Abstract
Background
Nephrogenic systemic fibrosis (NSF) is an incurable, debilitating disease found exclusively in patients with decreased kidney function and comprises a fibrosing disorder of the skin and systemic tissues. The disease is associated with exposure to Gadolinium based contrast agents (GBCA) used in magnetic resonance imaging (MRI). Tissue samples from many NSF patients contain micron-sized insoluble Gd-containing deposits. However, the precise composition and chemical nature of these particles is unclear.
Objectives
To clarify the precise chemical structure of the Gd-containing deposits in NSF tissues.
Methods
Autopsy skin tissues from a NSF patient are examined in situ using synchrotron x-ray fluorescence (SXRF) microscopy and extended absorption fine structure (EXAFS) spectroscopy and in correlation with light microscopy and the results of SEM/EDS analyses.
Results
The insoluble Gd deposits are shown to contain Gd no longer coordinated by GBCA chelator molecules but rather in a sodium calcium phosphate material. SXRF microscopy shows a clear correlation between Gd, Ca and P. EXAFS spectroscopy shows a very different spectrum from the GBCAs, with Gd-P distances at 3.11 Å and 3.72 Å as well as Gd-Gd distances at an average of 4.05 Å, consistent with a GdPO4 structure.
Conclusions
This is the first direct evidence for the chemical release of Gd from GBCA in human tissue. This supports the physical-chemical, clinical, and epidemiological data indicating a link between stability and dose of GBCA to the development of NSF.
Keywords: Nephrogenic systemic fibrosis, NSF; gadolinium; magnetic resonance imaging, MRI; gadolinium-based contrast agents, GBCA; extended x-ray absorption fine structure spectroscopy, EXAFS; x-ray micro-imaging; pathology; toxicology
Introduction
The potentially fatal disease, Nephrogenic Systemic Fibrosis (NSF), is associated with decreased kidney function and exposure to gadolinium-based contrast agents (GBCA) used in magnetic resonance imaging (MRI) 1,2. Scanning electron microscopy/energy dispersive spectroscopy (SEM/EDS) of patient tissues has consistently demonstrated micron-sized insoluble deposits containing Gd together with Ca, P, and Na 3,4. GBCAs are the only significant source of human Gd exposure. As Gd is toxic, they comprise Gd3+ bound to a chelator designed to both solubilize the metal ion and render it biologically inaccessible. Whether the deposits observed in tissues contain precipitated intact GBCA or mineralized Gd dissociated from the chelating ligand remains an intractable biomedical problem key to understanding the pathogenesis of NSF.
In this paper we use synchrotron x-ray fluorescence (SXRF) microscopy, and extended x-ray absorption fine structure (EXAFS) spectroscopy to chemically characterize the Gd deposits in skin from a NSF patient. SXRF rasters a focused monochromatic x-ray beam across a sample, collecting an x-ray fluorescence spectrum at each point and yielding images showing distributions of specific elements 5. EXAFS is a structural technique that yields numbers, types and distances of neighbouring atoms around a specific element within a radius of about 6 Å. Fourier transforms of EXAFS spectra are used to visualize atomic structure, as they comprise plots of intensity vs. distance with peaks corresponding to neighbouring atoms 6.
Materials and Methods
The 64-year-old patient had been diagnosed with end stage renal disease at age 43. While on peritoneal dialysis he had three MRI studies on one day, receiving 180 ml of Optimark® (Gadoversetamide 0.5 mol/L). NSF symptoms occurred within a few days, and NSF was diagnosed by skin biopsy 4 weeks later. His immediate cause of death 50 months later was multiple organ failure. Autopsy with consent of the family included permission for examination of tissues and access to clinical information for publication.
A formalin-fixed paraffin-embedded skin sample showing Gd deposits and fibrosis typical of NSF was selected. SXRF microscopy on 20 μm sections used Stanford Synchrotron Radiation Lightsource (SSRL) beamline 2-3 with a 2.8 × 2.0 μm beam. Fluorescence intensities were calibrated to element concentrations using appropriate standards (Micromatter). EXAFS measurements on a 3 × 1.5 × 2 mm tissue block used SSRL beamline 7-3. Omniscan™ (gadodiamide) (GE Healthcare) and Magnevist® (gadopentetate dimeglumine) (Bayer) were diluted to 20 mM Gd before measurement. These GBCAs and OptiMARK® are structurally analogous as they are all diethylene triamine pentaacetic acid (DTPA) derivatives. EXAFS analysis employed EXAFSPAK software 7. Detailed methods are in the supporting information.
Results
Microscopy
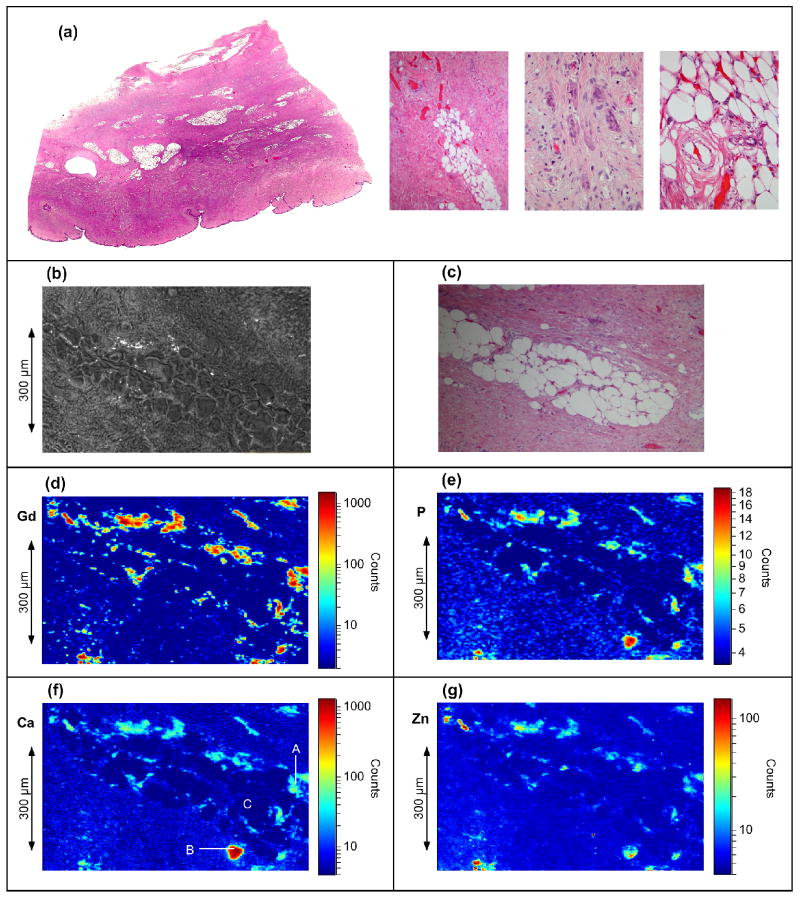
Figure 1 summarizes the microscopy results. Additional information is presented in Figures S1 to S4 in the supporting information. The tissue shows typical histopathology of NSF 4. The area studied by SXRF comprises a subcutaneous fat lobule surrounded by fibrotic bands. SEM reveals the presence of insoluble deposits while SEM/EDS confirms Gd associated with P, Ca and Na (Figure S1). The Gd SXRF image shows Gd deposits throughout the tissue especially surrounding the fat lobule. The maximum observed Gd concentration was 0.41 gcm-3 with mean and median Gd densities of 0.011 gcm-3 and 0.0012 gcm-3 respectively. The median concentration compares well with published gravimetric data 8. With the exception of a large Ca-rich region containing little Gd, the Gd, Ca and P images show almost identical distributions (see Figure S2) and analyses of the x-ray fluorescence intensities show a consistent ratio Gd : Ca : P of 1.0 : 0.43 : 1.6 when Gd is present (the P quantitation has ± 0.5 error). Other metals, such as Zn, are present at low concentrations (< 0.035 per Gd). These show no such trend, with the Zn image revealing an inhomogeneous distribution of Zn throughout the Gd and Ca deposits.
Figure 1.
Light and SXRF microscopy images of the skin tissue showing element distribution. (a) Light microscopy, Hematoxylin and eosin (H&E) stained section of skin with dense fibrosis involving the dermis, extending into the subcutaneous tissue, and containing areas of fibrocytes, osteoclast-like giant cells, and tissue calcification. (b-g) images of the tissue area studied by x-ray fluorescence: (b) SEM image, (c) light microscope image of same area in an adjacent tissue section with H&E stain (d) Gd Lα image, (e) P Kα image, for clarity this image has been processed by a 3 × 3 pixel smoothing function, (f) Ca Kα image, (g) Zn Kα image. For the SXRF images, the field of view is 766 μm horizontal by 482 μm vertical by with a pixel size of 2.8 μm × 2.0 μm. The sample had approximate thickness of 20 μm. The incident x-ray energy was 13.0 keV.
EXAFS Spectroscopy
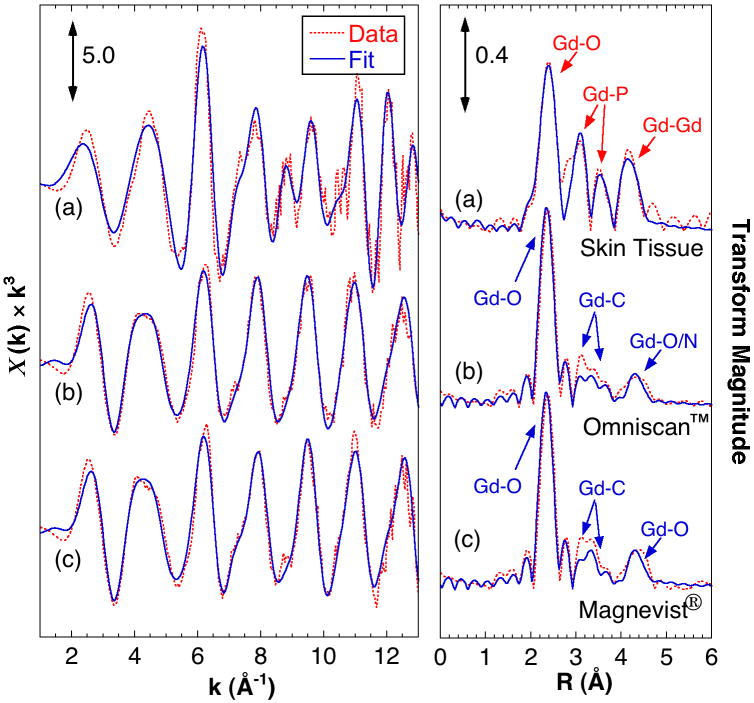
The Gd EXAFS from the skin sample is very different from those of the GBCAs and analysis indicates that tissue bound Gd is coordinated by phosphate. Figure 2 compares the Gd EXAFS spectra, Fourier transforms and simulations. Table 1 summarizes the simulation parameters. The data from both GBCAs are very similar and consistent with coordination to the DTPA chelator, with coordinating O atoms at 2.4 Å, a complex pattern of C atoms around 3.5 Å and a split peak at 4.2-4.5 Å from the outer O/N atoms. Analysis of the tissue EXAFS shows around 8 O atoms at 2.39 Å, about 3 P atoms at both 3.11 Å and 3.72 Å, with 3 Gd atoms at an average of 4.05 Å. These are consistent with the structures of hydrated GdPO4 and mixed metal salts like KCaGd(PO4)2 9,10. Although not required for a good fit, Gd-Ca interactions at approximately 4 Å and Gd-Na at shorter distances can also be included (see supporting information).
Figure 2.
EXAFS Gd L3-edge EXAFS and analysis of the tissue sample compared with that from selected GBCAs. (Left) EXAFS spectra and (right) Fourier transforms (FTs) with simulated fits of (a) skin tissue, (b) Omniscan™, (c) Magnevist®. The Fourier transforms are phase corrected assuming Gd-O interactions. Indicated are the atomic origins of the observed peaks.
Table 1.
Key EXAFS curve fitting parameters. N = number of backscattering atoms. R = interatomic distance. Parameters fixed in the fit are indicated with parentheses. Full parameters are in the supporting information.
| Interaction | (a) Skin Tissue | (b) Omniscan™ | (c) Magnevist® | |||
|---|---|---|---|---|---|---|
| N | R (Å) | N | R (Å) | N | R (Å) | |
| Gd-O | (8.0) | 2.385 | (6.0) | 2.391 | (6.0) | 2.382 |
| Gd-N | (3.0) | 2.641 | (3.0) | 2.634 | ||
| Gd-P | 2.55 | 3.111 | ||||
| Gd-C | (9.0) | 3.415 | (9.0) | 3.415 | ||
| (5.0) | 3.599 | (5.0) | 3.600 | |||
| Gd-P (long) | 2.43 | 3.715 | ||||
| Gd-Gd | 2.74 | 4.053 | ||||
| Gd-O (long) | (5.0) | 4.323 | (5.0) | 4.323 | ||
Discussion
These SXRF microscopy and EXAFS data provide the first atomic scale constraints on the molecular structure of these Gd deposits in an NSF tissue sample. The SXRF microscopy data show a strong correlation between Gd, P and Ca. These correlations are similar to those seen by SEM/EDS 4,11 and confirm, with substantially greater sensitivity, the co-distribution of Gd and Ca previously reported using secondary ion mass spectrometry (SIMS) 12 and more recently SXRF microscopy 13, neither of which reported imaging for P. The EXAFS analysis clearly shows Gd present in a GdPO4-like structure. Since Ca can substitute Gd sites in hydrated GdPO4 provided Na or K is also coordinated 9,10, we infer that the observed deposits comprise a predominately CaxNaxGd(1-x)PO4 material.
We conclude that the Gd deposits in this NSF case consist of a Gd-phosphate material, with little or no Gd still coordinated to the original organic chelator. That the structure of these deposits in this patient's skin is representative of those seen in other NSF cases is strongly supported by previously published analyses of thousands of similar deposits in tissues from NSF patients 11. The enormous thermodynamic stability of Gd phosphate structures (the solubility product for GdPO4.H2O has been estimated at 10-14) 14 may provide both an explanation for the formation of these deposits and a challenge to chemists and physicians hoping to treat NSF. It appears reasonable that these deposits in NSF are either causally related to the disease or at least represent a marker of release of Gd from the chelated GBCA.
Bullet Points.
What's already known about this topic
Nephrogenic systemic fibrosis (NSF) is an incurable, debilitating disease found exclusively in patients with decreased kidney function and comprises a fibrosing disorder of the skin and systemic tissues.
NSF is associated with exposure to Gadolinium based contrast agents (GBCA) in MRI procedures.
Tissue samples from NSF patients contain micron-sized insoluble deposits of Gd with P, Ca, and Na.
The chemical nature of these particles is unknown.
What does this study add
-
SXRF microscopy and EXAFS spectroscopy on skin autopsy samples from an NSF patient show:
nearly invariant ratios of Gd:Ca and Gd:P in the particles,
Gd is predominately coordinated by phosphate, and not by the GBCA.
Further evidence for a causal link between release of Gd from the GBCA and NSF.
State-of-the-art physical chemical analysis has solved an important and previously intractable biomedical problem.
Supplementary Material
Acknowledgments
Funding: This work was funded by the US National Institutes of Health (NIH) grants GM-65440 (SPC), EB-001962 (SPC), and the US Department of Energy (DOE) Office of Biological and Environmental Research (OBER) (SPC). Support was also provided by the Department of Pathology, SUNY Upstate Medical University (JLA).
Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource (SSRL), a national user facility operated by Stanford University on behalf of the DOE Office of Basic Energy Sciences (DOE OBES). The SSRL Structural Molecular Biology Program is supported by the DOE OBER, and the NIH, National Center for Research Resources, Biomedical Technology Program.
Lawrence Berkeley National Laboratory is supported by DOE OBES.
Footnotes
Conflict of Interest Disclosure: Two of the authors (SPC and JLA) have served as expert witnesses for plaintiffs in Gadolinium-related litigation. The investigations reported herein and previous studies by JLA were not performed for litigation.
References
- 1.Cowper SE, Robin HS, Steinberg SM, et al. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000–1. doi: 10.1016/S0140-6736(00)02694-5. [DOI] [PubMed] [Google Scholar]
- 2.Grobner T. Gadolinium - a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrology Dialysis Transplantation. 2006;21:1104–8. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 3.Boyd AS, Sanyal S, Abraham JL. Tissue Gadolinium Deposition and Fibrosis Mimicking Nephrogenic Systemic Fibrosis – Subclinical NSF? Journal of the American Acad Dermatol. 2009 doi: 10.1016/j.jaad.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Abraham JL, Thakral C, Skov L, et al. Dermal inorganic gadolinium concentrations: evidence for in vivo transmetallation and long-term persistence in nephrogenic systemic fibrosis. British Journal of Dermatology. 2008;158:273–80. doi: 10.1111/j.1365-2133.2007.08335.x. [DOI] [PubMed] [Google Scholar]
- 5.Pickering IJ, Prince RC, Salt DE, et al. Quantitative, chemically specific imaging of selenium transformation in plants. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10717–22. doi: 10.1073/pnas.200244597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George GN, Pickering IJ. X-ray Absorption Spectroscopy in Biology and Chemistry. In: Tsakanov V, Wiedemann H, editors. Brilliant Light in Life and Materials Sciences. Dordrecht, NL: Springer; 2007. pp. 97–119. [Google Scholar]
- 7.George GN, George SJ, Pickering IJ. EXAFSPAK: a Suite of Computer Programs for Analysis of X-ray Absorption Spectra. Stanford Synchrotron Radiation Lightsource. 1998. http://ssrl.slac.stanford.edu/exafspak.html.
- 8.Swaminathan S, High WA, Ranville J, et al. Cardiac and vascular metal deposition with high mortality in nephrogenic systemic fibrosis. Kidney International. 2008;73:1413–8. doi: 10.1038/ki.2008.76. [DOI] [PubMed] [Google Scholar]
- 9.Parent C, Bochu P, Daoudi A, et al. Nd3+, Eu3+, and Gd3+ ions as local probes in the NaXSr3-2XLnX(PO4)2 AND KCaLn(PO4)2 rare-earth phosphates. Journal of Solid State Chemistry. 1982;43:190–5. [Google Scholar]
- 10.Vlasse M, Bochu P, Parent C, et al. Structure determination of calcium neodymium potassium phosphate CaKNd(PO4)2. Acta Crystallographica Section B-Structural Science. 1982;38:2328–31. [Google Scholar]
- 11.Thakral C, Abraham JL. Automated scanning electron microscopy and x-ray microanalysis for in situ quantification of Gadolinium deposits in skin. Journal of Electron Microscopy. 2007;56:181–7. doi: 10.1093/jmicro/dfm020. [DOI] [PubMed] [Google Scholar]
- 12.Abraham JL, Chandra S, Thakral C, et al. SIMS imaging of gadolinium isotopes in tissue from Nephrogenic Systemic Fibrosis patients: Release of free Gd from magnetic resonance imaging (MRI) contrast agents. Applied Surface Science. 2008;255:1181–4. [Google Scholar]
- 13.High WA, Ranville JF, Brown M, et al. Gadolinium deposition in nephrogenic systemic fibrosis: An examination of tissue using synchrotron x-ray fluorescence spectroscopy. Journal of the American Academy of Dermatology. 2010;62:38–44. doi: 10.1016/j.jaad.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Lessing PA, Erickson AW. Synthesis and characterization of gadolinium phosphate neutron absorber. Journal of the European Ceramic Society. 2003;23:3049–57. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.