Abstract
Platelets are chief effector cells in hemostasis. In addition, however, their specializations include activities and intercellular interactions that make them key effectors in inflammation and in the continuum of innate and adaptive immunity. This review focuses on the immune features of human platelets and platelets from experimental animals and on interactions between inflammatory, immune, and hemostatic activities of these anucleate but complex and versatile cells. The experimental findings and evidence for physiologic immune functions include previously unrecognized biologic characteristics of platelets and are paralleled by new evidence for unique roles of platelets in inflammatory, immune, and thrombotic diseases.
Keywords: Platelets, Inflammation, Innate immunity, Adaptive immunity, Infection, Host defense
Introduction
Platelets are anucleate myeloid blood cells that are unique to mammals. They are extremely versatile effectors of hemostasis, inflammation, and immune activity with specialized roles in host defense, response to injury, and immune surveillance. The concept that platelets are essential for hemostasis and vascular integrity is a fundamental tenet in physiology and medicine that has progressively evolved from original observations of platelets in the blood of rodents and humans and in tissue samples by Bizzozero and by Osler [1, 2]. A concept that has taken longer to establish, but is now rapidly evolving, is that platelets are key effector cells in inflammation and the immune continuum [3, 4], which encompass innate and adaptive immune responses [5]. There is now compelling evidence that platelets have important recognition (“sentinel”) and surveillance activities in microbial invasion (Figs. 1 and 2) and antigen challenge. They also have signaling functions that trigger important responses of other myeloid leukocytes and lymphocytes, which are principal immune effector cells [5], and endothelial cells, which also contribute critical inflammatory and immune responses [6, 7]. Furthermore, signaling by platelets is a mechanism for information transfer in immune cell–cell interactions, and platelets have the potential to orchestrate complex immune and inflammatory events [4]. These functional capabilities likely evolved through specializations of ancient innate defensive cells, as suggested by features of hemocytes of Limulus and Drosophila and coelomocytes of sea urchins, worms, and other invertebrate species [3].
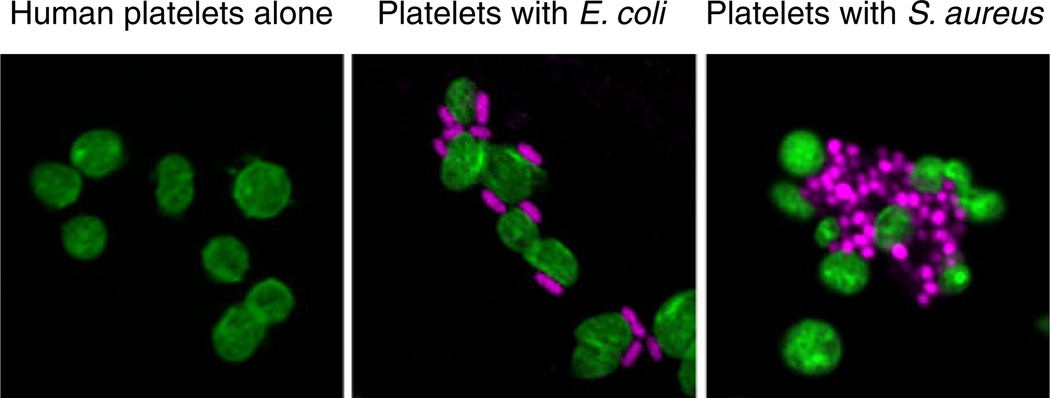
Fig. 1.
Human platelets recognize and interact with bacterial pathogens. Platelets (left panel) were incubated with E. coli (middle panel) or S. aureus (right panel) for 30 min in vitro followed by staining of platelet F-actin (green) and bacterial DNA (magenta) (from [149] with permission from the publisher). Platelets are sentinels that detect intravascular microbes of a variety of classes and respond with activities that contribute to the capture, containment, and elimination of pathogens and to complex innate and adaptive immune responses (Figs. 2 and 3). In some cases, platelet activation by bacteria or their toxins—for example, LPS released by E. coli or staphylococcal alpha toxin—leads to inflammatory vascular injury and thrombosis (see text and cited references for details)
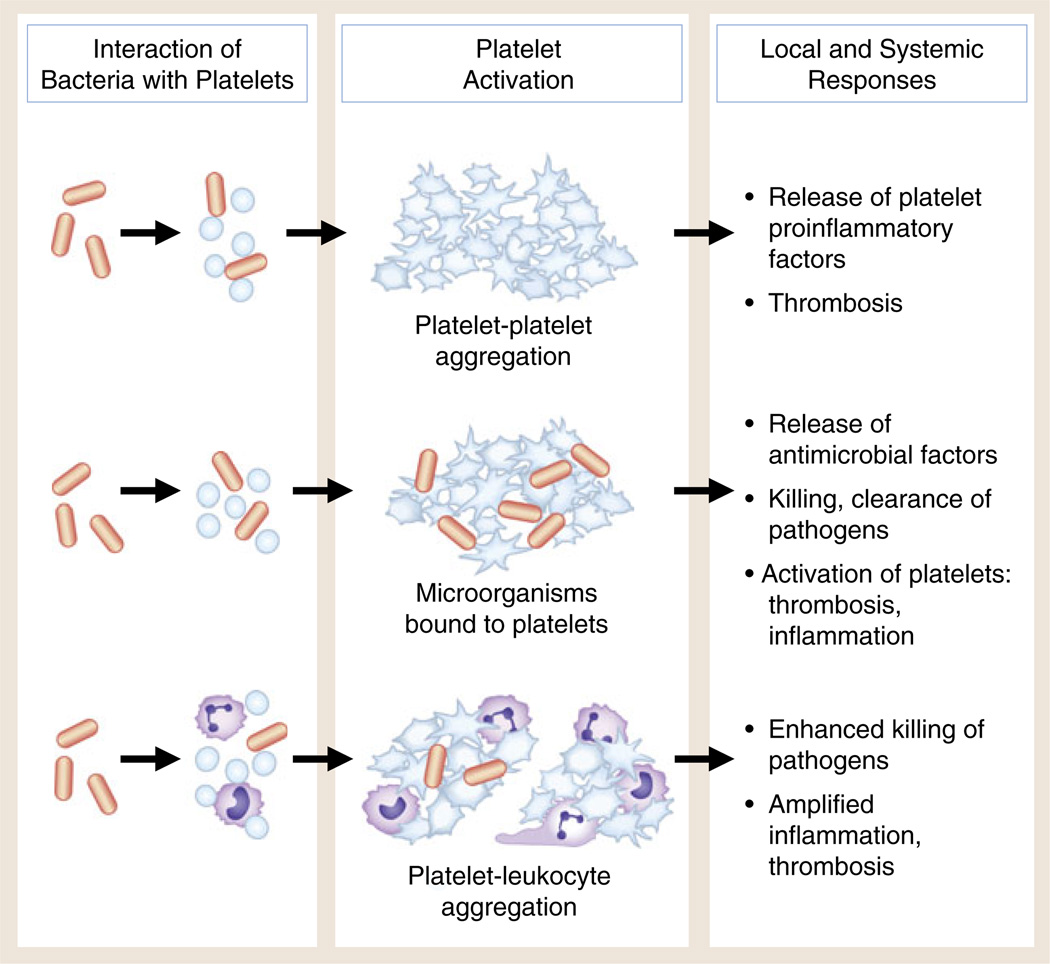
Fig. 2.
Interaction of bacteria with human platelets induces cellular activation and local and systemic thrombotic and inflammatory responses. Direct interaction of bacteria with platelets (see Fig. 1) can lead to aggregation, release of antimicrobial factors and proinflammatory molecules, and formation of platelet–leukocyte aggregates. Under some conditions, bacterial toxins of several classes can trigger these responses of human and murine platelets. Each functional activity is considered in detail in the text. There is evidence that these activities can mediate the containment, immobilization, and killing of bacteria and other pathogens. In addition, however, each can contribute to local thrombosis and inflammation in endovascular infectious syndromes such as bacterial endocarditis and to systemic infections with end-organ injury, including sepsis and complicated malaria
Unanticipated functional properties and molecular pathways have emerged from recent studies of the behavior of platelets, their responses to pathogens, and their activation by stimuli and agonists that are present in the internal milieu of the host. Recognition of this “new biology” of platelets [8, 9], although at times controversial in the field, is contributing to the evolution of our understanding of them as immune effector cells as well as to the reinterpretation of some of their more traditional roles in hemostasis and tissue repair. Approaches utilizing animal models, including mice and zebrafish, contribute relevant observations and also reveal interesting and important differences in the features of human platelets compared to cells from surrogate species ([10–13]; Rowley et al., manuscript submitted for publication). Furthermore, studies of isolated human platelets, megakaryocytes, and in vitro models of human thrombopoiesis continue to yield new discoveries relevant to the complex biology of these cells [13–18]. A corollary is that recent investigations also provide new insights into the potential roles of platelets in inflammatory and immune diseases and their potential for immune activities “in natura” [19]. This review will highlight some of these recent observations and evolving concepts and paradigms and will build on and amplify previously published summaries [3, 4, 20–35].
Platelets in hemostasis, coagulation, and vascular barrier function
As neutrophils (polymorphonuclear leukocytes; PMNs), monocytes, dendritic cells (DC), and lymphocytes of various classes are considered the chief effector cells of inflammation and immune activity, platelets are chief effector cells of hemostasis, coagulation, and pathologic thrombosis [1, 2, 36–42]. Platelets adhere avidly at sites of clinical or experimental vascular injury, a critical first step in hemostasis and thrombosis [36, 38, 39] (Fig. 3). Further amplification of platelet adhesion, triggering of platelet aggregation, secretion of fibrinogen, von Wille-brand Factor (vWF), and other prothrombotic mediators from intracellular granules and secondary recruitment of additional platelets contribute to the formation of the “hemostatic plug” [36, 38, 39] (Fig. 3). These are dynamic, receptor-mediated activation events that involve signaling mechanisms and adhesion molecules—including integrin αIIbβ3 (glycoprotein IIb/IIIa) and the glycoprotein Ibα/V/IX complex (gpIb/V/1X)—that have been intensely studied in addition to newly emerging pathways [2, 38– 42]. Platelet microvesicles, which are membrane-bound particles shed from activated platelets, can contribute to the formation of the hemostatic plug [43]. While these primary hemostatic and thrombotic activities of platelets will not be reviewed in detail here, there is also evidence that adhesion and local activation of platelets at the vessel wall are important early events in inflammatory and immune responses [27, 38, 44]. Therefore, we will refer to them again.
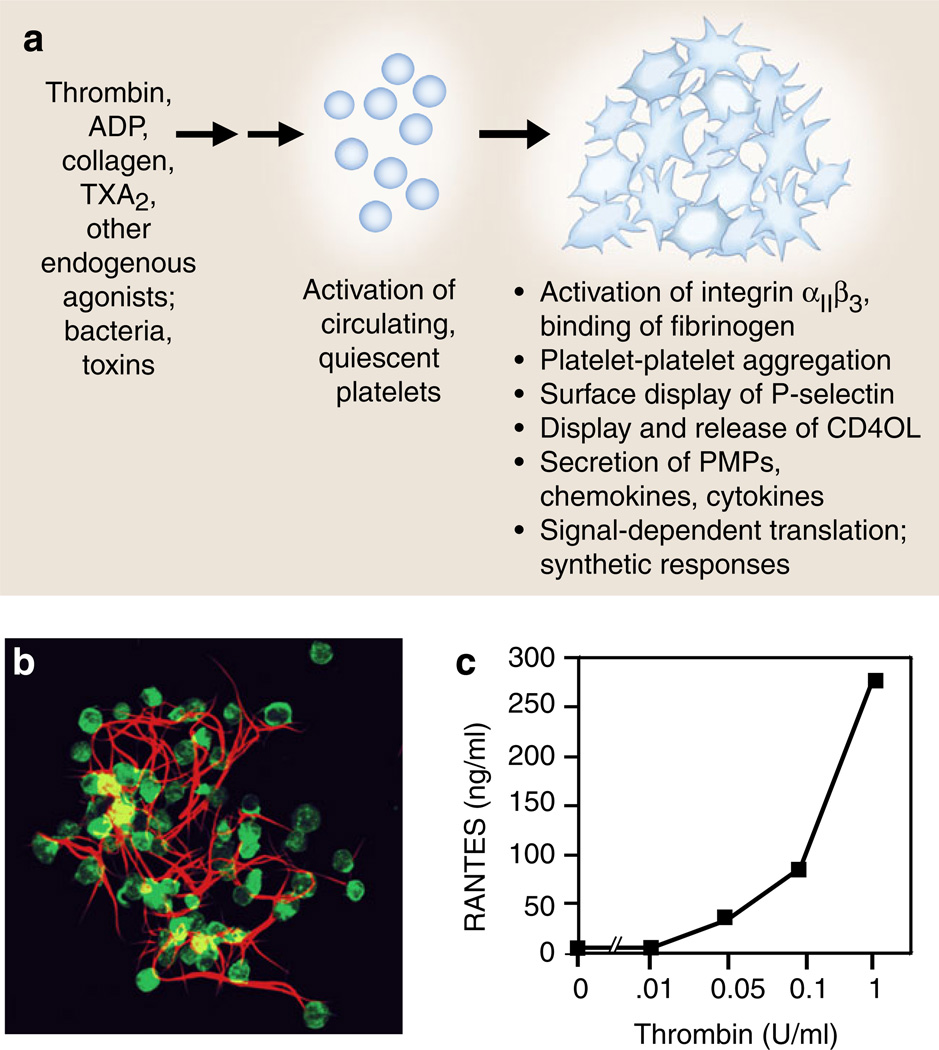
Fig. 3.
Activation responses of platelets mediate the critical events in hemostasis and inflammation. a Platelets activated by agonists recognized by G-protein-coupled receptors, including thrombin, ADP, and TxA2 or by immunoreceptors such as GPVI, a collagen receptor, deliver outside-in signals to human and murine platelets, resulting in rapid and, in some cases, sustained functional responses that mediate key events in hemostasis and inflammation, and often provide molecular links between hemostatic and immune pathways and responses. Activated platelets also release microvesicles that can initiate or amplify hemostasis and inflammation (see text for details). b Activated platelets bind fibrinogen via integrin αIIbβ3 and also bind other coagulation proteins, synthesize tissue factor, and promote the conversion of fibrinogen to fibrin as illustrated by this human platelet– fibrin clot formed in vitro. Activated platelets also retract fibrin, potentially consolidating and remodeling clots in vivo. The adhesive interactions of platelets with fibrinogen and fibrin can trigger additional activation and synthetic responses and inflammatory signaling (panel b is reproduced from [110] with permission from the publisher). c RANTES is an example of a preformed mediator with multiple inflammatory and immune activities that is secreted by activated human platelets (also see Table 1). In this experiment, RANTES was measured by ELISA in supernatants of human platelets activated with increasing concentrations of thrombin for 30 min as described in [171, 173] (panel c was modified from data in [173])
Platelets also facilitate the process of plasma coagulation and ultimate conversion of fibrinogen, which has both hemostatic and proinflammatory activities (see review by Akassoglou, this symposium) to fibrin. This complex biochemical cascade is triggered by the exposure of subendothelial collagen and catalyzed by tissue factor (TF) and the pivotal coagulant protease and inflammatory modulator, thrombin [37, 39, 45]. Platelet contributions to the coagulation cascade include the expression of high-affinity receptors for coagulation proteases and co-factors, providing surfaces for amplification of local thrombin generation, release of stored coagulation factors, and regulation of the activities of associated, surface-bound coagulation enzymes [37]. Human platelets were recently reported to synthesize TF [16, 46], suggesting that they can directly participate in clot propagation and stabilization [16] (also see later). Platelets contribute actively to clot retraction and remodeling [2], which—like thrombus formation—are dynamic features of hemostasis [40]. The interactions of activated platelets with leukocytes, particularly monocytes, amplify the clotting cascade in physiologic and pathologic hemostasis [37]. The intimate and daedal relationships between the coagulation cascade and inflammatory and immune responses are well known—although new features continue to be reported [47]—and are extensively explored in other reviews in this symposium.
Platelet number and activity are critical to vascular integrity during inflammation [48, 49]. In a mouse model of severe thrombocytopenia, there was spontaneous bleeding when inflammation was induced in the skin, brain, or lungs, but in contrast the animals did not have spontaneous hemorrhages in the absence of inflammation [48]. Genetically altered mice deficient in GPIb, β3 integrins, or vWF did not locally bleed in areas of immune complex-triggered skin inflammation [48], suggesting that individual “classic” platelet adhesion pathways [38] are not absolutely required to prevent inflammatory hemorrhage under these conditions. An interesting feature of this study was that only a small increase in platelet number (~10%) was sufficient to reduce the bleeding tendency. These observations raise intriguing mechanistic questions as well as potential therapeutic dilemmas since “antiplatelet” therapy is a major approach in inflammatory conditions that include atherosclerosis and many other syndromes that can include paradoxical bleeding as well as thrombosis [42, 45, 50]. Traditional and newly discovered mechanisms that alter platelet numbers [15, 18] have not been well characterized in the context of acute and chronic inflammation.
In addition to critical activities in primary hemostasis, platelets are required for the maintenance of basal endothelial barrier function. Increased vascular permeability as a consequence of altered barrier function occurs in physiologic and pathologic inflammatory and infectious syndromes [29, 51] and is one of the cardinal signs of inflammation. Vascular integrity is impaired by thrombocytopenia in experimental models, and thrombocytopenia may be a key factor in disrupted vascular barrier function in clinical sepsis, dengue, syndromes of acute lung injury, transplantation, and other conditions (reviewed in [29, 30]). The molecular mechanisms that account for the stabilizing effects of platelets on basal endothelial permeability are not completely defined and appear to be multi-factorial. A key feature may be the release of sphingosine-1-phosphate (S-1-P), which is an important vascular stabilizing molecule [29, 52, 53]. Signaling via the S-1-P pathway also controls lymphocyte egress from the vasculature [54] (also see the review by Hla, this symposium).
Platelets in antimicrobial surveillance and responses to invading pathogens
Platelets interact with, and respond to, invading microbes and pathogens (Fig. 1) [25, 28, 55–57]. Early studies demonstrated that platelets localize at sites of microbial invasion (reviewed in [28]). These observations complemented evidence that platelets accumulate in inflammatory exudates and in tissues involved in antigen-mediated inflammatory injury [21, 22] and, together with more recent studies [4, 58], indicate that platelets are among the “first responders” in defense of the host—although they are often overlooked in this group of effector cells [59]. Platelets are likely key cellular mediators in “intravascular immunity” in host–pathogen interactions [58]. They are, in addition, the earliest and most numerous cells to accumulate at sites of vascular infections involving a variety of pathogens [28, 60].
There is evidence that platelets have defensive roles in infections by bacteria, fungi, protozoa, viruses, and spirochetes (reviewed in [28]). Clinical studies indicate that deficiencies of platelet number and/or function are risk factors for serious infections [60]. The most intensely studied area of platelet”pathogen interactions is bacterial invasion [25, 28, 60], where they have been compared to other myeloid leukocytes in terms of their innate antibacterial effector responses [60] (Figs. 1 and 2). An interesting possibility is that the propensity to bacterial infection in some immunodeficiencies [19, 61]—including the recently identified variant of leukocyte adhesion deficiency syndrome in which there is a heritable defect in inside-out signaling of both leukocyte and platelet integrins [62]— involves impaired platelet surveillance.
Platelet responses to bacteria vary depending on the bacterium studied and include activation, adhesion, aggregation, and interaction of platelets with neutrophils, monocytes, and endothelial cells (Figs. 2, 3, and 4), each of which can contribute to the capture and containment of bacterial pathogens [4, 55, 60]. Human platelets have been reported to engulf bacteria and other microbes, potentially via the open cannalicular system [22, 55, 60, 63]. In parallel, many factors released from granules of activated platelets, or generated by active synthesis, have major antibacterial activities or can trigger secondary inflammatory responses that contribute to the elimination of bacteria [4, 22, 28] (Figs. 2 and 3; Table 1). Platelet microbicidal proteins (PMPs), including kinocidins and thrombocidins, are important examples of these antimicrobial factors [28, 60]. Importantly, PMPs potentiate the activities of antibiotic drugs [28]—an interaction between host defenses and introgenic antimicrobial therapies. Processing of some platelet antimicrobial proteins is catalyzed by thrombin [28], and it is suggested that a key mechanism by which activated platelets contribute to innate antimicrobial defenses is by providing a catalytic surface that favors the generation of thrombin [64]. Thrombin may also form a locally acting bactericidal complex with one or more plasma proteins [64].
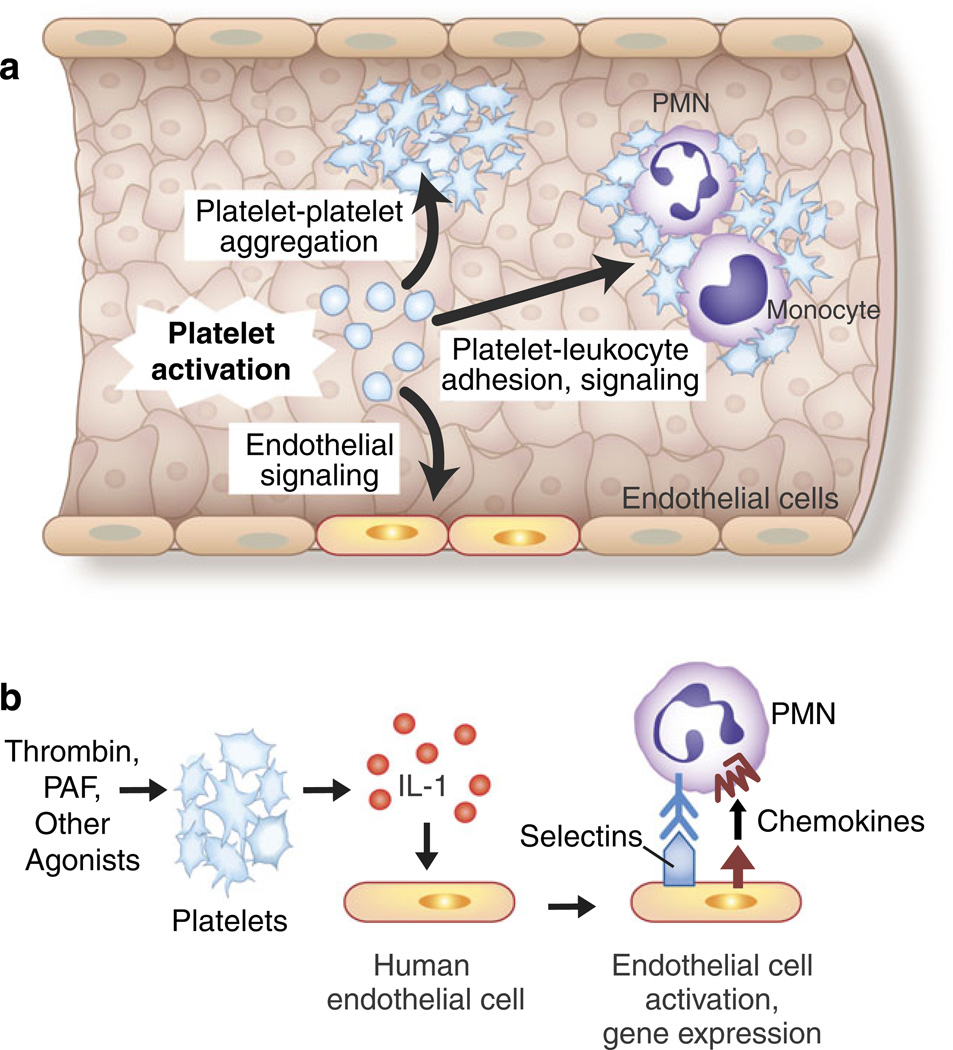
Fig. 4.
Adhesive and signaling mechanisms of activated platelets mediate critical cell–cell interactions in hemostasis, inflammation, and immune responses. a The activation of platelets mediates platelet– platelet aggregation, platelet–leukocyte adhesion and intercellular signaling, and interactions with inflamed and injured endothelial cells. Endothelial cells release factors that inhibit or modify these interactions, including prostacyclin and nitric oxide, under basal and stimulated conditions. The adhesion of platelets to exposed collagen and subendothelial matrix at sites of vascular injury is also a critical mechanism for platelet and leukocyte accumulation and for cell–cell interactions between platelets and leukocytes (not shown) (see text and Figs. 5, 6, 7, and 8 for additional details). b The release of IL-1 by activated platelets mediates platelet–endothelial interactions. The activated human and murine platelets release IL-1 in solution and associated with microvesicles. The release of both IL-1α and IL-1β has been reported, although in some studies IL-1 was only detected on the surfaces of activated platelets. Human endothelial cells release IL-8, MCP-1, IL-6, and GM-CSF and express E-selectin and increased levels of intercellular adhesion molecule 1 in response to juxtacrine signaling by IL-1 associated with activated platelets or IL-1 released by thrombin-stimulated platelets. IL-1β released in microvesicles from activated platelets also induces human endothelial cells to bind PMNs. This is consistent with many observations demonstrating that IL-1 is a prototypic agonist for “type II” activation of endothelial cells, which includes the synthesis and surface expression of E-selectin, IL-8, and other chemokines, leading to the adhesion and activation of PMNs and monocytes. IL-1 also induces the synthesis of tissue factor-dependent procoagulant activity by human endothelial cells. Thus, IL-1-mediated platelet-endothelial interactions have the potential to induce and drive key innate and adaptive immune responses and to link inflammation and hemostasis (see text, Table 1, and Fig. 5 for additional details and for references; Fig. 4 was adapted from [124])
Table 1.
Inflammatory, immune modulating, and antimicrobial factors and mediators released by activated human platelets and/or translocated to their plasma membranes
| Class of mediator | Factor | Stored or synthesized | Reported immune target cell(s) |
|---|---|---|---|
| Pleiotropic inflammatory and immune modulators |
Histamine | Stored | ECs, monocytes, PMNs, NK cells, T and B cells, eosinophils |
| Inflammatory and immunomodulatory lipids |
Serotonin (5-HT) | Stored | Monocytes, macrophages, DC |
| TXA2 | Synthesized | Platelets, T lymphocyte and macrophage subsets | |
| PAF | Synthesized | Platelets, PMNs, monocytes, macrophage and lymphocyte subsets |
|
| Growth factors with immune activities |
PDGF | Stored | Monocytes, macrophages, T lymphocytes |
| TGF-β | Stored | Monocytes, macrophages, T and B lymphocytes | |
| Chemokines | NAP2 (CXCL7) and related β-TG variants |
Proteolytic cleavage of stored precursors |
PMNs |
| PF4 (CXCL4) | Stored | PMNs, monocytes, macrophages | |
| GRO-α (CXCL1) | Stored | PMNs | |
| ENA-78 (CXCL5) | Stored | PMNs | |
| SDF-1 (CXCL12) | Stored | Bone marrow-derived progenitor cells (?) | |
| RANTES (CCL5) | Stored | Monocytes, eosinophils, basophils, NK cells, T lymphocyte and DC subsets |
|
| MIP-1α (CCL3) | Stored | Monocytes, eosinophils, basophils, NK cells lymphocyte and DC subsets |
|
| MCP-3 (CCL7) | Stored | Monocytes, basophils, NK cells lymphocyte and DC subsets |
|
| Cytokines | CD40L (CD154) | Stored | EC, monocytes, DC subtypes, lymphocyte subtypes |
| IL-1β | Synthesized | Monocytes, DC and macrophage subsets, T cell lines, EC, vascular smooth muscle cells, synoviocytes |
|
| IL-1α | Stored (?) | Same as IL-1β | |
| HMGB1 | Stored | Macrophages, PMNs, ECs | |
| GM-CSF | Stored | Eosinophils | |
| Antimicrobial peptides | Platelet microbicidal proteins (several classes) |
Stored; and in some cases protelytically cleaved |
No human cellular targets identified; microbicidal for several bacteria and fungi |
This table is modified from [4]. The list is not comprehensive, and more than 300 proteins, peptides, biologically active lipids, and eicosanoids have been reported to be translocated to the platelet surface and/or released from activated platelets. Recent observations demonstrate that platelets have several specialized storage granules containing differentially packaged preformed factors and previously unrecognized post-transcriptional pathways for the synthesis of mediators (see text and cited reviews for additional details)
Platelet activation, in concert with coupled procoagulant events including thrombin generation and fibrin formation, may be critical for the containment and killing of specific bacteria and prevention of their dissemination [47, 65]. As an example, genetic reduction in either the platelet or the plasma pool of coagulation factor V markedly increased mortality after group A streptococcal infection in mice [65]. Bacteria themselves can contribute to defensive platelet activation (Figs. 2 and 3), and a number of bacterial products that trigger platelet responses have been identified [60, 66]. Nevertheless, some of the same surveillance and containment mechanisms may contribute to the persistence of bacteria at sites of endocarditis and other endovascular infections [25]. This is a serious pathologic consequence that may be compounded by bacterial features that contribute to the subversion of their killing and clearance by platelets and leukocytes [25, 58, 60, 67].
As with essentially all innate and adaptive immune responses, the antibacterial activities of platelets can injure the host if they are induced inappropriately or are dysregulated. As an example, platelet aggregation triggered by bacteria or bacterial products may contribute to thrombotic events [55, 60]. As a second example, platelets were recently reported to trigger the formation of neutrophil extracellular traps (NETs) in in vitro experiments and in a murine model of bacterial sepsis [68]. NETs are extracellular lattices of decondensed chromatin studded with histones and antimicrobial proteins that are formed by neutrophils undergoing a unique pathway of cell death [69– 72], although other mechanisms may be involved [68]. The formation of NETs is a newly recognized mechanism of extracellular bacterial capture and killing [47, 58, 69, 73] that may be impaired in human infections and immunodeficiencies [72]. Thus, the ability of platelets to induce NET formation [68] may be an important intravascular immune activity, and platelets are suggested to be cellular “barometers” that sense the magnitude of bacteremia and regulate NET generation [58]. Nevertheless, NET formation was also reported to cause endothelial injury [68] and to be a mechanism of thrombosis and secondary platelet activation that could amplify pathologic hemostasis [74]. Therefore, NET formation mediated by platelets that are activated by bacterial signals may be an injurious response that harms the host in sepsis [58, 68]. It is possible that platelets also contribute to NET formation and vascular injury in non-infectious immune conditions, such as vasculitis [75, 76].
Although not as extensively studied as the interactions of platelets with bacteria and fungi, specific molecular and immune events are induced by encounters of platelets with protozoa, parasites, and viruses [28, 56, 57, 77]. In parallel with bacterial interactions, there is evidence for both host defense and immune-mediated injury by platelets when they respond to these classes of pathogens. As an example of host defense in protozoal infection, the adhesion of platelets to Leishmania promastigotes results in the formation of large aggregates; this is one of the earliest events after Leishmania invasion in vertebrates and may be a key innate immune defensive mechanism [78]. Additional observations indicate that human platelets kill a second important protozoan, Toxoplasma gondii, via a mechanism that involves thromboxane A2 synthesis [79].
In a more recent study, isolated human platelets were reported to kill Plasmodium falciparum in infected human red blood cells (RBC), and platelet-deficient or aspirin-treated mice had reduced survival compared to control animals in response to erythrocytic infection with a rodent malarial strain, Plasmodium chabaudi [80]. Earlier experiments demonstrated that human platelets inhibit the growth of P. falciparum in parasitized RBC in vitro [81]. In neither case was a mechanism identified, but the observations suggested that platelets are protective in the early stages of in vivo malarial blood stream infection [80, 81]. The biologic paradox is that platelets are, however, also implicated as key effector cells in clinical cerebral malaria, one of the most lethal and morbid consequences of human plasmodial infection, as well as in animal models of cerebral malaria and in other severe systemic complications of human and experimental malarial infection [82]. Together these examples of platelet responses to protozoan invasion illustrate the potential for collateral organ damage in addition to host defense when platelets and other inflammatory and immune effector cells engage invading pathogens.
Experimental and clinical viral infections also demonstrate that platelet–pathogen encounters are complex [28, 57] and that both host defense and injury can result. In a murine model of adenoviral infection, platelets were shown to facilitate antiviral immunity and dendritic cell maturation [83], an example of platelet signaling that facilitates adaptive immune responses [4]. In a second murine model, animals depleted of platelets had reduced viral clearance and an impaired virus-specific cytotoxic T lymphocyte response when infected with lymphocytic choriomeningitis virus (LCV) [84]. Platelet CD40 ligand (CD40L) was implicated in viral clearance and the prevention of a severe hemorrhagic anemia that occurred in platelet-deficient animals infected with LCV. In contrast, the same group reported that activated platelets contribute to cytotoxic T lymphocyte (CTL)-mediated liver injury and hepatic fibrin deposition in a mouse model of acute viral hepatitis [85]. Subsequently, platelet-delivered serotonin was identified as a mediator of liver injury in LCV-infected mice [86], even though platelet serotonin is reported to facilitate liver regeneration after injury (reviewed in [87]). These surrogate animal models demonstrate complex host–viral interactions mediated by platelets, and platelets interacting with other host immune effector cells, and again illustrate that apparently similar cellular mechanisms can result in pathogen clearance or host damage depending on the biologic context. Complexity in platelet-viral interactions is also suggested by clinical and experimental studies of infection with human immunodeficiency virus [63, 88–90], dengue virus [91, 92], hantavirus [93], hepatitis C virus [94–97], and influenza [98]. These syndromes cannot be reviewed in detail here, but some will be referred to again.
An aspect that has received scant attention is the interaction of pathogens with megakaryocytes as well as the mechanisms by which this influences platelet number, functions, and signaling in infectious syndromes. Many infections, particularly those with systemic manifestations, are complicated by thrombocytopenia, and impaired or disordered thrombopoiesis alone or in combination with peripheral platelet sequestration or destruction is often invoked [77]. The studies of primary megakaryocytes in clinical conditions is challenging because of their relative inaccessibility in the marrow, but hematopoietic stem cell models of megakaryopoiesis and thrombopoiesis now exist [14, 15, 18, 29, 99] and should be useful to better define the interactions of pathogens with megakaryocytes. Animal models may also be useful [100]. It seems highly likely that megakaryocytes recognize and respond to microbes and/or their products in a fashion that, in specific cases, alters thrombopoiesis and platelet number. Interactions with pathogens may also alter the transcriptomes and proteomes of megakaryocytes, proplatelets, and their platelet progeny [8], “reprogramming” them in effect. The phenotype of platelets is altered in non-infectious immune and inflammatory conditions associated with high platelet turnover [99], suggesting that transcriptional, post-transcriptional, and proteomic pathways of megakaryocytes and platelets may also be modified in infectious syndromes.
Platelet integrins and immunoreceptors
Platelet integrins are critical for their adhesive and intercellular signaling activities and have been extensively reviewed [2, 38, 101]. Integrin αIIbβ3 on the platelet surface is required for platelet aggregation, stable adhesion of platelets to exposed subendothelial matrix, and clot retraction in primary hemostasis (Fig. 3) and is a critical molecular target in pathologic thrombosis [2, 38, 39, 42, 45]. Maximal platelet hemostatic responses also depend on other surface integrins [101]. Integrin αIIbβ3 becomes competent to recognize ligands and mediate adhesive interactions in hemostasis through the intricate process of inside-out signaling and also delivers outside-in signals to critical intracellular cascades that alter platelet phenotype and function [2, 36, 38, 40, 62].
Platelets also express “immunoreceptors” on their surfaces, including Fc receptors that recognize immunoglobulins of the IgG, IgE, and IgA classes [21, 28, 101, 102]. One group of platelet immunoreceptors and related receptors, including FcγRIIA, GPVI, and C-type lectin-like receptor 2 (CLEC-2), functions through immunoreceptor tyrosine-based activation motifs (ITAMs) that are positioned in intracellular domains of the receptor or in an associated FcRγ subunit [101]. Signals are transmitted via ITAM and ITAM-like motifs in platelet interface with the intracellular actin-binding protein filamin A and the tyrosine kinase syk based on studies in mice [103]. Expression of human FcγRIIAwas reported to increase with human megakaryocyte maturation [104]. In contrast, mouse platelets do not express this immunoreceptor [101]. In humans, FcγRII may contribute to enhanced platelet reactivity and the propensity for thrombotic complications in diabetes mellitus [105]. In addition, FcγRIIA and integrin αIIbβ3 are key components in the recognition mechanism for Staphylococcus aureus that involves binding of IgG directed against the bacteria to FcγRIIA and parallel binding of fibrinogen to αIIbβ3, triggering platelet activation. This mechanism is thought to mediate the clinical syndrome of bacterial endocarditis [25] and may contribute to other endovascular infections. Platelet Fc receptors may also be important in inflammatory and hemostatic responses in immune-mediated nepropathy and other syndromes [101, 102].
GPVI and CLEC-2 trigger tyrosine phosphorylation, activation of αIIbβ3, and granular secretion by platelets [103]. Additional activities of CLEC-2 will be outlined in a later section. GPVI recognizes collagen, and platelets deficient in GPVI have impaired adhesion to collagen surfaces; therefore, it is a key collagen receptor in addition to a signaling activator of αIIbβ3 [40, 106]. The GPIb–1X–V complex has been reported to associate with GPVI and FcRγ, and engagement of GPIb-1X–V by VWF may trigger FcRγ ITAM signaling [101]. Mice deficient in GPVI had decreased inflammatory responses in an arthritis model reported to be dependent on platelets and collagen [107] (also see later), indicating that platelet immunoreceptors, like platelet integrins, influence both adhesion and inflammatory and immune responses. GPVI is also reported to be a receptor for hepatitis C virus [95].
Diverse platelet signaling receptors that are critical in hemostasis also have inflammatory or immunomodulatory functions
Platelets have a repertoire of signal-transducing receptors that have traditionally been studied because they trigger primary hemostatic responses [36, 41]. Many also induce inflammatory and immune responses. G-protein coupled receptors are important examples [41]. G-protein-coupled protease-activated receptors (PARs) are expressed by human and mouse platelets—although the complement of PAR subtypes is different in the two species—and activation of platelets by thrombin via PARs is one of the most potent and widely investigated signaling paradigms in the biology of these cells [108]. The activities of PARs in coagulation and inflammation are considered in other articles in this monograph (Dorling, Ruf) and will not be reviewed in detail here. Nevertheless, unexpected functional activities induced by thrombin and PAR signaling that are relevant to immune responses and immunopathology have been identified. For example, thrombin stimulates the activation of the mammalian target of rapamycin (mTOR) in human platelets, resulting in rapamycin-inhibited translation of a group of protein products [109]. One of these, Bcl-3, alters fibrin retraction by thrombin-stimulated platelets [8, 110]. The mTOR cascade mediates major immune responses and is a molecular target of immune modification [111]; this suggests that thrombin may have unrecognized functions in immunopathology via mTOR-dependent activities of platelets.
Adenosine diphosphate is, like thrombin, a classic platelet agonist. ADP is recognized by G-protein-coupled P2Y1 and P2Y12 purinergic receptors on platelets [36, 41, 112]. Signaling via P2Y12 amplifies platelet aggregation and secretion, and it is a major target for therapeutic intervention in thrombotic disorders [41, 112]. A clinically important antagonist of P2Y12 on platelets, clopidogrel, or its active metabolite were reported to inhibit mouse and human platelet–PMN and platelet–monocyte signaling (see below) in vitro [113], nephropathy in a murine model of systemic lupus erythematosus (SLE) [114], and platelet– leukocyte interactions and other indices of inflammation in human renal transplant patients [115]. In contrast, antagonism of P2Y1, but not P2Y12, was reported to inhibit platelet-mediated P. falciparum killing [80]. Thus, signaling via platelet purinergic receptors, like outside-in signaling triggered by the engagement of PARs, induces both hemostatic and immune effector activities of platelets. Furthermore, PAR activation triggers the release of ADP from platelet stores, linking the two pathways and mediating autocrine amplification [36, 41, 112].
Thromboxane A2 (TxA2) is synthesized by activated platelets by a sequential reaction catalyzed by cyclo-oxygenase 1—the molecular target of aspirin—and TxA2 synthase [42]. TxA2 is recognized by a G-protein-coupled receptor on the platelet plasma membrane and is an important autocrine and paracrine agonist for platelet activation [42, 116]. Human platelets also express other prostanoid receptors, which deliver signals that modify their functions [117]. TxA2 triggers platelet aggregation, adhesion, and recognition of fibrinogen and other ligands by integrin αIIbβ3 and potentiates platelet activation induced by thrombin [42]. TxA2-mediated signaling is particularly important in thrombotic events complicating atherosclerosis [36, 41, 42]. In addition, however, the activation of platelets or other cells that bear the TxA2 receptor by TxA2 induces inflammatory and immune responses [116, 118]. Inhibition of cyclooxygenase with aspirin, alone or in combination with blockade of P2Y12 pathways, is suggested to be an antiinflammatory strategy in atherosclerosis and its complications [42, 119]. TxA2 and prostanoid signaling are also important in other complex inflammatory and immune syndromes [116, 118]. As previously noted, TxA2 was reported to be a mechanism by which platelets kill T. gondii [79].
The G-protein-coupled receptor that recognizes platelet-activating factor (PAF) and certain oxidatively modified phospholipids is expressed on human platelets and, in addition, on other myeloid leukocytes [120]. Because of this distribution, PAF and PAF-like lipids activate platelets, neutrophils, monocytes, and other target cells in inflammatory and immune responses, and the PAF signaling system thus links inflammatory and hemostatic responses [120]. Interestingly, the PAFR is not expressed by mouse platelets, and its mRNA transcript is not present in these cells although it is expressed by murine neutrophils (Rowley J et al., manuscript submitted for publication). PAF is not considered to be a strong agonist for the activation and aggregation of isolated human platelets, and the PAFR is sometimes not mentioned in reviews of platelet signaling pathways. Nevertheless, PAF potently induces the formation of platelet–neutrophil and platelet–monocyte aggregates in whole human blood [121] and, at nanomolar concentrations, induces the synthesis of interleukin1 β (IL-1β) (Figs. 4 and 5)—a proinflammatory and procoagulant cytokine—by isolated human platelets [122]. Both of these responses will be discussed in more detail below. Thus, the responses of platelets to PAF may have been underestimated. Furthermore, PAF signaling synergistically augments platelet activation triggered by ADP or thrombin in whole human blood [123]. PAFR signaling and activities of PAF in sepsis, dengue, and anaphylaxis—systemic inflammatory responses in which platelets are targets and/or have key roles [91, 92, 124, 125]—were recently reviewed [126].
Fig. 5.
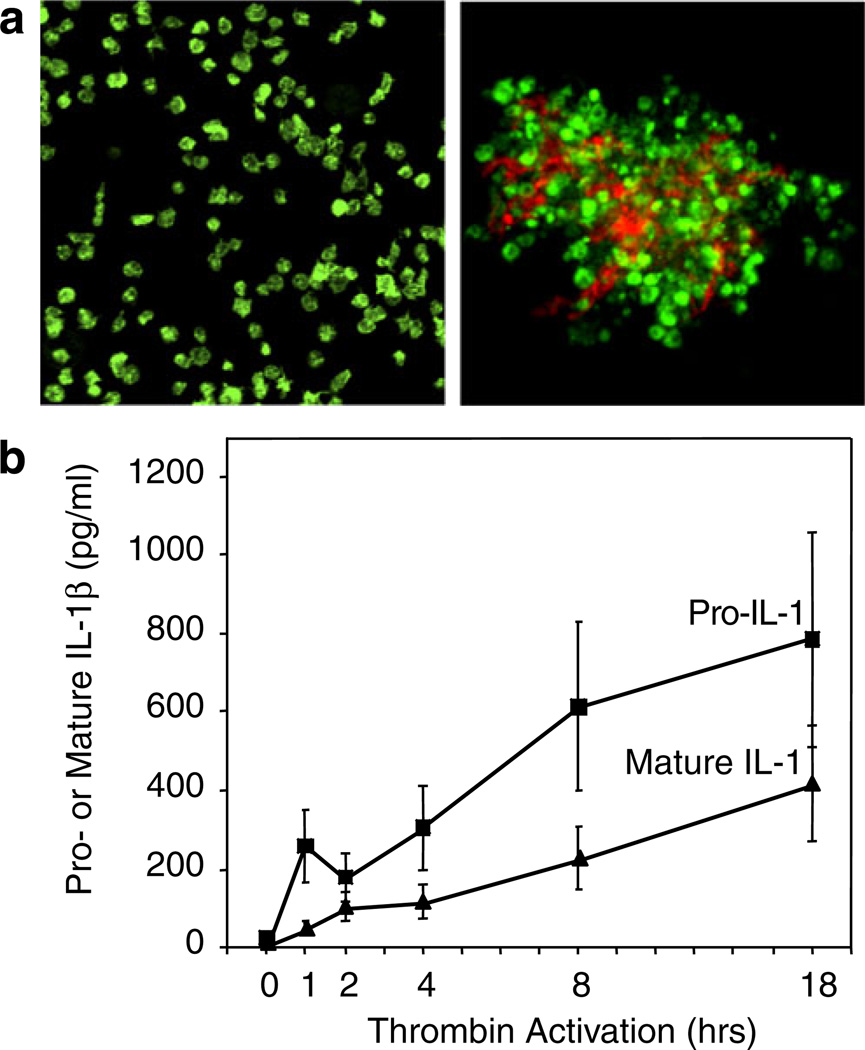
Activated human platelets synthesize IL-1β. IL-1β was not detected in freshly isolated unstimulated platelets by immunocyto-chemistry (aleft panel) or ELISA (b). Stimulation of platelets with thrombin (0.1 u/mL) in the presence of fibrinogen as a model of clot formation induced the rapid and sustained accumulation of the precursor and mature forms of IL-1β (b) that was blocked by the protein synthesis inhibitor puromycin [122]. Transcripts encoding IL-1β were present in resting, unactivated platelets and were found in polyribosomes of activated platelets. PAF, ADP, collagen, and epinephrine also induced IL-1β synthesis (not shown). IL-1β was detected in the fibrin lattice of platelet–fibrin complexes as well as associated with the platelets when platelet–fibrin complexes were examined by confocal microscopy (aright panel; IL-1β is stained in orange and platelet F-actin in green) and also in microvesicles shed from the activated platelets (not shown). Microvesicles from activated platelets induced cultured human endothelial monolayers to bind PMNs in an IL-1β-dependent fashion (Fig. 4b) (from [122] with permission from the publisher; see text and Fig. 9 for additional details of the mechanism of IL-1β synthesis)
A variety of additional receptors of the G-protein-coupled and other classes are expressed by human and/or mouse platelets [127–130]. Signaling cascades activated by these receptors are important in primary hemostasis [127] but may also have alternative activities. A recently identified example is a pathway mediated by the chemokine receptor CXCR4 and stromal cell-derived factor-1 (SDF-1; CXCL12) [26]. SDF-1 is both released by activated platelets and stimulated platelets, and triggers their interactions with progenitor cells [24, 26]. In addition to CXCR4, platelets are reported to express several other chemokine receptors [26, 128, 130]. Platelets also express semaphorins and their ligands, which have the potential for bidirectional signaling [129]. A unique group of receptors—members of the toll-like family—is considered in the next section.
Platelet toll-like receptors
Human and mouse platelets express several toll-like receptors (TLRs), a relatively recent observation now documented by many laboratories (reviewed in [31, 60, 130, 131]). TLRs are conserved sensors that are critical for microbial surveillance and regulation of inflammatory and immune responses. Their biology, signaling, and recognition of ligands with pathogen-, damage-, and danger-associated molecular pattern motifs have been extensively studied and reviewed [5, 132–134]. The expression of TLRs by platelets provides compelling evidence that these cells evolved not only as principal effectors of hemostasis but also as immune cells [3, 4] and reconciles earlier reports that platelets respond to lipopolysaccharide (LPS) and other microbial “endotoxins” [135–139].
Initial reports of TLRs on circulating human platelets and platelets in coronary thrombi [140, 141] were followed by experiments confirming TLR expression on murine and human platelets and indicating that they are functional [142, 143]. The availability of well-characterized, specific anti-TLR antibodies has been a limitation [141]. Furthermore, in most studies, anti-TLR antibodies have not been independently validated, and investigators accepted claims of specificity by commercial suppliers. Nevertheless, an analysis of human, murine, and bovine platelets and chicken thrombocytes now indicates that the expression and function of platelet TLRs are conserved and have physiologic relevance (reviewed in [31, 131]). The apparent pattern of expression of TLRs by human platelets is interesting: TLR1, 2, 4, 5, 6, and 9 have been detected at the mRNA and/or protein level [31, 131, 140–144]. There is an overlapping, but potentially distinct, pattern of expression by mouse platelets [31]. TLRs 2, 4, and 9 are common to both species. Of these, TLRs 2 and 4 have been studied the most [131]. Recent genomic analysis indicates that the TLR2 and TLR4 transcripts have altered expression levels in platelets from human subjects with clinical syndromes that have inflammatory components [144]. The most intriguing TLR expressed by human platelets is, however, TLR9, which recognizes microbial DNA [132, 133] and other pathogen-derived ligands [145]. TLR9 appears to be basally present on the plasma membrane and in intracellular compartments of human platelets and to be increased on the cell surface of platelets activated by thrombin [141, 143] (A. Vieira-de-Abreu, unpublished studies). This is remarkable because in other cell types TLR9 is localized to intracellular endosomal domains [132, 133]. The mechanisms that account for this distribution under resting and activated conditions, and its functional significance, remain to be defined.
Functional responses triggered by platelet TLRs and their downstream signaling pathways are also intriguing and incompletely defined. A paucity of well-characterized, specific, function-blocking anti-TLR antibodies is again a limitation, and small molecule inhibitors and genetically altered mice have been utilized. TLR4 signaling induced by LPS has been most intensively studied. In experiments utilizing wild-type and TLR4-deficient murine platelets, LPS induced adhesion to fibrinogen-coated surfaces (indicating inside-out signaling of integrin αIIbβ3), but not surface expression of P-selectin, and differential TLR-4-dependent sequestration in microvascular beds (lung, liver, spleen) [142]. In a different study, experiments with TLR4-mutant, LPS-resistant C3H/HEJ, and LPS-sensitive C3H/ HeN mice indicated that TLR4 signaling mediates thrombocytopenia and tumor necrosis factor α (TNFα) release in response to LPS administration, and depletion of platelets with anti-platelet antibodies substantially blunted TNFα release [143]. LPS also enhanced microvascular thrombosis in a TLR4-dependent fashion in a vessel injury model [146] and induced platelet–PMN interactions and the accumulation of both cell types in hepatic sinusoids when administered systemically to mice [68].
The analyses of TLR4 signaling of platelets in vitro have yielded conflicting and, to date, confusing functional response patterns that have been termed “atypical” [147] or “unconventional” [68]. Several of these studies report that LPS does not trigger rapid, “classic” activation responses of human platelets including shape change, aggregation, or enhanced expression of surface P-selectin, which is a marker of degranulation, and/or does not potentiate functional responses to agonists such as epinephrine, ADP, or PAF [68, 147–149]. This is similar to the patterns reported from in vitro and in vivo murine studies [142, 146]. In contrast, in other analyses, LPS induced platelet aggregation, P-selectin display, secretion, and/or association with a cultured endothelial cell line, and potentiated responses to thrombin or collagen [131, 147, 150–152]. LPS also induces the novel activities of human platelets, including triggering of NET formation by PMNs [68] and pre-messenger RNA (premRNA) splicing [147, 149] (see below). In one study, platelet TLR4 signaling was reported to be mediated by nitric oxide- and cyclic GMP-dependent protein kinase pathways [152]. Time dependency [147] and use of washed platelets versus platelet-rich plasma with the consequent absence of plasma factors [150] may contribute to some of the differences in these experimental outcomes, although these variables do not seem to be the entire explanation. Interestingly, fibrinogen is reported to be a ligand for TLR4 in other cell types [153, 154]; whether it is recognized by platelet TLR4 and influences binding and signaling by LPS is unknown, however.
Human platelets express key components of intracellular cascades associated with TLR4 [132], including MyD88, MD2, and CD14 [152]. Studies of human platelets using inhibitors of these associations [68, 149] and mice with genetic deficiency of TLR4 or MyD88 [152] indicate that these pathways participate in the LPS-triggered signaling mechanism(s) in platelets.
The evidence that TLR2 and its heterodimeric binding partners TLR1 and TLR6 are expressed by human and mouse platelets [31, 131] is accompanied by an evidence for functionality in vitro and in vivo [147, 155, 156]. Signaling of human platelets via TLR2 induces “thromboinflammatory” responses that are differentially regulated compared to activation induced by thrombin [155, 156].
Factors released by activated platelets or displayed on their surfaces: diverse mediators of inflammatory and immune activity
Activated platelets secrete or translocate to the plasma membrane a panoply of more than 300 proteins and lipids with remarkable diversity in structure, function, mechanism of release and/or surface localization, signaling activities, and cellular targets (Table 1; Fig. 3) [4, 27, 30]. Soluble factors with antimicrobial activities were mentioned earlier and have been reviewed in detail [28, 60]. In this section, we focus on a selected group of additional mediators that have major importance in immune responses and that are also particularly instructive in terms of the intricacy of the platelet repertoire.
Human and murine platelets basally express CD40 ligand (CD40L; also called CD154 and gp39), a transmembrane member of the tumor necrosis factor family, and its receptor, CD40 [4]. CD40L is translocated to the surface from storage sites, cleaved, and shed by activated platelets, and activated platelets are the principal source of plasma CD40L [4, 157]. Inflammatory and immune activities of platelet CD40L have been extensively reviewed [4, 26, 27, 31, 157]. Important examples include signaling of B cells, endothelial cells, and monocytes [31, 157]. CD40L signaling is a major mechanism that links acute inflammation, adaptive immune responses, and hemostasis [157]. Multiple examples indicate key activities of platelet CD40L in clinical syndromes and models of disease. As noted earlier, platelet CD40L was reported to mediate viral clearance in an experimental model of hemorrhagic anemia caused by LCV [84]. In humans, CD40L from platelets is reported to be associated with febrile responses to transfusion and other adverse events [157–159] and inflammation and thrombosis in rickettsial infection [160]. It was recently reported that platelets are activated by immune complexes and FcγRIIa signaling in patients with SLE, resulting in the sCD40L-dependent stimulation of plasmacytoid dendritic cells and interferon α secretion [114]. These examples indicate the potential spectrum of platelet CD40L activities in experimental and clinical immune and inflammatory conditions. Additional examples of CD40L signaling are mentioned below.
Platelet factor 4 (PF4; CXCL4) and β-thromboglobulin (βTbg) are CXC chemokines and the first members of this family to be identified [4]. PF4 is recognized by CXCR3A and CXCR3B and, potentially, by glycosaminoglycans on target cells [33]. PF4 is released in high local concentrations in the regions of evolving blood clots, alters fibrin polymerization, and consolidates thrombi [161]. Thus, it may have critical hemostatic functions. PF4 also has complex inflammatory activities that depend in part on the target cell(s) and model system [33]. In vitro, it is chemotactic for neutrophils and monocytes [162]. PF4 also promotes monocyte survival and differentiation to macrophages [163], one of only a limited number of factors known to have this activity, and induces a unique transcript profile in human monocyte-derived macrophages [164]. This suggests that PF4 may be important in signaling intravascular macrophage subsets [58]. In a mouse model of cerebral malaria, PF4 had proinflammatory activities including T-cell trafficking in the brain, increased CXCR3 expression, interferon α production, and monocyte trafficking and activation [165, 166]. The levels of both PF4 and βTbg are elevated in the plasma of human subjects with P. falciparum malaria [167]. As a clinical example, PF4 is a component of a complex antigen that induces activating antibodies in the introgenic syndrome of heparin-induced thrombocytopenia, an important cause of thrombosis in hospitalized patients that has implications for mechanisms of autoimmunity [168].
RANTES (“regulated upon activation normal T cell secreted”) is a member of the CC family of chemokines, in contrast to the CXC chemokine PF4 [4, 26]. RANTES is recognized by the CCR1, CCR3, and CCR5 chemokine receptors on target cells and, via these signaling pathways, mediates pleiotropic immune, angiogenic, and anti-viral responses [169]. Its first identified activity as a platelet chemokine was as a chemoattractant for human eosinophils [170]. Subsequently, it was shown to mediate coordinate signaling with platelet P-selectin in interactions of activated platelets and monocytes that trigger inflammatory gene expression [171] (Figs. 6, 7, and 8). RANTES is stored in platelet alpha granules [172] and is rapidly released in soluble form from thrombin-stimulated platelets [170, 171, 173] (Fig. 3) and also as a component of platelet micro-vesicles, which can act as delivery systems that mediate the recruitment of monocytes to endothelium [174]. In an in vitro system, CD40L-positive T cells engaged platelet CD40 and triggered the release of RANTES from the activated platelets, which associated with endothelial cells and mediated T-cell recruitment [175]. In an in vivo atherosclerosis model, RANTES and PF4 were deposited on inflamed endothelium and contributed to vascular injury and lesion size [26].
Fig. 6.
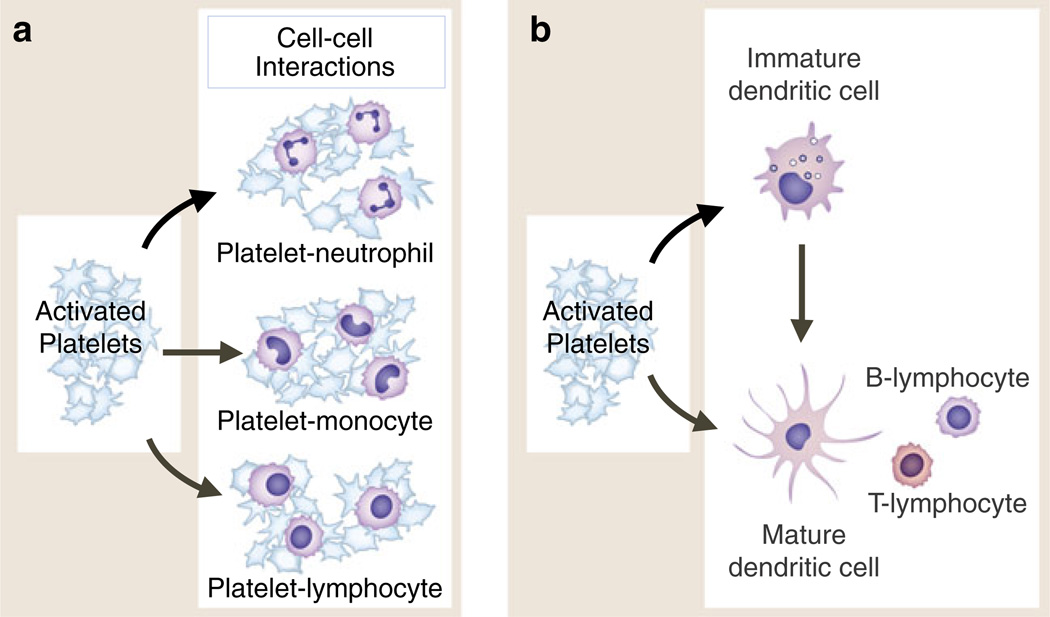
Activated platelets adhere to and signal circulating leukocytes and interact with dendritic cells and macrophages. Activated human platelets form heterotypic aggregates with neutrophils and other granulocytes (eosinophils, basophils), monocytes, and lymphocytes of several subclasses (a). In each interaction, binding of P-selectin on the activated platelet to PSGL-1 on the “target” leukocyte is critical. Additional adhesive mechanisms contribute to aggregate formation and to interactions with endothelial cells, subendothelial matrix, or other cell types depending on the specific leukocyte class involved. In addition to adhesion, intercellular signaling mediated by PSGL-1 engagement and other signal transduction pathways leads to altered functions of the leukocytes (see text and Fig. 7). Activated platelets also interact with and signal dendritic cells and, by these interactions, can indirectly alter lymphocyte activities (b). This may be a key mechanism by which activated platelets mediate information transfer between innate and adaptive components of the immune continuum and influence adaptive immune responses. Activated platelets interact with macrophages in addition to DC and may influence cell fate dermination when monocytes differentiate to immature dendritic cells or to macrophages (not shown). Activated platelets bind to monocytes and plasmacytoid DC in the circulation of patients with SLE and to PMNs and monocytes in a variety of other clinical conditions (see text for details)
Fig. 7.
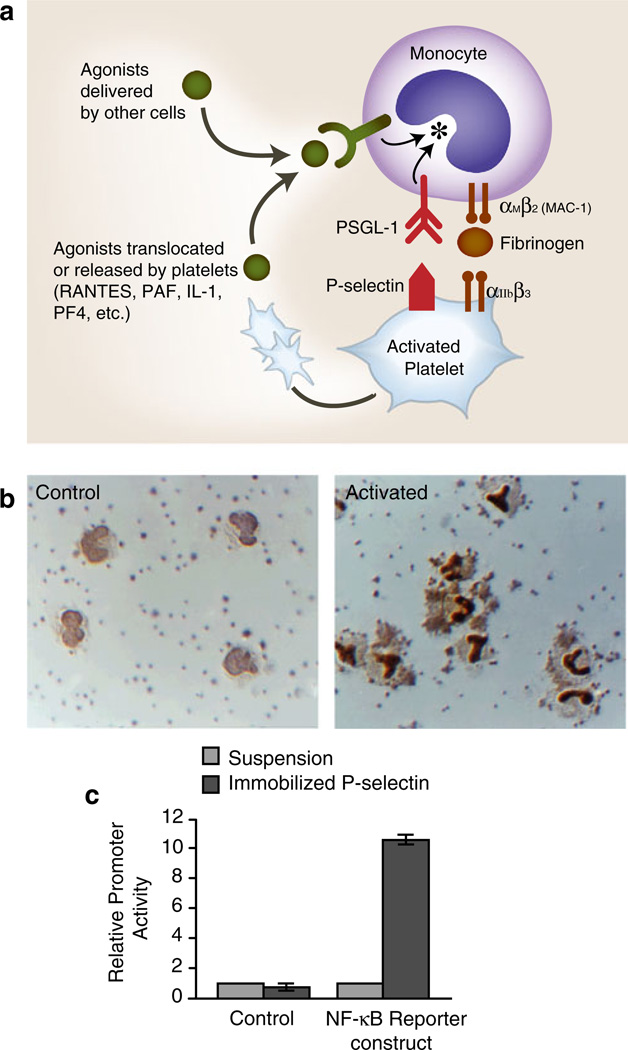
a Activated human platelets adhere to monocytes and deliver outside-in signals that induce altered functions. Activated human platelets adhere to monocytes in vivo and in vitro. Binding of P-selectin, which is translocated to the plasma membranes of activated platelets from alpha granules, to PSGL-1 basally present on the monocyte plasma membrane is critical for this interaction. The adhesion of activated platelets to monocytes is inhibited by blocking antibodies against P-selectin or PSGL-1 and by other approaches that interrupt this interaction. The engagement of PSGL-1 signals the activation of αMβ2and, likely, other members of the integrin family on monocytes, potentially amplifying the adhesion provided by P-selectin/PSGL-1 and contributing to other adhesive interactions with endothelium, subendothelial matrix, or other blood cells. Outside-in signals delivered by soluble or juxtacrine factors released or displayed by activated platelets (RANTES, PF4, IL-1, PAF, CD40L, etc.) can act in concert with signals delivered by the engagement of PSGL-1 (see Fig. 8) as can agonists delivered by other cell types. Signaling of monocytes by activated platelets alters transcriptional and post-transcriptional pathways in the leukocytes (see Fig. 8). b The activated human platelets induce the nuclear translocation of NF-kB in adherent monocytes. Under basal conditions in vitro, human platelets do not adhere to monocytes, and NF-kB is largely found in the monocyte cytoplasm (left panel, “control”). When platelets are activated with thrombin, platelet-monocyte aggregates rapidly form and NF-kB translocates to the nucleus as indicated by dark nuclear staining with an antibody against the p65 subunit of NF-kB. Both platelet-monocyte aggregate formation and NF-kB translocation are blocked by interrupting the binding of P-selectin on the activated platelets to PSGL-1 on the monocytes. In additional experiments, integrated signals delivered by platelet P-selectin and RANTES (see Fig. 3) were shown to induce the expression of the NF-kB-dependent gene products MCP-1 and IL-8 by the monocytes (see Fig. 8) (panel b is from [171] with permission from the publisher.) c Signaling by P-selectin induces NF-kB activation. U937 monocytic cells transfected with an NF-kB reporter or a control construct were incubated in suspension or allowed to adhere to purified, immobilized P-selectin and reporter activity was measured. Triggering of NF-kB reporter activity by adhesion to purified P-selectin is consistent with additional experiments, demonstrating that the engagement of PSGL-1 transmits outside-in signals to this pathway and that activated platelets deliver signals that alter patterns of inflammatory gene expression in monocytes (see also panels a, b, and Fig. 8; panel c is from [218] with permission from the publisher)
Fig. 8.
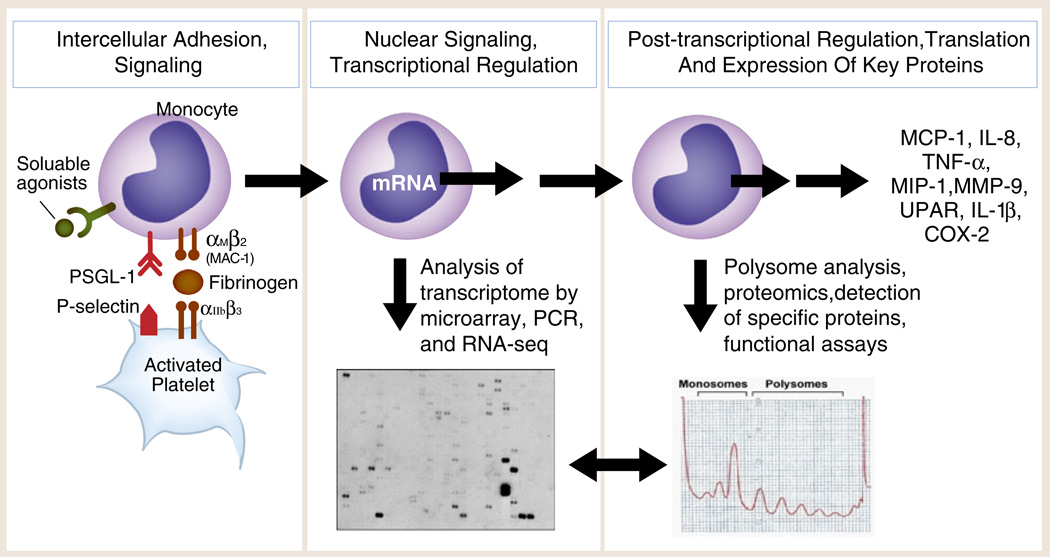
Outside-in signals delivered by activated, adherent human platelets induce the expression of inflammatory and thrombotic gene products by human monocytes. Experiments using thrombin-stimulated human platelets interacting with isolated monocytes as outlined in the left panel and in Fig. 7a demonstrate that activated platelets deliver outside-in signals to transcriptional (Fig. 7b, c) and post-transcriptional pathways (mRNA stabilization; mRNA association with RNA binding proteins; activation of mTOR, a regulator of specialized translation) via P-selectin/P-selectin engagement and parallel signaling by soluble or cell-associated platelet chemokines or cytokines. Assays of the monocyte transcriptome and transcrip-tional responses (middle panel) and proteomic analysis and functional assays (right panel) indicate that the protein products (far right) are synthesized by mechanisms that are time dependent and gene specific. Each protein product has important inflammatory and/or hemostatic activities based on many experimental and clinical studies. Two specific examples—expression of MCP-1 and COX-2—are outlined in the text (Fig. 8 was adapted from [277] with permission from the publisher)
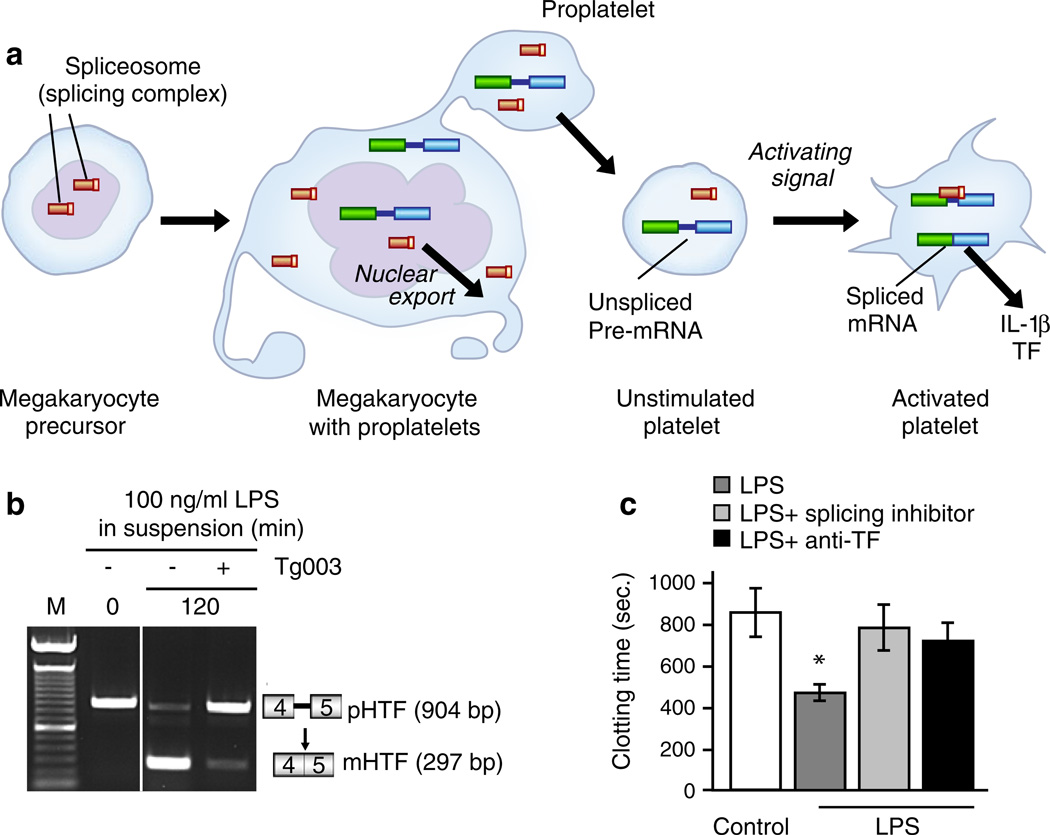
Activated human and mouse platelets mediate intercellular signaling with interleukin-1 (IL-1) in cell-associated, soluble, and microvesicle-associated forms in vivo and in vitro [4, 107, 122, 176–179]. IL-1α and IL-1β are evolutionarily conserved cytokines with pleiotropic immune and hemostatic activities that induce similar responses of target cells by ligating IL-1 receptor type I (IL-1RI), an interaction that can be interrupted by a naturally occurring soluble IL-1 receptor antagonist (IL-1Ra) [180]. IL-1α and IL-1β are transcribed by separate genes and are synthesized as precursor (pro-) forms followed by processing [180]. IL-1β has been detected in fluids and tissue biopsies from humans with a variety of inflammatory and immune diseases; in contrast, IL-1α may principally act as an autocrine cell-associated messenger [180]. The responses induced by platelet IL-1 include the expression of adhesion molecules and chemokines by human endothelial cells, endothelial–neutrophil interaction, signaling of Tcells, and expression of pro-inflammatory mediators by synoviocytes in vitro or when delivered to the arthritic articular space in microvesicles [107, 122, 176–178, 181] (Fig. 4). It was previously thought that IL-1 is preformed in platelets, and some reviews indicate that platelets store IL-1α and IL-1β [30, 33]. However, we did not detect pro-IL-1β or mature IL-1β in resting human platelets by ELISA or immunocytochemical analysis but instead found that it is synthesized by platelets activated by thrombin, PAF, ADP, or collagen [122] (Fig. 5). Newly synthesized IL-1β was associated with fibrin in addition to platelets in a clot model (Fig. 5), suggesting that thrombi are reservoirs for cytokines; IL-1β also stimulated target endothelial cells when translocated in microvesicles (Fig. 4) [122]. Subsequent studies identified a new post-transcriptional mechanism by which activated platelets splice IL-1β pre-messenger RNA (pre-mRNA), translate the spliced transcript, and synthesize IL-1β and other biologically active proteins [14], one of the several mechanisms by which platelets may actively produce key proteins in clots and thrombi [8]. Additional details of this mechanism are outlined below.
Cleavage of sCD40L, secretion of PF4 and RANTES from granular stores, and synthesis and microvesicle-associated release of IL-1β illustrate three mechanisms by which activated platelets can contribute biologically active mediators to the blood or to extravascular compartments in hemostatic and immune responses [4, 8, 9]. It is important to emphasize that some signaling factors also remain associated with the plasma membranes of activated platelets and can mediate cell–cell interactions in a juxtacrine signaling fashion when localized to the platelet surface [4]. CD40L [182], IL-1 [176–178], and PAF [183, 184] are examples of platelet factors that can activate target cells in a membrane-bound, juxtacrine manner. Thus, exclusive measurements of soluble factors and biomarkers may not detect signaling events mediated by activated platelets in immune or hemostatic responses.
The adhesion molecules on activated platelets can also deliver outside-in signals to cellular binding partners, alone or in combination with soluble platelet factors that act locally in a paracrine fashion or with other juxtacrine signals. P-selectin (formerly called GMP-140 and PADGEM protein)—which is translocated to the plasma membranes of activated platelets from alpha granules and is present in high local densities on their surfaces [4, 30, 185]—is an important example. P-selectin on activated platelets mediates both adhesion and intercellular signaling by binding to P-selectin glycoprotein ligand 1 (PSGL-1) on PMNs, monocytes, lymphocytes, and other leukocytes [4, 30, 185] as will be profiled below (Fig. 7). P-selectin also contributes to platelet aggregate stabilization and hemostasis under some conditions [27, 31].
Interactions of platelets with leukocytes and endothelial cells
Platelets interact with leukocytes of several classes in vivo and in vitro [3, 4] (Fig. 6). A number of examples have already been mentioned in this review. The interaction of leukocytes with adherent, activated platelets at sites of vascular injury or inflammation is a mechanism of cellular targeting and transfer to tissues and involves rolling, arrest, and in some cases transmigration mediated by selectins, chemokines and PAF, and integrins and their ligands depending on the specific leukocyte type and microvascular locale; these events parallel similar mechanisms in leukocyte–endothelial interactions (reviewed in [6, 7, 27, 30, 44]). Platelet–leukocyte aggregates formed in circulating blood (Fig. 6) are also involved in immune targeting and function. As an example, circulating platelet–leukocyte complexes requiring PSGL-1 engagement were reported to be essential for leukocyte activation and accumulation in mouse models of allergic and inflammatory responses [186]. Platelet–leukocyte interactions of this nature can enhance bacterial phagocytosis and killing [28] and may involve complex interactions involving integrins on both cell types and factors such as fibrinogen [62, 101, 187]. In another murine study, platelet–leukocyte aggregate formation (Fig. 6) mediated by P-selectin/PSGL-1 and local chemokine secretion by these complexes (Figs. 3, 7, and 8) controlled leukocyte recruitment in a model of the cutaneous arthus reaction [188]. There are also clinical parallels to these experiments. As an example, the formation of platelet–leukocyte aggregates in banked human blood may contribute to transfusion reactions and transfusion-related pathology [189]. Additional evidence for platelet–leukocyte dialogs in immune responses and diseases will be illustrated in the following profiles of interactions of activated platelets with specific leukocyte subtypes.
PMNs
P-selectin is central in platelet–PMN interactions as it is in the interactions of PMNs with inflamed endothelium (reviewed in [30, 44]). In each case, binding of P-selectin to PSGL-1 mediates both adhesion and signaling. Interruption of P-selectin/PSGL-1 binding by antibody blockade or genetic manipulation interrupts these events [30, 44]. Studies in murine models indicate that engagement of PSGL-1 on PMNs by P-selectin triggers the activation of leukocyte β2 integrins [30, 185, 190], a sequence of events predicted by earlier studies of human PMNs [191]. β2 integrins on the PMN plasma membrane that have been activated by signals from PSGL-1 and/or by parallel signals from G-protein-coupled receptors that recognize chemokines or PAF can then bind fibrinogen, platelet GP1bα, ICAM-2, JAM-3, and other ligands on platelets, enhancing adhesion-mediated P-selectin binding to PSGL-1 [27, 30, 44, 183, 184, 192–194]. In vitro studies of cellular activation in whole human blood indicate that there is reciprocal platelet activation in such interactions [195]. Additional responses of mouse or human PMNs are triggered by platelet signaling and are inhibited by blocking platelet activation [113]. Consistent with this, in vitro studies of human PMNs indicate that engagement of PSGL-1 by P-selectin induces the activation of kinase cascades and chemokine and cytokine synthesis pathways [196, 197]. In vitro models also suggest that human neutrophils enhance fibrin deposition under flow via mechanisms that are partially platelet dependent [198], thus potentially linking inflammatory signaling to hemostasis. Platelet–PMN aggregates (Fig. 6) may be vehicles for delivery of coagulation factors (see below), and PMNs may transport platelet microvesicles to extravascular sites [9, 107]. Platelet–PMN interactions of this nature contribute to pathologic inflammation and thrombosis in a variety of experimental models, and there is evidence that these interactions are also pathogenetic mechanisms in clinical syndromes such as sepsis and acute lung injury [29, 124]. It is unknown how molecular adhesion and signaling interactions between platelets and PMNs influence NET formation triggered by LPS [68]. P-selectin/PSGL-1 signaling, chemokines, and β2 integrins also mediate platelet interactions with eosinophils and basophils [199, 200] and therefore may be critical in immune defenses and allergic responses involving these granulocytes.
Monocytes
The interaction of activated platelets with monocytes occurs in patients with a variety of inflammatory, immune, and thrombotic diseases and in human cellular systems and rodent models (reviewed in [201, 202]). The adhesion of monocytes to inflamed or injured endothelial cells occurs by coordinated selectin-, signaling-, and integrin-mediated events similar to those originally identified in endothelial–neutrophil interactions, but with leukocyte-specific molecular variations [30, 202]. In vitro modeling suggests that platelets preferentially recruit monocytes to thrombi [203] and that platelets and platelet microparticles transfer PF4 and RANTES to the surfaces of inflamed endothelial cells, leading to shear-resistant local accumulation and activation of monocytes—a mechanism proposed to occur in atherosclerosis (reviewed in [26, 202]).
Heterotypic platelet–monocyte complexes (Fig. 6) form in vitro and in the blood of humans and experimental animals and are both markers and effectors of disease (reviewed in [201, 202]). Platelet–monocyte aggregates are reported to form more readily (i.e., at lower concentrations of platelet agonists), more rapidly, and in greater numbers compared with platelet–PMN or platelet–lymphocyte aggregates and to be more stable than platelet–neutrophil aggregates [204] (reviewed in [202, 205]). The differences in the pattern of formation of platelet–PMN and platelet–monocyte aggregates may be due to the differential modulation of PSGL-1 on activated PMNs versus monocytes [206, 207]. The formation of platelet–monocyte aggregates is a sensitive marker of platelet activation in vivo, and these complexes are durable over many hours based on studies in primates and in humans with acute coronary syndromes [208]. Studies using blocking antibodies and other approaches demonstrate that binding of P-selectin on activated platelets to PSGL-1 on monocytes (Fig. 7) is a key molecular axis in this adhesive interaction [171, 209, 210]. In addition, molecular interactions including bridging of CD34 on adjacent platelets and monocytes by thrombospondin, binding of monocyte αMβ2 to platelet GPIbα or JAM3, and bridging of platelet αIIbβ3 and αMβ2 on monocytes by fibrinogen have been suggested, based on extrapolation from studies of platelet–neutrophil interaction (see above), studies with THP-1 or U937 monocytic cells, or other strategies [192–194, 211, 212]. Furthermore, engagement of PSGL-1 on monocytes activates β1 and β2 integrins on monocytes [202, 213], suggesting that these leukocyte integrins may participate in adhesive interactions in platelet–monocyte aggregates (Fig. 7). Nevertheless, blocking studies in ex vivo whole blood from volunteers and subjects undergoing coronary intervention [214] and in platelet–monocyte suspensions [215] indicated that binding of P-selectin to PSGL-1 is dominant in platelet–monocyte aggregate formation, and it was recently concluded that other molecular interactions have secondary, additive roles [202]. Other adhesion molecules may be critical in more complex interactions involving platelet–monocyte aggregates and endothelial cells, exposed vascular matrix, or other blood cell types [26, 202, 216]. For example, P-selectin/PSGL-1-dependent adhesion was required for inclusion of platelets in aggregates of monocytes and reticulocytes in the blood of patients with sickle cell disease, an interaction that also involved integrin α4β1 and bridging fibronectin [217].
The adhesion of activated platelets to monocytes is a mechanism of intercellular signaling and information transfer and not simply a mechanical interaction and has been useful as a general model of human cell signaling and gene expression as well as one with immediate physiologic relevance in inflammation and thrombosis (Fig. 8). For example, the interaction of activated platelets with human monocytes induces nuclear translocation of NF-kB and induction of NF-kB-dependent inflammatory genes [171, 210, 218]. The engagement of PSGL-1 by P-selectin delivers outside-in signals to this transcriptional pathway (Fig. 7) as demonstrated by experiments with primary cells, purified immobilized P-selectin, transfected models, and reporter systems [171, 218]. RANTES, IL-1β, and PAF can act in concert with the engagement of PSGL-1 to amplify inflammatory gene expression, demonstrating signal integration and mechanisms for differential, time-dependent expression of key inflammatory products in a gene-specific fashion (Fig. 7) [171, 210, 216, 218]. Initial and subsequent studies demonstrated that monocyte chemotactic protein 1 (MCP-1), TNFα, interleukin 8 (IL-8), and other inflammatory proteins are synthesized by monocytes in this fashion [171, 210, 219] (Fig. 8). Each of these inflammatory gene products has major immune and/or hemostatic activities in vitro and in vivo. In a more recent example, we found that cyclooxygenase 2 (COX-2) expression and COX-2-dependent prostaglandin E2 (PGE2) synthesis by monocytes are induced by activated platelets in a complex, time-dependent mechanism that involves P-selectin/PSGL-1 signaling, transcriptional regulation, and subsequent time-dependent post-transcriptional stabilization of COX-2 mRNA mediated by endogenous IL-1 synthesis and signaling [218]. PGE2 can reciprocally modify platelet responses [117] and has multiple immune activities [118]. The synthesis of a number of other chemokines, cytokines, adhesion receptors, and proteases is induced by the signaling of monocytes by activated platelets and/or by PSGL-1 engagement in combination with soluble agonists [216, 219, 220] (Michetti et al., unpublished studies). These experiments also demonstrated that PSGL-1 engagement is linked to the activation of MAP kinase pathways and mammalian target of rapamycin [218, 220], consistent with earlier studies in PMNs [196, 197] and with more recent observations of PSGL-1 signaling mechanisms [185, 202]. Other laboratories have also reported the induction of inflammatory genes when monocytes interact with activated platelets [221–223]. Furthermore, preliminary studies indicate that platelets accelerate chemokine synthesis by monocytes in an in vitro clot model (Campbell, unpublished studies). Importantly, signaling of monocytes by activated platelets and purified P-selectin is reported to induce tissue factor [223–228] expression by monocytes, although the magnitude of TF activity varies with the experimental conditions [171]. Furthermore, platelet activation in whole blood induced binding of TF, factor Xa, and fibrinogen to the surfaces of monocytes and neutrophils in platelet–leukocyte aggregates, suggesting that these cellular complexes are vehicles for local delivery of coagulation factors [227]. Thus, platelet–monocyte interactions are platforms for intricate interplay in vascular inflammation and hemostasis [201, 202, 229, 230].
Monocytes that have formed heterotypic complexes with activated platelets are reported to transmigrate more efficiently, leaving adherent platelets behind as they move [202, 213]. This may be of major functional importance since monocytes are precursors of tissue macrophages, and soluble and membrane-associated platelet factors influence monocyte survival and differentiation [163, 231, 232]. Platelets may also deliver signals that favor differentiation of monocytes to DC rather than macrophages [233]. Platelet–DC interactions will be considered further below.
Endothelial cells
The interactions of platelets with endothelial cells (Fig. 4a) have been reviewed in detail [27, 202, 234], and the endothelial control of inflammation and coagulation is considered elsewhere in this symposium. Thus, these issues will not be discussed in detail here. Examples of direct or indirect platelet signaling of endothelial activation and other intercellular activities [26, 27, 29, 82, 122, 160, 176–178, 181, 182] illustrate the importance of these interactions, however. Signaling of human endothelial cells by IL-1 from activated platelets (Fig. 4b) is a prototype dialog between the two interacting cells.
Lymphocytes
Although less studied than interactions of platelets with myeloid leukocytes, activated platelets influence adhesion, homing, and activation of T helper, Tcytolytic, natural killer (NK), and B lymphocyte subsets (reviewed in [205]). P-selectin on platelets and PSGL-1 on lymphocytes are critical for these responses, as in platelet interactions with PMNs and monocytes. P-selectin-dependent adhesion of activated platelets mediates platelet–lymphocyte aggregate formation (Fig. 6) and association of T lymphocytes with high endothelial venues of peripheral lymph nodes, facilitating lymphocyte trafficking and induction of immunologic memory [235, 236]. Lymphocyte and eosinophil accumulation in the lung in response to allergen challenge required P-selectin-dependent aggregate formation [237] and platelets promoted T-cell recruitment to the brain in experimental cerebral malaria [165], indicating that platelet signaling may regulate lymphocyte homing and activities in specific tissues in pathologic conditions. Engagement of PSGL-1 on lymphocytes induces activation and/or clustering of β2 integrins, which can then recognize members of the ICAM family on platelets or other cells [205, 238, 239]. There is differential adhesiveness and binding affinity for P-selectin among lymphocyte subsets [205, 240]. Early reports indicated that platelets may also influence the proliferation and trigger the immunoregulatory activities of lymphocytes [176, 241]. Subsequent observations support this conclusion and indicate that there is reciprocal signaling and transcellular metabolism by the interacting cells (reviewed in [205]). As examples, PF4 regulates multiple T-cell responses [165, 205], and the IgE-mediated release of serotonin by platelets initiated T-cell-dependent contact sensitivity in a mouse model [242]. As previously noted, T cells can trigger RANTES release from platelets by a CD40-CD40L-dependent mechanism, amplifying lymphocyte recruitment [175]. There is also evidence for modification of NK cell adhesion and activity by platelet signaling and for modification of antibody responses of B cells (reviewed in [4, 205]) (also see below).
The platelet transcriptome and post-transcriptional repertoire
It has been known for many years that circulating platelets have a complement of stable, functional messenger RNAs and basal protein synthesis capability [243]. As an example, human platelets contain functional mRNA encoding HLA class I that can be translated into metabolically labeled protein [244]. More recent observations demonstrate that the human platelet transcriptome is much more complex than previously appreciated and includes pre-mRNAs and spliceosomal RNAs (U sn RNAs) in addition to mRNA transcripts [8, 14, 16, 109, 110, 147, 149]. The human platelet transcriptome also includes microRNAs, and human platelets have the Dicer/Ago2 microRNA processing pathway; micro-RNA control may regulate the expression of the P2Y12 receptor [17]. The human platelet transcriptome changes in disease, including inflammatory, immune, and thrombotic syndromes, based on a rapidly expanding body of observations [144, 149, 245–250]. It has also recently been discovered that human platelets translate some mature mRNAs and synthesize their corresponding proteins in response to activating signals [8]. Paralleling the complexity of the transcriptome, human platelets have an unexpectedly complex post-transcriptional repertoire for signal-dependent translation of mRNA transcripts, mediating synthesis of protein products that can alter platelet proteome and function after cellular activation [8, 30, 243]. There are both similarities and differences in the transcriptomes of human and mouse platelets and, presumably, patterns of newly expressed proteins after activation [110, 251] (Rowley and Weyrich, manuscript submitted for publication).
One pathway for signal-dependent translation by human platelets involves the activation of intracellular mTOR triggered by thrombin stimulation or engagement of integrin αIIbβ3 [8, 109, 110, 173]. mTOR controls the specialized translation of a subset of mRNAs with key sequence features in a variety of cells, including B cell lymphoma-3 (Bcl-3) in activated platelets [109]. Newly synthesized Bcl-3 protein binds to the intracellular kinase Fyn in human platelets and regulates in vitro clot retraction by activated mouse and human platelets [8, 109, 110] (Fig. 3). It may thus influence the consolidation and remodeling of thrombi in pathologic conditions. Bcl-3 is an index example of a group of proteins that are transcription factors in nucleated cells but have non-traditional functions and signaling activities in anucleate platelets [8, 252]. Alpha toxin from S. aureus triggers the synthesis of Bcl-3 by human platelets [253], a mechanism that may alter the natural history of microvascular thrombosis in staphylococcal sepsis [124].
A second signal-dependent post-transcriptional pathway in activated human platelets regulates the splicing of pre-mRNA transcripts present in circulating, quiescent platelets to spliced, translatable mRNAs in response to outside-in signals delivered via receptors for thrombin or other agonists, followed by the translation of the mature mRNAyielding a biologically active protein product (Fig. 9a). Experiments utilizing primary human platelets and a human megakaryocytic proplatelet model indicated that megakaryocytes transfer a functional spliceosome and key pre-mRNAs to platelets during throm-bopoiesis, establishing a molecular basis for this post-transcriptional mechanism [14, 16, 149]. As an example, the tissue factor pre-mRNA is present in resting human platelets and is spliced to the mature mRNA transcript in platelets activated by thrombin while adherent to fibrinogen, an in vitro thrombosis model [16]. This is accompanied by the synthesis of TF-dependent procoagulant activity that is inhibited by blocking a kinase, Cdc2-like kinase 1, upstream in the splicing pathway [16]. The time-dependent splicing of TF pre-mRNA and generation of TF procoagulant activity are also induced in platelets activated by LPS, staphylococcal alpha toxin, or live bacteria [149] (Figs. 1 and 9b, c). Consistent with this, platelets isolated from patients with sepsis express spliced TF mRNA and TF-dependent procoagulant activity [149], although it is not yet clear if this results from splicing of the TF transcripts by platelets activated by microbial and/or host factors (thrombin, PAF; reviewed in [124]) in the circulation or by reprogramming of megakar-yocytes in the septic milieu [100]. In other studies, TF pre-mRNA splicing and TF protein synthesis were induced by crosslinking of the immunoreceptor FcαRI on platelets, suggesting a new mechanism for IgA-mediated nepropathy and other IgA-induced inflammatory and thrombotic syndromes [102]. In addition, TF pre-mRNA splicing and synthesis of TF activity by platelets from patients with type 2 diabetes were reported to be resistant to inhibition by insulin and to be greater than these responses in platelets from matched control subjects, a feature that may contribute to thrombotic complications in diabetes [254]. Platelets from patients with acute coronary syndromes were reported to have higher levels of TF mRNA and TF protein than those from healthy subjects or patients with stable angina and to have a greater number of TF-positive platelet–monocyte aggregates [228]. A previous analysis suggested that platelets contribute TF in platelet–monocyte and platelet–neutrophil aggregates [227]. Thus, the synthesis of TF by activated human platelets may be a mechanism of thrombosis in disease [8, 46, 255]. Furthermore, experimental studies suggest that TF is also an inflammatory mediator [256] and thus may be another platelet-derived link between hemostasis and immune events.
Fig. 9.
Activated human platelets synthesize inflammatory and hemostatic proteins by mechanisms involving signal-dependent translation and pre-mRNA splicing. An analysis of primary human platelets, cultured megakaryocytic cells, and proplatelet models demonstrate that human platelets have a complex transcriptome and post-transcriptional mechanisms for activation-dependent (“signal-dependent”) translation of mRNA transcripts [8]. One mechanism is illustrated in a: In addition to stable, mature mRNAs, the tran-scriptome of quiescent circulating human platelets includes unspliced pre-mRNAs. In response to thrombin or other activating signals, specific pre-mRNAs are processed by a functional spliceosome yielding the spliced, translatable mRNA. The pre-mRNAs and spliceosomal components are delivered to proplatelets during throm-bopoiesis, and an in situ analysis demonstrates the presence of unspliced pre-mRNAs in proplatelets and unstimulated platelets and in spliced transcripts in activated platelets. Following signal-dependent splicing, the mature, spliced mRNA is translated to biologically active protein. This pathway was first discovered in studies of the mechanism of synthesis of IL-1β by activated human platelets (Figs. 4b and 5) [14] (Fig. 9a is based on an illustration in [278]). Tissue factor is also synthesized by signal-dependent splicing and translation in platelets stimulated by thrombin and fibrinogen ([16]; not shown), bacteria (Fig. 1), or bacterial toxins. b A PCR analysis of the region of the TF pre-mRNA spanning exons 4 and 5 and the intervening intron (box diagrams at right of panel) revealed only the unspliced pre-mRNA in the control condition (time 0, lane 2; lane 1 indicates molecular weight markers). Treatment of the platelets with LPS for 120 min induced the conversion of the pre-mRNA to the spliced transcript that was nearly complete (lane 3). An inhibitor of Cdc2-like kinase 1, which is upstream in the splicing pathway, almost completely blocked the splicing and accumulation of the mature TF mRNA (lane 4). Parallel assays of TF-dependent procoagulant activity (c) demonstrated the shortening of the clotting time by LPS-stimulated platelets (bar 2 compared to bar 1; p<0.05) that was reversed by the splicing pathway inhibitor (bar 3) and an antibody against TF (bar 4). Additional analysis and controls can be found in [149] (panels b and c are from [149] with permission from the publisher). Additional pathways mediate signal-dependent translation of other mRNA transcripts by activated human platelets [8] (see text for details and additional references)
IL-1β pre-mRNA is also present in unactivated human platelets, and IL-1β protein is synthesized by the signal-dependent splicing of the pre-mRNA and translation of the mature transcript [14]. As previously outlined, this occurs in platelets activated with a variety of soluble agonists with inflammatory and prothrombotic activities and is a mechanism for signaling and activation of target endothelial cells [122] (Fig. 4b). Splicing of IL-1β pre-mRNA is potently triggered by LPS and less robustly induced by a synthetic TLR2 agonist [147], indicating that this is a potential mechanism for IL-1β synthesis in septic syndromes [124]. LPS is also reported to be a direct agonist for the splicing-dependent synthesis of COX-2 by isolated platelets [147]. Cross-linking of FcαRI and human IgA also induced IL-1β pre-mRNA splicing and IL-1β synthesis by human platelets in vitro [102], indicating that this may be a mechanism of immunomodulation in syndromes involving platelet Fc receptor engagement.
Platelets in adaptive immune responses
In the continuum of physiologic and pathologic immune responses [4, 5], platelets are best known as innate immune effector cells with activities in acute inflammation and for interactions with myeloid leukocytes and endothelial cells in pathologic inflammatory syndromes such as atherosclerosis and sepsis [20–22, 24, 26, 31]. Recent observations, and the emerging new biology of platelets [8, 9], demonstrate that they also have adaptive immune effector functions and can link innate and adaptive immune responses [4, 23, 30, 31, 35, 60, 257]. Here again the repertoire of activities is much more diverse than previously expected for an anucleate cell that is deceptively simple in structure and, in traditional formulations, function [8, 9].
Platelets interact with DC (Fig. 6b), which are pivotal in pathogen and antigen recognition, orchestration of innate and adaptive responses, immune tolerance, and allergy and autoimmunity [258]. Platelets regulate differentiation, maturation, and activation of DC via both contact-dependent signaling and soluble products [83, 259–266]. In vitro experiments indicate that purified P-selectin can differentially enhance the differentiation of human monocytes to a dendritic-like cell while retarding differentiation to macrophages [233], suggesting that signaling of monocytes by P-selectin on activated platelets may influence this key cell fate pathway. Platelet CD40L is reported to promote DC differentiation and/or maturation [83, 257, 260–262] although this has not been a consistent result under all conditions [263, 266]. Serotonin, a major platelet release factor (Table 1), modulates monocyte differentiation to DC [267]. Platelets are reported to modify activation and proinflammatory cytokine secretion by DC [259, 263–266]. In vitro, human platelets recruit monocyte-derived DC under static and flow conditions by rolling and adhesion mediated by PSGL-1 and integrin αMβ2 on DC and JAM-C on the platelets [265]. Also, in vitro activated platelets enhanced DC-induced lymphocyte proliferation [263, 265]. In vivo activated platelets formed aggregates with monocytes and plasmacytoid DC in the blood of patients with SLE, an interaction thought to facilitate CD40L-triggered secretion of interferon α by plasmacytoid DC [114]. Platelet activation, which appeared to result from circulating immune complexes acting via FcγRIIA, correlated with disease severity in this report. These studies illustrate the potential for complex signaling between activated platelets, DC of dynamically variable phenotypes, and lymphocytes (Fig. 6) and the clinical relevance of these cellular interactions.
Additional newly discovered activities of platelets contribute to the functional anatomy and topography of the adaptive immune system. The separation of lymphatic vessels from blood vessels involves the engagement of CLEC-2 on platelets by a ligand on lymphatic EC, podoplanin, leading to SLP-76- and SYK-dependent platelet aggregation at separation zones between embryonic lymphatic vessels and veins [268–271] (reviewed in [272]). This newly recognized role for platelets in lymphatic development is based on murine models and is important because organization of the lymphatic system, lymphocyte, and other cellular trafficking via lymphatics and immune and inflammatory responses are intimately intertwined [272]. CLEC-2 is also required for stable platelet aggregation and thrombus stabilization in vivo and in vitro based on studies of genetically altered mice [269].
One of the aspects of “new biology” of platelets that has emerged from recent studies is that activated platelets induce functional responses of target cells in extravascular compartments, adding to their traditional signaling activities in thrombi and at intravascular sites [8]. There is evidence that platelets can influence the adaptive immune responses in this fashion. One mechanism is the direct release of soluble cytokines with immunomodulatory activities that transfer to extravascular compartments (Table 1) or the indirect induction of chemokine, cytokine, and eicosanoid synthesis by intercellular signaling (Figs. 5, 6, 7, and 8). A second mechanism involves microvesicle release from activated platelets. Studies in mice demonstrated that the transfer of platelet microvesicles bearing CD40L from wild-type animals to CD40L−/− mice together with adenoviral immunization induced antigen-specific IgG production and augmented germinal center (GC) formation, indicating signaling to the splenic B-cell compartment [273]. These observations were consistent with earlier reports from this group in which the same models were used to show that platelets induce DC maturation, B-cell responses, and specific IgG generation and enhanced T lymphocyte activities via CD40L-dependent mechanisms, and they suggested that platelets contribute to GC responses and the development of humoral adaptive immunity under conditions in which antigen-specific B and T cells are rare and limiting [83, 257, 274]. Together the studies suggest that the release of microvesicles from activated platelets at sites of thrombosis may induce adaptive immune activities at distant sites, a potentially novel function of these subcellular particles [275]. In a second example illustrating the concept of “remote” immune signaling by activated platelets, platelet micro-vesicles were detected in articular fluids from patients with rheumatoid arthritis (RA) and induced the synthesis of inflammatory mediators by cultured synoviocytes, potentially by delivering IL-1 [107]. Parallel murine studies indicated that this may occur in vivo and that the activation of platelets via GPVI or by signals from synoviocytes may trigger microvesicle formation and IL-1 delivery. Binding of platelet microvesicles to leukocytes in synovial fluid samples from RA patients was also detected [107], suggesting a mechanism for additional intercellular signaling that could amplify joint inflammation and injury as well as promote the extravascular transfer of microvesicles [9].
Throughout this review, we have cited many examples of platelet activities and interactions that strongly suggest that they have additional unrecognized functions in adaptive immunity and in the transition from innate to adaptive immune responses. Interactions with antigen-presenting cells—monocytes, macrophages, and DC—and lymphocyte subsets under conditions of tissue injury or infection may be fertile fields in which to search for new platelet activities of this nature [201, 202, 205, 261, 276]. Because of intimate connections between inflammatory and immune responses and hemostasis, as reviewed in this monograph, new mechanisms of coagulation and thrombosis mediated by platelets acting as immune effector cells are also likely to be discovered.
Acknowledgments
We thank our colleagues, co-investigators, and collaborators for many relevant observations and discussions and for contributions to work cited in this review. We also thank Jenny Pierce and Diana Lim for invaluable and creative efforts in the preparation of the manuscript and figures. Work cited in this review was supported by the National Institutes of Health (HL044525, HL066277, HL091754, HL092746, HL048872, HL090870, and K231440921), public health service grants ULI-RRO25764 and CO6-RR11234 from the National Center for Research Resources, a Deutsche Forschlungsgemenschaft (DFG) grant (SCHW 1167/1-2), and Western States Affiliate American Heart Association awards 0625098Y and 09BGIA2250381 and by Conselho Nacional de Pesquisa e Desenvolvimento Technológico (CNPq).
Contributor Information
Adriana Vieira-de-Abreu, Department of Medicine, University of Utah School of Medicine, Salt Lake City, UT 84112, USA; Program in Molecular Medicine, University of Utah School of Medicine, Salt Lake City, UT 84112, USA; Labrotorio de Immunofarmacologia, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil.
Robert A. Campbell, Department of Medicine, University of Utah School of Medicine, Salt Lake City, UT 84112, USA Program in Molecular Medicine, University of Utah School of Medicine, Salt Lake City, UT 84112, USA.
Andrew S. Weyrich, Department of Medicine, University of Utah School of Medicine, Salt Lake City, UT 84112, USA Program in Molecular Medicine, University of Utah School of Medicine, Salt Lake City, UT 84112, USA.
Guy A. Zimmerman, Department of Medicine, University of Utah School of Medicine, Salt Lake City, UT 84112, USA, guy.zimmerman@u2m2.utah.edu
References
- 1.Coller BS. A brief history of ideas about platelets in health and disease. In: Alan D, Michelson MD, editors. Platelets. 2nd edn. London: Elsevier; 2007. pp. xxiii–xlii. [Google Scholar]
- 2.Coller BS, Shattil SJ. The GPIIb/IIIa (integrin alphaIIb-beta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112:3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemost. 2003;1:1897–1905. doi: 10.1046/j.1538-7836.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- 4.Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol. 2004;25:489–495. doi: 10.1016/j.it.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J Immunol. 2007;178:6017–6022. doi: 10.4049/jimmunol.178.10.6017. [DOI] [PubMed] [Google Scholar]
- 7.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman GA, Weyrich AS. Signal-dependent protein synthesis by activated platelets: new pathways to altered phenotype and function. Arterioscler Thromb Vasc Biol. 2008;28:s17–s24. doi: 10.1161/ATVBAHA.107.160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerman GA, Weyrich AS. Immunology. Arsonists in rheumatoid arthritis. Science. 2010;327:528–529. doi: 10.1126/science.1185869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 11.Watkins NA, Gusnanto A, de Bono B, et al. A HaemAtlas: characterizing gene expression in differentiated human blood cells. Blood. 2009;113:e1–e9. doi: 10.1182/blood-2008-06-162958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor MN, Salles II, Cvejic A, et al. Functional genomics in zebrafish permits rapid characterization of novel platelet membrane proteins. Blood. 2009;113:4754–4762. doi: 10.1182/blood-2008-06-162693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weyrich AS, Zimmerman GA. Comparative genomics: fishing nets hemostatic catch. Blood. 2009;113:4479–4480. doi: 10.1182/blood-2009-02-203117. [DOI] [PubMed] [Google Scholar]
- 14.Denis MM, Tolley ND, Bunting M, et al. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel SR, Hartwig JH, Italiano JE., Jr The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115:3348–3354. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwertz H, Tolley ND, Foulks JM, et al. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J Exp Med. 2006;203:2433–2440. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landry P, Plante I, Ouellet DL, et al. Existence of a microRNA pathway in anucleate platelets. Nat Struct Mol Biol. 2009;16:961–966. doi: 10.1038/nsmb.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwertz H, Koster S, Kahr WH, et al. Anucleate platelets generate progeny. Blood. 2010;115:3801–3809. doi: 10.1182/blood-2009-08-239558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quintana-Murci L, Alcais A, Abel L, et al. Immunology in natura: clinical, epidemiological and evolutionary genetics of infectious diseases. Nat Immunol. 2007;8:1165–1171. doi: 10.1038/ni1535. [DOI] [PubMed] [Google Scholar]
- 20.Nachman RL, Polley M. The platelet as an inflammatory cell. In: Weissmann G, et al., editors. Advances in inflammation research. Vol. 1. New York: Raven; 1979. pp. 169–173. [Google Scholar]
- 21.Herd CM, Page CP. Do platelets have a role as inflammatory cells? In: Joseph M, editor. Immunopharmacology of platelets. San Diego: Academic; 1995. pp. 1–20. [Google Scholar]
- 22.Klinger MH. Platelets and inflammation. Anat Embryol (Berl) 1997;196:1–11. doi: 10.1007/s004290050075. [DOI] [PubMed] [Google Scholar]
- 23.Diaz-Gonzalez F, Ginsberg MH. Platelets and rheumatic diseases. In: Harris ED, et al., editors. Kelley’s textbook of rheumatology. Philadelphia: Elsevier Saunders; 2005. pp. 252–259. [Google Scholar]
- 24.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzgerald JR, Foster TJ, Cox D. The interaction of bacterial pathogens with platelets. Nat Rev Microbiol. 2006;4:445–457. doi: 10.1038/nrmicro1425. [DOI] [PubMed] [Google Scholar]
- 26.von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 27.Bergmeier W, Wagner DD. Inflammation. In: Alan D, Michelson MD, editors. Platelets. 2nd edn. London: Elsevier; 2007. pp. 713–726. [Google Scholar]
- 28.Yeaman MR, Bayer AS. Antimicrobial host defense. In: Alan D, Michelson MD, editors. Platelets. 2nd edn. London: Elsevier; 2007. pp. 727–755. [Google Scholar]
- 29.Bozza FA, Shah AM, Weyrich AS, et al. Amicus or adversary: platelets in lung biology, acute injury, and inflammation. Am J Respir Cell Mol Biol. 2009;40:123–134. doi: 10.1165/rcmb.2008-0241TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth SS, McEver RP, Weyrich AS, et al. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7:1759–1766. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 31.Semple JW, Freedman J. Platelets and innate immunity. Cell Mol Life Sci. 2010;67:499–511. doi: 10.1007/s00018-009-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peerschke EI, Yin W, Ghebrehiwet B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol. 2010;47:2170–2175. doi: 10.1016/j.molimm.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi G, Morrell CN. Platelets as initiators and mediators of inflammation at the vessel wall. Thromb Res. 2011;127:387–390. doi: 10.1016/j.thromres.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Yang F, Dunn S, et al. Platelets as immune mediators: their role in host defense responses and sepsis. Thromb Res. 2011;127:184–188. doi: 10.1016/j.thromres.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldwin WM, III, Kuo H-H, Morrell CN. Platelets: versatile modifiers of innate and adaptive immune responses to transplants. Curr Opin Org Trans. 2011;16:41–46. doi: 10.1097/MOT.0b013e3283425365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freedman JE. Molecular regulation of platelet-dependent thrombosis. Circulation. 2005;112:2725–2734. doi: 10.1161/CIRCULATIONAHA.104.494468. [DOI] [PubMed] [Google Scholar]
- 37.Bouchard BA, Tracy PB. Platelets, leukocytes, and coagulation. Curr Opin Hematol. 2001;8:263–269. doi: 10.1097/00062752-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 39.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 40.Rivera J, Lozano ML, Navarro-Nunez L, et al. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica. 2009;94:700–711. doi: 10.3324/haematol.2008.003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smyth SS, Woulfe DS, Weitz JI, et al. G-protein-coupled receptors as signaling targets for antiplatelet therapy. Arterioscler Thromb Vasc Biol. 2009;29:449–457. doi: 10.1161/ATVBAHA.108.176388. [DOI] [PubMed] [Google Scholar]
- 42.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Pujol S, Marker PH, Key NS. Platelet microparticles are heterogeneous and highly dependent on the activation mechanism: studies using a new digital flow cytometer. Cytometry A. 2007;71:38–45. doi: 10.1002/cyto.a.20354. [DOI] [PubMed] [Google Scholar]
- 44.McIntyre TM, Prescott SM, Weyrich AS, et al. Cell-cell interactions: leukocyte-endothelial interactions. Curr Opin Hem-atol. 2003;10:150–158. doi: 10.1097/00062752-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panes O, Matus V, Saez CG, et al. Human platelets synthesize and express functional tissue factor. Blood. 2007;109:5242–5250. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- 47.Massberg S, Grahl L, von Bruehl ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 48.Goerge T, Ho-Tin-Noe B, Carbo C, et al. Inflammation induces hemorrhage in thrombocytopenia. Blood. 2008;111:4958–4964. doi: 10.1182/blood-2007-11-123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Washington AV, Gibot S, Acevedo I, et al. TREM-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. J Clin Invest. 2009;119:1489–1501. doi: 10.1172/JCI36175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tucker EI, Marzec UM, Berny MA, et al. Safety and antithrombotic efficacy of moderate platelet count reduction by thrombopoietin inhibition in primates. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000697. 37ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.London NR, Zhu W, Bozza FA, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000678. 23ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaphorst KL, Chiang E, Jacobs KN, et al. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am J Physiol Lung Cell Mol Physiol. 2003;285:L258–L267. doi: 10.1152/ajplung.00311.2002. [DOI] [PubMed] [Google Scholar]
- 53.Camerer E, Regard JB, Cornelissen I, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 55.Clawson CC. Platelets in bacterial infections. In: Joseph M, editor. Immunopharmacology of platelets. San Diego: Academic; 1995. pp. 83–124. [Google Scholar]
- 56.Pancre V, Auriault C. Platelets in parasitic diseases. In: Joseph M, editor. Immunopharmacology of platelets. San Diego: Academic; 1995. pp. 125–135. [Google Scholar]
- 57.Zucker-Franklin D. Platelets in viral infections. In: Joseph M, editor. Immunopharmacology of platelets. San Diego: Academic; 1995. pp. 137–149. [Google Scholar]
- 58.Hickey MJ, Kubes P. Intravascular immunity: the host- pathogen encounter in blood vessels. Nat Rev Immunol. 2009;9:364–375. doi: 10.1038/nri2532. [DOI] [PubMed] [Google Scholar]
- 59.Mueller KL. Innate immunity. Recognizing the first responders. Introduction. Science. 2010;327:283. doi: 10.1126/science.327.5963.283. [DOI] [PubMed] [Google Scholar]
- 60.Yeaman MR. Platelets in defense against bacterial pathogens. Cell Mol Life Sci. 2010;67:525–544. doi: 10.1007/s00018-009-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med. 2000;343:1703–1714. doi: 10.1056/NEJM200012073432307. [DOI] [PubMed] [Google Scholar]
- 62.Zimmerman GA. LAD syndromes: FERMT3 kindles the signal. Blood. 2009;113:4485–4486. doi: 10.1182/blood-2009-01-198853. [DOI] [PubMed] [Google Scholar]
- 63.Youssefian T, Drouin A, Masse JM, et al. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029. doi: 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 64.Zander DM, Klinger M. The blood platelets contribution to innate host defense—what they have learned from their big brothers. Biotechnol J. 2009;4:914–926. doi: 10.1002/biot.200800362. [DOI] [PubMed] [Google Scholar]
- 65.Sun H, Wang X, Degen JL, et al. Reduced thrombin generation increases host susceptibility to group A streptococcal infection. Blood. 2009;113:1358–1364. doi: 10.1182/blood-2008-07-170506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McRedmond JP, Fitzgerald DJ. A growing set of platelet-activating bacterial proteins. Blood. 2002;99:387–388. doi: 10.1182/blood.v99.1.387. [DOI] [PubMed] [Google Scholar]
- 67.White JG. Why human platelets fail to kill bacteria. Platelets. 2006;17:191–200. doi: 10.1080/09537100500441234. [DOI] [PubMed] [Google Scholar]
- 68.Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 69.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 70.Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wartha F, Henriques-Normark B. ETosis: a novel cell death pathway. Sci Signal. 2008;1:e25. doi: 10.1126/stke.121pe25. [DOI] [PubMed] [Google Scholar]
- 72.Yost CC, Cody MJ, Harris ES, et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009;113:6419–6427. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30:513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 74.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kessenbrock K, Krumbholz M, Schonermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hakkim A, Furnrohr BG, Amann K, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22:913–922. doi: 10.1089/10799900260286623. [DOI] [PubMed] [Google Scholar]
- 78.Dominguez M, Torano A. Leishmania immune adherence reaction in vertebrates. Parasite Immunol. 2001;23:259–265. doi: 10.1046/j.1365-3024.2001.00380.x. [DOI] [PubMed] [Google Scholar]
- 79.Yong EC, Chi EY, Fritsche TR, et al. Human platelet-mediated cytotoxicity against Toxoplasma gondii: role of thromboxane. J Exp Med. 1991;173:65–78. doi: 10.1084/jem.173.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McMorran BJ, Marshall VM, de Graaf C, et al. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 2009;323:797–800. doi: 10.1126/science.1166296. [DOI] [PubMed] [Google Scholar]
- 81.Peyron F, Polack B, Lamotte D, et al. Plasmodium falciparum growth inhibition by human platelets in vitro. Parasitology. 1989;99(Pt 3):317–322. doi: 10.1017/s0031182000059011. [DOI] [PubMed] [Google Scholar]
- 82.Cox D, McConkey S. The role of platelets in the pathogenesis of cerebral malaria. Cell Mol Life Sci. 2010;67:557–568. doi: 10.1007/s00018-009-0211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elzey BD, Tian J, Jensen RJ, et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 84.Iannacone M, Sitia G, Isogawa M, et al. Platelets prevent IFN-alpha/beta-induced lethal hemorrhage promoting CTL-dependent clearance of lymphocytic choriomeningitis virus. Proc Natl Acad Sci U S A. 2008;105:629–634. doi: 10.1073/pnas.0711200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iannacone M, Sitia G, Isogawa M, et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11:1167–1169. doi: 10.1038/nm1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lang PA, Contaldo C, Georgiev P, et al. Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med. 2008;14:756–761. doi: 10.1038/nm1780. [DOI] [PubMed] [Google Scholar]
- 87.Lisman T, Porte RJ. The role of platelets in liver inflammation and regeneration. Semin Thromb Hemost. 2010;36:170–174. doi: 10.1055/s-0030-1251501. [DOI] [PubMed] [Google Scholar]
- 88.Boukour S, Masse JM, Benit L, et al. Lentivirus degradation and DC-SIGN expression by human platelets and megakaryocytes. J Thromb Haemost. 2006;4:426–435. doi: 10.1111/j.1538-7836.2006.01749.x. [DOI] [PubMed] [Google Scholar]
- 89.Chaipan C, Soilleux EJ, Simpson P, et al. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80:8951–8960. doi: 10.1128/JVI.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Torre D, Pugliese A. Platelets and HIV-1 infection: old and new aspects. Curr HIV Res. 2008;6:411–418. doi: 10.2174/157016208785861140. [DOI] [PubMed] [Google Scholar]
- 91.Ghosh K, Gangodkar S, Jain P, et al. Imaging the interaction between dengue 2 virus and human blood platelets using atomic force and electron microscopy. J Electron Microsc (Tokyo) 2008;57:113–118. doi: 10.1093/jmicro/dfn007. [DOI] [PubMed] [Google Scholar]
- 92.Mourao MP, Lacerda MV, Macedo VO, et al. Thrombocytopenia in patients with dengue virus infection in the Brazilian Amazon. Platelets. 2007;18:605–612. doi: 10.1080/09537100701426604. [DOI] [PubMed] [Google Scholar]
- 93.Gavrilovskaya IN, Brown EJ, Ginsberg MH, et al. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta3 integrins. J Virol. 1999;73:3951–3959. doi: 10.1128/jvi.73.5.3951-3959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hamaia S, Li C, Allain JP. The dynamics of hepatitis C virus binding to platelets and 2 mononuclear cell lines. Blood. 2001;98:2293–2300. doi: 10.1182/blood.v98.8.2293. [DOI] [PubMed] [Google Scholar]
- 95.Zahn A, Jennings N, Ouwehand WH, et al. Hepatitis C virus interacts with human platelet glycoprotein VI. J Gen Virol. 2006;87:2243–2251. doi: 10.1099/vir.0.81826-0. [DOI] [PubMed] [Google Scholar]
- 96.de Almeida AJ, Campos-de-Magalhaes M, Brandao-Mello CE, et al. Detection of hepatitis C virus in platelets: evaluating its relationship to viral and host factors. Hepatogastroenterology. 2007;54:964–968. [PubMed] [Google Scholar]
- 97.Pugliese A, Gennero L, Cutufia M, et al. HCV infective virions can be carried by human platelets. Cell Biochem Funct. 2004;22:353–358. doi: 10.1002/cbf.1113. [DOI] [PubMed] [Google Scholar]
- 98.Tareda H, Baldini M, Ebbe S, et al. Interaction of influenza viruses with blood platelets. Blood. 1966;28:213–227. [PubMed] [Google Scholar]
- 99.Rocca B, Secchiero P, Ciabattoni G, et al. Cyclooxygenase-2 expression is induced during human megakaryopoiesis and characterizes newly formed platelets. Proc Natl Acad Sci U S A. 2002;99:7634–7639. doi: 10.1073/pnas.112202999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Freishtat RJ, Natale J, Benton AS, et al. Sepsis alters the megakaryocyte-platelet transcriptional axis resulting in granzyme B-mediated lymphotoxicity. Am J Respir Crit Care Med. 2009;179:467–473. doi: 10.1164/rccm.200807-1085OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kasirer-Friede A, Kahn ML, Shattil SJ. Platelet integrins and immunoreceptors. Immunol Rev. 2007;218:247–264. doi: 10.1111/j.1600-065X.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 102.Qian K, Xie F, Gibson AW, et al. Functional expression of IgA receptor FcalphaRI on human platelets. J Leukoc Biol. 2008;84:1492–1500. doi: 10.1189/jlb.0508327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Falet H, Pollitt AY, Begonja AJ, et al. A novel interaction between FlnA and Syk regulates platelet ITAM-mediated receptor signaling and function. J Exp Med. 2010;207:1967–1979. doi: 10.1084/jem.20100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tomer A. Human marrow megakaryocyte differentiation: multiparameter correlative analysis identifies von Willebrand factor as a sensitive and distinctive marker for early (2N and 4N) megakaryocytes. Blood. 2004;104:2722–2727. doi: 10.1182/blood-2004-02-0769. [DOI] [PubMed] [Google Scholar]
- 105.Calverley DC, Hacker MR, Loda KA, et al. Increased platelet Fc receptor expression as a potential contributing cause of platelet hypersensitivity to collagen in diabetes mellitus. Br J Haematol. 2003;121:139–142. doi: 10.1046/j.1365-2141.2003.04233.x. [DOI] [PubMed] [Google Scholar]
- 106.Moroi M, Jung SM. Platelet glycoprotein VI: its structure and function. Thromb Res. 2004;114:221–233. doi: 10.1016/j.thromres.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 107.Boilard E, Nigrovic PA, Larabee K, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 109.Weyrich AS, Dixon DA, Pabla R, et al. Signal-dependent translation of a regulatory protein, Bcl-3, in activated human platelets. Proc Natl Acad Sci U S A. 1998;95:5556–5561. doi: 10.1073/pnas.95.10.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weyrich AS, Denis MM, Schwertz H, et al. mTOR-dependent synthesis of Bcl-3 controls the retraction of fibrin clots by activated human platelets. Blood. 2007;109:1975–1983. doi: 10.1182/blood-2006-08-042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thomson AW, Turnquist HR, Raimondi G. Immunoregu-latory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Michelson AD. P2Y12 antagonism: promises and challenges. Arterioscler Thromb Vasc Biol. 2008;28:s33–s38. doi: 10.1161/ATVBAHA.107.160689. [DOI] [PubMed] [Google Scholar]
- 113.Evangelista V, Manarini S, Dell’Elba G, et al. Clopidogrel inhibits platelet-leukocyte adhesion and platelet-dependent leukocyte activation. Thromb Haemost. 2005;94:568–577. [PubMed] [Google Scholar]
- 114.Duffau P, Seneschal J, Nicco C, et al. Platelet CD154 potentiates interferon-alpha secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3001001. 47ra63. [DOI] [PubMed] [Google Scholar]
- 115.Graff J, Harder S, Wahl O, et al. Anti-inflammatory effects of clopidogrel intake in renal transplant patients: effects on platelet-leukocyte interactions, platelet CD40 ligand expression, and proinflammatory biomarkers. Clin Pharmacol Ther. 2005;78:468–476. doi: 10.1016/j.clpt.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 116.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108:25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Petrucci G, De Cristofaro R, Rutella S, et al. Prostaglandin E2 differentially modulates human platelet function through the prostanoid EP2 and EP3 receptors. J Pharmacol Exp Ther. 2011;336:391–402. doi: 10.1124/jpet.110.174821. [DOI] [PubMed] [Google Scholar]
- 118.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Muhlestein JB. Effect of antiplatelet therapy on inflammatory markers in atherothrombotic patients. Thromb Haemost. 2010;103:71–82. doi: 10.1160/TH09-03-0177. [DOI] [PubMed] [Google Scholar]
- 120.Prescott SM, Zimmerman GA, Stafforini DM, et al. Platelet-activating factor and related lipid mediators. Annu Rev Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 121.Groscurth P, Huracek J, Filgueira L, et al. Effects of platelet activating factor (PAF) on human citrated whole blood. Eur J Haematol. 1988;41:37–416. doi: 10.1111/j.1600-0609.1988.tb00866.x. [DOI] [PubMed] [Google Scholar]
- 122.Lindemann S, Tolley ND, Dixon DA, et al. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Keating FK, Schneider DJ. The influence of platelet activating factor on the effects of platelet agonists and antiplatelet agents in vitro. J Thromb Thrombolysis. 2009;28:38–45. doi: 10.1007/s11239-008-0239-5. [DOI] [PubMed] [Google Scholar]
- 124.Harris ES, Rondina MT, Schwertz H, et al. Pathogenesis of sepsis and sepsis-induced acute lung injury. In: Choi AMK, editor. Acute respiratory distress syndrome. 2nd edn. Informa, Zug; 2010. pp. 369–419. [Google Scholar]
- 125.Coyle AJ, Vargaftig BB. Animal models for investigating the allergic and inflammatory properties of platelets. In: Joseph M, editor. Immunopharmacology of platelets. San Diego: Academic; 1995. pp. 21–30. [Google Scholar]
- 126.Yost CC, Weyrich AS, Zimmerman GA. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochimie. 2010;92:692–697. doi: 10.1016/j.biochi.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brass LF, Stalker TJ, Zhu L, et al. Signal transduction during platelet plug formation. In: Michelson AD, editor. Platelets. 2nd edn. London: Elsevier; 2007. pp. 319–346. [Google Scholar]
- 128.Boehlen F, Clemetson KJ. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice? Transfus Med. 2001;11:403–417. doi: 10.1046/j.1365-3148.2001.00340.x. [DOI] [PubMed] [Google Scholar]
- 129.Brass LF, Zhu L, Stalker TJ. Novel therapeutic targets at the platelet vascular interface. Arterioscler Thromb Vasc Biol. 2008;28:s43–s50. doi: 10.1161/ATVBAHA.107.161026. [DOI] [PubMed] [Google Scholar]
- 130.Clemetson JK, Clemetson JM. Platelet receptors. In: Michelson AD, editor. Platelets. 2nd edn. London: Elsevier; 2007. pp. 117–143. [Google Scholar]
- 131.Garraud O, Cognasse F. Platelet Toll-like receptor expression: the link between “danger” ligands and inflammation. Inflamm Allergy Drug Targets. 2010;9:322–333. doi: 10.2174/187152810793937991. [DOI] [PubMed] [Google Scholar]
- 132.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 133.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Des Prez RM, Horowitz HI, Hook EW. Effects of bacterial endotoxin on rabbit platelets. I. Platelet aggregation and release of platelet factors in vitro. J Exp Med. 1961;114:857–874. doi: 10.1084/jem.114.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ginsberg MH, Henson PM. Enhancement of platelet response to immune complexes and IgG aggregates by lipid A-rich bacterial lipopolysaccharides. J Exp Med. 1978;147:207–217. doi: 10.1084/jem.147.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Romano M, Hawiger J. Interaction of endotoxic lipid A and lipid X with purified human platelet protein kinase C. J Biol Chem. 1990;265:1765–1770. [PubMed] [Google Scholar]
- 138.Berg M, Offermanns S, Seifert R, et al. Synthetic lipopeptide Pam3CysSer(Lys)4 is an effective activator of human platelets. Am J Physiol. 1994;266:C1684–C1691. doi: 10.1152/ajpcell.1994.266.6.C1684. [DOI] [PubMed] [Google Scholar]
- 139.Zhao L, Ohtaki Y, Yamaguchi K, et al. LPS-induced platelet response and rapid shock in mice: contribution of O-antigen region of LPS and involvement of the lectin pathway of the complement system. Blood. 2002;100:3233–3239. doi: 10.1182/blood-2002-01-0252. [DOI] [PubMed] [Google Scholar]
- 140.Shiraki R, Inoue N, Kawasaki S, et al. Expression of Tolllike receptors on human platelets. Thromb Res. 2004;113:379–385. doi: 10.1016/j.thromres.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 141.Cognasse F, Hamzeh H, Chavarin P, et al. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol. 2005;83:196–198. doi: 10.1111/j.1440-1711.2005.01314.x. [DOI] [PubMed] [Google Scholar]
- 142.Andonegui G, Kerfoot SM, McNagny K, et al. Platelets express functional Toll-like receptor-4. Blood. 2005;106:2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 143.Aslam R, Speck ER, Kim M, et al. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood. 2006;107:637–641. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 144.Freedman JE, Larson MG, Tanriverdi K, et al. Relation of platelet and leukocyte inflammatory transcripts to body mass index in the Framingham Heart Study. Circulation. 2010;122:119–129. doi: 10.1161/CIRCULATIONAHA.109.928192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Coban C, Igari Y, Yagi M, et al. Immunogenicity of whole-parasite vaccines against Plasmodium falciparum involves malarial hemozoin and host TLR9. Cell Host Microbe. 2010;7:50–61. doi: 10.1016/j.chom.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 146.Rumbaut RE, Bellera RV, Randhawa JK, et al. Endotoxin enhances microvascular thrombosis in mouse cremaster venules via a TLR4-dependent, neutrophil-independent mechanism. Am J Physiol Heart Circ Physiol. 2006;290:H1671–H1679. doi: 10.1152/ajpheart.00305.2005. [DOI] [PubMed] [Google Scholar]
- 147.Shashkin PN, Brown GT, Ghosh A, et al. Lipopolysaccharide is a direct agonist for platelet RNA splicing. J Immunol. 2008;181:3495–3502. doi: 10.4049/jimmunol.181.5.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ward JR, Bingle L, Judge HM, et al. Agonists of toll-like receptor (TLR)2 and TLR4 are unable to modulate platelet activation by adenosine diphosphate and platelet activating factor. Thromb Haemost. 2005;94:831–838. [PubMed] [Google Scholar]
- 149.Rondina MT, Schwertz H, Harris ES, et al. The septic milieu triggers expression of spliced tissue factor mRNA in human platelets. J Thromb Haemost. 2011;9:748–758. doi: 10.1111/j.1538-7836.2011.04208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stahl AL, Svensson M, Morgelin M, et al. Lipopolysaccharide from enterohemorrhagic Escherichia coli binds to platelets through TLR4 and CD62 and is detected on circulating platelets in patients with hemolytic uremic syndrome. Blood. 2006;108:167–176. doi: 10.1182/blood-2005-08-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cognasse F, Hamzeh-Cognasse H, Lafarge S, et al. Tolllike receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol. 2008;141:84–91. doi: 10.1111/j.1365-2141.2008.06999.x. [DOI] [PubMed] [Google Scholar]
- 152.Zhang G, Han J, Welch EJ, et al. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol. 2009;182:7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 154.Kuhns DB, Priel DA, Gallin JI. Induction of human monocyte interleukin (IL)-8 by fibrinogen through the toll-like receptor pathway. Inflammation. 2007;30:178–188. doi: 10.1007/s10753-007-9035-1. [DOI] [PubMed] [Google Scholar]
- 155.Blair P, Rex S, Vitseva O, et al. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res. 2009;104:346–354. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rex S, Beaulieu LM, Perlman DH, et al. Immune versus thrombotic stimulation of platelets differentially regulates signalling pathways, intracellular protein-protein interactions, and alpha-granule release. Thromb Haemost. 2009;102:97–110. doi: 10.1160/TH08-08-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Blumberg N, Spinelli SL, Francis CW, et al. The platelet as an immune cell--CD40 ligand and transfusion immunomodulation. Immunol Res. 2009;45:251–260. doi: 10.1007/s12026-009-8106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Phipps RP, Kaufman J, Blumberg N. Platelet derived CD154 (CD40 ligand) and febrile responses to transfusion. Lancet. 2001;357:2023–2024. doi: 10.1016/s0140-6736(00)05108-4. [DOI] [PubMed] [Google Scholar]
- 159.Blumberg N, Gettings KF, Turner C, et al. An association of soluble CD40 ligand (CD154) with adverse reactions to platelet transfusions. Transfusion. 2006;46:1813–1821. doi: 10.1111/j.1537-2995.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 160.Damas JK, Jensenius M, Ueland T, et al. Increased levels of soluble CD40L in African tick bite fever: possible involvement of TLRs in the pathogenic interaction between Rickettsia africae, endothelial cells, and platelets. J Immunol. 2006;177:2699–2706. doi: 10.4049/jimmunol.177.4.2699. [DOI] [PubMed] [Google Scholar]
- 161.Amelot AA, Tagzirt M, Ducouret G, et al. Platelet factor 4 (CXCL4) seals blood clots by altering the structure of fibrin. J Biol Chem. 2007;282:710–720. doi: 10.1074/jbc.M606650200. [DOI] [PubMed] [Google Scholar]
- 162.Deuel TF, Senior RM, Chang D, et al. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci U S A. 1981;78:4584–4587. doi: 10.1073/pnas.78.7.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Scheuerer B, Ernst M, Durrbaum-Landmann I, et al. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood. 2000;95:1158–1166. [PubMed] [Google Scholar]
- 164.Gleissner CA, Shaked I, Little KM, et al. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J Immunol. 2010;184:4810–4818. doi: 10.4049/jimmunol.0901368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Srivastava K, Cockburn IA, Swaim A, et al. Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe. 2008;4:179–187. doi: 10.1016/j.chom.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Srivastava K, Field DJ, Aggrey A, et al. Platelet factor 4 regulation of monocyte KLF4 in experimental cerebral malaria. PLoS One. 2010;5:e10413. doi: 10.1371/journal.pone.0010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Essien EM, Ebhota MI. Platelet secretory activities in acute malaria (Plasmodium falciparum) infection. Acta Haematol. 1983;70:183–188. doi: 10.1159/000206720. [DOI] [PubMed] [Google Scholar]
- 168.Greinacher A. Heparin-induced thrombocytopenia. J Thromb Haemost. 2009;7(Suppl 1):9–12. doi: 10.1111/j.1538-7836.2009.03385.x. [DOI] [PubMed] [Google Scholar]
- 169.Levy JA. The unexpected pleiotropic activities of RANTES. J Immunol. 2009;182:3945–3946. doi: 10.4049/jimmunol.0990015. [DOI] [PubMed] [Google Scholar]
- 170.Kameyoshi Y, Dorschner A, Mallet AI, et al. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992;176:587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Weyrich AS, Elstad MR, McEver RP, et al. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Klinger MH, Wilhelm D, Bubel S, et al. Immunocytochemical localization of the chemokines RANTES and MIP-1 alpha within human platelets and their release during storage. Int Arch Allergy Immunol. 1995;107:541–546. doi: 10.1159/000237097. [DOI] [PubMed] [Google Scholar]
- 173.Pabla R, Weyrich AS, Dixon DA, et al. Integrin-dependent control of translation: engagement of integrin alphaIIbbeta3 regulates synthesis of proteins in activated human platelets. J Cell Biol. 1999;144:175–184. doi: 10.1083/jcb.144.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mause SF, von Hundelshausen P, Zernecke A, et al. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. 2005;25:1512–1518. doi: 10.1161/01.ATV.0000170133.43608.37. [DOI] [PubMed] [Google Scholar]
- 175.Danese S, de la Motte C, Reyes BM, et al. Cutting edge: T cells trigger CD40-dependent platelet activation and granular RANTES release: a novel pathway for immune response amplification. J Immunol. 2004;172:2011–2015. doi: 10.4049/jimmunol.172.4.2011. [DOI] [PubMed] [Google Scholar]
- 176.Hawrylowicz CM, Santoro SA, Platt FM, et al. Activated platelets express IL-1 activity. J Immunol. 1989;143:4015–4018. [PubMed] [Google Scholar]
- 177.Hawrylowicz CM, Howells GL, Feldmann M. Platelet-derived interleukin 1 induces human endothelial adhesion molecule expression and cytokine production. J Exp Med. 1991;174:785–790. doi: 10.1084/jem.174.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kaplanski G, Porat R, Aiura K, et al. Activated platelets induce endothelial secretion of interleukin-8 in vitro via an interleukin-1-mediated event. Blood. 1993;81:2492–2495. [PubMed] [Google Scholar]
- 179.Loppnow H, Bil R, Hirt S, et al. Platelet-derived interleukin-1 induces cytokine production, but not proliferation of human vascular smooth muscle cells. Blood. 1998;91:134–141. [PubMed] [Google Scholar]
- 180.Braddock M, Quinn A. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat Rev Drug Discov. 2004;3:330–339. doi: 10.1038/nrd1342. [DOI] [PubMed] [Google Scholar]
- 181.Gawaz M, Brand K, Dickfeld T, et al. Platelets induce alterations of chemotactic and adhesive properties of endothelial cells mediated through an interleukin-1-dependent mechanism. Implications for atherogenesis. Atherosclerosis. 2000;148:75–85. doi: 10.1016/s0021-9150(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 182.Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 183.Weber C, Springer TA. Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to alphaIIbbeta3 and stimulated by platelet-activating factor. J Clin Invest. 1997;100:2085–2093. doi: 10.1172/JCI119742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ostrovsky L, King AJ, Bond S, et al. A juxtacrine mechanism for neutrophil adhesion on platelets involves platelet-activating factor and a selectin-dependent activation process. Blood. 1998;91:3028–3036. [PubMed] [Google Scholar]
- 185.Zarbock A, Muller H, Kuwano Y, et al. PSGL-1-dependent myeloid leukocyte activation. J Leukoc Biol. 2009;86:1119–1124. doi: 10.1189/jlb.0209117. [DOI] [PubMed] [Google Scholar]
- 186.Kornerup KN, Salmon GP, Pitchford SC, et al. Circulating platelet-neutrophil complexes are important for subsequent neutrophil activation and migration. J Appl Physiol. 2010;109:758–767. doi: 10.1152/japplphysiol.01086.2009. [DOI] [PubMed] [Google Scholar]
- 187.Flick MJ, Du X, Witte DP, et al. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113:1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hara T, Shimizu K, Ogawa F, et al. Platelets control leukocyte recruitment in a murine model of cutaneous arthus reaction. Am J Pathol. 2010;176:259–269. doi: 10.2353/ajpath.2010.081117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Keating FK, Fung MK, Schneider DJ. Induction of platelet white blood cell (WBC) aggregate formation by platelets and WBCs in red blood cell units. Transfusion. 2008;48:1099–1105. doi: 10.1111/j.1537-2995.2008.01692.x. [DOI] [PubMed] [Google Scholar]
- 190.Evangelista V, Pamuklar Z, Piccoli A, et al. Src family kinases mediate neutrophil adhesion to adherent platelets. Blood. 2007;109:2461–2469. doi: 10.1182/blood-2006-06-029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lorant DE, Patel KD, McIntyre TM, et al. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J Cell Biol. 1991;115:223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Simon DI, Chen Z, Xu H, et al. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Exp Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Santoso S, Sachs UJ, Kroll H, et al. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counter-receptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196:679–691. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ehlers R, Ustinov V, Chen Z, et al. Targeting platelet-leukocyte interactions: identification of the integrin Mac-1 binding site for the platelet counter receptor glycoprotein Ibalpha. J Exp Med. 2003;198:1077–1088. doi: 10.1084/jem.20022181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li N, Hu H, Lindqvist M, et al. Platelet-leukocyte cross talk in whole blood. Arterioscler Thromb Vasc Biol. 2000;20:2702–2708. doi: 10.1161/01.atv.20.12.2702. [DOI] [PubMed] [Google Scholar]
- 196.Hidari KI, Weyrich AS, Zimmerman GA, et al. Engagement of P-selectin glycoprotein ligand-1 enhances tyrosine phosphorylation and activates mitogen-activated protein kinases in human neutrophils. J Biol Chem. 1997;272:28750–28756. doi: 10.1074/jbc.272.45.28750. [DOI] [PubMed] [Google Scholar]
- 197.Lindemann SW, Yost CC, Denis MM, et al. Neutrophils alter the inflammatory milieu by signal-dependent translation of constitutive messenger RNAs. Proc Natl Acad Sci U S A. 2004;101:7076–7081. doi: 10.1073/pnas.0401901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Goel MS, Diamond SL. Neutrophil enhancement of fibrin deposition under flow through platelet-dependent and -independent mechanisms. Arterioscler Thromb Vasc Biol. 2001;21:2093–2098. doi: 10.1161/hq1201.100255. [DOI] [PubMed] [Google Scholar]
- 199.McCarty OJ, Tien N, Bochner BS, et al. Exogenous eosinophil activation converts PSGL-1-dependent binding to CD18-dependent stable adhesion to platelets in shear flow. Am J Physiol Cell Physiol. 2003;284:C1223–C1234. doi: 10.1152/ajpcell.00403.2002. [DOI] [PubMed] [Google Scholar]
- 200.de Bruijne-Admiraal LG, Modderman PW, Von dem Borne AE, et al. P-selectin mediates Ca(2+)-dependent adhesion of activated platelets to many different types of leukocytes: detection by flow cytometry. Blood. 1992;80:134–142. [PubMed] [Google Scholar]
- 201.Michetti N, Weyrich AS, Zimmerman GA. Platelet- leukocyte interactions in inflammation and thrombosis. US Hematology. 2009;2:24–27. [Google Scholar]
- 202.van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endo-thelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. 2009;85:195–204. doi: 10.1189/jlb.0708400. [DOI] [PubMed] [Google Scholar]
- 203.Kirchhofer D, Riederer MA, Baumgartner HR. Specific accumulation of circulating monocytes and polymorphonuclear leukocytes on platelet thrombi in a vascular injury model. Blood. 1997;89:1270–1278. [PubMed] [Google Scholar]
- 204.Rinder HM, Bonan JL, Rinder CS, et al. Dynamics of leukocyte-platelet adhesion in whole blood. Blood. 1991;78:1730–1737. [PubMed] [Google Scholar]
- 205.Li N. Platelet-lymphocyte cross-talk. J Leukoc Biol. 2008;83:1069–1078. doi: 10.1189/jlb.0907615. [DOI] [PubMed] [Google Scholar]
- 206.Rinder HM, Tracey JL, Rinder CS, et al. Neutrophil but not monocyte activation inhibits P-selectin-mediated platelet adhesion. Thromb Haemost. 1994;72:750–756. [PubMed] [Google Scholar]
- 207.Lorant DE, McEver RP, McIntyre TM, et al. Activation of polymorphonuclear leukocytes reduces their adhesion to P-selectin and causes redistribution of ligands for P-selectin on their surfaces. J Clin Invest. 1995;96:171–182. doi: 10.1172/JCI118018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Michelson AD, Barnard MR, Krueger LA, et al. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104:1533–1537. doi: 10.1161/hc3801.095588. [DOI] [PubMed] [Google Scholar]
- 209.Larsen E, Celi A, Gilbert GE, et al. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989;59:305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- 210.Weyrich AS, McIntyre TM, McEver RP, et al. Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion. Signal integration and NF-kappa B translocation. J Clin Invest. 1995;95:2297–2303. doi: 10.1172/JCI117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Silverstein RL, Asch AS, Nachman RL. Glycoprotein IV mediates thrombospondin-dependent platelet-monocyte and platelet-U937 cell adhesion. J Clin Invest. 1989;84:546–552. doi: 10.1172/JCI114197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gawaz MP, Loftus JC, Bajt ML, et al. Ligand bridging mediates integrin alpha IIb beta 3 (platelet GPIIB-IIIA) dependent homotypic and heterotypic cell-cell interactions. J Clin Invest. 1991;88:1128–1134. doi: 10.1172/JCI115412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.da Costa Martins PA, van Gils JM, Mol A, et al. Platelet binding to monocytes increases the adhesive properties of monocytes by up-regulating the expression and functionality of beta1 and beta2 integrins. J Leukoc Biol. 2006;79:499–507. doi: 10.1189/jlb.0605318. [DOI] [PubMed] [Google Scholar]
- 214.Fernandes LS, Conde ID, Smith CW, et al. Platelet- monocyte complex formation: effect of blocking PSGL-1 alone, and in combination with alphaIIbbeta3 and alphaMbeta2, in coronary stenting. Thromb Res. 2003;111:171–177. doi: 10.1016/j.thromres.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 215.Ahn KC, Jun AJ, Pawar P, et al. Preferential binding of platelets to monocytes over neutrophils under flow. Biochem Biophys Res Commun. 2005;329:345–355. doi: 10.1016/j.bbrc.2005.01.146. [DOI] [PubMed] [Google Scholar]
- 216.Galt SW, Lindemann S, Medd D, et al. Differential regulation of matrix metalloproteinase-9 by monocytes adherent to collagen and platelets. Circ Res. 2001;89:509–516. doi: 10.1161/hh1801.096339. [DOI] [PubMed] [Google Scholar]
- 217.Brittain JE, Knoll CM, Ataga KI, et al. Fibronectin bridges monocytes and reticulocytes via integrin alpha4beta1. Br J Haematol. 2008;141:872–881. doi: 10.1111/j.1365-2141.2008.07056.x. [DOI] [PubMed] [Google Scholar]
- 218.Dixon DA, Tolley ND, Bemis-Standoli K, et al. Expression of COX-2 in platelet–monocyte interactions occurs via combinatorial regulation involving adhesion and cytokine signaling. J Clin Invest. 2006;116:2727–2738. doi: 10.1172/JCI27209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Weyrich AS, Denis MM, Kuhlmann-Eyre JR, et al. Dipyridamole selectively inhibits inflammatory gene expression in platelet–monocyte aggregates. Circulation. 2005;111:633–642. doi: 10.1161/01.CIR.0000154607.90506.45. [DOI] [PubMed] [Google Scholar]
- 220.Mahoney TS, Weyrich AS, Dixon DA, et al. Cell adhesion regulates gene expression at translational checkpoints in human myeloid leukocytes. Proc Natl Acad Sci U S A. 2001;98:10284–10289. doi: 10.1073/pnas.181201398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Neumann FJ, Marx N, Gawaz M, et al. Induction of cytokine expression in leukocytes by binding of thrombin-stimulated platelets. Circulation. 1997;95:2387–2394. doi: 10.1161/01.cir.95.10.2387. [DOI] [PubMed] [Google Scholar]
- 222.Eligini S, Barbieri SS, Arenaz I, et al. Paracrine up-regulation of monocyte cyclooxygenase-2 by platelets: role of transforming growth factor-beta1. Cardiovasc Res. 2007;74:270–278. doi: 10.1016/j.cardiores.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 223.Christersson C, Johnell M, Siegbahn A. Tissue factor and IL8 production by P-selectin-dependent platelet-monocyte aggregates in whole blood involves phosphorylation of Lyn and is inhibited by IL10. J Thromb Haemost. 2008;6:986–994. doi: 10.1111/j.1538-7836.2008.02956.x. [DOI] [PubMed] [Google Scholar]
- 224.Celi A, Pellegrini G, Lorenzet R, et al. P-selectin induces the expression of tissue factor on monocytes. Proc Natl Acad Sci U S A. 1994;91:8767–8771. doi: 10.1073/pnas.91.19.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lindmark E, Tenno T, Siegbahn A. Role of platelet P-selectin and CD40 ligand in the induction of monocytic tissue factor expression. Arterioscler Thromb Vasc Biol. 2000;20:2322–2328. doi: 10.1161/01.atv.20.10.2322. [DOI] [PubMed] [Google Scholar]
- 226.Steiner S, Seidinger D, Huber K, et al. Effect of glycoprotein IIb/IIIa antagonist abciximab on monocyte-platelet aggregates and tissue factor expression. Arterioscler Thromb Vasc Biol. 2003;23:1697–1702. doi: 10.1161/01.ATV.0000087035.46547.89. [DOI] [PubMed] [Google Scholar]
- 227.Barnard MR, Linden MD, Frelinger AL, 3rd, et al. Effects of platelet binding on whole blood flow cytometry assays of monocyte and neutrophil procoagulant activity. J Thromb Hae-most. 2005;3:2563–2570. doi: 10.1111/j.1538-7836.2005.01603.x. [DOI] [PubMed] [Google Scholar]
- 228.Brambilla M, Camera M, Colnago D, et al. Tissue factor in patients with acute coronary syndromes: expression in platelets, leukocytes, and platelet-leukocyte aggregates. Arterioscler Thromb Vasc Biol. 2008;28:947–953. doi: 10.1161/ATVBAHA.107.161471. [DOI] [PubMed] [Google Scholar]
- 229.Elstad MR, McIntyre TM, Prescott SM, et al. The interaction of leukocytes with platelets in blood coagulation. Curr Opin Hematol. 1995;2:47–54. doi: 10.1097/00062752-199502010-00007. [DOI] [PubMed] [Google Scholar]
- 230.Freedman JE, Loscalzo J. Platelet-monocyte aggregates: bridging thrombosis and inflammation. Circulation. 2002;105:2130–2132. doi: 10.1161/01.cir.0000017140.26466.f5. [DOI] [PubMed] [Google Scholar]
- 231.Ammon C, Kreutz M, Rehli M, et al. Platelets induce monocyte differentiation in serum-free coculture. J Leukoc Biol. 1998;63:469–476. doi: 10.1002/jlb.63.4.469. [DOI] [PubMed] [Google Scholar]
- 232.Lang D, Dohle F, Terstesse M, et al. Down-regulation of monocyte apoptosis by phagocytosis of platelets: involvement of a caspase-9, caspase-3, and heat shock protein 70-dependent pathway. J Immunol. 2002;168:6152–6158. doi: 10.4049/jimmunol.168.12.6152. [DOI] [PubMed] [Google Scholar]
- 233.Li G, Kim YJ, Mantel C, et al. P-selectin enhances generation of CD14 + CD16+ dendritic-like cells and inhibits macrophage maturation from human peripheral blood monocytes. J Immunol. 2003;171:669–677. doi: 10.4049/jimmunol.171.2.669. [DOI] [PubMed] [Google Scholar]
- 234.Chen J, Lopez JA. Interactions of platelets with subendothelium and endothelium. Microcirculation. 2005;12:235–246. doi: 10.1080/10739680590925484. [DOI] [PubMed] [Google Scholar]
- 235.Diacovo TG, Puri KD, Warnock RA, et al. Platelet-mediated lymphocyte delivery to high endothelial venules. Science. 1996;273:252–255. doi: 10.1126/science.273.5272.252. [DOI] [PubMed] [Google Scholar]
- 236.Diacovo TG, Catalina MD, Siegelman MH, et al. Circulating activated platelets reconstitute lymphocyte homing and immunity in L-selectin-deficient mice. J Exp Med. 1998;187:197–204. doi: 10.1084/jem.187.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pitchford SC, Momi S, Giannini S, et al. Platelet P-selectin is required for pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation. Blood. 2005;105:2074–2081. doi: 10.1182/blood-2004-06-2282. [DOI] [PubMed] [Google Scholar]
- 238.Diacovo TG, deFougerolles AR, Bainton DF, et al. A functional integrin ligand on the surface of platelets: intercellular adhesion molecule-2. J Clin Invest. 1994;94:1243–1251. doi: 10.1172/JCI117442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Atarashi K, Hirata T, Matsumoto M, et al. Rolling of Th1 cells via P-selectin glycoprotein ligand-1 stimulates LFA-1-mediated cell binding to ICAM-1. J Immunol. 2005;174:1424–1432. doi: 10.4049/jimmunol.174.3.1424. [DOI] [PubMed] [Google Scholar]
- 240.Li N, Ji Q, Hjemdahl P. Platelet-lymphocyte conjugation differs between lymphocyte subpopulations. J Thromb Haemost. 2006;4:874–881. doi: 10.1111/j.1538-7836.2006.01817.x. [DOI] [PubMed] [Google Scholar]
- 241.Katz IR, Hoffmann MK, Zucker MB, et al. A platelet-derived immunoregulatory serum factor with T cell affinity. J Immunol. 1985;134:3199–3203. [PubMed] [Google Scholar]
- 242.Matsuda H, Ushio H, Geba GP, et al. Human platelets can initiate Tcell-dependent contact sensitivity through local serotonin release mediated by IgE antibodies. J Immunol. 1997;158:2891–2897. [PubMed] [Google Scholar]
- 243.Weyrich AS, Schwertz H, Kraiss LW, et al. Protein synthesis by platelets: historical and new perspectives. J Thromb Haemost. 2009;7:241–246. doi: 10.1111/j.1538-7836.2008.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Santoso S, Kalb R, Kiefel V, et al. The presence of messenger RNA for HLA class I in human platelets and its capability for protein biosynthesis. Br J Haematol. 1993;84:451–456. doi: 10.1111/j.1365-2141.1993.tb03100.x. [DOI] [PubMed] [Google Scholar]
- 245.Wang L, Erling P, Bengtsson AA, et al. Transcriptional down-regulation of the platelet ADP receptor P2Y(12) and clusterin in patients with systemic lupus erythematosus. J Thromb Haemost. 2004;2:1436–1442. doi: 10.1111/j.1538-7836.2004.00854.x. [DOI] [PubMed] [Google Scholar]
- 246.Healy AM, Pickard MD, Pradhan AD, et al. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation. 2006;113:2278–2284. doi: 10.1161/CIRCULATIONAHA.105.607333. [DOI] [PubMed] [Google Scholar]
- 247.Potti A, Bild A, Dressman HK, et al. Gene-expression patterns predict phenotypes of immune-mediated thrombosis. Blood. 2006;107:1391–1396. doi: 10.1182/blood-2005-07-2669. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 248.Raghavachari N, Xu X, Harris A, et al. Amplified expression profiling of platelet transcriptome reveals changes in arginine metabolic pathways in patients with sickle cell disease. Circulation. 2007;115:1551–1562. doi: 10.1161/CIRCULATIONAHA.106.658641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Gnatenko DV, Zhu W, Xu X, et al. Class prediction models of thrombocytosis using genetic biomarkers. Blood. 2010;115:7–14. doi: 10.1182/blood-2009-05-224477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lood C, Amisten S, Gullstrand B, et al. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up-regulation of the type I interferon system is strongly associated with vascular disease. Blood. 2010;116:1951–1957. doi: 10.1182/blood-2010-03-274605. [DOI] [PubMed] [Google Scholar]
- 251.Pawlinski R, Wang JG, Owens AP, 3rd, et al. Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood. 2010;116:806–814. doi: 10.1182/blood-2009-12-259267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Spinelli SL, Maggirwar SB, Blumberg N, et al. Nuclear emancipation: a platelet tour de force. Sci Signal. 2010;3:e37. doi: 10.1126/scisignal.3144pe37. [DOI] [PubMed] [Google Scholar]
- 253.Schubert S, Schwertz H, Weyrich AS, et al. Staphylococcus aureus alpha-toxin triggers the synthesis of B-cell lymphoma 3 by human platelets. Toxins. 2011;3:120–133. doi: 10.3390/toxins3020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gerrits AJ, Koekman CA, van Haeften TW, et al. Platelet tissue factor synthesis in type 2 diabetic patients is resistant to inhibition by insulin. Diabetes. 2010;59:1487–1495. doi: 10.2337/db09-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Mezzano D, Matus V, Saez CG, et al. Tissue factor storage, synthesis and function in normal and activated human platelets. Thromb Res. 2008;122(Suppl 1):S31–S36. doi: 10.1016/S0049-3848(08)70016-1. [DOI] [PubMed] [Google Scholar]
- 256.Anthoni C, Russell J, Wood KC, et al. Tissue factor: a mediator of inflammatory cell recruitment, tissue injury, and thrombus formation in experimental colitis. J Exp Med. 2007;204:1595–1601. doi: 10.1084/jem.20062354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Elzey BD, Sprague DL, Ratliff TL. The emerging role of platelets in adaptive immunity. Cell Immunol. 2005;238:1–9. doi: 10.1016/j.cellimm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 258.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 259.Hilf N, Singh-Jasuja H, Schwarzmaier P, et al. Human platelets express heat shock protein receptors and regulate dendritic cell maturation. Blood. 2002;99:3676–3682. doi: 10.1182/blood.v99.10.3676. [DOI] [PubMed] [Google Scholar]
- 260.Kaneider NC, Kaser A, Tilg H, et al. CD40 ligand-dependent maturation of human monocyte-derived dendritic cells by activated platelets. Int J Immunopathol Pharmacol. 2003;16:225–231. doi: 10.1177/039463200301600307. [DOI] [PubMed] [Google Scholar]
- 261.Czapiga M, Kirk AD, Lekstrom-Himes J. Platelets deliver costimulatory signals to antigen-presenting cells: a potential bridge between injury and immune activation. Exp Hematol. 2004;32:135–139. doi: 10.1016/j.exphem.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 262.Martinson J, Bae J, Klingemann HG, et al. Activated platelets rapidly up-regulate CD40L expression and can effectively mature and activate autologous ex vivo differentiated DC. Cytotherapy. 2004;6:487–497. doi: 10.1080/14653240410005249. [DOI] [PubMed] [Google Scholar]
- 263.Hagihara M, Higuchi A, Tamura N, et al. Platelets, after exposure to a high shear stress, induce IL-10-producing, mature dendritic cells in vitro. J Immunol. 2004;172:5297–5303. doi: 10.4049/jimmunol.172.9.5297. [DOI] [PubMed] [Google Scholar]
- 264.Kissel K, Berber S, Nockher A, et al. Human platelets target dendritic cell differentiation and production of proinflam-matory cytokines. Transfusion. 2006;46:818–827. doi: 10.1111/j.1537-2995.2006.00802.x. [DOI] [PubMed] [Google Scholar]
- 265.Langer HF, Daub K, Braun G, et al. Platelets recruit human dendritic cells via Mac-1/JAM-C interaction and modulate dendritic cell function in vitro. Arterioscler Thromb Vasc Biol. 2007;27:1463–1470. doi: 10.1161/ATVBAHA.107.141515. [DOI] [PubMed] [Google Scholar]
- 266.Hamzeh-Cognasse H, Cognasse F, Palle S, et al. Direct contact of platelets and their released products exert different effects on human dendritic cell maturation. BMC Immunol. 2008;9:54. doi: 10.1186/1471-2172-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Katoh N, Soga F, Nara T, et al. Effect of serotonin on the differentiation of human monocytes into dendritic cells. Clin Exp Immunol. 2006;146:354–361. doi: 10.1111/j.1365-2249.2006.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Suzuki-Inoue K, Fuller GL, Garcia A, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 269.Suzuki-Inoue K, Inoue O, Ding G, et al. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem. 2010;285:24494–24507. doi: 10.1074/jbc.M110.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Uhrin P, Zaujec J, Breuss JM, et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115:3997–4005. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- 271.Bertozzi CC, Schmaier AA, Mericko P, et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 273.Sprague DL, Elzey BD, Crist SA, et al. Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood. 2008;111:5028–5036. doi: 10.1182/blood-2007-06-097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Elzey BD, Grant JF, Sinn HW, et al. Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation. J Leukoc Biol. 2005;78:80–84. doi: 10.1189/jlb.1104669. [DOI] [PubMed] [Google Scholar]
- 275.Italiano JE, Jr, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010;17:578–584. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Elzey BD, Schmidt NW, Crist SA, et al. Platelet-derived CD154 enables T-cell priming and protection against Listeria monocytogenes challenge. Blood. 2008;111:3684–3691. doi: 10.1182/blood-2007-05-091728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lindemann SW, Weyrich AS, Zimmerman GA. Signaling to translational control pathways: diversity in gene regulation in inflammatory and vascular cells. Trends Cardiovasc Med. 2005;15:9–17. doi: 10.1016/j.tcm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 278.Meshorer E, Misteli T. Splicing misplaced. Cell. 2005;122:317–318. doi: 10.1016/j.cell.2005.07.016. [DOI] [PubMed] [Google Scholar]