Abstract
ADAM17 is believed to be a tractable target in various diseases including cancer and rheumatoid arthritis; however, it is not known whether glycosylation of ADAM17 expressed in healthy cells differs from the one found in a diseased tissue and, if so, whether glycosylation affects inhibitor binding. We expressed human ADAM17 in mammalian and insect cells and compared their glycosylation, substrate kinetics, and inhibition profiles. We found that ADAM17 expressed in mammalian cells was more heavily glycosylated than its insect-expressed analog. To determine whether differential glycosylation modulates enzymatic activity, we performed kinetic studies with both ADAM17 analogs and various TNFα-based substrates. The mammalian form of ADAM17 exhibited 10–30 fold lower kcat values than the insect analog, while the KM was unaffected, suggesting that glycosylation of ADAM17 can potentially play role in the regulating enzyme activity in vivo. Finally, we tested ADAM17 forms for inhibition by several well-characterized inhibitors. Active site zinc-binding small molecules did not exhibit differences between the two ADAM17 analogs, while a non-zinc-binding exosite inhibitor of ADAM17 showed significantly lower potency towards the mammalian-expressed analog. These results suggest that glycosylation of ADAM17 can affect cell signaling in disease and might provide opportunities for therapeutic intervention using exosite inhibitors.
Keywords: ADAM17, metalloprotease, exosites, glycosylation
INTRODUCTION
A Disintegrin And Metalloproteases (ADAMs) are membrane proteases belonging to the metzincin family. The metzincin family includes astacin, matrix metalloproteinase (MMP), serralysin, and pappalysin, which are all zinc metalloproteases [1]. Metzincins owe their proteolytic activity to their conserved catalytic motif “HEXXHXXGXXH…..M”, in which the histidine residues bind to the zinc ion and the glutamine/methionine residues are involved in the “Met-turn” and contribute to the overall protein integrity.
ADAMs are all membrane anchored proteases, and are synthesized as inactive zymogens, which are subsequently cleaved by protease convertase PC7 and furin [2] to generate the mature active enzyme [3]. Most of the ADAMs proteins are enzymatically active; however it was reported that a few ADAMs do not exhibit proteolytic activity probably due to mutations present in the catalytic motif [4]. Active ADAMs mediate ectodomain shedding of a large variety of membrane proteins involved in cell-cell interactions and cell communication [5, 6]. Disregulation of shedding has been found to be associated with autoimmune and cardiovascular diseases, infection, inflammation, and cancer. During the past decades ADAM17, also known as TNFα converting enzyme, has been reported to be overexpressed in brain, breast, colon, gastric, kidney, liver, lung, ovarian, pancreatic, and prostate cancers [7, 8] which makes ADAM17 an attractive target for cancer therapy.
ADAM17 is a multidomain type I transmembrane protein, therefore the N-terminus which contain the catalytic site is exposed to the extracellular space. ADAM17 shares 49.8% homology with ADAM10 (also known as Kuzbanian) with respect to the amino acid sequence [9]. However, the level of homology with other members of the ADAM family is poor.
During the past decades, most of the reported inhibitors of the metzincin family were non-specific zinc-binding inhibitors [10]. However, preliminary animal studies showed a high level of toxicity for this class of inhibitors, which lead to their discontinuation. It is only recently that Tape et al. [11] reported the inhibition of ADAM17 by specific antibody, and our group [12] reported a non-zinc binding selective inhibitor of ADAM17, referred to as compound #15.
Even though the expression of ADAM17 has been originally reported by Black et al., using a mammalian cell line (293/EBNA, [13]), most of the studies investigating the inhibition of ADAM17 have been performed using recombinant human ADAM17 proteins expressed in insect cells using baculoviral systems [11, 12]. However, it is known that the glycosylation patterns and types observed in mammalian and insect cells differ [14], and that the glycosylation can influence the substrate and the inhibitor binding as well as catalytic activity of various enzymes [15, 16] including metalloproteinases [17]. Therefore, in this study we evaluated the influence of the expression system on production of recombinant ADAM17 with regards to its proteolytic activity and binding to inhibitors.
We cloned and expressed human ADAM17 variant proteins in mammalian cells. Recombinant expression of ADAM17 catalytic domain and ADAM17 ectodomain in HEK293 cells enabled us to obtain secreted mature proteins. The recombinant expression of ADAM17 variants in the absence of serum lead to 99% pure proteins after Ni-NTA purification. Kinetic studies in the presence of several commonly used substrates and inhibitors showed that the catalytic properties of ADAM17 were dependent on whether ADAM17 variants were produced in insect or mammalian cells. Based on our results, we report that ADAM17 glycosylation may influence substrate and inhibitor binding. This research highlights the need for a careful attention to the expression model used to produce recombinant human proteins, especially when intending to discover and design specific inhibitors.
MATERIALS AND METHODS
Cloning of ADAM17 truncated versions
The open reading frame (ORF) of the human ADAM17 (NM_003183.4) ligated into pCMV6-XL5 was purchased from Origene (cat# SC316426). Using the forward primer 5 -GAGGCGATCGCCATGAGGCAGTCTCTCC-3′, and the reverse primers: 5′-GCGACGCGTAACTTTATTGCTGCGTTCTTG-3′, 5′-GCGACGCGTTCGTTCAATTACATCCTGTAC-3′, and 5′-GCGACGCGTGTTGTCTGCTAAAAACTTTCC-3′, respectively, ADAM17 catalytic domain (ADAM17_CatD, Met1-Val477), ADAM17 truncated ectodomain (ADAM17_EctoD, Met1-Arg651), and ADAM17 full-length ectodomain (ADAM17_extEctoD, Met1-Asn671) were obtained by applying 30 cycles of PCR (94°C, 30 sec; 55°C, 30 sec; 72°C, 2 min) in the presence of high fidelity polymerase (Pwo DNA polymerase, Roche). The DNA fragments obtained by PCR were ligated into pCMV6-AC-His (Origene, Cat# PS100002) using AsiSI and MluI restriction sites. The ligation mix was used to transform E. coli DH5α (NEB) according to the manufacturer’s instructions. Due to DNA instability observed during the cloning of the human ADAM17 gene1, E. coli DH5α ligation mix and stocks had to be grown at 30°C in the presence of carbenicillin (100 μg/ml) as a selection agent, and always had to be freshly streaked on the plate in order to limit DNA recombination. Positive clones were sent for sequencing, and subsequently plasmids were isolated (Qiagen maxi prep kit) prior to use for transfecting mammalian cells. Purity and concentration of the DNA was assessed using the Nanodrop spectrophotometer (ThermoFisher).
Culture of HEK293 cells and transient and stable transfection
The recombinant expression of ADAM17 was performed in the HEK293 cell line (ATCC, Cat# CRL-1573). HEK293 cells were grown at 37°C and in a CO2 regulated incubator in the presence of DMEM implemented with fetal bovine serum (FBS) and streptomycin/penicillin (strep/pen). When HEK293 cells reached about 70% of confluency, fresh DMEM media was added to the cells prior to the transient transfection using XtremeGENE-HP transfection reagent (Roche) according to the manufacturer’s protocol. The recombinant protein expression was pursued in the presence of serum. Protein expression in the cell extract and in the conditioned media was assessed after 12, 24, 48, and 72 h of incubation. To obtain a higher yield of recombinant ADAM17, stable cell lines were established using Geneticin (Invitrogen) as a selection reagent. Cells were transfected using XtremeGENE-HP transfection reagent (Roche) as previously mentioned. Within 48 h following the transfection, cells were harvested and split to a larger plate containing selection media (DMEM, FBS, strep/pen, and 0.25 mg/ml Geneticin). Geneticin was used instead of neomycin sulfate as the former does not cross the cell membrane of mammalian cells. The media was replaced regularly, and after about 3 weeks of incubation, resistant HEK293 colonies were harvested individually using a cloning cylinder and were grown in 48 well plates. When reaching full confluency, the cells were harvested and transferred into a plate with a larger surface until sufficient cell material was obtained to propagate the cell growth and evaluate the expression of recombinant ADAM17. Selection conditions were maintained.
The integrity of the overexpressed protein was also investigated by RNA extraction (Qiagen), followed by cDNA synthesis by reverse transcription (Qiagen) and PCR-amplified using high fidelity polymerase, prior to be sent for sequencing (Retrogen). The integrity of the overexpressed ADAM17 proteins was confirmed.
Recombinant protein expression and purification
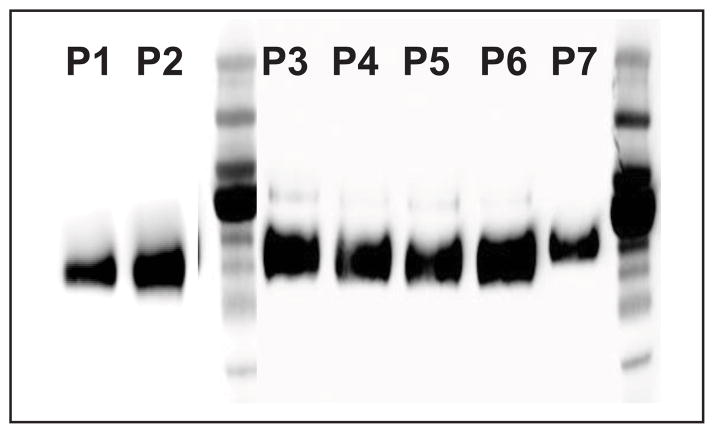
The incubation conditions enabling the production of satisfactory levels of recombinant protein in the absence of serum were investigated. The HEK293 cell line is known to require the presence of serum to grow. However, it was observed that after reaching full confluency, HEK293 were able to be maintained alive and stayed attached to the plate for about 10 to 12 d when incubated in the absence of serum. Every 48–72 h, the media (DMEM, strep/pen, and geneticin) was replaced and the production of recombinant ADAM17 was followed (see Results section) (Fig. 1).
Figure 1. Western blotting of the hADAM17_ECD recovered from the conditioned media after Ni-NTA agarose purification.
P1 and P2, HEK293 are incubated in the presence of serum (FBS (+)). P3-P6, HEK293 are incubated in the absence of serum (FBS (−)).
The pCMV6-AC-His vector enables the expression of protein with a 6 His C-terminal tag which facilitates the purification procedure. To purify recombinant ADAM proteins, 30 ml of conditioned media was mixed with 2 ml of 50% Ni-NTA agarose slurry (Qiagen), 10 mM imidazole, and complete EDTA-free Protease Inhibitor Cocktail (Roche). The mixture was incubated at 4 °C on a gyratory shaker for 1 h. The purification was performed by washing the resin with phosphate buffer (50 mM NaH2PO4 and 300 mM NaCl, pH 8) in the presence of increasing imidazole concentrations (from 10 mM to 20 mM). To elute the recombinant ADAM17, 250 mM of imidazole was added to the elution buffer. The first 4 eluted fractions (1 ml) were pooled and then desalted by dialysis against Tris buffer (25 mM Tris, 2.5 μM ZnCl2, 0.005% Brij-35 (v/v), pH 9). After 24 h dialysis at 4 °C, ADAM17 protein was aliquoted and stored at −80 °C. Larger volumes of condition media were purified using the same procedure.
Purity of the dialyzed fraction was monitored by staining the membrane after western blot transfer (7.5% or 10% SDS-PAGE gel) using MemCode reversible protein stain kit (Thermo scientific). Due to the low yield of ADAM17, the protein maturation (based on protein molecular weight) and the glycosylation were evaluated by western blotting. For that purpose, horseradish peroxidase coupled with mouse antibody (Genescript) against His-tag antibody, or rabbit antibody (Sigma) against specific ADAM17 antibodies targeting the prodomain (Abcam # ab39161) or the catalytic domain (Abcam # ab28233, recognizes a neoepitope sequence starting at R2152 resulting from cleavage by furin-like protease) were used. The detection was done using SuperSignal West Pico Chemiluminescent Substrate (Thermo scientific).
Since only N-glycosylation sites were predicted for ADAM17, deglycosylation of ADAM17 was performed using N-glycosidase F (PNGase F) and endoglycosidase H (Endo H) according to the provider’s instructions (New England BioLabs; NEB).
Kinetic parameters evaluation
In order to evaluate the influence of the expression system on the proteolytic activity and inhibition of ADAM17, we compared the kinetic parameters of commercially available ADAM17 ectodomain (ADAM17 ECD, R&D Systems, Cat# 930-ADB) and ADAM17 catalytic domain (ADAM17 CD, Enzo Life Bioscience, # BML-SE268-0010) expressed in insect cells, with the kinetic parameters of ADAM17 ECD and CD constructs expressed in mammalian cells from our laboratory.
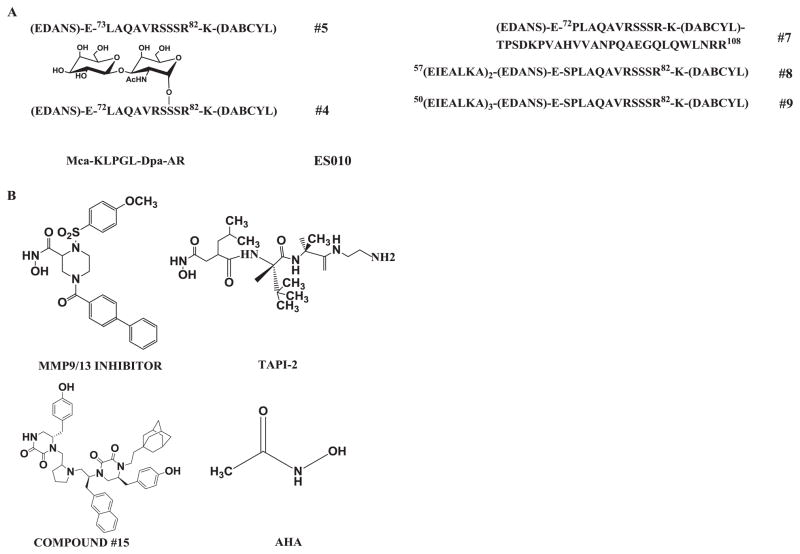
All the kinetic experiments were performed in solid bottom white 384 well low volume plates (Nunc #264706) using commercial (R&D Systems # ES010 and ES003) and “in-house” substrates (#4 and #5) (Fig. 3A). Reactions were monitored by following the increase of fluorescence using the microplate reader Synergy H4 (Biotek Instruments, Winooski, VT) set up with the following measurement parameters: λexcitation = 324 nm, λemission = 405 nm for ES010 and ES003 and λexcitation = 360 nm, λemission = 460 nm for #4 and #5. The rates of hydrolysis were calculated as previously described [12]. All the kinetic parameters were calculated using GraphPad Prism version 5.01 (GraphPad, Software, Inc., La Jolla, CA).
Figure 3. Structures of substrates and inhibitors used to evaluate ADAM17 expressed in insect and mammalian cells.
(A) Structures of substrates; (B) Structures of small molecular weight inhibitors. Substrate numbering according to [25].
ADAM17 active site titration
In order to determine the concentration of active ADAM17, N-hydroxy-1-(4-methoxyphenyl)sulfonyl-4-(4-biphenylcarbonyl)piperazine-2-carboxamide (a.k.a. MMP-9/-13 inhibitor, 496 Da) (Calbiochem 444252) was used. The substrate hydrolysis was followed in a total volume of 15 μl. ADAM17 was incubated for 45 min at 37 °C in the presence of a range of inhibitor concentration (5 μl of inhibitors; concentrations used are described in the legend to the figures). Then, the substrate (5 μl of 30 μM solution) was added to the 10 μl pre-incubated enzyme/inhibitor mix, and the hydrolysis of the substrate was followed in order to obtain the initial velocities.
Inhibition kinetics
Enzymes were pre-incubated with range of inhibitor concentrations after which a range of substrates from 0 to 50 μM was added. Fluorescence was measured as described above. Rates of hydrolysis were obtained from plots of fluorescence versus time, using data points from only the linear portion of the hydrolysis curve. All kinetic parameters were calculated using GraphPad Prism version 5.01 (GraphPad Software, Inc., La Jolla, CA). All Ki values were determined by non-linear regression (hyperbolic equation) analysis using the mixed inhibition model which allows for simultaneous determination of mechanism of inhibition (13). Mechanism of inhibition was determined using the “alpha” parameter (alpha = Ki/Ki′) derived from a mixed-model inhibition by GraphPad Prism. The mechanism of inhibition was additionally confirmed by Lineweaver-Burke plots and for each data set, Michaelis-Menten fitting was performed in order to evaluate the influence of the inhibitor concentration on the Vmax and KM.
Inhibition Studies
Substrate and ADAM17 working solutions were prepared in R&D Systems recommended assay buffer. 5 μL of 3x ADAM17 solution in assay buffer were added to solid bottom white 384 low volume plates (Nunc cat# 264706). Next, 5 μL of test compounds (TAPI-2, AHA, MMP-9/-13 inhibitor, or compound #15) were added to corresponding wells. After 30 min incubation at RT the reactions were started by addition of 5 μL of 3x solutions of respective substrates (30 μM). Fluorescence was measured every 30 min for 2 h using the microplate reader using λexcitation = 360 nm and λemission = 460 nm. Rates of hydrolysis were obtained from plots of fluorescence versus time, and inhibition was calculated using rates obtained from wells containing substrates only (100% inhibition) and substrates with enzyme (0% inhibition).
RESULTS AND DISCUSSION
Cloning of ADAM17
As mentioned in the Material and Methods section, human ADAM17 DNA exhibited stability problems when cloned in E. coli. It was observed that the 5′-end of the adam17 gene is responsible for the instability. Indeed, we could not successfully clone adam17 full ORF (adam17fullORF_2475 bp). However, the use of truncated versions of adam17 (adam17ΔTm _2013 bp, adam17EctoD _1953 bp, and adam17CatD_1431 bp) enhanced cloning efficiency and reduced DNA recombination. Also, in order to optimize the cloning and overcome the instability problem, E. coli plated on carbenicillin (100 μg/ml) plates was incubated at 30 °C instead of 37 °C, and fresh streaked plates were utilized each time.
Recombinant protein expression and purification
The transient expression of ADAM17 catalytic domain (ADAM17_CatD; Met1-Val477) and ADAM17 ectodomain (ADAM17_EctoD; Met1-Arg651) was done in HEK293 and confirmed by western blot. HEK293 cells were grown in the presence of serum and the conditioned media was harvested after 48 h. In the case of stable transfection, the production lasted around 14 to 16 d and was conducted in 2 production phases. The stable cell line was first grown in the presence of serum until reaching 100% confluency (phase 1 ~ 4 d), then recombinant protein expression was pursued in the absence of serum. To increase the production yield, the conditioned media was replaced every 48 h (phase 2 ~ 10–12 d). Comparable production yields were obtained in the presence or in the absence of serum. The purification of the serum-free batches using Ni-NTA resin was straightforward and enabled the recovery of 99% pure ADAM17 recombinant proteins based on membrane staining after protein transfer (Fig. 2). The purity of recombinant ADAM17 after NiNTA purification was assessed by staining the membrane with MemCode (Pierce) after the protein transfer. The absence of protein bands other than ADAM17 in the purified samples suggested that recombinant ADAM17 purity was greater than 95%.
Figure 2. Deglycosylation of insect and mammalian ADAM17 using PNGase F glycosidase.
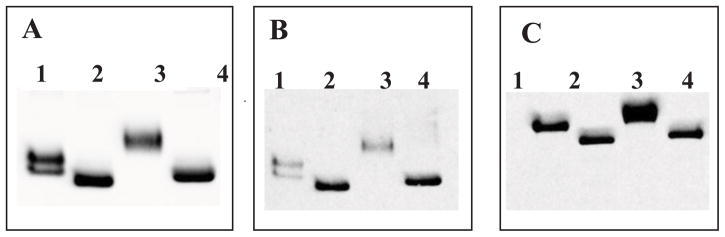
(A) His-Tag antibody and ADAM17_CD B). ADAM17-specific antibody and ADAM17_CD. C). ADAM17-specific antibody and ADAM17_ECD.
1 - insect non-treated; 2 - insect treated; 3 - mammalian non-treated; 4- mammalian treated.
The maturation of ADAM17_CatD and ADAM17_EctoD was analyzed by western blot using a primary antibody targeting either the pro-domain or the catalytic domain of ADAM17. Secreted ADAM17_CatD and ADAM17_EctoD did not react with ADAM17 pro-domain-specific antibody, suggesting that ADAM17 recombinant proteins are activated inside the cells (data not shown). Purified and desalted ADAM17_CatD and ADAM17_EctoD treated with endoglycosidase H (Endo H) did not show changes in their molecular weight (data not shown), while treatment with N-glycosidase F (PNGase F) reduced the molecular weight to that predicted for the non-glycosylated protein, suggesting glycosylation of recombinant ADAM17. It is known that PNGase F cleaves a broader substrate spectrum than Endo H. Indeed, Endo H only removes the oligomannose and hybrid N-glycans, but it does not catalyze the removal of complex N-glycans. On the contrary, PNGase F is known to catalyze the removal of oligomannose, hybrid, and complex N-glycans attached to asparagine. Thus, according to our results, ADAM17_EctoD is apparently only glycosylated with complex N-glycans.
Prior to the deglycosylation step, the molecular weight of recombinant ADAM17s expressed in insect cells and the ones expressed in mammalian cells was different. After PNGase treatment, ADAM17 proteins showed the same apparent molecular weight on western blot. This confirms that the glycosylation occurring in mammalian cells is more complex than in insect cells and therefore engender a higher molecular weight (Fig. 2).
Determination of the concentration of active ADAM17
Several approaches can be used in order to assess the amount of enzyme or protein present in a sample, but most of them do not allow assessment of the amount of active enzyme. It has been reported that the titration of the active site of an enzyme can be done using tight binding inhibitors [18]. This strategy is commonly used to assess the concentration of MMPs [19]. However, the only tight binding inhibitor reported for ADAM17, IK682, is not commercially available [20]. In the absence of tight inhibitors we used inhibitors and substrates that are commonly used in ADAM17 research to compare inhibition and kinetic profiles of insect- and mammalian-produced ADAM17 constructs (Fig. 3). Substrates #4, #5, and ES003 are based on TNFα, ADAM17’s canonical substrate [13], whereas ES010 is a generic metalloproteinase substrate cleaved by a wide repertoire of enzymes [21] (Fig. 3). We used three previously reported small hydroxamate-based zinc-binding inhibitors of ADAM17 [22, 23] (Fig. 3B) and a selective non-Zn-binding inhibitor of ADAM17 discovered by our group [24].
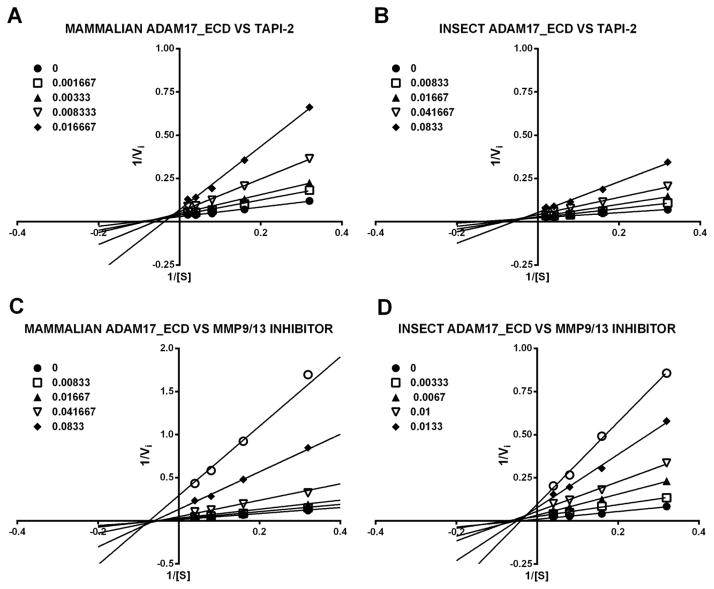
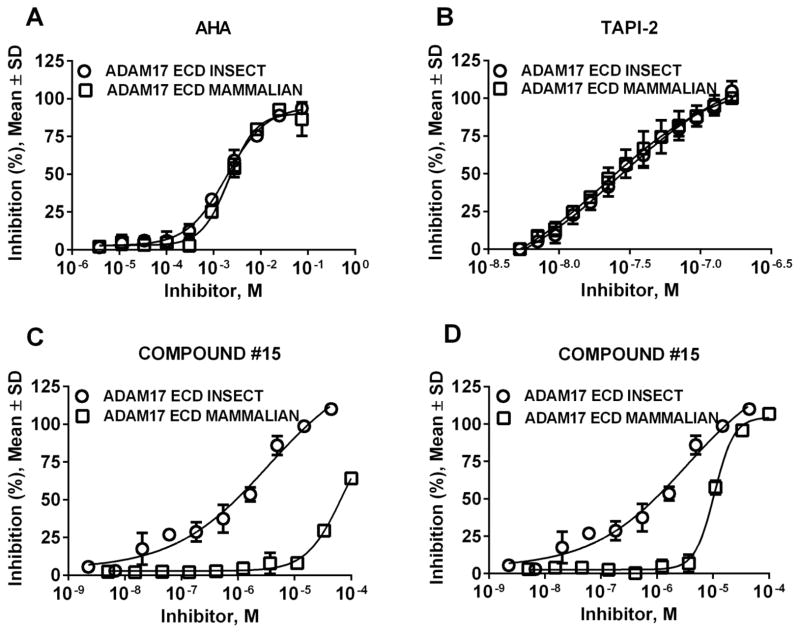
Obtained Ki values for insect-produced ADAM17 were in agreement with ones reported in the literature (TAPI-2 Ki = 31 ± 1.4 nM versus 120 ± 30 nM, MMP-9/-13 inhibitor Ki = 3.1 ± 0.2 nM versus 0.6 ± 0.3 nM) [22]. Next, we compared inhibition modalities of ADAM17 ECD produced in insect and mammalian cells by TAPI-2 and MMP-9/-13 inhibitor. Non-linear regression analysis suggested pure non-competitive inhibition mechanisms for MMP-9/-13 inhibitor with both insect- and mammalian-produced ADAM17 (Ki/Ki′ = 0.91 ± 0.26 and 0.99 ± 0.34 for insect- and mammalian-produced ADAM17, respectively) and mixed inhibition for TAPI-2 (Ki/Ki′ = 1.76 ± 0.42 and 2.27 ± 0.55 for insect- and mammalian-produced ADAM17, respectively). Examination of Lineweaver-Burke plots revealed lines of best fit crossing above the X-axis in the case of TAPI-2 (Fig. 4A and B) and on X-axis in the case of MMP-9/-13 inhibitor for both insect- and mammalian-produced ADAM17 (Fig. 4C and D), confirming pure non-competitive and mixed inhibition mechanisms for MMP-9/-13 inhibitor and TAPI-2, respectively. Additionally, Ki values of both inhibitors were similar for either insect- or mammalian-produced ADAM17 (TAPI-2 Ki = 31 ± 1.4 nM versus 32 ± 5 nM and MMP-9/-13 inhibitor Ki = 3.1 ± 0.2 nM versus 1.8 ± 0.1 nM for insect- and mammalian-produced ADAM17, respectively). These results suggested that both zinc-binding inhibitors interact in similar fashion with either insect- or mammalian-produced ADAM17. Therefore, we utilized the MMP-9/-13 inhibitor to determine the concentration of both active insect- and mammalian-produced ADAM17.
Figure 4. Lineweaver-Burke plots of insect- and mammalian-produced ADAM17 ECD in presence of small molecule inhibitors.
(A) ADAM17_ECD expressed in mammalian cells and TAPI-2 (B). ADAM17_ECD expressed in insect cells and TAPI-2. (C) ADAM17_ECD expressed in mammalian cells and CalBiochem MMP9/13 (D). ADAM17_EctoD expressed in insect cells and CalBiochem MMP9/13. Legend units are inhibitor concentration in μM.
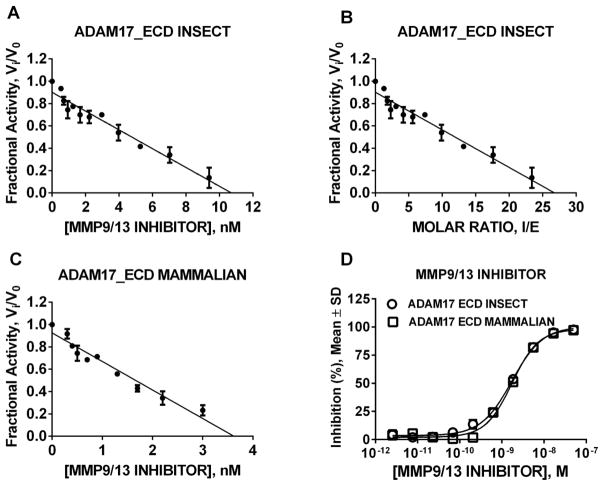
First, we titrated the insect-produced ADAM17 (R&D Systems). The titration yielded the concentration of MMP-9/-13 inhibitor necessary to produce 100% inhibition of ADAM17 (Fig. 5A, X = 10.5 nM). In order to obtain stoichiometry of ADAM17_ECD and MMP-9/-13 inhibitor, we divided 10.5 nM by the known enzyme concentration (0.4 nM) which yielded the factor of 26.3 (Fig. 5B, X = 26.3). To allow quantitative comparison between insect- and mammalian-produced ADAM17, we titrated the stock of mammalian-produced ADAM17 using the same approach as with insect-derived ADAM17 and applied the stoichiometric ratio of 26.3 (Fig. 5C, X = 3.5.nM × 26.3 = 92 nM mammalian ADAM17 stock). Additionally, a dose response study with both insect- and mammalian-produced ADAM17 and MMP-9/-13 inhibitor yielded superimposable sigmoidal curves. These results in combination with similar Ki values and identical inhibition mechanism of MMP-9/-13 inhibitor for both insect- and mammalian-produced ADAM17 suggested the validity of our approach to determine the concentration of active ADAM17 thus enabling further quantitative studies.
Figure 5. Determination of concentration of active ADAM17_ECD using MMP9/13 inhibitor.
(A) Titration of insect-produced ADAM7_ECD from R&D Systems; (B) Stoichiometry determination for insect-produced ADAM7_ECD from R&D Systems and MMP9/13 inhibitor; (C) Titration of mammalian-produced ADAM7_ECD; (D) Dose response study with insect- and mammalian-produced ADAM7_ECD and MMP9/13 inhibitor. Once the concentration of inhibitor for which Vi/V0 is determined, the stoichiometric coefficient was calculated by dividing the label amount of R&D Systems enzyme present in the reaction. To determine the concentration of mammalian-produced ADAM17_ECD the stoichiometry of insect-produced ADAM17_ECD and MMP9/13 inhibitor interaction was used.
Comparison of insect- and mammalian-produced ADAM17 enzyme kinetics and inhibition
To assess whether there are differences in the way ADAM17 from insect and mammalian sources interact with their substrates we utilized several TNFα-based substrates reported previously (Fig. 3A) [24, 25]. Interestingly, no significant differences in KM values between enzymes were observed. However, kcat values of insect-produced ADAM17 were 10–30 fold greater than mammalian-produced ADAM17 (Table 1). Since both insect- and mammalian-produced ADAM17 have the same sequence and tag and differ only in the utilized expression system, it is reasonable to attribute the differences in kinetic parameters to the differences in mammalian and insect glycosylation.
Table 1.
Kinetic parameters for hydrolysis of TNFα-based substrates by insect- and mammalian-expressed human ADAM17. Substrate numbering from Fig. 3A.
| Substrate | kcat/KM, M−1 s−1 | kcat, s−1 | KM, μM | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Insect | Mammalian | Insect | Mammalian | Insect | Mammalian | |
| 7 | 1.5±0.2×104 | 0.2±0.0×104 | 0.34±0.03 | 0.03±0.001 | 24±6 | 15±1 |
| 8 | 0.9±0.04×104 | 0.03±0.0×104 | 0.064±0.01 | 0.002±0.0001 | 7.5±1.3 | 5.5±1 |
| 9 | 0.2±0.1×104 | 0.001±0.00×104 | 0.003±0.001 | 0.0003±0.000 | 2±0.1 | 4.1±0.3 |
Results reported as an average of 3 experiments ± standard deviation.
To determine the effect of glycosylation on inhibition of ADAM17 we performed dose response experiments with various zinc- and non-zinc-binding inhibitors of ADAM17. Unsurprisingly, zinc-binding inhibitors AHA and TAPI-2 behaved similarly to MMP-9/-13 inhibitor whereby the dose response curves for inhibition of either insect- or mammalian-expressed ADAM17 were superimposable (Fig. 6A and B). Compound #15, a non-zinc-binding exosite inhibitor of ADAM17, exhibited significant differences between inhibition of insect- and mammalian-expressed ADAM17 in assays utilizing two different substrates (Fig. 6C and D). In an assay with TNFα-based substrate #4 (Fig. 6C, (EDANS)-E-PLAQAVRSS*S-K (DABCYL)), * = glycosylation) the IC50 values were 69 ± 2 μM versus 3.4 ± 0.2 μM for inhibition of mammalian and insect ADAM17, respectively. In an assay with commercial generic metalloproteinase substrate ES010 (Fig. 6D, Mca-KPLGL-Dpa-AR-NH2) the IC50 values were 11 ± 0.9 μM versus 3.4 ± 0.3 μM for inhibition of mammalian and insect ADAM17, respectively. This suggests that glycosylation of ADAM17 interferes with binding of exosite inhibitors but does not interfere with binding of active site inhibitors of ADAM17. This possibly occurs due to the proximity of glycosylation to the exosite where compound #15 binds.
Figure 6. Inhibition profile of insect- and mammalian-expressed ADAM17_ECD.
(A) ADAM17_ECD inhibition by N-acetohydroxamic acid; (B) ADAM17_ECD inhibition by TAPI-2; (C) ADAM17_ECD inhibition by compound #15 using substrate #4 ((EDANS) E-PLAQAVRSS*S-K (DABCYL). Note: * - glycosylation); (D) ADAM17_ECD inhibition by compound #15 using substrate ES010 (Mca-KPLGL-Dpa-AR-NH2).
We previously observed the differences in inhibition of ADAM17_ECD and CD constructs expressed in insect cells [24] which suggested that an exosite is located on the interface of catalytic and non-catalytic domains. This can potentially mean that glycosylation occurring closest to the interface of catalytic and non-catalytic domains can be the cause of differences in inhibition of mammalian- and insect-expressed ADAM17. We will test this hypothesis in future studies.
SIGNIFICANCE.
Results presented herein suggest that potential differential glycosylation of ADAM17 in diseased cells can affect processing of its cell surface substrates in vivo (cytokines, growth factors, receptors, etc.) thus leading to the changes of paracrine and autocrine signaling. Application of an exosite inhibitor revealed differences between mammalian- and insect-expressed ADAM17. This suggests that potential differences in ADAM17 glycosylation of diseased and normal cells might provide opportunities for therapeutic intervention and greater therapeutic window for targeting ADAM17 with exosite inhibitors.
Acknowledgments
Funding Sources: This work was supported by the James and Esther King Biomedical Research Program (2KN05 to DM), the National Institutes of Health (DA033985 to DM, CA098799 to GBF, DA031370 to RAH), the Multiple Sclerosis National Research Institute (to GBF), and the State of Florida, Executive Office of the Governor’s Office of Tourism, Trade, and Economic Development.
ABBREVIATIONS
- ADAM17
A Disintegrin And Metalloprotease 17
- TNFα
tissue necrosis factor α
- AHA
N-acetohydroxamic acid
- MMP
matrix metalloprotease
Footnotes
Observed in our laboratory and confirmed by verbal communication with Origene scientists.
Confirmed by Abcam technical support.
Author Contributions: The manuscript was written by AC and DM. All authors have given approval to the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:932–941. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- 2.Endres K, Anders A, Kojro E, Gilbert S, Fahrenholz F, Postina R. Tumor necrosis factor-α converting enzyme is processed by proprotein-convertases to its mature form which is degraded upon phorbol ester stimulation. European Journal of Biochemistry. 2003;270:2386–2393. doi: 10.1046/j.1432-1033.2003.03606.x. [DOI] [PubMed] [Google Scholar]
- 3.Moss ML, Jin SLC, Milla ME, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Warner J, Willard D, Becherer JD. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-[alpha] Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 4.Primakoff P, Myles DG. The ADAM gene family: surface proteins with adhesion and protease activity. Trends in Genetics. 2000;16:83–87. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- 5.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Molecular Aspects of Medicine. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiss K, Saftig P. The “A Disintegrin And Metalloprotease” (ADAM) family of sheddases: Physiological and cellular functions. Seminars in Cell & Developmental Biology. 2009;20:126–137. doi: 10.1016/j.semcdb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:929–941. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- 8.Borrell-Pages M, Rojo F, Albanell J, Baselga J, Arribas J. TACE is required for the activation of the EGFR by TGF-alpha in tumors. EMBO J. 2003;22:1114–1124. doi: 10.1093/emboj/cdg111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Condon TP, Flournoy S, Sawyer GJ, Baker BF, Kishimoto TK, Bennett CF. ADAM17 but Not ADAM10 Mediates Tumor Necrosis Factor-α and L-Selectin Shedding from Leukocyte Membranes ADAM17 but Not ADAM10 Mediates Tumor Necrosis Factor-α and L-Selectin Shedding from Leukocyte Membranes. 2001;11:107–116. doi: 10.1089/108729001750171353. [DOI] [PubMed] [Google Scholar]
- 10.Georgiadis D, Yiotakis A. Specific targeting of metzincin family members with small-molecule inhibitors: progress toward a multifarious challenge. Bioorg Med Chem. 2008;16:8781–8794. doi: 10.1016/j.bmc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 11.Tape CJ, Willems SH, Dombernowsky SL, Stanley PL, Fogarasi M, Ouwehand W, McCafferty J, Murphy G. Cross-domain inhibition of TACE ectodomain. Proceedings of the National Academy of Sciences. 2011;108:5578–5583. doi: 10.1073/pnas.1017067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minond D, Cudic M, Bionda N, Giulianotti M, Maida L, Houghten RA, Fields GB. Discovery of Novel Inhibitors of A Disintegrin And Metalloprotease 17 (ADAM17) Using Glycosylated and Non-Glycosylated Substrates. Journal of Biological Chemistry. 2012 doi: 10.1074/jbc.M112.389114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 14.Ajit V, Richard CD, Jeffrey DE, Hudson FH, Pamela S, Carolyn BR, Gerald HW, Marilynn EE. Essentials of Glycobiology. 2. Cold Spring Harbor NY: 2009. [Google Scholar]
- 15.Rolain T, Bernard E, Beaussart A, Degand H, Courtin P, Egge-Jacobsen W, Bron PA, Morsomme P, Kleerebezem M, Chapot-Chartier MP, Dufrene YF, Hols P. O-glycosylation as a novel control mechanism of peptidoglycan hydrolase activity. J Biol Chem. 2013 doi: 10.1074/jbc.M113.470716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liebminger E, Grass J, Altmann F, Mach L, Strasser R. Characterizing the link between glycosylation state and enzymatic activity of the endo-beta1,4-glucanase KORRIGAN1 from Arabidopsis thaliana. J Biol Chem. 2013 doi: 10.1074/jbc.M113.475558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit Rev Biochem Mol Biol. 2013;48:222–272. doi: 10.3109/10409238.2013.770819. [DOI] [PubMed] [Google Scholar]
- 18.Copeland RA. Enzymes. 2. Wiley-VCH, Inc; 2000. [Google Scholar]
- 19.Wei S, Xie Z, Filenova E, Brew K. Drosophila TIMP is a potent inhibitor of MMPs and TACE: similarities in structure and function to TIMP-3. Biochemistry. 2003;42:12200–12207. doi: 10.1021/bi035358x. [DOI] [PubMed] [Google Scholar]
- 20.Niu X, Umland S, Ingram R, Beyer BM, Liu YH, Sun J, Lundell D, Orth P. IK682, a tight binding inhibitor of TACE. Arch Biochem Biophys. 2006;451:43–50. doi: 10.1016/j.abb.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 21.Neumann U, Kubota H, Frei K, Ganu V, Leppert D. Characterization of Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2, a fluorogenic substrate with increased specificity constants for collagenases and tumor necrosis factor converting enzyme. Anal Biochem. 2004;328:166–173. doi: 10.1016/j.ab.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 22.Moss ML, Rasmussen FH. Fluorescent substrates for the proteinases ADAM17, ADAM10, ADAM8, and ADAM12 useful for high-throughput inhibitor screening. Anal Biochem. 2007;366:144–148. doi: 10.1016/j.ab.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 23.Moss ML, Rasmussen FH, Nudelman R, Dempsey PJ, Williams J. Fluorescent substrates useful as high-throughput screening tools for ADAM9. Comb Chem High Throughput Screen. 2010;13:358–365. doi: 10.2174/138620710791054259. [DOI] [PubMed] [Google Scholar]
- 24.Minond D, Cudic M, Bionda N, Giulianotti M, Maida L, Houghten RA, Fields GB. Discovery of Novel Inhibitors of a Disintegrin and Metalloprotease 17 (ADAM17) Using Glycosylated and Non-glycosylated Substrates. J Biol Chem. 2012;287:36473–36487. doi: 10.1074/jbc.M112.389114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stawikowska R, Cudic M, Giulianotti M, Houghten RA, Fields GB, Minond D. Activity of a disintegrin and metalloprotease 17 (ADAM17) is Regulated by its non-catalytic domains and secondary structure of its substrates. J Biol Chem. 2013 doi: 10.1074/jbc.M113.462267. [DOI] [PMC free article] [PubMed] [Google Scholar]