IL-24 from injected tumors can inhibit the growth of distant tumors by suppressing proliferation and angiogenesis.14,33 In recent years, breast cancer-initiating/stem cells have been confirmed as integral components of cancer recurrence after chemotherapy. The mechanisms involved in chemoresistance are multifaceted and can be due to overexpression of ABC transporters, detoxification enzymes (aldehyde dehydrogenase), low cell turnover rates and/or the ability to activate a DNA checkpoint response.12,43 Our observations suggest a means of overriding the significant therapeutic roadblock of chemoresistance associated with cancer stem cells and current conventional therapies and provide further validation of the potential use of MDA-7/IL-24 as an innovative therapy for enhanced and long-term cure rates in breast cancer.
Supplementary Material
What’s new?
Cancer stem cells, or tumor-initiating cells, are the small population of cells within a tumor that promote the growth and spread of the tumor. These self-renewing cells produce high levels of proteins that thwart apoptosis, such as Bcl-2, NF- κB, and Akt. In this paper, the authors investigated a protein, MDA-7/IL-24, which has been shown to slow tumor growth and induce apoptosis. They found that it specifically targets the cancer stem cells and stops them from self-renewing, which may help prevent recurrence of the disease.
Acknowledgments
The present studies were supported in part by National Institutes of Health grants R01 CA097381 and P01 CA104177 (P.B.F.); NCI Cancer Center Support Grant to VCU Massey Cancer Center (P.B.F., D.S., X.Y.W.); and VCU MCC (P.B.F.). D.S. and X.-Y. Wang are Harrison Scholars in the VCU Massey Cancer Center. P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center.
Footnotes
Additional Supporting Information may be found in the online version of this article
Conflicts of interest: Nothing to report
References
- 1.Goldhirsch A, Colleoni M, Domenighetti G, et al. Systemic treatments for women with breast cancer: outcome with relation to screening for the disease. Ann Oncol. 2003;14:1212–14. doi: 10.1093/annonc/mdg327. [DOI] [PubMed] [Google Scholar]
- 2.Charafe-Jauffret E, Monville F, Ginestier C, et al. Cancer stem cells in breast: current opinion and future challenges. Pathobiology. 2008;75:75–84. doi: 10.1159/000123845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106:13820–5. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han JS, Crowe DL. Tumor initiating cancer stem cells from human breast cancer cell lines. Int J Oncol. 2009;34:1449–53. [PubMed] [Google Scholar]
- 6.Ponti D, Costa A, Zaffaroni N, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7:86–95. doi: 10.1186/bcr1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Li Y, Semenov M, et al. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–47. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 10.Korkaya H, Paulson A, Charafe-Jauffret E, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/β-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruyt FA, Schuringa JJ. Apoptosis and cancer stem cells: implications for apoptosis targeted therapy. Biochem Pharmacol. 2010;80:423–30. doi: 10.1016/j.bcp.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Huang M, Li Y, Wu G, et al. Whole genome expression profiling reveals a significant role for the cell junction and apoptosis pathways in breast cancer stem cells. Mol Biotechnol. 2010;45:39–48. doi: 10.1007/s12033-010-9241-1. [DOI] [PubMed] [Google Scholar]
- 13.Jiang H, Lin JJ, Su ZZ, et al. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–86. [PubMed] [Google Scholar]
- 14.Dash R, Bhutia SK, Azab B, et al. mda-7/IL-24: a unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine Growth Factor Rev. 2010;2:381–91. doi: 10.1016/j.cytogfr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pestka S, Krause CD, Sarkar D, et al. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–79. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 16.Fisher PB, Gopalkrishnan RV, Chada S, et al. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther. 2003;2:S23–S37. [PubMed] [Google Scholar]
- 17.Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res. 2005;65:10128–38. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham CC, Chada S, Merritt JA, et al. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11:149–59. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Tong AW, Nemunaitis J, Su D, et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–72. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Lebedeva IV, Emdad L, Su ZZ, et al. mda-7/IL-24, novel anticancer cytokine: focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience. Int J Oncol. 2007;31:985–1007. [PubMed] [Google Scholar]
- 21.Sarkar D, Lebedeva IV, Gupta P, et al. Melanoma differentiation associated gene-7 (mda-7)/IL-24: a ‘magic bullet’ for cancer therapy? Expert Opin Biol Ther. 2007;7:577–86. doi: 10.1517/14712598.7.5.577. [DOI] [PubMed] [Google Scholar]
- 22.Ramesh R, Mhashilkar AM, Tanaka F, et al. Melanoma differentiation- associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003;63:5105–13. [PubMed] [Google Scholar]
- 23.Miyahara R, Banerjee S, Kawano K, et al. Melanoma differentiation associated gene-7 (mda-7)/interleukin (IL)-24 induces anticancer immunity in a syngeneic murine model. Cancer Gene Ther. 2006;13:753–61. doi: 10.1038/sj.cgt.7700954. [DOI] [PubMed] [Google Scholar]
- 24.Su ZZ, Lebedeva IV, Sarkar D, et al. Ionizing radiation enhances therapeutic activity of mda-7/IL-24: overcoming radiation- and mda-7/IL-24-resistance in prostate cancer cells overexpressing the antiapoptotic proteins bcl-xL or bcl-2. Oncogene. 2006;25:2339–48. doi: 10.1038/sj.onc.1209271. [DOI] [PubMed] [Google Scholar]
- 25.Yacoub A, Hamed H, Emdad L, et al. MDA-7/IL-24 plus radiation enhance survival in animals with intracranial primary human GBM tumors. Cancer Biol Ther. 2008;7:917–33. doi: 10.4161/cbt.7.6.5928. [DOI] [PubMed] [Google Scholar]
- 26.Sauane M, Su ZZ, Gupta P, et al. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc Natl Acad Sci USA. 2008;105:9763–8. doi: 10.1073/pnas.0804089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su Z, Emdad L, Sauane M, et al. Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene. 2005;24:7552–66. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- 28.Gupta P, Walter MR, Su ZZ, et al. BiP/GRP78 is an intracellular target for MDA-7/IL-24 induction of cancer-specific apoptosis. Cancer Res. 2006;66:8182–91. doi: 10.1158/0008-5472.CAN-06-0577. [DOI] [PubMed] [Google Scholar]
- 29.Su ZZ, Madireddi MT, Lin JJ, et al. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci USA. 1998;95:14400–5. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKenzie T, Liu Y, Fanale M, et al. Combination therapy of Ad-mda7 and trastuzu-mab increases cell death in Her-2/neu-overex-pressing breast cancer cells. Surgery. 2004;136:437–42. doi: 10.1016/j.surg.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Suh YJ, Chada S, McKenzie T, et al. Synergistic tumoricidal effect between celecoxib and adenoviral-mediated delivery of mda-7 in human breast cancer cells. Surgery. 2005;138:422–30. doi: 10.1016/j.surg.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 32.Bocangel D, Zheng M, Mhashilkar A, et al. Combinatorial synergy induced by adenoviral-mediated mda-7 and Herceptin in Her-2+ breast cancer cells. Cancer Gene Ther. 2006;13:958–68. doi: 10.1038/sj.cgt.7700972. [DOI] [PubMed] [Google Scholar]
- 33.Sarkar D, Su ZZ, Vozhilla N, et al. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci USA. 2005;102:14034–9. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mhashilkar AM, Stewart AL, Sieger K, et al. MDA-7 negatively regulates the beta-catenin and PI3K signaling pathways in breast and lung tumor cells. Mol Ther. 2003;8:207–19. doi: 10.1016/s1525-0016(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 35.Chada S, Bocangel D, Ramesh R, et al. mda-7/IL24 kills pancreatic cancer cells by inhibition of the Wnt/PI3K signaling pathways: identification of IL-20 receptor-mediated bystander activity against pancreatic cancer. Mol Ther. 2005;11:724–33. doi: 10.1016/j.ymthe.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Zhang T, Korkaya H, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580–90. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison H, Farnie G, Howell SJ, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–18. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hedgepeth CM, Conrad LJ, Zhang J, et al. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol. 1997;185:82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- 39.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 40.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26:2813–20. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakariassen PO, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia. 2007;9:882–92. doi: 10.1593/neo.07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dean M, Fojo T, Bates S. Tumor stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 43.Chuthapisith S, Eremin J, El-Sheemey M, et al. Breast cancer chemoresistance: emerging importance of cancer stem cells. Surg Oncol. 2010;19:27–32. doi: 10.1016/j.suronc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 44.McDermott SP, Wicha MS. Targeting breast cancer stem cells. Mol Oncol. 2010;4:404–19. doi: 10.1016/j.molonc.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahmani M, Mayo M, Dash R, et al. MDA-7/IL-24 potently induces apoptosis in human myeloid leukemia cells through a process regulated by ER stress. Mol Pharmacol. 2010;78:1096–104. doi: 10.1124/mol.110.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.You L, He B, Xu Z, et al. An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res. 2004;64:5385–9. doi: 10.1158/0008-5472.CAN-04-1227. [DOI] [PubMed] [Google Scholar]
- 47.Kawasaki BT, Hurt EM, Mistree T, et al. Targeting cancer stem cells with phytochemicals. Mol Interv. 2008;8:174–84. doi: 10.1124/mi.8.4.9. [DOI] [PubMed] [Google Scholar]
- 48.Zolochevska O, Yu G, Gimble JM, et al. Pigment epithelial-derived factor and melanoma differentiation associated gene-7 cytokine gene therapies delivered by adipose-derived stromal/mesenchymal stem cells are effective in reducing prostate cancer cell growth. Stem Cells Dev. 2012;21:1112–23. doi: 10.1089/scd.2011.0247. [DOI] [PubMed] [Google Scholar]
- 49.Hingtgen S, Kasmieh R, Elbayly E, et al. A first generation multi-functional cytokine for simultaneous optical tracking and tumor therapy. PLoS One. 2012;7:e40234. doi: 10.1371/journal.pone.0040234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


