Abstract
Background
Cytokines play an important role in human immune responses to malaria and variation in their production may influence the course of infection and determine the outcome of the disease. The differential production of cytokines has been linked to single nucleotide polymorphisms in gene promoter regions, signal sequences, and gene introns. Although some polymorphisms play significant roles in susceptibility to malaria, gene polymorphism studies in Brazil are scarce.
Methods
A population of 267 individuals from Brazilian Amazon exposed to malaria was genotyped for five single nucleotide polymorphisms (SNPs), IFNG + 874 T/A, IL10A-1082G/A, IL10A-592A/C, IL10A-819 T/C and NOS2A-954G/C. Specific DNA fragments were amplified by polymerase chain reaction, allowing the detection of the polymorphism genotypes. The polymorphisms IL10A-592A/C and IL10A-819 T/C were estimated by a single analysis due to the complete linkage disequilibrium between the two SNPs with D’ = 0.99. Plasma was used to measure the levels of IFN-γ and IL-10 cytokines by Luminex and nitrogen radicals by Griess reaction.
Results
No differences were observed in genotype and allelic frequency of IFNG + 874 T/A and NOS2A-954G/C between positive and negative subjects for malaria infection. Interesting, the genotype NOS2A-954C/C was not identified in the study population. Significant differences were found in IL10A-592A/C and IL10A-819 T/C genotypes distribution, carriers of IL10A -592A/-819 T alleles (genotypes AA/TT + AC/TC) were more frequent among subjects with malaria than in negative subjects that presented a higher frequency of the variant C allele (p < 0.0001). The presence of the allele C was associated with low producer of IL-10 and low parasitaemia. In addition, the GTA haplotypes formed from combinations of investigated polymorphisms in IL10A were significantly associated with malaria (+) and the CCA haplotype with malaria (−) groups. The IL10A-1082G/A polymorphism showed high frequency of heterozygous AG genotype in the population, but it was not possible to infer any association of the polymorphism because their distribution was not in Hardy Weinberg equilibrium.
Conclusion
This study shows that the IL10A-592A/C and IL10A-819 T/C polymorphisms were associated with malaria and decreased IL-10 levels and low parasite density suggesting that this polymorphism influence IL-10 levels and may influence in the susceptibility to clinical malaria.
Keywords: Malaria disease, Cytokines, Nitric oxide synthase, Polymorphism
Background
Malaria is an infectious disease that affects millions of people each year worldwide. The species of Plasmodium that affect humans have different pathogenic potential. However, beyond the pathogenic potential of the parasite, there are environmental factors, host genetics and parasite virulence associated to susceptibility and resistance to malaria [1,2]. The identification of host factors may increase the understanding of the interactions between the parasite and host, as well as the mechanisms involved in the pathology and immunity. In human malaria, a link between enhanced IFN-γ, TNF, IL-6, IL-10 and nitric oxide (NO) levels and severity of the disease have long been reported [3-7], although this is not a consistent finding [8-10]. In recent years, several studies have demonstrated that the presence of polymorphisms in IFN-γ, IL-10 and NO gene have been associated with susceptibility or resistance to various diseases [11-15].
The main polymorphism in the gene encoding IFN-γ (IFNG + 874 T/A polymorphism) is located in its first intron at position +874 and studies have reported only weak associations between IFNG SNPs and susceptibility to severe malaria [16,17]. In Brazil, there is one study showing that IFNG + 874 T/A polymorphism are associated with reduced levels of IFN-γ in patients with the homozygote mutant AA genotype while carriers of the wild alleles (AT and TT) were associated with higher levels of this cytokine [18].
IL-10 cytokine has an important regulatory role and polymorphisms in the promoter region of IL10A impair the production of this cytokine [19,20], and may contribute to the pathogenesis of diseases. In malaria, the role of IL-10 in regulating the inflammatory response remain conflicting since several studies suggest that enhanced IL-10 is associated with increased pathogenesis while others associate with protection [10,21-23]. The coding gene of IL-10 cytokine contains a promoter region with at least 5 kb, which were described over 27 polymorphisms [19]. In malaria, polymorphisms in the promoter region (IL-10A-1082A/G, −819 T/C and -592A/C) were associated with reduced IL-10 plasma levels and with the development of acute anaemia in Kenyan children with P. falciparum malaria in holoendemic areas [20].
Nitric oxide synthase 2 is the critical enzyme involved in the synthesis of nitric oxide (NO), a molecule with diverse functions. There has been much speculation about the part played by nitric oxide in malaria, both as an antiparasitic agent and as a potential cause of cerebral malaria [24-27]. A report from Gabon suggests that a single nucleotide polymorphism in the inducible nitric oxide synthase (NOS2A) promoter is associated with protection from all forms of severe malaria, including susceptibility to reinfection while other study report an association with the risk of fatal cerebral malaria [28]. Recently, an association was found between mutation of a nucleotide at position 84 in the gene of the enzyme, NOS2A and a higher risk of cerebral malaria [29]. Moreover, associations between protection against severe malaria and polymorphic forms of the promoter region in African children have also been described [26]. However, few studies evaluate the effect of the gene polymorphism in the promoter of the NOS2A gene in NO production. Although some polymorphisms play significant roles in susceptibility to malaria, several findings are inconclusive and contradictory and studies that explore the influence of these polymorphisms in Brazil is scarce [18,30-32]. Thus, cytokine gene polymorphisms have an unquestionable role in the orchestration of the immune response, leading to different functional scenario, which in turn influence the outcome of disease establishment and evolution.
The hypothesis is that SNP polymorphisms may result in changes in recognition sites of some transcription factors that influence the levels of pro-and anti-inflammatory cytokines in malaria infection and may lead to imbalance between these molecules that could favor the host susceptibility to Plasmodium and increase the risk for clinical malaria in individuals naturally exposed to infections. Therefore, this study examined the SNPs polymorphisms that affect the expression of genes encoding IFN-γ (−874 T/A), IL-10 (−1082A/G, −819 T/C and -592A/C) and iNOS (−954G/C) from a Brazilian Amazonian population living in malaria endemic area of Brazil.
Methods
Subjects and methods
The present study included 267 individuals (malaria-exposed group) from Porto Velho, Rondônia State, malaria endemic area in the southwestern Brazilian Amazon. Among these individuals 73 (27.3%) were positive for malaria infection (malaria (+) group) and 194 (72.7%) individuals living in the same area were negative for malaria infection (malaria (−) group). Malaria diagnosis was performed in Giemsa-stained thin and thick blood smears and parasitological evaluation was done by examination of 200 fields at 1,000X magnification under oil-immersion. The parasitaemia was expressed as the number of parasites/μl of blood in the thick blood smear. The number of parasites/μl of blood was calculated by multiplying the number of parasites counted against 500 leucocytes, and the number of leukocytes of the subject and dividing the product by 500. A researcher expert in malaria diagnosis examined all slides. To confirm the parasitological diagnosis, molecular analyses of all samples was performed using primers specific for genus (Plasmodium sp.) and species (Plasmodium falciparum and Plasmodium vivax). The amplification protocols were described previously by Snounou et al. [33]. Subjects were considered to have malaria if they were positive in the thick blood smear and/or polymerase chain reaction (PCR). Positive volunteers for P. vivax and/or P. falciparum at the time of blood collection were subsequently treated using the chemotherapeutic regimen recommended by the Brazilian Ministry of Health [34]. Written informed consent was obtained from all volunteers and the study was reviewed and approved by the Fundação Oswaldo Cruz Ethical Committee and the Brazilian National Ethical Committee. All volunteers were clinically evaluated and answered an epidemiological questionnaire, including data as age, gender, time of residence in endemic area, number of past infections, past and last Plasmodium species infection and time since last infection.
DNA extraction and genotyping
Genomic DNA was extracted using the kit QIAamp® DNA Blood Midi/Maxi (QIAgen, Hilden, Germany), quantified using the NanoDrop ND-1000 and stored at −20°C until use. Amplification Refractory Mutation System (ARMS-PCR), first described by Newton et al. [35], analysed single nucleotide polymorphisms (SNPs) for the IFNG + 874 T/A, IL-10A-1082A/G, IL10A-819 T/C and IL10A-592A/C, and NOS2A-954G/C polymorphism by Restriction fragment length polymorphism (RFLP) [26]. The polymorphisms IL10A-592A/C and IL10A-819 T/C were estimated by a single analysis due to the complete linkage disequilibrium between the two SNPs with D’ = 0.99. Amplifications were performed in a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA) using 2.5UI for IFNG and IL10A and 1.5UI for NOS2A of Taq DNA polymerase (5U/ μL, Invitrogen). Cycling PCR conditions for IFNG + 874 T/A were 95°C (3 minutes), 10 cycles of 95°C (15 seconds), 65°C (50 seconds), 72°C (40 seconds) followed by 20 cycles of 95°C (20 seconds), 55°C (50 seconds) and 72°C (50 seconds), 72°C (7 minutes) 4°C until use. Cycling PCR conditions for IL10A polymorphisms were 95°C (1 minute), 10 cycles of 95°C (15 seconds), 65°C (50 seconds), 72°C (40 seconds) followed by 20 cycles of 95°C (20 seconds), 59°C (50 seconds) and 72°C (50 seconds), 4°C until use. Cycling PCR conditions for NOS2A-954G/C were 95°C (3 minutes), 30 cycles of 94°C (10 seconds), 60°C (30 seconds), 72°C (30 seconds); 72°C (7 minutes) and 4°C until use. The NOS2A-954G/C amplified product was subsequently digested with BSAI restriction enzyme in the following condition: 50°C (60 minutes), 65°C (20 minutes) and 4°C until use. All amplified products were evaluated by electrophoresis on a 1.5% (IFNG and IL10A) and 2.5% (NOS2A) agarose gel containing ethidium bromide (0.5 μg/mL). After electrophoresis, the fragments were displayed and the images were photographed on a transilluminator Multi Doc-It™ Digital Imaging System (UVP, Upland, CA) (Figure 1).
Figure 1.
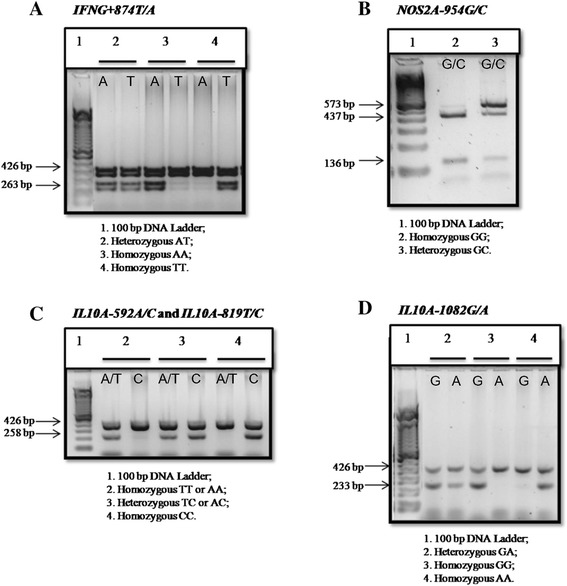
Visualization of amplified fragments on agarose gel. 426 bp fragment: band corresponding to internal control using a pair of primers designed from the nucleotide sequence of human growth hormone; A. 263 bp fragment: specific bands for T and A alleles of IFNG + 874 T/A polymorphism; B. 437 and 136 bp fragments: specific bands for G allele of NOS2A-954G/C polymorphism; 573 bp fragment: specific band for C allele of NOS2A-954G/C polymorphism; C. 258 bp fragment: specific bands for A/T and C alleles of IL10A-592A/C and IL10A-819 T/C polymorphisms were estimated by a single analysis due to the complete linkage disequilibrium of the two SNPs (D’ = 0.99), D. 233 bp fragment: specific bands for G and A alleles of IL10A-1082G/A polymorphism.
Analysis of plasma cytokines concentration
Levels of IFN-γ and IL-10 were detected in plasma samples by a multiplex assay (Bio-Plex assay, Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions using the Luminex system (Luminex Corporation, Austin, TX, USA) and analysed with a Bio-Plex suspension array system (Bio-Rad Laboratories). Fluorescence intensity was transformed into cytokine concentration using the Bio-Plex manager software (version 3.0). A minimum of 100 beads per region were analysed. A curve fit was applied to each standard curve according to the manufacturer’s manual and sample concentrations were interpolated from the standard curves. The limit of detection was 0.79 pg/mL for IFN-γ and 2 pg/mL for IL-10.
Griess microassay detection of nitrite and nitrate
A modified Griess reaction was used to detect nitrite and nitrate [36,37]. The NO levels in samples were indirectly measured after first converting nitrates to nitrites with a nitrate reductase treatment (Aspergillus species NAD [P] H, Sigma, UK) and NADPH β-nicotinamide adenine dinucleotide phosphate (Sigma Diagnostics, St. Louis, USA). Griess reagent [5% phosphoric acid, 1% sulphanilic acid and 0.1% N-(1-naphthyl-1)-ethylendiaminedihydrochloride, all from Sigma, UK, dissolved in 100 mL deionized water] was added and proteins were subsequently precipitated by trichloroacetic acid (BDH, England). The tube contents were mixed and centrifuged (Eppendorf centrifuge 5415 C, Germany); two samples of each supernatant were transferred to a flat-bottomed microplate and their absorbances were read at 520 nm using a microplate reader (SpectraMax, Molecular Devices Inc). NO values were calculated from standard calibration plots.
Statistical analysis
Epidemiological and experimental data were stored in the Epi- Info 3.5.1 (CDC, Atlanta, USA). Allele and genotype frequencies were estimated by gene counting and differences between groups by χ2 test. The risk of malaria associated with polymorphisms was estimated using odds ratios (OR) and confidence interval of 95% (CI) with and without adjustment by age, gender, length of residence in an endemic area and number of previous episodes of malaria. Differences between malaria (+) and malaria (−) groups were estimated by Mann–Whitney and t tests. Relationships between epidemiological factors and cytokine levels were assessed by Spearman’s correlation The Hardy-Weinberg equilibrium (HWE) was assessed by χ2 test and Fisher Person. The genetic analyses correspond to codominant logistic regression model (homozygous major allele VS. Heterozygote + homozygote secondary). The linkage disequilibrium was calculated by D statistic and frequency haplotypes by calculating the maximum likelihood estimator via the EM algorithm (Expectation Maximization) using two-step, step “E” (Hope) and step “M” (max) until convergence is achieved. Statistical analyses were performed using Graph Pad Prism software and SNPStats 5.0 (San Diego, California, USA). Statistically significant values of p < 0.05 was considered.
Results
Description of the studied population
The epidemiological surveillance shows that a significant proportion (93.16%) of the inhabitants reported a prior infection with P. vivax and/or P. falciparum indicating that the majority of the individuals were exposed to malaria parasite throughout the years. Among studied participants, 55.4% were male and the mean age was 30.99 ± 14.37 years. Comparing both groups, a higher frequency of males was observed in malaria (+) group. However, no statistical differences were identified regarding age, the length of residence in an endemic area and number of previous episodes of malaria between groups. Yet, the time since last infection was higher in malaria (−) group (Table 1). In malaria (+) group, 53 (72.6%) individuals were infected with P. vivax and 20 (27.4%) with P. falciparum, consistent with Plasmodium species distribution reported by the Brazilian Ministry of Health [34]. No cases of severe malaria were seen and fever and headache were the main reported symptoms in all patients. Parasitaemia and symptoms were similar in patients infected with both plasmodial species (Table 2).
Table 1.
Characteristics of the study participants
| Malaria diagnosis | ||||
|---|---|---|---|---|
| Positive N = 73 | Negative N = 194 | Total N = 267 | *P value | |
| Gender n (%) | ||||
| ♀ | 21 (28.8%) | 98 (50.5%) | 119 (44.6%) | 0.001 |
| ♂ | 52 (71.2%) | 96 (49.5%) | 148 (55.4%) | 0.001 |
| Age | 28 (9–54) | 30 (4–71) | 29 (4–71) | 0.531 |
| TREA (years) | 25 (1–33) | 24 (2–32) | 24 (1–63) | 0.079 |
| NPE | 5 (0–50) | 5 (0–99) | 5 (0–99) | 0.072 |
| TLI (Months) | 6 (0–360) | 12.5 (0–420) | 12 (0–420) | 0.016 |
Data expressed as n (%) of patients; Time of Residence in years TREA; Number of Previous Episodes NPE; Time since Last Infection TLI; Age, TREA, NPE and TLI expressed as median (minimum-maximum); *Statistical significance determined by Mann Whitney U and Chi-square tests.
Table 2.
Malaria symptoms and parasitaemia according to plasmodial species
| Plasmodial species (n = 73) | ||||
|---|---|---|---|---|
| P. vivax | P. falciparum | Total | *P value | |
| Number of infected n (%) | 53 (72.6%) | 20 (27.4%) | 73 | <0.0001 |
| Parasitaemia | 2286 (50–17933) | 1214 (52–12623) | 2097 (50–17933) | 0.334 |
| Days since the onset of symptoms | 2 (1–5) | 3 (1–10) | 3 (1–10) | |
| Fever | 38 (71.7%) | 15 (75%) | 53 (72.6%) | 0.779 |
| Headache | 40 (75.5%) | 15 (75%) | 55 (75.3%) | 0.968 |
| Chill | 32 (60.4%) | 13 (65%) | 45 (61.6%) | 0.719 |
| Myalgia | 31 (58.5%) | 14 (70%) | 45 (61.6%) | 0.368 |
| Nausea | 20 (37.7%) | 11 (55%) | 31 (42.5%) | 0.184 |
Data expressed as n (%) of patients; Parasitaemia (number of parasites/μL of blood) and onset of symptoms expressed as median (minimum-maximum); *Statistical significance determined by Mann Whitney U and Chi-square tests.
IFN-γ, IL-10 and NO plasma levels
The means levels of IFN-γ (66.71 pg/mL [2.00-3629.00]) and IL-10 (795.34 pg/mL [1.15-16782.93]), were higher in plasma of malaria (+) when compared with malaria (−) group (IFN-γ 19.44 pg/mL [1.46-3919.00] and IL-10 1.29 pg/mL [0.40-989.00]). The levels of NO did not differ significantly when the groups were compared (malaria (+) 25.49 μM [11.65-75.85] and malaria (−) 35.41 μM [0.52-110.95]) (Figure 2A). No differences were observed in the levels of IFN-γ (58.96 pg/mL [2–3629]), IL-10 (627.39 pg/mL [1.15-16782.9]) and NO levels (23.98 μM [11.65-75.85]) in P. vivax (n = 53) infected individuals when compared with the levels of IFN-γ (98.40 pg/mL [2.00-548.15]), IL-10 (1149.88 pg/mL [3.02-6540.45]) and NO levels (28.49 μM [12.99-40.31]) in P. falciparum (n = 20) infected individuals (Figure 2B). Although both IL-10 and IFN-γ were higher in malaria (+) group, Figure 3 shows that the correlation between cytokine levels and parasite density were only observed for IL-10 (rho = 0.58 p < 0.0001).
Figure 2.
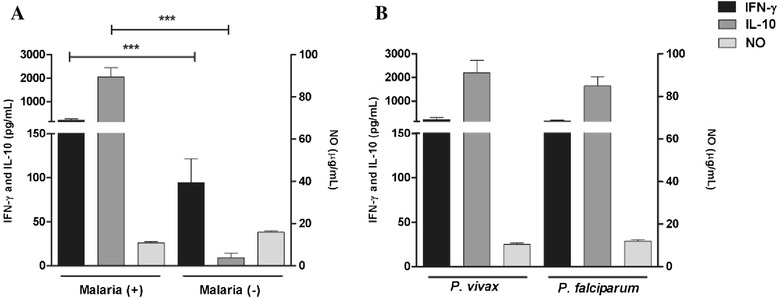
Levels of IFN-γ (pg/mL), IL-10 (pg/mL) and NO (μg/mL) were compared in Malaria (+) and Malaria (−) groups and according to the plasmodial species. Levels of IFN-γ, IL-10 and NO in: A. Malaria (+) and Malaria (-) groups and B. Plasmodium vivax and Plasmodium falciparum infections. Data are expressed as means levels (means ± SEM) on a logarithmic scale; Statistical significance determined by Mann Whitney U test; ***P value < 0.0001.
Figure 3.
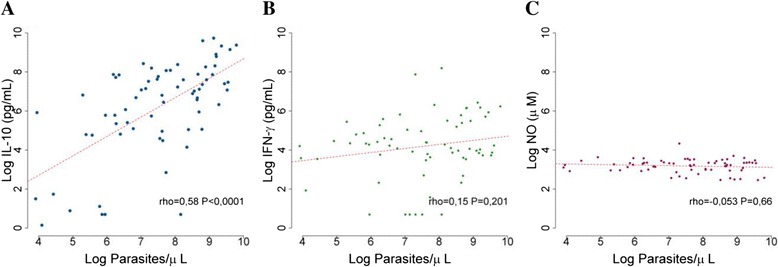
Correlation of cytokines and nitrogen radicals levels with parasite density in malaria patients. A. IL-10 levels, B. IFN-γ levels and C. NO levels. High titers of IL-10 serum levels were correlated with parasitaemia by Spearman correlation (rho = 0.58, p < 0.0001).
Alleles, genotypes and haplotypes frequencies obtained for IFNG, IL10A and NOS2A genes
The SNPs investigated, their location in the gene, the genotype and allele frequencies observed in malaria (+) and malaria (−) groups are presented in Table 3. The groups fell within Hardy-Weinberg equilibrium with non-significant values by χ2 test for the genotype observed and expected for all polymorphism except for the IL10A-1082G/A (χ2 = 41.76, p < 0.0001, χ2 = 32.33, p < 0.0001). The IFNG + 874 T/A genotype and allelic frequencies did not differ between malaria (+) and malaria (−) groups. In both groups, the most frequent genotype for IFNG + 874 T/A was the homozygous mutant AA followed by AT heterozygous and homozygous wild T allele. The variant allele +874 A was more frequent than wild T. In contrast, the distribution of the IL10A-1082G/A genotype differed between malaria (+) and malaria (−) groups while allelic frequencies were similar in both groups. Although the heterozygous AG genotype were more frequent in both groups, this genotype was significantly higher in malaria (+) (p < 0.001). The polymorphisms IL10A-592A/C and IL10A-819 T/C were estimated by a single analysis due to the complete linkage disequilibrium between the two SNPs with D’ = 0.99. The A allele of IL10A-592A/C was always linked with the T allele of and IL10A-819 T/C and C with C. IL10A-592A/A and IL10A-819 T/T genotypes were more frequently observed in malaria (+) compared with malaria (−) group (p < 0.001), even when adjusted by sex and age. Similarly the wild alleles A/T were more frequent in malaria (+) than malaria (−) groups (OR = 0.45; p < 0.0001). Stratification of the individuals into haplotypic groups based on the tree promoter IL10A polymorphisms at positions −592/-819/-1082 yielded the following haplotypes distribution: Hap1 (CCG), Hap2 (ATA), Hap3 (CCA) and Hap4 (ATG) with frequencies that varied from 7% to 38% in both malaria (+) and malaria (−) groups. As shown in Table 4, Hap1 and Hap2 were the most frequent haplotypes with similar distribution in the groups. The distribution of the haplotypes Hap3 and Hap4 were significantly different between malaria (+) and malaria (−) groups, while the Hap1 and Hap2 were similar between the groups. The Hap3 haplotype was more frequent in malaria (−) while the Hap4 were more frequent in malaria (+) group. The distribution of NOS2A-954G/C genotype in the population showed that the homozygous GG was more frequent in both groups and the genotype NOS2A-954C/C was not identified in the study population. The G allele was also the most frequent allele with similar distribution in both groups.
Table 3.
Genotypic and allelic frequencies of single nucleotide polymorphisms in malaria (+) and malaria (−) groups
| Studied polymorphisms | Malaria (+) (n = 73) | Malaria (−) (n = 194) | OR (95% CI) | * P value | |
|---|---|---|---|---|---|
| IFNG + 874 T/A | |||||
| Genotypes | A/A | 38 (52%) | 101 (52.1%) | 1.00 | 0.92 |
| A/T | 30 (41.1%) | 77 (39.7%) | 0.97 (0.55-1.70) | ||
| T/T | 5 (6.8%) | 16 (8.2%) | 1.20 (0.41-3.51) | ||
| Alleles | A | 106 (73%) | 279 (72%) | 0.16 (0.68 - 1.58) | 0.87 |
| T | 40 (27%) | 109 (28%) | |||
| NOS2A-952G/C | |||||
| Genotypes | G/G | 70 (95.9%) | 183 (94.3%) | 1.00 | 0.6 |
| G/C | 3 (4.1%) | 11 (5.7%) | 1.40 (0.38-5.18) | ||
| C/C | - | - | - | ||
| Alleles | G | 143 (98%) | 377 (97%) | 1.39 (0.38 - 5.05) | 0.6 |
| C | 3 (2%) | 11 (3%) | |||
| IL10A-1082G/A | |||||
| Genotypes | A/A | 3 (4.1%) | 40 (20.6%) | 1.00 | 0.0009 |
| G/A | 64 (87.7%) | 135 (69.6%) | 0.16 (0.05-0.53) | ||
| G/G | 6 (8.2%) | 19 (9.8%) | 0.24 (0.05-1.05) | ||
| Alleles | A | 70 (48%) | 215 (62%) | 0.74 (0.50 - 1.08) | 0.12 |
| G | 76 (52%) | 173 (45%) | |||
| IL10A-592A/C and IL10A-819 T/C | |||||
| Genotypes | C/C | 13 (17.8%) | 70 (36.1%) | 1.00 | 0.0002 |
| A/C; T/C | 36 (49.3%) | 100 (51.1%) | 0.52 (0.26-1.04) | ||
| A/A; T/T | 24 (32.9%) | 24 (12.4%) | 0.19 (0.08-0.42) | ||
| Alleles | C | 62 (42%) | 240 (62%) | 0.45 (0.30 - 0.67) | 0.0001 |
| A/T | 84 (58%) | 148 (38%) |
IFNG + 874 T/A, NOS2A-954G/C, IL10A-1082G/A, IL10A-592A/C and IL10A-819 T/C polymorphisms: Alleles, n (%); Genotypes n (%); OR (95% CI), calculating odds ratios with confidence interval (CI) of 95%; *Analysis by χ2 test using the Fisher model codominance.
Table 4.
Haplotypes frequencies of IL10A in malaria (+) and malaria (−) groups
| Haplotypes frequencies | |||||||
|---|---|---|---|---|---|---|---|
| −592 | −819 | −1082 | Malaria (+) | Malaria (−) | OR (95% CI) | * P value | |
| Hap1 | C | C | G | 0.3257 | 0.37 | 1.00 | - |
| Hap2 | A | T | A | 0.3805 | 0.3056 | 0.85 (0.40 – 1.77) | 0.66 |
| Hap3 | C | C | A | 0.0989 | 0.2486 | 0.36 (0.14 – 0.93) | 0.036 |
| Hap4 | A | T | G | 0.1948 | 0.0759 | 3.83 (1.40 – 10.45) | 0.009 |
-592/-819/-1082: Positions of polymorphisms in the promoter region of the interleukin 10 gene; OR (95% CI), calculating odds ratios with confidence interval (CI) of 95%; *Analysis by calculating EMV via EM algorithm using a logistic regression model.
Association between IFNG, IL-10 and NOS2A genotypes and their products
No association was observed between IFNG-874 T/A, NOS2A-954G/C and IL10A-1082G/A SNPs and the levels of their products in plasma (Figure 4A-C). However, individuals with IL10A-592A/C and IL10A-819 T/C genotypes were strongly associated with the plasma levels of IL-10. The IL-10 levels were lower in subjects who carried the homozygous variant IL10A-592CC and -819CC (1.19 pg/mL [0.4-2241]) compared to subjects with IL10A-592 AC and -819TC (2.3 pg/mL [0.4-11233]) and wild homozygous individuals with IL10A-592AA/-819TT (18.4 pg/mL [0.4-16782]) (p < 0.0001). Wild homozygous individuals with IL10A-592AA/-819TT presented IL-10 levels three times higher than individuals carrying variant allele C, indicating an association between the AA/TT genotypes with clinical malaria risk (Figure 4D).
Figure 4.
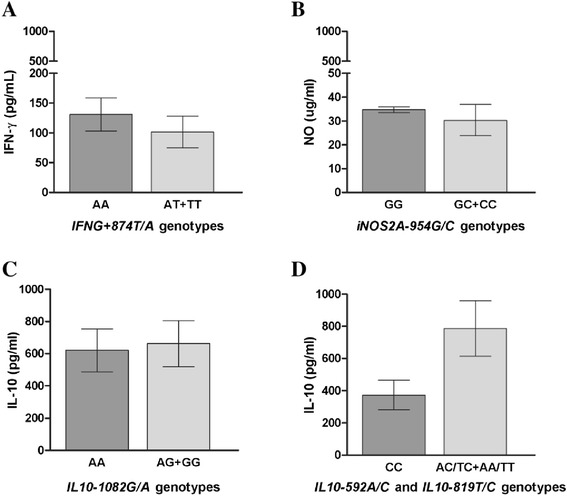
IFN-γ, IL-10 and nitrogen radicals levels by different alleles carried of polymorphisms. A. IFN-γ levels in IFNG-874 T/A genotypes, B. NO levels in NOS2A-954G/C genotypes, C. IL-10 levels in IL10A-1082G/A genotypes, D. IL-10 levels in IL10A-592A/C and IL10A-819 T/C genotypes. Data are expressed as means levels (means ± SEM) on a logarithmic scale; Statistical significance determined by Mann Whitney U test; **P value < 0.001.
Polymorphisms and parasitaemia levels association
The parasite densities among the different genotypes and carried alleles were evaluated and the parasitaemia was not influenced by the IFNG + 874 T/A, NOS2A-954G/C and IL10A-1082G/A polymorphisms (Figure 5). Among the IFNG + 874 T/A genotypes, the median parasitaemia was 37.85 (50–17933) parasites/μL for heterozygous genotype AT, 11.72 (199–6518) parasites/μL for homozygous wild TT and 1861 (52–11546) parasites/μL for homozygous variant AA (Figure 5A). Among the NOS2A-954G/C polymorphism, the homozygous CC variant was not detected and the median parasite density was 1758 (524–8888) parasites/μL for GC genotype and 2120 (50–17933) parasites/μL for homozygous GG (Figure 5B). The IL10A-1082G/A genotypes, the median parasitaemia was 1485 (510–3210) parasites/μL for the homozygous AA variant, 2200 (50–17933) parasites/μL for the heterozygous AG and 1906 (84–6000) parasites/μL for homozygous GG wild (Figure 5C). The IL10A-592AA and IL10A-819TT genotypes presented 2440 (52–17933) parasites/μL while the heterozygous IL10A-592 AC and IL10A-819TC presented 2280 (84–14891) parasites/μL (Figure 5D). These genotypes (carriers of the wild type) presented higher parasites densities when compared with the homozygous variant IL10A-592CC and IL10A-819CC 1108 (50–6000) parasites/μL.
Figure 5.
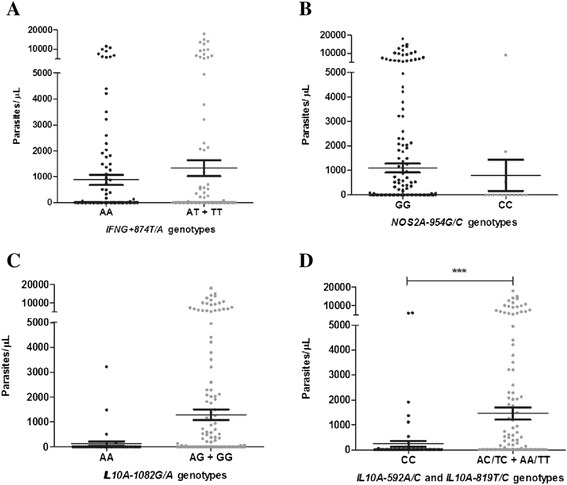
Parasite density and allelic carried frequencies of polymorphisms. Parasitaemia levels in: A. IFNG-874 T/A genotypes, B. NOS2A-954G/C genotypes, C. IL10A-1082G/A genotypes, D. IL10A-592A/C and IL10A-819 T/C genotypes. Data are expressed as means levels (means ± SEM) on a logarithmic scale; Statistical significance determined by Mann Whitney U test; **P value < 0.001.
Discussion
Human populations display differences in susceptibility to many diseases and the basis for this differential susceptibility is, at least in part, genetically determined [38,39]. Significant associations between cytokine polymorphism and diseases support that cytokine gene polymorphisms have an unquestionable role in the orchestration of the immune response, leading to different functional scenario, which in turn influence the outcome of disease establishment and evolution [38,40]. Thus, the present study aimed at exploring cytokine and NO polymorphism in populations naturally exposed to malaria, residents in Rondônia State, southwestern of Brazilian Amazon in order to establish the possible implications of these polymorphisms in malaria infection as well as analysing whether the alleles and genotypes are associated with their expression. In recent years, evaluation of SNPs have been considered a common approach for testing human genetic variation [37].
The SNPs investigated include IFNG (−874 T/A), IL10A (−1082A/G, −819 T/C and -592A/C) and NOS2A (−954G/C). Firstly, genotypic and allelic frequencies of the IFNG + 874 T/A polymorphism showed that the variant allele A was more common in the population as well as the corresponding homozygous AA genotype. The frequencies found in the study population, are in agreement with previous studies conducted in other Brazilian [18] and Colombian Amazon regions populations [41]. However, studies conducted in Brazil in non-Amazonian areas the homozygous AA genotype was predominant in patients with tuberculosis, while the control group of individuals from the same region had the heterozygous genotype predominantly AT, demonstrating a heterogeneous composition in different geographic region of Brazil, beyond the significant association of this polymorphism with tuberculosis [11,13].
In the study population, no difference in the allelic and genotypic distribution of IFNG + 874 T/A polymorphism was observed between malaria (+) or malaria (−) groups. Few studies find associations between severe malaria and IFNG + 874 T/A polymorphism and these associations were weak and not significant after correction for multiple comparisons [42,43]. In malaria-infected individuals, the relationships between IFNG + 874 T/A polymorphism and IFN-γ serum levels were not observed in this study, although high levels of IFN-γ were detected in malaria (+) subjects. The results of this study were different from Medina et al. that reported in a population of an endemic area in Brazil, a concentration of IFN-γ significantly lower in the serum of patients with AA individuals compared with T wild allele carriers [18]. The possibility that there exist functional IFNG polymorphisms that were not effectively tagged by this marker cannot be excluded, and further studies of the locus are warranted.
In the IL10A gene, investigation of three polymorphisms at positions −592, −819 and 1082 of the promoter region, were associated with the actual production of IL-10, which has an important role in the immune response to malaria infection [21,44,45]. The IL10A gene polymorphism at position −1082, have been associated with decreased production of IL-10 and clinical and severe malaria [7,20,46]. However, no association was found between this polymorphism and the levels of IL-10 nor with the occurrence of the disease and this polymorphism were not in Hardy-Weinberg equilibrium in the studied population, demonstrating the need for a representative sample to verify a possible association of this polymorphism with IL-10 levels in malaria patients. In the studied population the heterozygous GA was the most frequent genotype in both malaria (+) and malaria (−) groups. Medina et al. [18] reported the homozygous AA variant as the most common genotype in the population of Belem (Para state) but showed weak association between IL-10 concentration and parasite density [18], while a study in the Amazonas state revealed an association between IL-10A-1082G/A polymorphism with reduced risk to clinical malaria [32]. Some studies that have addressed this polymorphism showed variation in the allele and genotype distribution according to ethnicity [18,20,47]. In the case of the IL10A-592A/C and -819 T/C polymorphisms, it was observed association with IL-10 production and parasite density. Interestingly, a high prevalence of the C alleles and homozygous variant -592CC/-819CC was found in malaria (−) group while a higher prevalence of wild alleles A/T and homozygous genotype -592AA/-819TT in malaria (+), suggesting that the C allele were at lower risk to have malaria due to its prevalence in the malaria (−) group. The homozygous genotype -592CC/-819CC was associated with a reduced IL-10 levels and low parasite density compared to other genotypes. Indeed, carriers of the C allele variant were low producers of IL-10 and presented low parasite density while carriers of A/T wild alleles were high producer of IL-10 and presented high parasite density. The presence of the CC genotype may create site for enhanced binding of repressor that favor reduced IL-10 production.
Considering the haplotypes of IL10A at positions −592/-819/-1082, no association was observed between the most frequent haplotypes CCG and ATA with the risk of having malaria. However, carriers of the less frequent haplotype GTA were more prevalent in malaria (+) group while carriers of the ACC haplotype were more prevalent in malaria (−) group. The haplotype analysis of these three polymorphism in the Amazonas state, the GCT allelic combination were associated with low risk of any form of malaria, this haplotype was not present in the study population in Rondônia State neither in Para State, both Amazonian endemic areas [31]. The differences in haplotypes distribution in the same region are consistent with the heterogeneous genetic profile of Brazilian population [48]. Study in Kenia reported relationship between common Africa IL10A promoter variants and protection against severe malarial anaemia and increased production of IL-10 [20]. However, other studies have shown no evidence of association between the polymorphisms in the IL-10 gene and malaria severity [18]. A study in Gambia showed an association between the haplotype of five SNPs (+4949G, +919C, −627G, −1117C, −3585 T), not evaluated in this study, and resistance to cerebral malaria and severe anaemia [49]. In Brazil, this is the first report that investigate the frequency of the promoter region haplotypes in IL10A gene associated with malaria infection, IL-10 levels and parasite density. Similar IL10A haplotypes distribution were reported in a population from the State of Para another malaria endemic area in Brazil. However, the authors did not evaluate the IL-10 levels and did not find any influence of these haplotypes in susceptibility to malaria [31].
Finally, it was not found association between NOS2A-954G/C polymorphism and susceptibility to malaria, NO levels or parasitaemia. Indeed, the G-954-C C allele was present in less than 4% of the study population, it is absent in Caucasian and is found at low frequency in Asia. [26]. In contrast, in African population this genotype is present in high frequency where most of the associations with malaria outcome were reported. Even though, there has been much speculation about the role played by nitric oxide (NO) in malaria, both as an antiparasitic agent and as a potential cause of cerebral malaria [37]. In Brazil, the NOS2A-954G/C polymorphism have been reported in studies with tuberculosis and leprosy. In both studies, the allelic and genotypic frequencies were similar to the one found in this study, even though their population were from South and Southeast region [50,51]. Although no association has been found, this study is the first to report NOS2A-954G/C polymorphism and NO levels in malaria exposed individuals in endemic region of Brazil. Indeed, the NOS2A gene polymorphism have been associated with susceptibility to P. falciparum malaria and conflicting results have been obtained in studies that associate the presence of the G-954-C C allele and either risk of cerebral malaria or NO production [26,52]. It should be noted that individual differences in the levels of the cytokines and NO measured at a specific moment may not only result from host genetic factors predisposing to high or low production, but also for a great part from the physiological condition at that time, as well as from general immunity. The present findings reinforce the role of mediators of inflammation in malaria susceptibility and future studies in different setting with large samples numbers are warranted. Furthermore, it should be observed that not merely one genetic alteration but rather the combination of a set of genetic factors might influence the susceptibility or resistance to malaria.
Conclusions
This study shows that IFNG + 874 T/A, IL10A-1082G/A and NOS2A-954G/C polymorphisms was not associated with the occurrence of malaria or with the production of its respective cytokine and nitric oxide products. The IL10A-592A/C and IL10A-819 T/C polymorphisms were associated with malaria and decreased IL-10 levels and low parasite density suggesting that this polymorphism influence IL-10 levels and may influence in the susceptibility to clinical malaria in Amazonian population.
Acknowledgements
This work was supported by PRONEX Malaria/CNPq/FAPERJ. JOF is recipient of Research Productivity Fellowships from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). VAR and JCSA are recipients of a fellowship from CNPq and Instituto Oswaldo Cruz, respectively. We thank the Secretary of Health of Rondônia State, the Laboratório Central – LACEN of Rondônia for fieldwork support. We are grateful to all individuals that participated in this study for their cooperation and generous donation of blood, which made this study possible.
Abbreviations
- SNP
Single nucleotide polymorphism
- PCR
Polymerase chain reaction
- DNA
Deoxyribonucleic acid
- IFN-γ
Interferon-gamma
- NO
Nitric oxide
- TNF
Tumour necrosis factor
- IL-10
Interleukin 10
- iNOS
Enzyme nitric oxide synthase
- NADPH
β-nicotinamide adenine dinucleotide phosphate
- OR
Odds ratio
- CI
Confidence interval
- HWE
Hardy Weinberg equilibrium
- EM
Expectation Maximization
- E
Hope
- M
Max
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JOF conceived the idea for this study. JOF, MGBA and JCLJ designed the study. Fieldwork and sample collection was done by JCSA, DSPS, MPAV, CAML, CJLA, DMB and AT. VAP, JCSA, DSPS, RNRS and DOS performed the experiments. Data was collected and analysed by VAP with support from JCSA, MGBA, JOF and JCLJ. The first draft of this manuscript was written by VAP and JOF and MGBA critically read and advised on the manuscript. All authors read and approved the final version.
Contributor Information
Virginia A Pereira, Email: virginia@ioc.fiocruz.br.
Juan C Sánchez-Arcila, Email: jucasaar@ioc.fiocruz.br.
Antonio Teva, Email: teva@ioc.fiocruz.br.
Daiana S Perce-da-Silva, Email: daiana@ioc.fiocruz.br.
Mariana PA Vasconcelos, Email: marianapvasconcelos@hotmail.com.
Cleoni AM Lima, Email: cleoniml@yahoo.com.br.
Cesarino JL Aprígio, Email: cesarinovet@hotmail.com.
Rodrigo N Rodrigues-da-Silva, Email: rodrigo.nunes@bio.fiocruz.br.
Davi O Santos, Email: davisantos007@bol.com.br.
Dalma M Banic, Email: banic@ioc.fiocruz.br.
Maria G Bonecini-Almeida, Email: gloria.bonecini@ipec.fiocruz.br.
Josué C Lima-Júnior, Email: josue@ioc.fiocruz.br.
Joseli Oliveira-Ferreira, Email: lila@ioc.fiocruz.br.
References
- 1.Luzzatto L. Genetic factors in malaria. Bull World Health Organ. 1974;50:195–202. [PMC free article] [PubMed] [Google Scholar]
- 2.Driss A, Hibbert JM, Wilson NO, Iqbal SA, Adamkiewicz TV, Stiles JK. Genetic polymorphisms linked to susceptibility to malaria. Malar J. 2011;10:271. doi: 10.1186/1475-2875-10-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark IA, Budd AC, Alleva LM, Cowden WB. Human malarial disease: a consequence of inflammatory cytokine release. Malar J. 2006;5:85. doi: 10.1186/1475-2875-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade BB, Reis-Filho A, Souza-Neto SM, Clarencio J, Camargo LM, Barral A, Barral-Netto M. Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar J. 2010;9:13. doi: 10.1186/1475-2875-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akanmori BD, Kurtzhals JA, Goka BQ, Adabayeri V, Ofori MF, Nkrumah FK, Behr C, Hviid L. Distinct patterns of cytokine regulation in discrete clinical forms of Plasmodium falciparum malaria. Eur Cytokine Netw. 2000;11:113–8. [PubMed] [Google Scholar]
- 6.Kern P, Hemmer CJ, Van Damme J, Gruss HJ, Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989;87:139–43. doi: 10.1016/S0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- 7.Hugosson E, Montgomery SM, Premji Z, Troye-Blomberg M, Bjorkman A. Higher IL-10 levels are associated with less effective clearance of Plasmodium falciparum parasites. Parasite Immunol. 2004;26:111–7. doi: 10.1111/j.0141-9838.2004.00678.x. [DOI] [PubMed] [Google Scholar]
- 8.Lopansri BK, Anstey NM, Weinberg JB, Stoddard GJ, Hobbs MR, Levesque MC, Mwaikambo ED, Granger DL. Low plasma arginine concentrations in children with cerebral malaria and decreased nitric oxide production. Lancet. 2003;361:676–8. doi: 10.1016/S0140-6736(03)12564-0. [DOI] [PubMed] [Google Scholar]
- 9.Wilson NO, Bythwood T, Solomon W, Jolly P, Yatich N, Jiang Y, Shuaib F, Adjei AA, Anderson W, Stiles JK. Elevated levels of IL-10 and G-CSF associated with asymptomatic malaria in pregnant women. Infect Dis Obstet Gynecol. 2010;2010. [DOI] [PMC free article] [PubMed]
- 10.Rodrigues-da-Silva RN, Lima-Junior J d C, Fonseca BP, Antas PR, Baldez A, Storer FL, Santos F, Banic DM, De Oliveira-Ferreira J. Alterations in cytokines and haematological parameters during the acute and convalescent phases of Plasmodium falciparum and Plasmodium vivax infections. Mem Inst Oswaldo Cruz. 2014;109:154–62. doi: 10.1590/0074-0276140275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacheco AG, Cardoso CC, Moraes MO. IFNG +874 T/A, IL10–1082G/A and TNF -308G/A polymorphisms in association with tuberculosis susceptibility: a meta-analysis study. Hum Genet. 2008;123:477–84. doi: 10.1007/s00439-008-0497-5. [DOI] [PubMed] [Google Scholar]
- 12.Matos GI, Covas Cde J, Bittar Rde C, Gomes-Silva A, Marques F, Maniero VC, Amato VS, Oliveira-Neto MP, Mattos Mda S, Pirmez C, et al. IFNG +874 T/A polymorphism is not associated with American tegumentary leishmaniasis susceptibility but can influence Leishmania induced IFN-gamma production. BMC Infect Dis. 2007;7:33. doi: 10.1186/1471-2334-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallinoto AC, Graca ES, Araujo MS, Azevedo VN, Cayres-Vallinoto I, Machado LF, Ishak MO, Ishak R. IFNG +874 T/A polymorphism and cytokine plasma levels are associated with susceptibility to Mycobacterium tuberculosis infection and clinical manifestation of tuberculosis. Hum Immunol. 2010;71:692–6. doi: 10.1016/j.humimm.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Cardoso CC, Pereira AC, Brito-de-Souza VN, Dias-Baptista IM, Maniero VC, Venturini J, Vilani-Moreno FR, de Souza FC, Ribeiro-Alves M, Sarno EN, Pacheco AG, Moraes MO. IFNG +874 T > A single nucleotide polymorphism is associated with leprosy among Brazilians. Hum Genet. 2010;128:481–90. doi: 10.1007/s00439-010-0872-x. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro CS, Visentainer JE, Moliterno RA. Association of cytokine genetic polymorphism with hepatitis B infection evolution in adult patients. Mem Inst Oswaldo Cruz. 2007;102:435–40. doi: 10.1590/S0074-02762007005000043. [DOI] [PubMed] [Google Scholar]
- 16.Koch O, Rockett K, Jallow M, Pinder M, Sisay-Joof F, Kwiatkowski D. Investigation of malaria susceptibility determinants in the IFNG/IL26/IL22 genomic region. Genes Immun. 2005;6:312–8. doi: 10.1038/sj.gene.6364214. [DOI] [PubMed] [Google Scholar]
- 17.Cabantous S, Poudiougou B, Traore A, Keita M, Cisse MB, Doumbo O, Dessein AJ, Marquet S. Evidence that interferon-gamma plays a protective role during cerebral malaria. J Infect Dis. 2005;192:854–60. doi: 10.1086/432484. [DOI] [PubMed] [Google Scholar]
- 18.Medina TS, Costa SP, Oliveira MD, Ventura AM, Souza JM, Gomes TF, Vallinoto AC, Povoa MM, Silva JS, Cunha MG. Increased interleukin-10 and interferon-gamma levels in Plasmodium vivax malaria suggest a reciprocal regulation which is not altered by IL-10 gene promoter polymorphism. Malar J. 2011;10:264. doi: 10.1186/1475-2875-10-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordani L, Bruzzi P, Lasalandra C, Quaranta M, Schittulli F, Della Ragione F, Iolascon A. Association of breast cancer and polymorphisms of interleukin-10 and tumor necrosis factor-alpha genes. Clin Chem. 2003;49:1664–7. doi: 10.1373/49.10.1664. [DOI] [PubMed] [Google Scholar]
- 20.Ouma C, Davenport GC, Were T, Otieno MF, Hittner JB, Vulule JM, Martinson J, Ong’echa JM, Ferrell RE, Perkins DJ. Haplotypes of IL-10 promoter variants are associated with susceptibility to severe malarial anemia and functional changes in IL-10 production. Hum Genet. 2008;124:515–24. doi: 10.1007/s00439-008-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jason J, Archibald LK, Nwanyanwu OC, Bell M, Buchanan I, Larned J, Kazembe PN, Dobbie H, Parekh B, Byrd MG, Eick A, Han A, Jarvis WR. Cytokines and malaria parasitemia. Clin Immunol. 2001;100:208–18. doi: 10.1006/clim.2001.5057. [DOI] [PubMed] [Google Scholar]
- 22.Keller CC, Davenport GC, Dickman KR, Hittner JB, Kaplan SS, Weinberg JB, Kremsner PG, Perkins DJ. Suppression of prostaglandin E2 by malaria parasite products and antipyretics promotes overproduction of tumor necrosis factor-alpha: association with the pathogenesis of childhood malarial anemia. J Infect Dis. 2006;193:1384–93. doi: 10.1086/503047. [DOI] [PubMed] [Google Scholar]
- 23.May J, Lell B, Luty AJ, Meyer CG, Kremsner PG. HLA-DQB1*0501-restricted Th1 type immune responses to Plasmodium falciparum liver stage antigen 1 protect against malaria anemia and reinfections. J Infect Dis. 2001;183:168–72. doi: 10.1086/317642. [DOI] [PubMed] [Google Scholar]
- 24.Clark IA, Cowden WB, Rockett KA. Nitric oxide in cerebral malaria. J Infect Dis. 1995;171:1068–9. doi: 10.1093/infdis/171.4.1068. [DOI] [PubMed] [Google Scholar]
- 25.el-Nashar TM, el-Kholy HM, el-Shiety AG, Al-Zahaby AA. Correlation of plasma levels of tumor necrosis factor, interleukin-6 and nitric oxide with the severity of human malaria. J Egypt Soc Parasitol. 2002;32:525–35. [PubMed] [Google Scholar]
- 26.Kun JF, Mordmuller B, Perkins DJ, May J, Mercereau-Puijalon O, Alpers M, Weinberg JB, Kremsner PG. Nitric oxide synthase 2(Lambarene) (G-954C), increased nitric oxide production, and protection against malaria. J Infect Dis. 2001;184:330–6. doi: 10.1086/322037. [DOI] [PubMed] [Google Scholar]
- 27.Anstey NM, Granger DL, Hassanali MY, Mwaikambo ED, Duffy PE, Weinberg JB. Nitric oxide, malaria, and anemia: inverse relationship between nitric oxide production and hemoglobin concentration in asymptomatic, malaria-exposed children. Am J Trop Med Hyg. 1999;61:249–52. doi: 10.4269/ajtmh.1999.61.249. [DOI] [PubMed] [Google Scholar]
- 28.Kun JF, Mordmuller B, Lell B, Lehman LG, Luckner D, Kremsner PG. Polymorphism in promoter region of inducible nitric oxide synthase gene and protection against malaria. Lancet. 1998;351:265–6. doi: 10.1016/S0140-6736(05)78273-8. [DOI] [PubMed] [Google Scholar]
- 29.Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit MR. A new allele (eNOS4e) in the intron 4 (VNTR) of eNOS gene in malaria infected individuals of the population of Orissa (an eastern Indian state) Nitric Oxide. 2010;22:58–9. doi: 10.1016/j.niox.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Jha AN, Singh VK, Kumari N, Singh A, Antony J, van Tong H, Singh S, Pati SS, Patra PK, Singh R, Toan NL, Song le H, Assaf A, Messias-Reason IJ, Velavan TP, Singh L, Thangaraji K. IL-4 haplotype -590 T, −34 T and intron-3 VNTR R2 is associated with reduced malaria risk among ancestral indian tribal populations. PLoS One. 2012;7:e48136. doi: 10.1371/journal.pone.0048136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sortica VA, Cunha MG, Ohnishi MD, Souza JM, Ribeiro-Dos-Santos AK, Santos NP, Callegari-Jacques SM, Santos SE, Hutz MH. IL1B, IL4R, IL12RB1 and TNF gene polymorphisms are associated with Plasmodium vivax malaria in Brazil. Malar J. 2012;11:409. doi: 10.1186/1475-2875-11-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos SD, Clark TG, Campino S, Suarez-Mutis MC, Rockett KA, Kwiatkowski DP, Fernandes O. Investigation of host candidate malaria-associated risk/protective SNPs in a Brazilian Amazonian population. PLoS One. 2012;7:e36692. doi: 10.1371/journal.pone.0036692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–20. doi: 10.1016/0166-6851(93)90077-B. [DOI] [PubMed] [Google Scholar]
- 34.Saúde MD, Saúde SV. Guia Prático de Tratamento da Malária no Brasil. Brasília-DF: Annual Report; 2010. [Google Scholar]
- 35.Newton CR, Graham A, Heptinstall LE, Powell SJ, Summers C, Kalsheker N, Smith JC, Markham AF. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS) Nucleic Acids Res. 1989;17:2503–16. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rockett KA, Awburn MM, Rockett EJ, Clark IA. Tumor necrosis factor and interleukin-1 synergy in the context of malaria pathology. Am J Trop Med Hyg. 1994;50:735–42. doi: 10.4269/ajtmh.1994.50.735. [DOI] [PubMed] [Google Scholar]
- 37.Nahrevanian H, Dascombe MJ. Nitric oxide and reactive nitrogen intermediates during lethal and nonlethal strains of murine malaria. Parasite Immunol. 2001;23:491–501. doi: 10.1046/j.1365-3024.2001.00406.x. [DOI] [PubMed] [Google Scholar]
- 38.Bidwell J, Keen L, Gallagher G, Kimberly R, Huizinga T, McDermott MF, Oksenberg J, McNicholl J, Pociot F, Hardt C, D’Alfonso S. Cytokine gene polymorphism in human disease: on-line databases, supplement 1. Genes Immun. 2001;2:61–70. doi: 10.1038/sj.gene.6363733. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez Lopez R. [Human susceptibility to complex diseases. Genetic analysis in large populations](in Portuguese). Rev Derecho Genoma Hum. 2004;227–239. [PubMed]
- 40.Hill AV. Aspects of genetic susceptibility to human infectious diseases. Annu Rev Genet. 2006;40:469–86. doi: 10.1146/annurev.genet.40.110405.090546. [DOI] [PubMed] [Google Scholar]
- 41.Torres OA, Calzada JE, Beraun Y, Morillo CA, Gonzalez A, Gonzalez CI, Martin J. Role of the IFNG +874 T/A polymorphism in chagas disease in a Colombian population. Infect Genet Evol. 2010;10:682–5. doi: 10.1016/j.meegid.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangano VD, Clark TG, Auburn S, Campino S, Diakite M, Fry AE, Green A, Richardson A, Jallow M, Sisay-Joof F, Pinder M, Griffths MJ, Newton C, Peshu N, Williams TN, Marsh K, Molyneux ME, Taylor TE, Modiano D, Kwaitkowski DP, Rockett KA. Lack of association of interferon regulatory factor 1 with severe malaria in affected child-parental trio studies across three African populations. PLoS One. 2009;4:e4206. doi: 10.1371/journal.pone.0004206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naka I, Patarapotikul J, Hananantachai H, Tokunaga K, Tsuchiya N, Ohashi J. IFNGR1 polymorphisms in Thai malaria patients. Infect Genet Evol. 2009;9:1406–9. doi: 10.1016/j.meegid.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 45.Kariuki SM, Rockett K, Clark TG, Reyburn H, Agbenyega T, Taylor TE, Birbeck GL, Williams TN, Newton CR. The genetic risk of acute seizures in African children with falciparum malaria. Epilepsia. 2013;54:990–1001. doi: 10.1111/epi.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang G, Manaca MN, McNamara-Smith M, Mayor A, Nhabomba A, Berthoud TK, Khoo SK, Wiertsema S, Aguilar R, Barbosa A, Quintó L, Candelaria P, Schultz EN, Hayden CM, Goldblatt J, Guinovart C, Alonso PL, Lesouëf PN, Dobaño C. Interleukin-10 (IL-10) polymorphisms are associated with IL-10 production and clinical malaria in young children. Infect Immun. 2012;80:2316–22. doi: 10.1128/IAI.00261-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohashi J, Naka I, Patarapotikul J, Hananantachai H, Looareesuwan S, Tokunaga K. Lack of association between interleukin-10 gene promoter polymorphism, −1082G/A, and severe malaria in Thailand. Southeast Asian J Trop Med Public Health. 2002;33(Suppl 3):5–7. [PubMed] [Google Scholar]
- 48.Lins TC, Vieira RG, Abreu BS, Grattapaglia D, Pereira RW. Genetic composition of Brazilian population samples based on a set of twenty-eight ancestry informative SNPs. Am J Hum Biol. 2010;22:187–92. doi: 10.1002/ajhb.20976. [DOI] [PubMed] [Google Scholar]
- 49.Wilson JN, Rockett K, Jallow M, Pinder M, Sisay-Joof F, Newport M, Newton J, Kwiatkowski D. Analysis of IL10 haplotypic associations with severe malaria. Genes Immun. 2005;6:462–6. doi: 10.1038/sj.gene.6364227. [DOI] [PubMed] [Google Scholar]
- 50.Leandro AC, Rocha MA, Lamoglia-Souza A, VandeBerg JL, Rolla VC, Bonecini-Almeida Mda G. No association of IFNG + 874 T/A SNP and NOS2A-954G/C SNP variants with nitric oxide radical serum levels or susceptibility to tuberculosis in a Brazilian population subset. Biomed Res Int. 2013;2013:901740. doi: 10.1155/2013/901740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Messias-Reason IJT, van Tong H, Velavan TP. Analysis of polymorphic sites in the promoter of the nitric oxide synthase 2 gene in Brazilian patients with leprosy. Int J Immunogenet. 2014;41:231–5. doi: 10.1111/iji.12108. [DOI] [PubMed] [Google Scholar]
- 52.Ohashi J, Naka I, Patarapotikul J, Hananantachai H, Looareesuwan S, Tokunaga K. Significant association of longer forms of CCTTT Microsatellite repeat in the inducible nitric oxide synthase promoter with severe malaria in Thailand. J Infect Dis. 2002;186:578–81. doi: 10.1086/341779. [DOI] [PubMed] [Google Scholar]


