Abstract
Computational modeling predicts that the hippocampus plays an important role in the ability to apply previously learned information to novel problems and situations (referred to as the ability to generalize information or simply as ‘transfer learning’). These predictions have been tested in humans using a computer-based task on which individuals with hippocampal damage are able to learn a series of complex discriminations with two stimulus features (shape and color), but are impaired in their ability to transfer this information to newly configured problems in which one of the features is altered. This deficit occurs despite the fact that the feature predictive of the reward (the relevant information) is not changed. The goal of the current study was to develop a mouse analog of transfer learning and to determine if this new task was sensitive to pathological changes in a mouse model of AD. We describe a task in which mice were able to learn a series of concurrent discriminations that contained two stimulus features (odor and digging media) and could transfer this learned information to new problems in which the irrelevant feature in each discrimination pair was altered. Moreover, we report age-dependent deficits specific to transfer learning in APP+PS1 mice relative to nontransgenic littermates. The robust impairment in transfer learning may be more sensitive to AD-like pathology than traditional cognitive assessments in that no deficits were observed in the APP+PS1 mice on the widely used Morris water maze task. These data describe a novel and sensitive paradigm to evaluate mnemonic decline in AD mouse models that has unique translational advantages over standard species-specific cognitive assessments (e.g. water maze for rodent and delayed paragraph recall for humans).
Keywords: aging, AD, APP+PS1, hippocampus, transfer Learning, Spatial Learning, cognitive decline
1. Introduction
Recent estimates indicate that one in ten men and one in six women who live to be 55 years will be diagnosed with Alzheimer’s disease (AD, Alzheimer’s Association, 2008). Despite substantial research efforts focused on uncovering the neurobiological underpinnings of this disease, there is a current lack of behavioral assays that both identify individuals at early preclinical stages and that translate well between rodent models, from which the vast majority of the neurobiological data are derived, and humans who suffer from the disease. For example, one early neuropsychological change in AD is performance on a delayed paragraph recall test. This assessment predicts with near 90% accuracy which non-demented elderly individuals will progress into subsequent cognitive decline (Kluger et al., 1999; Lowndes eta al., 2008). However, it is difficult to develop analogs of such verbal tasks for use with rodent models. The development of novel assessments of hippocampal/medial temporal lobe function using non-verbal tasks that are sensitive to mild decrements in this circuitry and that are well-suited for use in rodents and humans would be of great benefit in terms of greater comparative power within and between species, and in their ability to serve as superior preclinical models in which to assess novel therapies.
Based on information from animal lesion studies (Ikonen et al., 2002), Myers, Gluck, and colleagues have generated unique computational models of hippocampal function that postulate that this structure is critically involved in encoding new information during learning so as to support the transfer of important stimulus features when familiar information is presented in novel recombinations (hereon referred to as ‘transfer learning’; Gluck and Myers, 1993; Gluck and Myers, 1995; Gluck at el., 2001; Johnson et al., 2008; Myers and Gluck, 1996). The prediction of the computational model that the hippocampus helps encode initial learning in such a way that it will support subsequent transfer is similar to the theoretical proposal that the hippocampus is involved in learning relations between stimuli that can be used flexibly later (Bunsey and Eichenbaum, 1996; Eichenbaum et al., 1989; Gluck and Myers, 1993; Gluck and Myers, 1995; Gluck and Myers, 2001). In the absence of hippocampal-region mediation, learning may still be possible, but will be restricted to simpler stimulus-response learning that does not support transfer to new contexts/recombination well. This idea finds empirical support in studies showing that individuals with bilateral hippocampal damage may “unitize” stimuli (or stimulus features) during learning (e.g. Barense et al., 2005; Quamme et al., 2007), conferring impairments when aspects of the trained stimuli are altered (see also Schacter, 1985).
Similarly, although animals with hippocampal damage often show impairments on configural learning (Sutherland & Rudy, 1989; Rudy & Sutherland, 1995), under conditions that arguably favor stimulus unitization, hippocampal-lesioned animals can learn configural tasks as well as controls (see, e.g., O’Reilly & Rudy, 2001; Moses & Ryan, 2006). The lesioned animals do not, however, perform well when the trained stimulus features are presented in novel recombinations (e.g., Eichenbaum et al., 1989).
More recently, a computer-based associative learning task was developed to assess transfer learning in humans with hippocampal damage and aged individuals at risk for cognitive decline (Myers et al., 2002; Myers et al., 2008a). As illustrated in Figure 1B, this task involves a series of 2 item visual discrimination problems in which subjects learn via trial-and-error to choose the correct object from each pair presented. Objects have two stimulus features (shape and color) but within each pair, objects differ with respect to color or shape but not both. In other words, only one of the features (color or shape) is relevant to the correct choice within a particular pair, whereas the other feature is identical between the two items. Once a subject learns all of the discrimination pairs (presented pseudo-randomly) to criterion (learning phase), an unsignaled transfer test is given in which the irrelevant feature of each object pair is changed but the relevant features remain the same and are still predictive of the correct choice (transfer phase). The model predicts that subjects with an intact hippocampus during initial learning should transfer learned information from the initial discrimination trials to the trials in which the irrelevant feature was altered. In contrast, subjects with hippocampal damage would be expected to view the altered objects as novel stimuli about which nothing is known, despite the fact that these objects retain the stimulus feature relevant to the correct choice.
Figure 1.
Left panel shows the test apparatus for transfer learning in mice. Cylinders represent terracotta pots which were filled with a variety of digging media and scented with different odorants. In this schematic, the rose odor is positive (+) and is predictive of a food reward buried in the pot. Middle panel shows examples of visual discrimination pairs used on in the human computer-based version of the transfer learning task. In each discrimination pair, either the shape or color is relevant to the correct choice (+), but not both. During both the learning and transfer phases of the task, each pair of shapes is presented sequentially and pseudo randomly and correct choices are rewarded with a smiley face icon. After reaching criterion performance on the learning phase, without signaling, subjects are presented with the reconfigured stimuli shown under the “Transfer Phase”. Note the positive feature predictive of correct choice (+) in the learning phase remains the same but the irrelevant feature is altered. Right panel shows examples of discrimination pairs used in the mouse transfer learning task. In each pair, either the odor or digging media in the pot is relevant to the correct choice, but not both. During the learning and transfer phases of the task, each discrimination pair is presented sequentially and pseudo randomly and correct choices are rewarded with a food reward buried in the pot (See Methods for Details). After reaching criterion performance on the learning phase, mice were immediately presented with the reconfigured stimuli showing under the “Transfer Phase”. Note that just as in the human version of the task, the positive feature (+) remained predictive of the food reward but the irrelevant feature for each discrimination problem was altered.
This task confirmed computational predictions regarding hippocampal mediation of transfer learning in a group of amnesic patients who had bilateral hippocampal damage. These patients could learn the initial discrimination pairs normally, but performed near chance on the test of transfer learning (Myers et al., 2008a), suggesting that they treated the reconfigured stimuli as new problems and failed to transfer the relevant stimulus features of the discrimination pairs learned initially. When the same task was tested in aged subjects, elderly individuals who were non-demented but who did have mild hippocampal atrophy showed a similar pattern of performance (i.e. preserved learning of the initial discrimination pairs, followed by poor transfer learning; Myers et al., 2002). Interestingly, these individuals were not impaired relative to non-atrophied controls on delayed paragraph recall, suggesting that performance on tests of transfer learning may be a more sensitive or an earlier marker of hippocampal dysfunction. Indeed, a small-scale longitudinal study suggests that, in non-demented elderly individuals, poor performance in transfer learning may be predictive of short-term (2 year) cognitive decline (Myers et al., 2008b).
These data in human subjects suggest that transfer learning is sensitive to hippocampal/medial temporal lobe dysfunction (Johnson et al., 2008) and that this nonverbal task may be well-suited as an assessment that translates across species. The spatial version of the Morris water maze is the cognitive assessment tool most widely used to evaluate hippocampal function in aged rodents (Bizon et al., 2009; Gallagher et al., 2003; LaSarge et al., 2007; Morris et al., 1982; Squire et al., 2004). However, this task has some limitations for within-subject pharmacological intervention studies and results acquired from transgenic mouse models of AD using water maze have proven inconsistent (Bizon et al., 2007; Dumont et al. 2004; Lassalle et al., 2008; Reiserer et al., 2007). However, like humans, rodents also perform well at discriminating objects that contain more than one stimulus feature and deficits on such tasks are reliable across multiple problems (Barense et al., 2002; Bissonette et al., 2008; LaSarge et al., 2007).
The goal of the present study was to develop an analogous mouse version of the human transfer learning task developed by Myers, Gluck and colleagues (Gluck and Myers, 1993; Gluck and Myers, 1995; Gluck and Myers, 2001; Myers et al., 2002). The task design was a modified version of a naturalistic odor discrimination task (Eichenbaum and Matthews, 1989; Lasarge et al., 2007) with odors and digging media used as the two stimulus features in the discrimination problems. Double transgenic (APP+PS1) mice of different ages were assessed in this new task to determine if performance was sensitive to age-related hippocampal pathology.
2. Methods
2.1. Subjects
For all experiments, mice were individually-housed in the AAALAC-accredited vivarium in the Psychology Building (Texas A&M University, College Station, TX). Mice were maintained on a 12-h light/dark cycle (lights on at 0800) and climate controlled at 25°C. All testing was conducted during the light cycle and mice in the study were screened daily for health problems, including but not limited to cataracts, jaundice, food and water intake, and the appearance of tumors. Sentinel mice housed in the same room as experimental mice were further screened for diseases such as pinworms, parvo and other pathogens. Blood work was negative for all health screens throughout testing. All animal procedures were conducted in accordance with approved institutional animal care procedures and NIH guidelines. All mice were given at least two weeks and received ad lib access to food and water. Mice continued to have free access to food and water with the exception that mice were food-restricted to 85% of their free-feeding weight prior to testing. After completion of all discrimination testing, mice were immediately returned to ad lib food.
2.1.1. Experiment 1: validation of the transfer learning task
Subjects used for validation of the transfer task were C57BL/6J (n= 5 in each of two groups, 5 months old) female retired breeders from The Jackson Laboratory, Maine, USA.
2.1.2. Experiment 2: transfer learning task with aged APP+PS1 mice
Subjects were a cohort of 12 month old, female Tg (APPswe, PSEN1dE9; APP+PS1; n=11) and age-matched non-transgenic littermates of B6C3F1/J background strain (NTg; n=7) obtained from The Jackson Laboratory, Maine, USA. The APP+PS1 double transgenic mice express a chimeric mouse/human amyloid precursor protein (Mo/HuAPP695swe) and the mutant human presenilin 1 (PS1-dE9). This presenilin mutation is related to the early onset of AD. The Swedish mutation (K595N/M596L), which is linked to familial AD, increases the number of Aβ deposits by stimulating the β-secretase pathway (Hardy et al., 2002). These mice develop Aβ deposits throughout the brain, including hippocampus, by 6 months of age, with numerous deposits reported by 12 months of age (Burgess et al., 2006; Jankowsky et al., 2004).
2.1.3. Experiment 3: longitudinal assessment of transfer learning in APP+PS1 mice
Subjects were a second cohort of female APP+ PS1 (n= 8) and age-matched NTg littermates of B6C3F1/J background (n= 9). Mice were initially tested at 3 months (learning and transfer phases) and then were retested in the transfer task (learning and transfer phases) at 12 months of age using all novel stimuli.
2.2. Transfer learning task
2.2.1. Apparatus and task parameters
The testing apparatus for the mouse transfer learning task is illustrated in Figure 1A. It consists of an open-topped black Plexiglas box (12 inches width; 18 inches length; 8 inches deep) with two small terra cotta flower pots (1.7 inches diameter; 1.3 inches deep) securely attached to the floor. There were two stimulus features in each discrimination problem: an odor that was applied directly to the rim of the pots and the digging media that filled the pots and hid a food reward (one chocolate-flavored food pellet, 20 mg, AIN-76A from TestDiet), placed on the bottom of the pot. Table 1 lists all complex discrimination pairs used in the study.
Table 1.
Odor and digging media pairs as paired in the transfer learning task
| Discrimination Pairs | Corresponding Irrelevant Dimension |
|
|---|---|---|
| Learning phase | Transfer phase | |
| beads × rubber string | jasmine | vanilla |
| patchouli × mulberry | pompoms | cork |
| paper × cut balloon | chocolate | watermelon |
| jasmine × vanilla | beads | rubber string |
| pompons × cork | patchouli | mulberry |
| chocolate × watermelon | paper | cut balloon |
| Cut straws × cheese cloth | pumpkin | cucumber |
| cucumber × pumpkin | cut straws | cheese cloth |
| Cut paper × guinea pig bedding | lemon | rosemary |
| bergamot × sage | sequins | raffia |
| Cut bench pad × wood bedding | lavender | cinnamon |
| vetiver × geranium | yarn | alphadri bedding |
| raffia × sponge | bergamot | sage |
| cinnamon × lavender | wood bedding | cut bench pad |
| alphadri bedding × yarn | vetiver | geranium |
Salience and difficulty of odor and digging medium problems appeared comparable as no significant differences in performance on odor and digging media discriminations were observed in any group. Additionally, analyses of individual pairings did not result in any differences, indicating comparable salience within individual pairs.
To disguise the odor of the reward, crushed chocolate food pellets were sprinkled over the surface of each pot. The position (left or right) of the rewarded pot varied pseudo-randomly across trials. For the first four trials of every new discrimination problem, mice were allowed to dig in both pots until they obtained the reward (i.e. they were allowed to self-correct if they dug in the incorrect pot). On these trials, only their first choice was scored (as correct or incorrect). On trials thereafter, mice were removed from the test chamber after only one dig (either correct or incorrect). A dig in a pot was scored if a mouse displaced the digging media with either its paws or nose.
Shaping took place in the box described above. Initially mice were presented with two empty pots containing a chocolate pellet in the bottom of each. The re-baiting of the pots was contingent on the mouse consuming both pellets. After 12 trials of successively retrieving both pellets, mice were then presented with two pots containing chocolate pellets in the bottom but filled with progressively more mixed digging media (12 trials per 33, 50 and 100% full). The day after successfully retrieving both pellets with full pots, mice received one simple discrimination to habituate them to the learning aspect of the task.
In developing the mouse transfer learning task and confining it to a single behavioral session (to closely mimic the human version), satiation and motivation were initial concerns. A series of pilot experiments, in which food-restricted C57BL/6J mice were allowed free access to the chocolate food pellets used in this task, revealed that mice would consume many more pellets than were ever needed for a full testing session. Moreover, all mice shaped, learned all problems to criterion, and completed all 30 trials in the transfer portion of the task irrespective of genotype or age, supporting that there we no motivational differences within sessions or between experimental groups.
2.2.2. Testing
Just as in the human version of the transfer learning task (Gluck and Myers, 1993; Myers et al., 2002), all testing was performed within a single session. Figure 1C and Table 2 illustrate an example of the order and sequence of presentation of the two feature stimuli in the learning and transfer phases of the task. Initially, mice were trained on a series of three concurrent discrimination problems. As in the human version of the task, new discrimination pairs were progressively introduced intermixed with learned pairs as criterion performance was reached on each pair.
Table 2.
Representative example of the order and sequence of complex stimuli presented the mouse transfer learning paradigm
| Dimensions | Combinations | |||
|---|---|---|---|---|
| Discriminations: | Relevant | Irrelevant | Pot 1 (+) | Pot 2 (−) |
| Learning phase (pair 1) | Odor | Media | O1 with M1 | O2 with M1 |
| Learning phase (pair 2) | Media | Odor | M2 with O3 | M3 with O3 |
| Learning phase (pair 3) | Odor | Media | O4 with M4 | O5 with M4 |
| Transfer phase (pair 1) | Odor | Media | O1 with M5 | O2 with M5 |
| Transfer phase (pair 2) | Media | Odor | M2 with O6 | M3 with O6 |
| Transfer phase (pair 3) | Odor | Media | O4 with M6 | O5 with M6 |
Abbreviations: O=odor ; M= digging media. Stimulus features predictive of reward are italicized.
Each number indicates novel stimulus.
As in the human version of the task, new discrimination pairs were progressively introduced intermixed with learned pairs as criterion performance was reached on each pair.
After reaching criterion in pair 1, the pot with O1 remained baited and O2 unbaited but the irrelevant dimension was changed from M1 to M5.
Note that all odors and digging medias were used only once throughout training (O1-O6; M1-M6).
For each problem, mice learned to discriminate between pots with two stimulus features (odor and digging media). One of the two pots was baited, with either the odor or the digging media as the relevant feature (Fig. 1C and Table 2). For example, if odor was the relevant feature, the pots differed in odor (with one odor always predictive of the food reward; e.g. rose+ versus citrus- as shown in Figure 1C) but contained the same digging media (e.g. sequins) that was thus irrelevant to the correct choice. For other discrimination problems, the digging media was the relevant feature that differed between pots and predicted the reward (e.g., yarn+ versus beads-; as shown in Fig. 1C), and the pots were scented with the same odor (e.g. vanilla), the irrelevant feature.
The positive and negative features in each pair and the sequence of discrimination problems were randomized across mice, although each mouse received discrimination problems in which the relevant feature was alternated between odor and digging media. As in the human task, initially several problems (2 for the mice) were presented in pseudorandom order and after criterion was reached on these problems (6 correct in a row, including 3 of each problem set), a third problem was introduced and the three pairs were presented in a pseudorandom fashion until mice reached criterion performance (6 correct in a row, including 2 of each problem). This design ensured that each discrimination pair was learned prior to the transfer (See Table 2).
After reaching criterion performance, mice were immediately assessed for transfer learning. The mice were presented with 30 trials in which only the stimulus feature in each discrimination pair that was irrelevant to the reward was changed, and the 3 new combinations were presented pseudo-randomly (including 10 each of the three discriminations; see Fig. 1C, Table 2). As in the human version of the task, this design affords an opportunity to assess the ability of the mice to transfer the predictive value of a previously learned relevant feature (e.g. a particular odor) to a food reward in a new context (e.g. pots containing a novel digging media).
2.3. Odor Detection Threshold Testing
Anosmia has been shown to emerge as a consequence of chronological age and in Alzheimer’s disease (e.g., Djordjevic et al., 2008). Thus, after evaluating transfer learning, mice in the second and third experiments (i.e., all APP+PS1 and NTg mice) were assessed for their ability to detect and respond to decreasing concentrations of odorants. Mice were tested at 13 months in cohort 2 and at 4 months in cohort 3. Odor detection threshold testing was performed in the same apparatus used for the transfer learning. First, mice were presented with a novel odor discrimination problem (sandalwood vs. mineral oil) using full strength odorant applied directly to the rims of two pots filled with mixed digging media. Sandalwood was the positive odor that predicted the food reward and mice were trained until reaching criterion performance (6 consecutive correct trials). Once achieving criterion performance, the mice were tested in a series of discrimination problems during which the sandalwood odor was systematically diluted (1/10, 1/100, 1/1000, 1/10000). Mice were given 16 trials at each dilution and the percent error was used as the measure of performance at each dilution.
2.4. Reversal learning
Previous work has shown that reversal learning is impaired in the Tg2576 mouse model of AD (Zhuo et al. 2007). Thus, in Experiment 3, mice were assessed for reversal learning performance after transfer learning testing.
On a separate day, mice were presented with a novel two-odor discrimination problem with mixed digging media. After reaching criterion performance (6 consecutive correct trials), the odor-reward contingencies were reversed (such that the pot scented with the previously non-rewarded odor now contained the reward), and errors to criterion were measured.
2.5. Water maze assessment
The spatial reference memory version of the Morris water maze is a standard task used in rodents to assess hippocampal/medial temporal lobe function. Although these brain regions are known to be particularly vulnerable to dysfunction in aging and AD, the results from assessments of spatial learning in mouse models of AD have been mixed, with some studies reporting progressive deficits that correlate with increasing hippocampal pathology (Gordon et al., 2001; Heikkinen et al., 2004; Liu et al., 2002; Puoliväli et al., 2002), but others reporting subtle or no deficits on this task despite known widespread neuropathology in critical brain regions (Bizon et al., 2007; Dumont et al., 2004; Lassalle et al., 2008; Reiserer et al., 2007). To directly compare the sensitivity of the rodent assessment of transfer learning described here to spatial learning ability, after all other testing, the APP+PS1 and NTg mice from both cohorts (at approximately 13 mo of age) were tested in hidden (hippocampal-dependent) and visible cued (non-hippocampal-dependent) versions of the Morris water maze task.
2.5.1. Apparatus
The water maze consisted of a 4-foot diameter circular tank filled with water (24 to 27°C) made opaque by the addition of nontoxic white tempera paint. The tank was surrounded by black curtains, to which large (15 × 15 inches) white geometric cues (made with fabric) were affixed. The tank was divided into four imaginary quadrants, each with a platform position equidistant from the center to the wall. During cue training, the tank was filled to 1 cm below a black visible platform. Each mouse’s swim was tracked and analyzed using a computer-based video tracking system (Water 2020, HVS Image, UK). On each trial, mice were carefully placed into the water facing the wall of the tank at one of four start points (N, S, E, or W). Throughout the experiment, mice that failed to reach the platform within 60 s were guided to it by hand. During the spatial reference memory assessment (hidden platform training), a retractable escape platform (12 cm diameter, HVS Image, UK) was located in the southwest quadrant of the maze and submerged 1.2 cm below the water’s surface.
Initially, mice were trained in the non-hippocampal dependent cued version of the task in order to assess visual acuity and sensorimotor abilities, as described previously (Bizon et al. 2007; Brody and Hotzman, 2006; Montgomery et al., 2008; Puoliväli et al., 2002).
During cue training, the platform position and start positions were varied on each trial such that the platform position and extra-maze cues were made explicitly irrelevant to the mouse’s escape. Training consisted of 2 days (6 trials per day). On each trial mice were given 60 seconds to find the visible escape platform. After finding the platform or being guided there by an experimenter, mice remained on the platform for 30 seconds before being removed from the tank. At the conclusion of each trial, mice were returned to a heated holding cage (about 30°C) for a 10 minute inter-trial interval.
Beginning the day immediately following completion of cue training, mice received 6 consecutive days of training (4 trials per day) to find a hidden, stationary platform, to assess hippocampal dependent spatial reference memory. The start position (N, S, E or W) was pseudo-randomly varied across trials such that mice needed to rely on the position of the platform relative to the extra-maze cues to effectively escape across trials. During each trial, mice were given 60 seconds to search for the hidden platform, followed by a 30 second post-trial period in which they were allowed to remain on the platform. After each trial, mice were placed in the holding cage for a 10 minute inter-trial interval.
The fourth swim of days 2 and 6 of hidden platform training were probe trials, during which the platform was retracted to the bottom of the tank for the first 30 sec of the 60 sec trial. Rodents with a good memory for the platform location concentrate their search in that area even when the platform is removed from the tank and performance on probe trials is particularly sensitive to detecting hippocampal impairment in a variety of aging models (Bizon et al., 2007; Bizon et al., 2009; Gallagher et al., 1993; LaSarge et al., 2007).
2.5.2. Water maze analysis
The water maze data were analyzed using a computer-based video tracking system, Water 2020, developed by HVS Image (Hampton, UK). Primary performance measures analyzed were path length (the total distance from the start position to the platform in centimeters) on hidden and visible training trials and percent time in the target quadrant on probe trials. Separate two-factor repeated measures ANOVA (genotype × day) were used to evaluate differences across visible platform training trials, hidden platform training trials and probe trials. Finally, swim speed (centimeters per second) between groups was also analyzed using a one-factor ANOVA (genotype) on hidden and visible trials respectively.
2.6. Statistical Analyses
Statistics were performed as described above for each task using StatView software (Version 5.01). For all discrimination learning, simple discrimination and reversal problems, trials and errors to criterion were analyzed. Because, in every case, data were identical by both measures, for simplicity of presentation, only errors are reported and shown. For the transfer phase and odor detection threshold testing, in which a fixed number of trials were presented, percent error was the performance measure analyzed. One-factor ANOVAs were used unless stated otherwise. In all cases p<0.05 was considered significant.
3. Results
3.1. Experiment 1
The goal of Experiment 1 was to confirm that mice could learn the transfer learning task, including learning a series of concurrent discriminations (learning phase), and that mice could perceive and respond to an unsignaled recombination of stimuli in the transfer phase of the task. C57BL/B6 mice (n=10) were trained on 3 concurrent discrimination problems as described above. Immediately after reaching criterion performance, half of these mice (n=5) received 30 trials in which the irrelevant feature within each discrimination problem not predictive of food was altered (“test group”) and half of the mice (n=5) received 30 trials that were identical to those learned initially (“control group”). Assignments to control and test groups were made such that group means on initial discrimination learning were equivalent (Figure 2A, F (1, 8) = 00, ns). During the transfer phase, the test group performed significantly worse than the control group (Figure 2B, F (1, 8) = 8.73, p < 0.05). These data demonstrate that, similar to subjects in the human version of the task, mice are sensitive to alterations of the irrelevant stimulus feature.
Figure 2.
Bar graphs show performance of young C57BL/6J mice (n=10) during learning of three concurrent discriminations with multiple stimulus features (A; errors to criterion) and the immediate recall of those problems both with (n=5; “test group”; black bar) and without (n=5; “control group”; open bar) reconfiguration of the irrelevant stimuli (B; percent error). Panel A shows that although the two groups of C57BL/6J mice performed identically during the initial discrimination learning (mean errors to criterion ± S.E.M), the “test group” (black bar) that received a change in the irrelevant stimulus feature made significantly more errors than the “control group” that received problems identical to those in the initial discriminations (percent error). See text for statistical analysis.
3.2. Experiment 2
The goal of experiment 2 was to determine if aged APP+PS1 mice showed impaired transfer learning performance relative to age-matched NTg controls, as would be expected if the mouse task is sensitive to age-related pathological changes associated with AD.
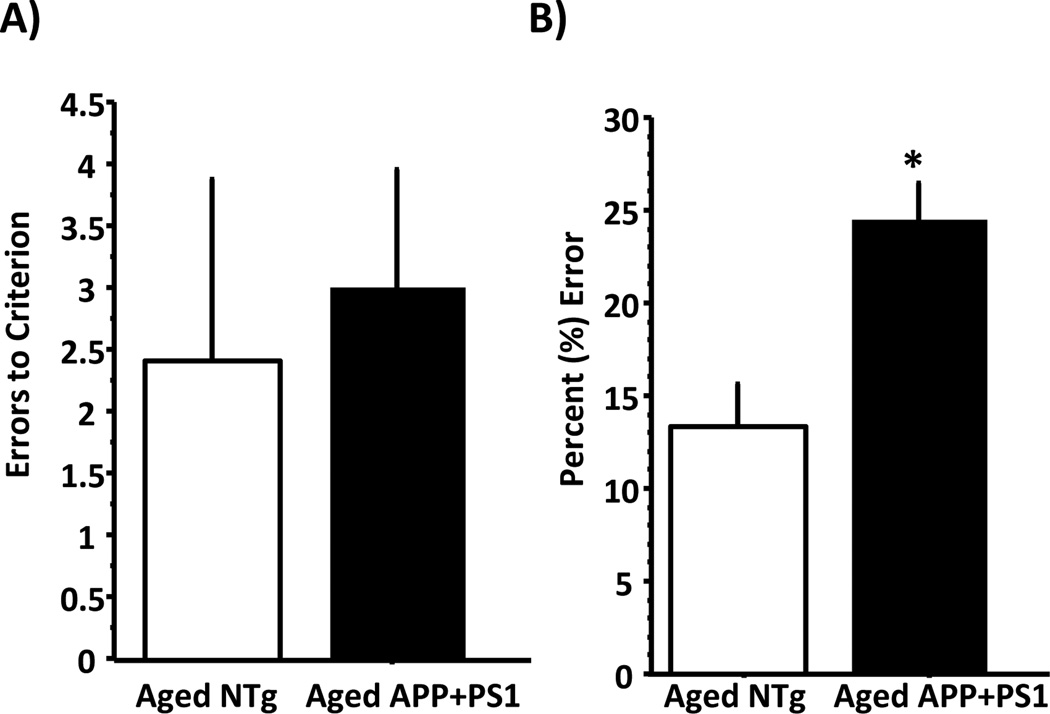
A one-factor ANOVA revealed no difference due to genotype on the initial discrimination learning phase of the task (Figure 3A; F (1, 17) = 0.118, ns). However, the APP+PS1 mice were significantly impaired relative to age-matched NTg mice in their ability to perform the discrimination problems when the irrelevant feature was changed during the transfer phase of the task (Figure 3B, main effect of genotype; F (1, 17) = 12.16, p <0.005).
Figure 3.
Bar graphs show performance of aged (12 mo) APP+PS1 (black bars) and age-matched NTg mice (open bars) on the transfer learning task. Panel (A) shows errors to criterion (mean ± S.E.M) in the concurrent discrimination learning phase of the task and panel (B) shows percent error (mean ± S.E.M) in the transfer phase of the task. Note that while there is no difference in performance between NTg and APP+PS1 mice in number of errors on the initial discriminations, APP+PS1 were significantly impaired relative to NTg mice on the transfer phase of the task. See text for statistical analysis.
3.3. Experiment 3
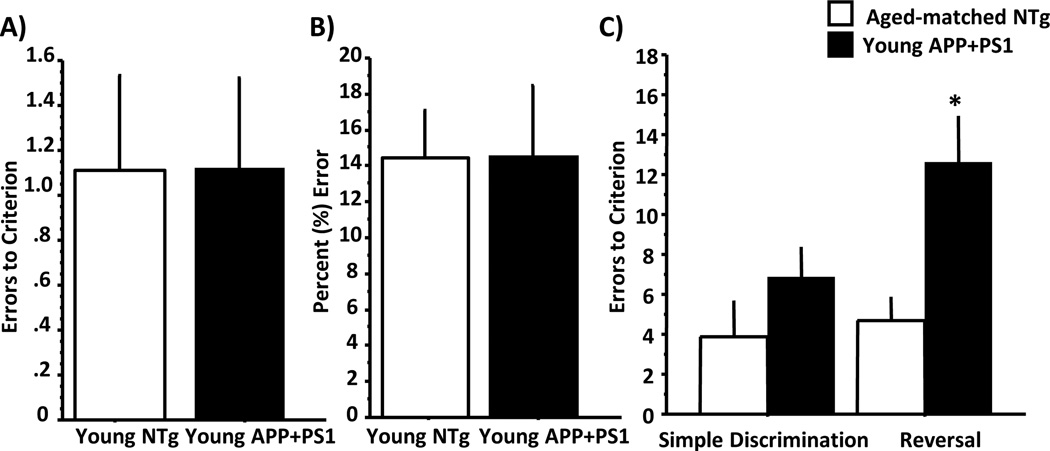
A second cohort of APP+PS1 and NTg mice was assessed longitudinally for transfer and reversal learning. At 3 months of age, there were no differences due to genotype on the initial discriminations (Figure 4A; F (1, 15) = 0.001, ns), nor on the transfer test (Figure 4B, F (1, 15) = 0.001, ns).
Figure 4.
Bar graphs show concurrent discrimination learning (A), transfer learning (B) and reversal learning (C) performance of young APP+PS1 (black bars) and age-matched NTg mice (open bars). No significant differences were observed in errors to criterion (mean ± S.E.M) during initial discrimination learning (A) or in percent error (mean ± S.E.M) during transfer learning (B). However, when subsequently tested on for reversal learning, young APP+PS1 performed comparably to NTg in acquiring the new simple odor discrimination problem but were impaired when the odor predictive of reward in this new problem was reversed. See text for statistical analyses.
The same young cohort of APP+PS1 and NTg mice also demonstrated comparable learning in a simple odor discrimination pair presented on a separate day (Figure 4C; F (1,15)=2.09, ns). However, in agreement with previously reported data in Tg2576 mice (Zhuo et al., 2007), the APP+PS1 mice were impaired when odors predictive of the reward were reversed (Figure 4C, repeated-measures ANOVA; F (1, 15) = 6.34, p<0.05).
These mice were retested for transfer learning at 12 months of age and data is shown in figure 5. As in Experiment 2, APP+PS1 mice learned the initial discriminations on par with NTg mice (Figure 5A; F (1, 15) =0.018, ns) but the 12 mon APP+PS1 mice were significantly impaired on the transfer phase of the task (Figure 5B; F (1, 15) = 4.69, p<0.05). Notably, however, the deficit in reversal learning that was present at 3 months of age was absent upon retesting at 12 months (Figure 5C; F (1, 15) = 0.36, ns), nor was there a difference in learning of the novel odor pair (Figure 5C; F (1, 15) = 1.05, ns).
Figure 5.
Bar graphs show performance of the same APP+PS1 (black bars) and NTg mice (open bars) mice shown in Figure 4 re-tested for transfer and reversal learning at 12 mo. Panel (A) shows errors to criterion (mean ± S.E.M) in the concurrent discrimination learning and panel (B) shows percent error (mean ± S.E.M) in the transfer phase of the task. No significant differences were observed in errors to criterion (mean ± S.E.M) during initial discrimination learning (A); however, a significant and robust deficit in transfer learning was observed in APP+PS1 mice at this age. In contrast, the reversal learning deficit observed at young ages (Fig. 4C) was not observed when aged mice were re-tested using a novel problem at 12 mo (C). See text for statistical analyses.
3.4. Odor Detection Threshold Testing
In order to determine whether a decreased ability to detect odors with age and/or transgene was a factor in the transfer deficit observed in the APP+PS1 mice at 12 months of age, odor detection abilities were assessed at different ages in mice from Experiment 2 (13 mo) and Experiment 3 (4 mo) using identical procedures. In a separate session following transfer learning testing, mice were trained to criterion on one additional olfactory discrimination problem. Both young and aged APP+PS1 and NTg mice learned the simple discrimination problem comparably (Figure 6A); a repeated measures ANOVA revealed no significant differences due to age (F (1,30) = 0.71, ns) or genotype (F (1,30) = 0.20, ns); and, no interaction was observed between age and genotype (F (1,30) = 0.77, ns). Moreover, Figure 6B shows that the odor detection threshold did not differ across groups, with all groups showing similar declines in accuracy as concentrations of the odorant were decreased (repeated-measures ANOVA; age: F (1,30) = 0.38, ns; genotype: F (1,30) = 0.22, ns; age × genotype interaction: F (1,30) = 0.057, ns).
Figure 6.
Graphs show odor detection threshold testing in young and aged APP+PS1 (black bar) and NTg (open bar) mice. Errors to criterion (mean ± S.E.M) to learn a novel odor discrimination pair are shown in (A) and percent error (mean ± S.E.M) of responses to decreasing dilutions of the odorant (B). As expected, all groups’ ability to detect the odors decreased with diminishing concentrations of the odorants, nearing chance performance at a 1:10000 dilution of that used during training. No effects of age or genotype were observed in the ability to detect odorants. See text for statistical analysis.
3.6. Experiment 4
Following all other testing procedures, the aged mice from both Experiments 2 and 3 were trained in visible (cued) and hidden platform versions of the water maze. Repeated-measures ANOVAs were used for all analysis. As shown in Fig. 7A, aged (12 mo) APP+PS1 and NTg mice showed comparable path length to reach the visible platform across days (F (1, 18) = 0.083, ns) and there was no interaction between genotype and day (F (1, 18) = 0.158, ns). Figure 7B shows path length across training days 1 through 6. Both groups improved performance across training (F (5, 90) = 2.921, p<0.05) but there was no main effect of genotype (F (1, 18) = 0.169, ns) and no interaction between day and genotype (F (5, 90) = 0.563, ns). Probe trial performance is show in Fig. 7C. Percent time in the target quadrant significantly increased for both groups from the early probe trial on day 2 and the last probe trial on day 6 (F (1, 18) = 12.164, p=0.003) but no main effect of genotype (F (1, 18) = 0.547, ns) nor interaction between genotype and probe trial (F (1, 18) = 0.260, ns) was observed.
Figure 7.
Water maze performance in aged APP+PS1 (black) and NTg (open) mice. Panel (A) shows that both groups were able to find a visible platform comparably, demonstrating a lack of sensorimotor or motivational differences between groups. Panel (B) shows that the path from the start position to the stationary hidden platform decreased across the 6 training days for both groups, although there was no main effect of genotype nor any interaction of day with genotype. Panel (C) shows performance on probe trials early (probe 1) and late (probe 2) in the spatial memory version of the task. All mice spent significantly more time in the target quadrant (containing the platform) with increased training although in agreement with training trial data, no effect of genotype was observed. See text for statistical analysis.
Finally, no main effect of genotype was found on swim speed during the first trial of cue training (F (1, 18) = 1.349, ns) nor on the first hidden platform training trial (F (1, 18) = 2.022, ns), two measures not confounded by learning.
4. Discussion
The goals of the current studies were to develop a mouse analogue of a computerized assessment of transfer learning and to determine if transgenic mice that have pathology associated with AD show deficits in this type of learning. First, we validated the mouse version of the transfer learning assessment. Young adult C57B6 mice counterbalanced for comparable discrimination learning abilities were sensitive to the change in the irrelevant feature. This was evident by significantly decreased accuracy of performance in a test condition during which the irrelevant feature was altered compared to recall when parameters were unchanged from the initial learning problems. Such performance in the test portion of the transfer learning task involves cognitive processes (and likely neural substrates) beyond those required for simple recall of discrimination information acquired during the learning phase of this task. Also notable from this initial experiment is that although young adult mice performed significantly worse when the irrelevant stimulus feature was altered, these mice still demonstrated relatively proficient transfer learning, providing evidence that this task should be useful for the detection of a range of decrements -mild to severe- associated with disease.
Indeed, in subsequent studies, aged (12 mo) APP+PS1 mice were selectively impaired in transfer learning in comparison to age-matched NTg mice. Even at the most advanced ages tested here (12–13 mo), APP+PS1 mice were able to learn three compound discriminations concurrently that included combinations across perceptual sets (i.e., on some problems odor was relevant to the correct choice and on others digging media signaled the correct choice). These data show that APP+PS1 mice are able to form perceptual sets across at least two stimulus features. In contrast, when the irrelevant stimulus feature was altered, aged APP+PS1 mice performed significantly worse than did NTg age-matched controls. This deficit was observed both in the initial cross-sectional study conducted on 12 mo. old mice (Experiment 2) and in the longitudinal study in which transfer learning in APP+PS1 mice was comparable to NTgs at young ages (3 mo.) but deficits of a remarkably similar magnitude as in Experiment 2 emerged in the APP+PS1 mice compared to NTgs when these groups were re-tested at 12 mo. of age (Experiment 3). As anosmia has been reported at advanced age and in AD, and odor was a key stimulus feature in this task, mice were assessed for odor detection threshold. There were no age- or pathology- associated differences in olfactory detection abilities (Fig. 6). These data confirm that procedural aspects of the transfer task did not account for the robust age-related deficits observed in the APP+PS1 mice.
The APP+PS1 mouse strain was chosen for this study because this model exhibits significant hippocampal pathology; the presenilin mutation exacerbates the plaque pathology in mice with the APPswe mutation (Burgess et al., 2006; Hardy et al., 2002; Jankowsky et al., 2004). Similarly, these initial experiments were confined to females as Aβ pathology is reportedly greater in female than male mice in this and other AD mouse models. Indeed, epidemiological studies have reported a higher incidence of AD in women (Andersen et al., 1999; Jorm and Jolley, 1998; Schafer et al., 2006; Xu et al., 2006), and female transgenic mice presenting the APP mutation develop higher plaque numbers than male mice (Bayer et al., 2003; Callahan et al., 2001; Lewis et al., 2001; Sturchler et al., 2000). Future work includes evaluating other transgenic models of AD, including those that model distinct aspects of AD (eg., tau pathology and synaptic/neuronal loss), to determine the reliability and robustness of the transfer deficit across mouse models of AD and to define the specific genetic and neurobiological factors responsible for this deficit.
Changes in ovarian hormones including estrogen have been linked to cognitive changes in young and aged subjects (Gree et al., 2005; Golub et al., 2008). At all ages tested here, female mice were likely cycling, but the possibility of an interaction between estrogen levels, pathology and transfer learning should be acknowledged. However, as there is no evidence to our knowledge to indicate that pathology alters entry into di- or anestrous which reportedly occurs at 14 months of age in this mouse strain (Callahan et al., 2001), it is not likely that changes in ovarian hormones alone are sufficient in 12 mon APP+PS1 mice to produce transfer learning deficits observed here. Ultimately, it will be of significant interest to determine the relationship between transfer learning proficiency and levels of estrogen and other hormones that are sensitive to alterations with age and cognition.
Studies from humans tested in the analogous transfer task (see Fig. 1) strongly suggest that the age-dependent deficits in APP+PS1 mice observed here are at least in part mediated by progressive hippocampal pathology that is pervasive in the 12 mon APP+PS1 mice (Burgess et al., 2006; Jankowsky et al., 2004). Specifically, humans with hippocampal damage are able to learn a series of compound discriminations on par with intact control subjects but demonstrate impaired transfer learning (Myers et al., 2008a; Myers et al., 2008c). Moreover, in aged individuals, deficits selective to transfer learning correlate with hippocampal atrophy and are predictive of further cognitive decline (Myers et al., 2002; Myers et al., 2008b). Previous animal studies suggest that the hippocampus is important for learning of contextual information and binding of stimulus cues, sometimes called structural learning or relational learning (Aggleton et al., 2007; Chun & Phelps, 1999; Eichenbaum et al., 2007; Haskins et al., 2008; Johnson et al., 2008; Pascalis et al., 2009). Moreover, relational learning encompasses plasticity of learning and memory for relationships among stimuli, allowing for reconfiguration of these in new contexts (Aggleton et al., 2007). The nature of these stimuli is variable across these studies making a direct and reliable comparison between relational learning and transfer learning difficult, but these data to further implicate the hippocampus as a critical structure mediating transfer learning. With the establishment of this new task, lesions specifically targeting the hippocampus are underway to determine if the role and necessity of this structure in transfer learning.
Notably, we failed to observe deficits in the spatial reference version of the water maze in the aged APP+PS1 mice conducted after transfer learning assessment. One interpretation of these data would suggest that transfer learning is not solely dependent on the hippocampus, as hippocampal lesions dramatically impair water maze performance (Arns et al., 1999; Kirwan et al., 2005; Morris et al., 1982). However, it is notable that while deficits in water maze performance have been reported in mice with Aβ pathology (Gordon et al., 2001; Heikkinen et al., 2004; Liu et al., 2002; Puoliväli et al., 2002), many studies fail to observe such impairment (Bizon et al., 2007; Dumont et al., 2004; Lassalle et al., 2008; Reiserer et al., 2007). One possibility then is that transfer learning may tax a different aspect of potentially hippocampal-dependent cognition that is sensitive to more modest changes in pathology than is spatial reference memory.
Certainly, in addition, brain regions beyond hippocampus (e.g., prefrontal cortex, striatum, basal forebrain) may also be important for accurate transfer learning. With respect to prefrontal cortex, however, it would be expected that age/pathology associated changes in this region might largely influence the initial learning phase of the task that requires the ability to discriminate across perceptual sets, as lesions of prefrontal cortex impair this type of learning (Birrell and Brown, 2000). Notably, some deficits related to prefrontal cortical pathology were detected in young but not aged APP+PS1 mice in the current study. Despite performing on par with NTgs in transfer learning, young APP+PS1 mice were impaired in their ability to reverse the relevant contingency of a simple discrimination problem. Interestingly, this reversal deficit in APP+PS1 mice was not observed when retested at advanced age, when the transfer learning impairment was pronounced. Our reversal data agree with those from previous studies using aged rats (Schoenbaum et al., 2006) and in studies from rodents with prefrontal cortical lesions (Schoenbaum et al., 2002). In all cases, reversal learning deficits were present initially but were ameliorated with additional testing or on re-tests later in the lifespan. Reversal learning at least initially depends upon prefrontal cortical function, one of the earliest regions to develop Aβ plaques (Sgaramella et al., 2001; Watz et al., 2004; Zhuo et al., 2007). Early reversal deficits in APP+PS1 mice and those reported here previously described in Tg2576 mice appear to be an early indicator of Aβ pathology (Zhuo et al., 2007; Zhuo et al., 2008). In agreement with Schoenbaum 2006, in the APP+PS1 mouse model, there appears to be a compensatory mechanism that allows such deficits to be erased later in the lifespan (Compare Fig. 4C and 5C). Alternatively, as shown in rodents with orbitofrontal cortex lesions (Schoenbaum et al., 2002), while acquiring the rule of reversal learning may be impaired in the APP+PS1 mice, once acquired, the mice may simply be able to perform well on subsequent problems. As only one reversal test was performed at each age in the current study, we are unable to distinguish between these two possibilities.
It is somewhat curious that young APP+PS1 mice demonstrated deficits on reversal learning but not on the concurrent discrimination learning problems in the first phase of the transfer task, which could be conceived as having components of a set-shifting task known to depend on intact prefrontal cortical regions (Birrell and Brown, 2000). There are several possible explanations for these results. First, it may be the case that the acquisition of the initial discrimination problems, while analogous to shifting of attentional sets, does not necessarily require such abilities (i.e., the task could be learned simply as a series of discrimination problems). Second, reversal learning and the ability to shift across perceptual sets depend on two distinct neuroanatomical regions of prefrontal cortex, orbital and medial prefrontal cortex, respectively (Birrell and Brown, 2000; McAlonan and Brown, 2003; Schoenbaum et al., 2002). Again, ongoing lesion studies are directed at identifying the precise role of the orbitofrontal and prefrontal cortex in the rodent transfer learning task.
The current series of experiments show that we have successfully established the parameters of a rodent task to assess transfer learning, which, like the human analogue of the task, is sensitive to age-related pathology. This task revealed robust deficits in cognitive function in animals that did not show deficits on more standard assessments of basal forebrain/medial temporal lobe function (i.e., the spatial reference memory version of the Morris water maze task). These data and the highly similar parameters of the human and rodent tasks provide a behavioral paradigm that should be useful for translational research between rodents and humans, resolving a high-priority need for current AD research. One of the impediments to translational research has been an inability to extend cross-sectional experimental designs traditionally used in rodent experimentation to the longitudinal experimental designs typically used in human clinical trials. Tasks such as the water maze are not easily adaptable for re-assessment due to significant savings across test sessions, even in animals with significant memory impairments (LaSarge and Nicolle, 2009; Volz et al., 2001). In contrast, the longitudinal study presented here demonstrates that the transfer learning task can be used in a within subject, test-retest manner in mice. Therefore, this new assessment should be an excellent pre-clinical tool for evaluation of therapeutic interventions that could halt and even reverse cognitive deficits associated with age and age-related disease.
Acknowledgements
We thank Dr. Barry Setlow, Candi Lynn LaSarge, Nicholas Simon, and Ian Mendez for comments on the manuscript. This work was supported by R01 AG029421 to J.L. Bizon and Institute for the Study of Aging (ISOA) to M.A. Gluck.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors declare no conflict of interest.
References
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurol. 1999;53:1992–1997. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Sanderson DJ, Pearce JM. Structural Learning and the Hippocampus. Hipp. 2007;17:723–734. doi: 10.1002/hipo.20323. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. Alzheimer’s disease. Facts and Figures, published in. 2008;4(Issue 2) doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Arns M, Sauvage M, Steckler T. Excitotoxic hippocampal lesions disrupt allocentric spatial learning in mice: effects of strain and task demands. Beh. Brain Res. 1999;106:151–164. doi: 10.1016/s0166-4328(99)00103-5. [DOI] [PubMed] [Google Scholar]
- Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn. Mem. 2002;94:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Bussey TJ, Lee AC, Rogers TT, Davies RR, Saksida LM, Murray EA, Graham KS. Functional specialization in the human medial temporal lobe. J. Neurosci. 2005;25:10239–10246. doi: 10.1523/JNEUROSCI.2704-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer TA, Schafer S, Simons A, Kemmling A, Kamer T, Tepest R, Eckert A, Schussel K, Eikenberg O, Sturchler-Pierrat C, Abramowski D, Staufenbiel M, Multhaup G. Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Abeta production in APP23 transgenic mice. Proc. Natl. Acad. Sci. USA. 2003;100:14187–14192. doi: 10.1073/pnas.2332818100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J. Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Prescott S, Nicolle MM. Intact spatial learning in adult Tg2576 mice. Neurobiol. Aging. 2007;28:440–446. doi: 10.1016/j.neurobiolaging.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol. Aging. 2009;30:646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- Burgess BL, McIsaac SA, Naus KE, Chan JY, Tansley GH, Yang J, Miao F, Ross CJ, van Eck M, Hayden MR, van Nostrand W, St George-Hyslop P, Westaway D, Wellington CL. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer's disease mouse models with abundant A beta in plasma. Neurobiol. Dis. 2006;24:114–127. doi: 10.1016/j.nbd.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC. Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am. J. Pathol. 2001;158:1173–1177. doi: 10.1016/s0002-9440(10)64064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J. Bio. Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nat. Neurosci. 1999;2:844–847. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Golomb J, George AE, Convit A, Tarshish CY, McRae T, De Santi S, Smith G, Ferris SH, Noz M. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. Am. J. Neuroradiol. 1993;14:897–906. [PMC free article] [PubMed] [Google Scholar]
- Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging. 2008;29:693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Dumont M, Strazielle C, Staufenbiel M, Lalonde R. Spatial learning and exploration of environmental stimuli in 24-month-old female APP23 transgenic mice with the Swedish mutation. Brain Res. 2004;1024:113–121. doi: 10.1016/j.brainres.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Mathews P, Cohen N. Further studies of hippocampal representation during odor discrimination learning. Behav. Neurosci. 1989;3:1207–1216. doi: 10.1037//0735-7044.103.6.1207. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The Medial Temporal Lobe and Recognition Memory. Annu. Rev. Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav. Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Bizon JL, Hoyt EC, Helm KA, Lund PK. Effects of aging on the hippocampal formation in a naturally occurring animal model of mild cognitive impairment. Exp. Gerontol. 2003;38:71–77. doi: 10.1016/s0531-5565(02)00159-6. [DOI] [PubMed] [Google Scholar]
- Gluck MA, Myers C. Hippocampal mediation of stimulus representation: A computational theory. Hippocampus. 1993;3:491–516. doi: 10.1002/hipo.450030410. [DOI] [PubMed] [Google Scholar]
- Gluck MA, Myers CE. Representation and association in memory: A neurocomputational view of hippocampal function. Curr. Dir. Psychol. Sci. 1995;4:23–29. [Google Scholar]
- Gluck MA, Myers CE. Gateway to Memory: An Introduction to Neural Network Models of the Hippocampus and Learning. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- Gluck MA, Myers CE, Nicolle MM, Johnson S. Computational models of the hippocampal region: implications for prediction of risk for Alzheimer's disease in non-demented elderly. Curr. Alzheimer Res. 2006;3:247–257. doi: 10.2174/156720506777632826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MN, King DL, Diamond DM, Jantzen PT, Boyett KV, Hope CE, Hatcher JM, DiCarlo G, Gottschall WP, Morgan D, Arendash GW. Correlation between cognitive deficits and Abeta deposits in transgenic APP+PS1 mice. Neurobiol. Aging. 2001;22:377–385. doi: 10.1016/s0197-4580(00)00249-9. [DOI] [PubMed] [Google Scholar]
- Green PS, Bales K, Paul S, Bu G. Estrogen therapy fails to alter amyloid deposition in the PDAPP model of Alzheimer's disease. Endocrinology. 2005;146:2774–2781. doi: 10.1210/en.2004-1433. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Haskins AL, Yonelinas AP, Quamme JR, Ranganath C. Perirhinal Cortex Supports Encoding and Familiarity-Based Recognition of Novel Associations. Neuron. 2008;59:554–560. doi: 10.1016/j.neuron.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Kalesnykas G, Rissanen A, Tapiola T, Iivonen S, Wang J, Chaudhuri J, Tanila H, Miettinen R, Puoliväli J. Estrogen treatment improves spatial learning in APP+ PS1 mice but does not affect beta amyloid accumulation and plaque formation. Exp. Neurol. 2004;187:105–117. doi: 10.1016/j.expneurol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Ikonen S, McMahan R, Gallagher M, Eichenbaum H, Tanila H. Cholinergic system regulation of spatial representation by the hippocampus. Hippocampus. 2002;12:386–397. doi: 10.1002/hipo.1109. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum. Mol. Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- Jauhiainen AM, Pihlajamäki M, Tervo S, Niskanen E, Tanila H, Hänninen T, Vanninen RL, Soininen H. Discriminating accuracy of medial temporal lobe volumetry and fMRI in mild cognitive impairment. Hippocampus. 2009;19:166–175. doi: 10.1002/hipo.20494. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Asthana S, Gluck MA, Myers CE. Associative learning over trials activates the hippocampus in healthy elderly but not mild cognitive impairment. Aging, Neuropsychology, and Cognition. 2008;15:129–145. doi: 10.1080/13825580601139444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728–733. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Gilbert PE, Kesner RP. The role of the hippocampus in the retrieval of a spatial location. Neurobiol. Learn. Mem. 2005;83:65–71. doi: 10.1016/j.nlm.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kluger A, Ferris SH, Golomb J, Mittelman MS, Reisberg B. Neuropsychological Prediction of Decline to Dementia in Nondemented Elderly. J. Geriatr. Psychiatry Neurol. 1999;12:168–179. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- LaSarge CL, Montgomery KS, Tucker C, Slaton S, Griffith WH, Setlow B, Bizon JL. Deficits across multiple cognitive domains in a subset of aged Fischer 344 rats. Neurobiol. Aging. 2007;28:928–936. doi: 10.1016/j.neurobiolaging.2006.04.010. [DOI] [PubMed] [Google Scholar]
- LaSarge CL, Nicolle M. Comparison of different cognitive rat models of human aging. In: Bizon JL, Woods AG, editors. Animal Models of Human Cognitive Aging. New York: Humana Press; 2009. pp. 73–102. [Google Scholar]
- Lassalle JM, Halley H, Dumas S, Verret L, Francés B. Effects of the genetic background on cognitive performances of TG2576 mice. Behav. Brain Res. 2008;191:104–110. doi: 10.1016/j.bbr.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Liu L, Ikonen S, Heikkinen T, Heikkilä M, Puoliväli J, van Groen T, Tanila H. Effects of fimbria-fornix lesion and amyloid pathology on spatial learning and memory in transgenic APP+PS1 mice. Behav. Brain Res. 2002;134:433–445. doi: 10.1016/s0166-4328(02)00058-x. [DOI] [PubMed] [Google Scholar]
- Lowndes GJ, Saling MM, Ames D, Chiu E, Gonzalez LM, Savage GR. Recall and recognition of verbal paired associates in early Alzheimer's disease. J. Int. Neuropsychol. Soc. 2008;14:591–600. doi: 10.1017/S1355617708080806. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav. Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Montgomery KS, Mackey J, Thuett K, Ginestra S, Bizon JL, Abbott LC. Chronic, low-dose prenatal exposure to methylmercury impairs motor and mnemonic function in adult C57/B6 mice. Behav. Brain Res. 2008;191:55–61. doi: 10.1016/j.bbr.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moses SN, Ryan JD. A comparison and evaluation of the predictions of relational and conjunctive accounts of hippocampal function. Hippocampus. 2006;16:43–65. doi: 10.1002/hipo.20131. [DOI] [PubMed] [Google Scholar]
- Myers CE, Gluck MA. Cortico-hippocampal representations in simultaneous odor discrimination: a computational interpretation of Eichenbaum, Mathews, and Cohen (1989) . Behav. Neurosci. 1996;110:685–706. doi: 10.1037//0735-7044.110.4.685. [DOI] [PubMed] [Google Scholar]
- Myers CE, Kluger A, Golomb J, Ferris SH, de Leon MJ, Schnirman G, Gluck MA. Hippocampal atrophy disrupts transfer generalization in nondemented elderly. J. Geriatr. Psychiatry Neurol. 2002;15:82–90. doi: 10.1177/089198870201500206. [DOI] [PubMed] [Google Scholar]
- Myers CE, Hopkins R, DeLuca J, Moore N, Wolansky LJ, Sumner J, Gluck MA. Learning and generalization deficits in patients with memory impairments due to anterior communicating artery aneurysm rupture or hypoxic brain injury. Neuropsychol. 2008a;22:681–686. doi: 10.1037/0894-4105.22.5.681. [DOI] [PubMed] [Google Scholar]
- Myers CE, Kluger A, Golomb J, Gluck MA, Ferris SH. Learning and generalization tasks predict short-term outcome in non-demented elderly. J. Geriatr. Psychiatry Neurol. 2008b;21:93–103. doi: 10.1177/0891988708316858. [DOI] [PubMed] [Google Scholar]
- Myers CE, Hopkins RO, DeLuca J, Moore NB, Wolansky LJ, Gluck MA. Learning and generalization deficits in patients with memory impairments due to anterior communicating artery aneurysm rupture or hypoxic brain injury. Neuropsychology. 2008c;22:681–686. doi: 10.1037/0894-4105.22.5.681. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW. Conjunctive representations in learning and memory: Principles of cortical and hippocampal function. Psychological Review. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Hunkin NM, Bachevalier J, Mayes AR. Change in background context disrupts performance on visual paired comparison following hippocampal damage. Neuropsychologia. 2009;47:2107–2113. doi: 10.1016/j.neuropsychologia.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Puoliväli J, Wang J, Heikkinen T, Heikkilä M, Tapiola T, van Groen T, Tanila H. Hippocampal A beta 42 levels correlate with spatial memory deficit in APP and PS1 double transgenic mice. Neurobio. Dis. 2002;9:339–347. doi: 10.1006/nbdi.2002.0481. [DOI] [PubMed] [Google Scholar]
- Quamme JR, Yonelinas AP, Norman KA. Effect of unitization on associative recognition in amnesia. Hippocampus. 2007;17:192–200. doi: 10.1002/hipo.20257. [DOI] [PubMed] [Google Scholar]
- Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer's disease. Genes Brain Behav. 2007;6:54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- Rudy J, Sutherland R. Configural association theory and the hippocampal formation: An appraisal and reconfiguration. Hippocampus. 1995;5:375–398. doi: 10.1002/hipo.450050502. [DOI] [PubMed] [Google Scholar]
- Schacter D. Multiple forms of memory in humans and animals. In: Weinberger N, McGaugh J, Lynch G, editors. Memory Systems of the Brain: Animal and Human Cognitive Processes. New York: Guildford Press; 1985. pp. 351–379. [Google Scholar]
- Schoenbaum G, Nugent S, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding changes in orbitofrontal cortex in reversal-impaired aged rats. J. Neurophysiol. 2006;95:1509–1517. doi: 10.1152/jn.01052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgaramella TM, Borgo F, Mondini S, Pasini M, Toso V, Semenza C. Executive deficits appearing in the initial stage of Alzheimer’s disease. Brain Cogn. 2001;46:264–268. doi: 10.1016/s0278-2626(01)80080-4. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CS, Clark RE. The medial temporal lobe. Annu. Rev. Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Staufenbiel M. Pathogenic mechanisms of Alzheimer’s disease analyzed in the APP23 transgenic mouse model. Ann. N. Y. Acad. Sci. 2000;920:134–139. doi: 10.1111/j.1749-6632.2000.tb06915.x. [DOI] [PubMed] [Google Scholar]
- Sutherland R, Rudy J. Configural association theory: The role of the hippocampal formation in learning, memory and amnesia. Psychobiology. 1989;17:129–144. [Google Scholar]
- Volz HP, Nenadic I, Gaser C, Rammsayer T, Häger F, Sauer H. "Time estimation in schizophrenia: an fMRI study at adjusted levels of difficulty". Neuroreport. 2001;12(2):313–316. doi: 10.1097/00001756-200102120-00026. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Knowlton BJ, Holyoak KJ, Boone KB, Back-Madruga C, McPherson S, Masterman D, Chow T, Cummings JL, Miller BL. Relational integration and executive function in Alzheimer’s disease. Neuropsychology. 2004;18:296–305. doi: 10.1037/0894-4105.18.2.296. [DOI] [PubMed] [Google Scholar]
- Zhuo JM, Prescott SL, Murray ME, Zhang HY, Baxter MG, Nicolle MM. Early discrimination reversal learning impairment and preserved spatial learning in a longitudinal study of Tg2576 APPsw mice. Neurobiol. Aging. 2007;28:1248–1257. doi: 10.1016/j.neurobiolaging.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Zhuo JM, Prakasam A, Murray ME, Zhang HY, Baxter MG, Sambamurti K, Nicolle MM. An increase in Abeta42 in the prefrontal cortex is associated with a reversal-learning impairment in Alzheimer's disease model Tg2576 APPsw mice. Current Alz. Res. 2008;5:385–391. doi: 10.2174/156720508785132280. [DOI] [PubMed] [Google Scholar]