Abstract
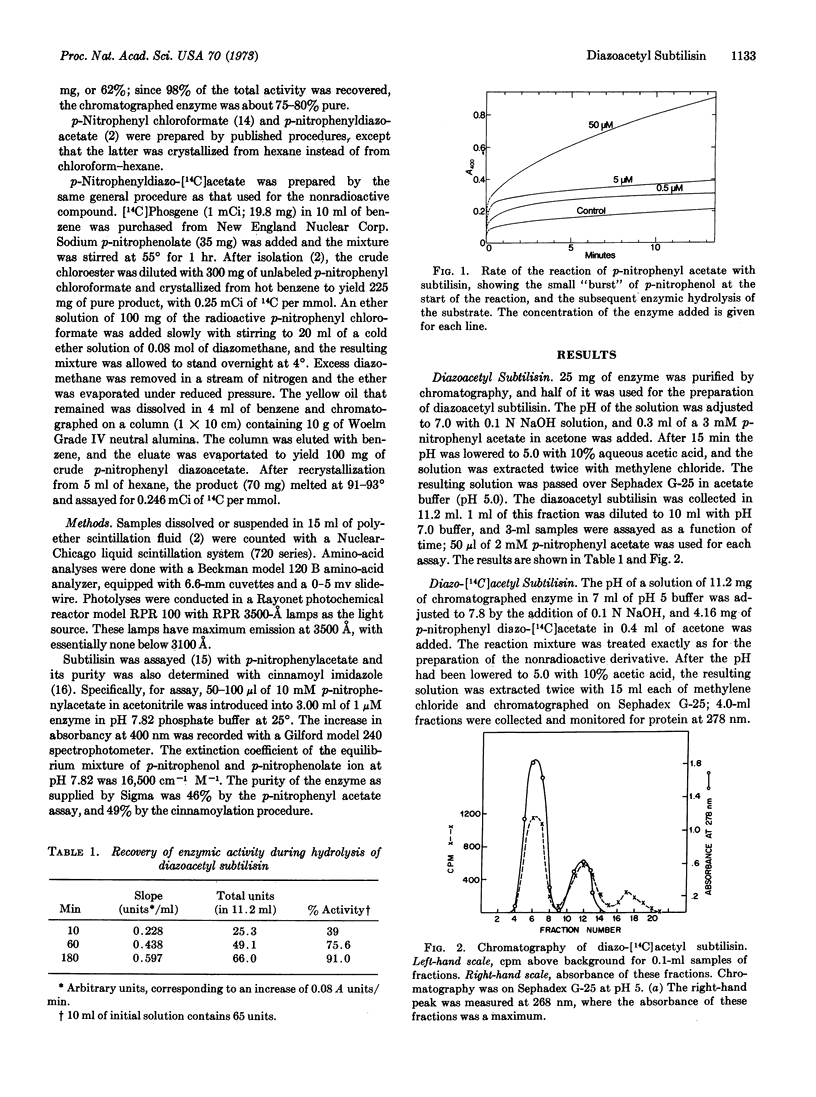
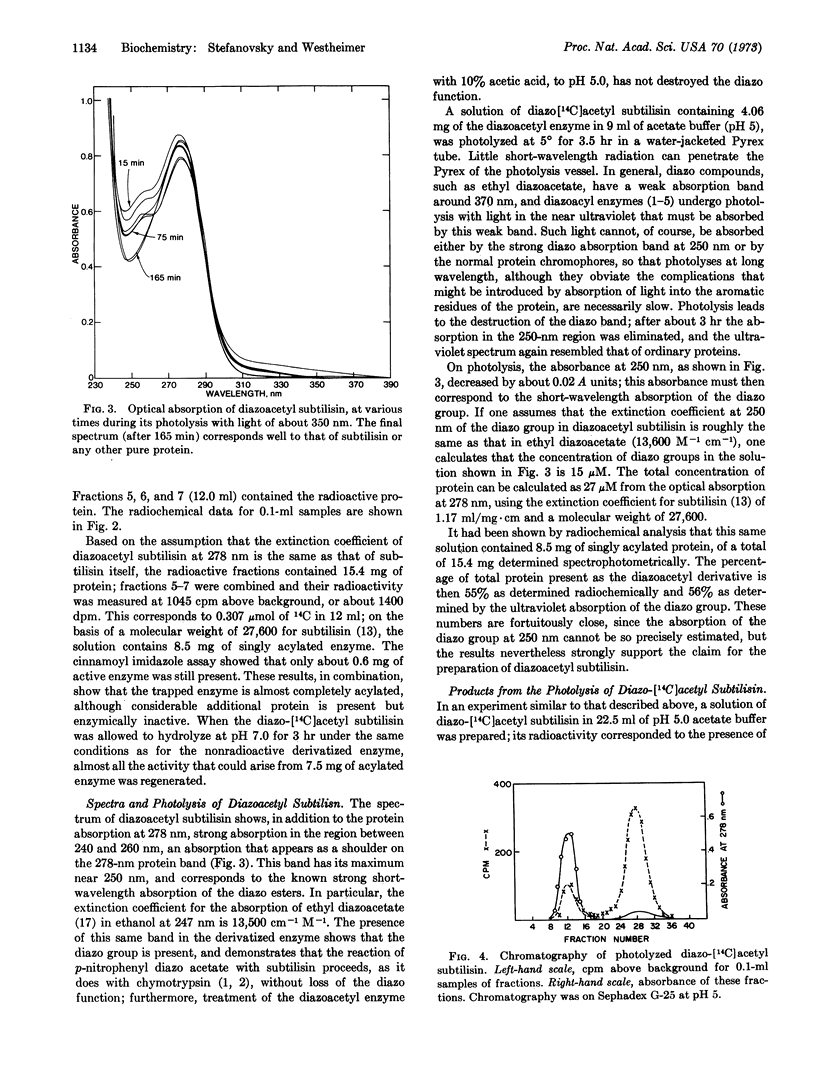
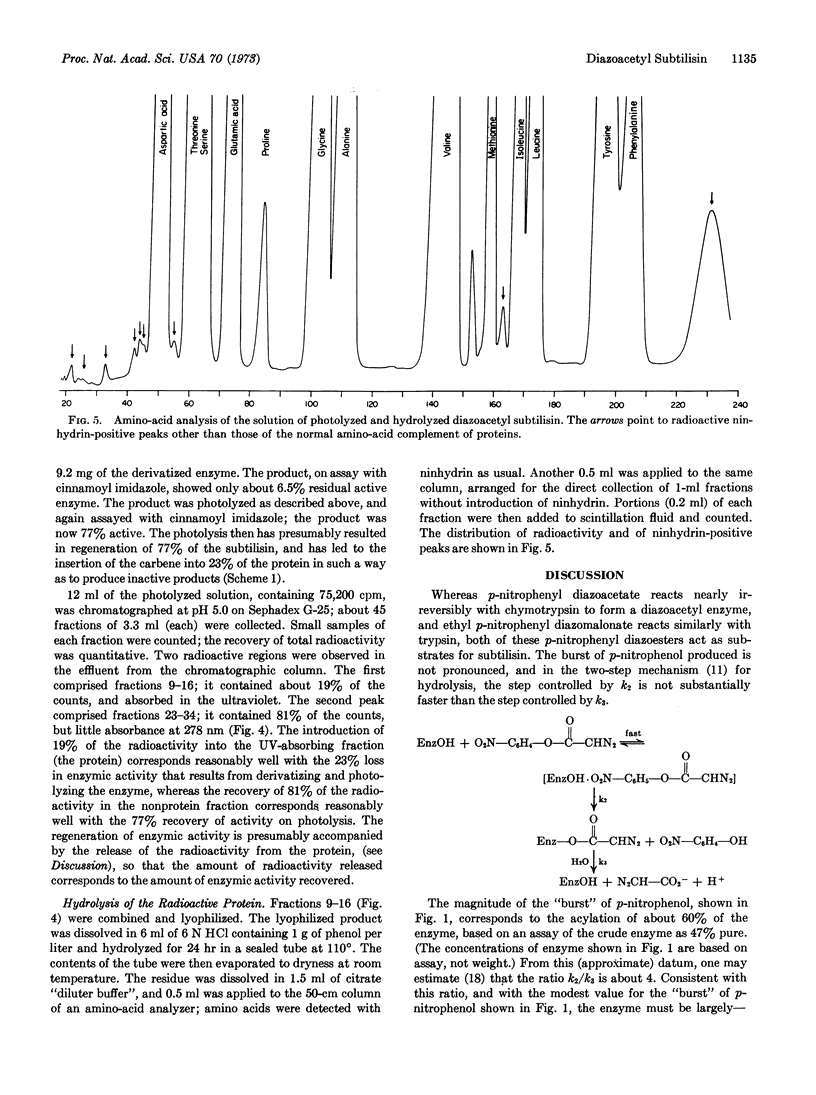
Subtilisin reacts at pH 6.8-7.8 with p-nitrophenyl diazoacetate to release p-nitrophenol and form diazoacetyl subtilisin. Although at pH 7.8 this derivative rapidly undergoes hydrolytic cleavage to regenerate active enzyme, the derivative can be trapped by rapidly lowering the pH to 5. Similarly, with 14C-labeled p-nitrophenyl diazoacetate, the corresponding radiochemically labeled diazoacetyl enzyme can be prepared. Photolysis of this radioactive derivative incorporates radioactivity into the protein, and subsquent hydrolysis gives rise to several radioactive components, which, however, have not yet been identified.
Keywords: labeled enzyme, photolyses
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balls A. K., Aldrich F. L. ACETYL-CHYMOTRYPSIN. Proc Natl Acad Sci U S A. 1955 Apr 15;41(4):190–196. doi: 10.1073/pnas.41.4.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. L., Begué-Cantón M. L., Blakeley R. L., Brubacher L. J., Feder J., Gunter C. R., Kézdy F. J., Killheffer J. V., Jr, Marshall T. H., Miller C. G. The determination of the concentration of hydrolytic enzyme solutions: alpha-chymotrypsin, trypsin, papain, elastase, subtilisin, and acetylcholinesterase. J Am Chem Soc. 1966 Dec 20;88(24):5890–5913. doi: 10.1021/ja00976a034. [DOI] [PubMed] [Google Scholar]
- Browne D. T., Hixson S. S., Westheimer F. H. A diazo compound for the photochemical labeling of yeast alcohol dehydrogenase. J Biol Chem. 1971 Jul 25;246(14):4477–4484. [PubMed] [Google Scholar]
- Brunswick D. J., Cooperman B. S. Photo-affinity labels for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1801–1804. doi: 10.1073/pnas.68.8.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Converse C. A., Richards F. F. Two-stage photosensitive label for antibody combining sites. Biochemistry. 1969 Nov;8(11):4431–4436. doi: 10.1021/bi00839a031. [DOI] [PubMed] [Google Scholar]
- HARTLEY B. S., KILBY B. A. The reaction of p-nitrophenyl esters with chymotrypsin and insulin. Biochem J. 1954 Feb;56(2):288–297. doi: 10.1042/bj0560288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hexter C. S., Westheimer F. H. Intermolecular reaction during photolysis of diazoacetyl -chymotrypsin. J Biol Chem. 1971 Jun 25;246(12):3928–3933. [PubMed] [Google Scholar]
- Hexter C. S., Westheimer F. H. S-carboxymethylcysteine from the photolysis of diazoacyl trypsin and chymotrypsin. J Biol Chem. 1971 Jun 25;246(12):3934–3938. [PubMed] [Google Scholar]
- Kiefer H., Lindstrom J., Lennox E. S., Singer S. J. Photo-affinity labeling of specific acetylcholine-binding sites on membranes. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1688–1694. doi: 10.1073/pnas.67.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar L., Bender M. L. The reactivity of thiol-subtilisin, an enzyme containing a synthetic functional group. Biochemistry. 1967 Feb;6(2):610–620. doi: 10.1021/bi00854a032. [DOI] [PubMed] [Google Scholar]
- SCHONBAUM G. R., ZERNER B., BENDER M. L. The spectrophotometric determination of the operational normality of an alpha-chymotrypsin solution. J Biol Chem. 1961 Nov;236:2930–2935. [PubMed] [Google Scholar]
- SINGH A., THORNTON E. R., WESTHEIMER F. H. The photolysis of diazoacetylchymotrypsin. J Biol Chem. 1962 Sep;237:3006–3008. [PubMed] [Google Scholar]
- Shafer J., Baronowsky P., Laursen R., Finn F., Westheimer F. H. Products from the photolysis of diazoacetyl chymotrypsin. J Biol Chem. 1966 Jan 25;241(2):421–427. [PubMed] [Google Scholar]
- Vaughan R. J., Westheimer F. H. A method for marking the hydrophobic binding sites of enzymes. An insertion into the methyl group of an alanine residue of trypsin. J Am Chem Soc. 1969 Jan 1;91(1):217–218. doi: 10.1021/ja01029a055. [DOI] [PubMed] [Google Scholar]



