Abstract
Metastatic spread of melanoma to the central nervous system (CNS) is a common and devastating manifestation of disease progression, which, despite its clinical importance, remains poorly understood with respect to underlying molecular mechanisms. Using a recently developed preclinical model of spontaneous melanoma CNS metastasis, we have identified alterations in expression of endothelin receptor B (EDNRB) as a potential factor that influences brain metastatic potential. Induced overexpression of this gene mediated enhanced overall metastatic disease, and resulted in an increased incidence of spontaneous CNS metastases. In contrast, the overexpression of other highlighted genes, such as BCL2A1, did not affect the incidence of CNS metastases but nevertheless appears to facilitate intracranial tumor growth. The prometastatic effect in the CNS associated with EDNRB appears to be mediated by the interaction with its ligands resulting in enhanced tumor cell proliferation and thus intracranial melanoma growth. That EDNRB contributes to melanoma metastasis is underscored by the fact that its therapeutic inhibition by the EDNRB-specific inhibitor A192621 translated into improved outcomes when treating mice with either visceral metastases or intracranial tumors. The identification of an influential role of EDNRB in CNS melanoma spontaneous metastasis may provide both a target for therapeutic intervention as well as a potential prognostic marker for patients having an increased predisposition for incidence of CNS melanoma metastases.
Introduction
Melanoma metastasis to the central nervous system (CNS) is a common end-stage manifestation of malignant progression for this type of cancer and remains a significant clinical treatment challenge. The incidence of brain metastases in melanoma patients, which is among the highest in all tumor types, highlights a particular predilection of metastasis to the brain by this tumor type (1), and is invariably associated with poor prognosis. Lack of efficacy, likely in large part, occurs as a result of poor penetration of these agents across the blood-brain barrier (BBB; 2, 3). The recent success achieved with vemurafenib for melanoma represents an important step toward improving the prognosis of patients (4, 5) and highlights the importance of identifying molecules that are specifically relevant to this cancer type that can in turn be used as effective targets for novel therapies.
Ironically, however, as the ability to treat systemic visceral metastatic disease in other cancer improves, the problem of CNS metastatic disease is becoming more common (6–9). Indeed, the importance of CNS as sanctuary site for cancers such as metastatic melanoma is underlined by the fact that even when patients achieve long-term remission, 50% of them will experience CNS metastases as the only site of relapse (10–12).
With the aim of studying the biology and treatment of melanoma brain metastasis, we recently reported the generation of stable variant human melanoma cell lines capable of metastasizing spontaneously to CNS from a primary orthotopic tumor transplant (13). This model of spontaneous metastasis presents a rigorous challenge to tumor cell spread in a manner that closely recapitulates the multistep dissemination and clinical presentation of melanoma metastasis. Here we report our efforts using these unique brain metastatic lines (named 131/4-5B1 and 131/4-5B2) to elucidate molecular alterations that appear to contribute to the progression to the brain metastatic phenotype, one of which is endothelin receptor-B (EDNRB).
Materials and Methods
Cell lines
The human melanoma cell line WM239 was kindly provided by Dr. Meenhard Herlyn (The Wistar Institute) and used to develop the visceral metastatic variant 113/6-4L as well as the brain metastatic variants 131/4-5B1 and 131/4-5B2. The methodology used is outlined in Supplementary Fig. S1. All variants were karyotyped and then Illumina genotyped to ensure lack of mouse genomic contamination.
Microarray analysis
The gene expression profiles of cell lines were assessed on the HEEBO human genome set (44K Agilent-like oligo set from Invitrogen). Both brain metastatic cell lines (131/4-5B1 and 131/4-5B2) were compared with the poorly metastatic parental cell line WM239A and a derived highly metastatic variant 113/6-4L. Additional comparisons examined the expression profile of 113/6-4L relative to WM239A using 2 different passage numbers, incorporating a dye swap.
Confirmation of clinical and functional relevance
The expression of EDNRB and BCL2A1 in brain metastatic variants and in clinical samples was conducted as described in “Supplementary Materials and Methods.”
Effect of gene upregulation on intracranial melanoma growth
EDNRB or BCL2A1 cDNA was transduced into the 113/6-4L parental cell line and implanted intracranially. To this end, 25,000 6-4EDNRB cells were delivered using stereostatic set up. Control mice were implanted with the 113/6-4L-cell line transduced with the empty vector (6-4vector). Mice were monitored regularly and sacrificed when they developed signs of distress (e.g., lethargy, scruffiness, body weight loss >12%). Brains were sectioned and immunostained with HMB45 antibody to detect the presence of intracranial tumors. The cross-sectional area of these tumors was measured using Axiovision 4.6 software. Cross-sections from 6-4EDNRB and 6-4 vector were further immunostained for Ki67.
Effect of EDNRB inhibitor A192621 on lung metastases and intracranial melanoma growth
Mice were implanted orthotopically with 131/4-5B2 melanoma cell line and primary tumors resected, as described above. Mice were treated with either 60 mg/Kg A192621 or vehicle by daily gavage for over 5 months (n = 4). Treatment was initiated 1 week postprimary tumor resection. At the end of treatment period, mice were sacrificed and lungs excised, fixed, sectioned, and immunostained for HMB45 to detect the presence of melanoma metastases.
To examine whether A192621 could have an effect on intracranial melanoma tumors, mice were implanted as above with 131/4-5B2 melanoma cells. Mice were treated with 60 mg/Kg A192621 or vehicle. Treatment was initiated 4 days posttumor cell inoculation and continued until mice began to show signs of distress as above. Brains were sectioned and immunostained for HMB45. To examine whether cyclosporin A could improve the penetration of A192621 across the BBB, mice were treated by means of daily gavage with either cyclosporin A (50 mg/Kg daily gavage) + A192621 vehicle or cyclosporin A (50 mg/Kg) + A192621 (60 mg/Kg). Mice were treated for 2 weeks, brains were excised, immunostained, and cross-sectional area measured as above.
Results
Gene expression profile changes in the spontaneous brain-metastasizing variant cell lines
Microarray analysis was conducted to discern the nature of possible molecules/pathways that underlie the alterations responsible for the unique spontaneous brain metastatic phenotype of the 131/45B1 and 131/4-5B2 variant cell lines. Our model allows the examination of the sequence of transcriptional changes that occurs in the transition from poorly metastatic (i.e., WM239A parental cell line) to highly visceral metastatic variant (i.e., the 113/6-4L variant that metastasizes to sites such as lung and liver) and finally to the brain metastatic phenotype (i.e., the 131/4-5B1 and 131/4-5B2 variant cell lines). The lineage relationship of these cell lines is shown in Supplementary Fig. S1. The nature of expression changes noted in metastatic variants relative to poorly metastatic variants is shown in Table 1.
Table 1. List of genes differentially expressed in 131/4-5B1, 131/4-5B2, and 113/6-4L versus WM239.
| Average fold change | t test P-value | Common | Description |
|---|---|---|---|
| Upregulated | |||
| 5.2 | 3.7E-06 | D2S448 | Melanoma-associated gene |
| 4.2 | 2.0E-06 | IGFBP3 | Insulin-like growth factor binding protein 3 |
| 4.0 | 1.6E-08 | XAGE1 | X antigen family, member 1 |
| 3.7 | 1.5E-09 | GAGE7 | G antigen 7 |
| 3.5 | 1.1E-09 | GAGE7B | G antigen 7B |
| 3.3 | 1.0E-09 | GAGES | G antigen 5 |
| 3.2 | 5.0E-08 | PAGES | P antigen family, member 5 (prostate associated) |
| 3.2 | 8.7E-09 | GAGE4 | G antigen 4 |
| 2.7 | 1.6E-06 | SFRP1 | Secreted frizzled-related protein 1 |
| 2.5 | 1.5E-06 | GAGE1 | G antigen 1 |
| 2.5 | 4.3E-06 | GAGEE3 | G antigen, family E, 3 |
| 2.3 | 1.1E-05 | TM4SF19 | Transmembrane 4 L 6 family member 19 |
| 2.3 | 1.4E-05 | GAGES | G antigen 3 |
| 2.2 | 2.5E-05 | TMSL2 | Thymosin-like 2 |
| 2.2 | 5.6E-08 | HLA-B | Major histocompatibility complex, class I, B |
| 2.2 | 5.5E-06 | TMSLB | Thymosin-like 6 |
| 2.1 | 2.5E-05 | GAGE6 | G antigen 6 |
| 2.1 | 1.1E-05 | TMSL3 | Thymosin-like 3 |
| 2.1 | 1.3E-04 | TMSB4X | Thymosin, beta 4, X-linked |
| 2.1 | 1.6E-04 | TMSL4 | Thymosin-like 4 |
| 2.0 | B.0E-05 | TMSL1 | Thymosin-like 1 |
| 2.0 | 1.6E-05 | HLA-H | Major histocompatibility complex, class I, H (pseudogene) |
| 1.6 | 1.7E-05 | HLA-C | Major histocompatibility complex, class I, C |
| 1.8 | 4.9E-05 | SCML4 | sex comb on midleg-like 4 (Drosophila) |
| 1.6 | 1.5E-04 | TM7SF1 | Transmembrane 7 superfamily member 1 (upregulated in kidney) |
| 1.5 | 1.4E-04 | DKFZP566N034 | Hypothetical protein DKFZp566N034 |
| Downregulated | |||
| 0.65 | 2.9E-05 | SMS | Spermine synthase |
| 0.64 | 3.4E-05 | PLOD1 | Procollagen-lysine 1, 2-oxoglutarate 5-dioxygenase 1 |
| 0.62 | 8.0E-05 | ASPH | Aspartate beta-hydroxylase |
| 0.62 | 1.7E-05 | ATP2B1 | ATPase, Ca++ transporting, plasma membrane 1 |
| 0.59 | 9.7E-07 | C19orl28 | Chromosome 19 open reading frame 28 |
| 0.5B | 1.2E-04 | LY96 | Lymphocyte antigen 96 |
| 0.52 | 3.8E-04 | FABP7 | Fatty acid binding protein 7, brain |
| 0.52 | 4.4E-06 | RND3 | Rho family GTPase 3 |
| 0.48 | 4.9E-05 | CDH19 | Cadherin 19, type 2 |
| 0.43 | 1.4E-04 | SPP1 | Secreted phosphoprotein 1 (osteopontin, bone sialoprotein I) |
| 0.43 | 1.1E-05 | DKFZp761 D112 | Hypothetical protein DKFZp761D112 |
| 0.43 | 9.4E-07 | OVOS2 | Ovostatin 2 |
| 0.39 | 5.4E-06 | MGST1 | Microsomal glutathione S-transferase 1 |
| 0.31 | 3.9E-07 | na | Hypothetical gene supported by BC034933; BC068085 |
Relevant to the brain metastatic phenotype, our analysis shows that the 131/4-5B1 and 131/4-5B2 cell lines have similar expression profiles to one another but these are different from those of their visceral (lung/liver) metastatic parental cell line 113/6-4L, when using 113/6-4L or WM239 as a baseline for all comparisons (Supplementary Fig. S2A). The brain metastatic cell lines showed enrichment of a number of genes involved in cell development, neurogenesis, locomotion, and cell localization. Analysis of genes present in the comparison of 131/4-5B1 or 131/4-5B2 versus 113/6-4L, but not in the comparison of 11/6-4L versus WM239, showed differential expression in 87 genes in the variant brain metastatic cell lines (44 genes upregulated and 43 downregulated). Among these, 28 showed an upregulation of 1.5-fold or more and 24 showed a downregulation greater than or equal to 0.67-fold (Table 2). Quantitative real-time PCR (qRT-PCR) was used to confirm the changes in gene expression noted by microarray analysis. To this end, multiple passages of brain metastatic variants and parental highly metastatic (visceral metastatic only) 113/6-4L were compared. To ensure that alterations in gene expression were caused by the therapeutic regimen used to treat the mice from which the brain metastatic variants were derived (i.e., metronomic chemotherapy using the combination of vinblastine and cyclophosphamide), 131/4-5B1 and 131/4-5B2 were further compared with other visceral metastatic variants (named 113/7-4L and 113/8-2L). Both 7-4L and 8-2L were derived from mice that had been similarly implanted with 113/6-4L and exposed to the same therapeutic regimen. Some genes such as dopachrome tautomerase (DCT) and candidate of metastasis (COM1) showed the expected upregulation in 131/4-5B1 and B2 in comparison with to 113/6-4L, but were also upregulated in the visceral metastatic 113/7-4L and 113/8-2L cell lines. Genes chosen for further studies were those that showed significant upregulation in both 131/4-5B1 and B2 versus the 113/6-4L cells (but not in 113/7-4L and 113/8-2L). These genes included, among others, EDNRB and BCL2A1 (Supplementary Fig. S2B and S2C and Fig. S3). Below we describe the efforts undertaken to examine the relevance of these genes to CNS metastatic disease.
Table 2. List of genes differentially expressed in 131/4-5B1 and B2 versus 113/6-4L but not 113/6-4L versus WM239.
| Normalized | t test P-value | Common | Description |
|---|---|---|---|
| Upregulated | |||
| 5.3 | 8.9E-06 | VEX | Variable charge, X-linked |
| 4.8 | 7.9E-05 | VCX3A | Variable charge, X-linked 3A |
| 3.5 | 6.1E-04 | na | Hypothetical gene supported by BC056506; NM 004679 |
| 2.7 | 5.2E-04 | NELL1 | NEL-like 1 (chicken) |
| 2.7 | 5.9E-06 | C10orf33 | Chromosome 10 open reading frame 33 |
| 2.1 | 2.1E-03 | EDNRB | Endothelin receptor type B |
| 2.1 | 3.7E-04 | DCT | Dopachrome tautomerase (dopachrome delta-isornerase) |
| 2.0 | 5.0E-03 | P8 | p8 protein (candidate of metastasis 1) |
| 1.9 | 1.1E-02 | FLJ32942 | Hypothetical protein FLJ32942 |
| 1.7 | 4.2E-03 | CXorf4B | Chromosome X open reading frame 43 |
| 1.7 | 2.1E-03 | VCY | Variable charge, Y-linked |
| 1.7 | 3.9E-04 | PLXNC1 | Plexin C1 |
| 1.7 | 4.4E-03 | BCL2A1 | BCL2-related protein A1 |
| 1.7 | 5.7E-04 | KBTBD9 | Kelch repeat and BTB (POZ) domain containing 9 |
| 1.6 | 4.4E-05 | GPM6B | Glycoprotein M6B |
| 1.6 | 1.4E-04 | GPR143 | G protein-coupled receptor 143 |
| 1.6 | 2.SE-03 | ZFYVE16 | Zinc finger, FYVE domain containing 16 |
| 1.6 | 1.3E-04 | CDH3 | Cadherin 3, type 1, P-cadherin (placental) |
| 1.6 | 6.7E-04 | MCOLN3 | Mucolipin 3 |
| 1.6 | 5.5E-04 | LOC255313 | Hypothetical protein LOC255313 |
| 1.5 | 1.9E-05 | ATP6V1B2 | ATPase, H+ transporting, lysosomal 56/58kDa, V1 subunit B |
| 1.5 | 1.2E-03 | ITIH5 | Interalpha (globulin) inhibitor H5 |
| 1.5 | 9.0E-04 | ETS1 | v-ets erythroblastosis virus E26 oncogene homolog 1 (avian) |
| 1.5 | 1.9E-03 | C3orf6 | Chromosome 3 open reading frame 6 |
| 1.5 | 2.1E-02 | CXorf48 | Chromosome X open reading frame 48 |
| 1.5 | 1.4E-03 | KIAA0220 | PI-3-kinase–related kinase SMG-1-like |
| 1.5 | 3.1E-03 | MY05A | Myosin VA (heavy polypeptide 12, rnyoxin) |
| 1.5 | 7.7E-04 | SDCBP | Syndecan binding protein (syntenin) |
| Downregulated | |||
| 0.67 | 8.SE-04 | CDKN1A | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) |
| 0.67 | 2.8E-04 | KIAA1914 | KIAA1914 |
| 0.67 | 9.2E-02 | IGFBP5 | Insulin-like growth factor binding protein 5 |
| 0.66 | 9.0E-04 | CREG1 | Cellular repressor of E1 A-stimulated genes 1 |
| 0.66 | 5.9E-03 | PRDX1 | Peroxiredoxin 1 |
| 0.66 | 1.2E-03 | ADM | Adrenomedullin |
| 0.66 | 8.7E-03 | BCAS3 | Breast carcinoma amplified sequence 3 |
| 0.65 | 6.4E-05 | PPP2R4 | Protein phosphatase 2A, regulatory subunit B1 (PR 53) |
| 0.65 | 2.1E-04 | L1 CAM | L1 cell adhesion molecule |
| 0.65 | 8.0E-02 | TNC | Tenascin C (hexabrachion) |
| 0.65 | 2.2E-03 | COL9A3 | Collagen, type IX, alpha 3 |
| 0.63 | 2.7E-03 | NEDD9 | Neural precursor cell expressed |
| 0.63 | 2.1E-03 | TJP1 | Tight junction protein 1 (zona occludens 1) |
| 0.63 | 6.5E-03 | LOC2054O1 | Hypothetical protein LOC285401 |
| 0.62 | 6.3E-03 | ATP1B1 | ATPase, Na+/K+ transporting, beta 1 polypeptide |
| 0.62 | 1.3E-03 | PFN2 | Profilin 2 |
| 0.61 | 1.1E-01 | THBS1 | Thrombospondin 1 |
| 0.56 | 4.1E-02 | CD74 | CD74 antigen |
| 0.56 | 2.4E-04 | GPR126 | G protein-coupled receptor 126 |
| 0.55 | 1.3E-03 | BIRC7 | Baculoviral IAP repeat-containing 7 (livin) |
| 0.53 | 4.2E-03 | SERPINA3 | Serine (or cysteine) proteinase inhibitor, clade A |
| 0.53 | 3.4E-04 | MTUS1 | Mitochondrial tumor suppressor 1 |
| 0.52 | 4.5E-03 | FAM38B | Family with sequence similarity 38, member B |
| 0.32 | 1.1E-03 | MPZ | Myelin protein zero (Charcot-Marie-Tooth neuropathy 1 B) |
EDNRB is expressed in clinical samples of melanoma CNS metastases
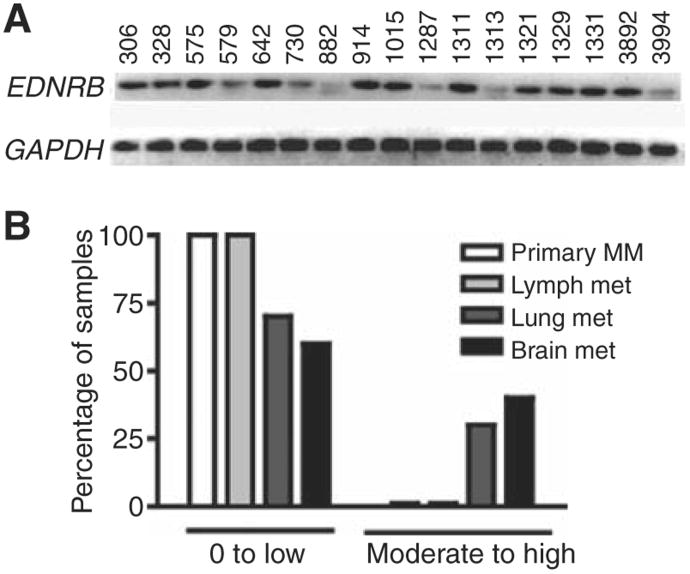
To discern the possible relevance of EDNRB to the clinical brain metastatic phenotype, we used RT-PCR to examine whether this gene was expressed in a panel of 17 clinical samples of CNS melanoma metastases (Fig. 1A). Eleven of the samples showed strong EDNRB expression. The remaining samples showed lower levels of expression. In contrast, the expression of the other endothelin receptor subtype, EDNRA, was not upregulated in the brain metastatic cell lines and showed significantly lower levels of expression in the panel of clinical samples of brain metastases when compared with EDNRB (Supplementary Fig. A and S4B, respectively).
Figure 1.
Confirmation of EDNRB expression in melanoma clinical tissue samples. A, RT-PCR examination of expression of EDNRB in clinical samples of melanoma CNS metastases. B, examination of EDNRB immunostaining in independent clinical samples showed lower levels of expression in primary and lymph node metastases than lung and brain metastases.
We conducted immunohistochemistry on independent clinical samples to compare the levels of EDNRB expression in melanoma brain metastases relative to visceral metastases (lymph node and lung) and primary melanoma. Moderate to high levels of cytoplasmic EDNRB immunostaining (≥20% of positively stained cells in any given sample) were only noted in lung and brain metastases (3 out of 10 lung and 4 out of 10 brain metastases). This stands in contrast to primary melanomas or lymph node metastases for which all showed immunostaining levels that were none to low (0% –10% positively stained cells in sample; Fig. 1B and Supplementary Fig. S4C–S4H).
EDNRB overexpression enhances metastatic potential
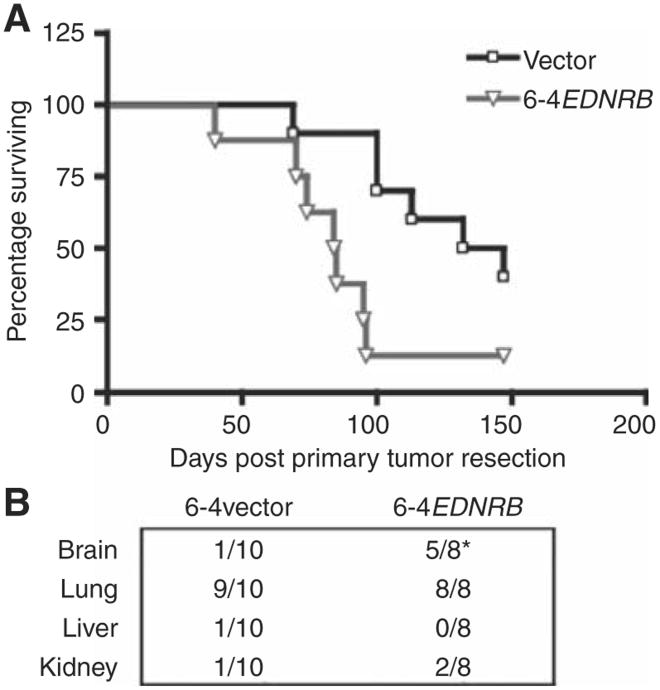
To examine the significance of EDNRB upregulation to the process of spontaneous metastatic disease (both visceral and CNS), we made use of lentiviral vectors to induce stable alterations of EDNRB expression in the visceral metastatic 113/6-4L cell line (Supplementary Fig. S5A–S5C). We used these cells to examine the effect of EDNRB upregulation on the spontaneous metastasis. The induced overexpression of this gene resulted in enhanced metastatic potential, leading to more rapid progression of extensive lung metastasis that was characterized symptomatically as breathing difficulty and that resulted both in a significantly shorter median survival of mice that had been implanted orthotopically with the 6-4EDNRB cells (84.5 days) compared with control cells transduced with empty lentiviral vector (6-4vector; 134.5 days; Logrank test P < 0.05; Fig. 2A) and a higher hazard ratio (HR = 3.26). More pertinent with respect to the role of this gene to its contribution to spontaneous CNS metastatic disease, we noted a higher incidence of brain metastases in the mice orthotopically implanted with 6-4EDNRB cells (5 out of 8 mice) compared with the 6-4vector-control group (1 out of 10 mice) (Fisher's exact test P < 0.05). Importantly, this enhanced incidence of metastatic disease was limited to the CNS because other organs such as liver and kidney failed to show any alterations in frequency of metastatic disease between EDNRB overexpressing cells and the vector control (Fig. 2B). Overall, these results suggest that EDNRB upregulation contributesto a prometastatic role, which can ultimately result in increased melanoma seeding/colonization of the CNS.
Figure 2.
Confirmation of functional relevance of EDNRB. A, orthotopic implantation of cells overexpressing ENDRB (6-4EDNRB) leads to more aggressive spontaneous metastatic disease and shorter median survival (P < 0.05) when compared with controls. B, a higher incidence of brain metastases in mice implanted with cell lines overexpressing EDNRB was also noted (6-4EDNRB; 5 out of 8 mice) when compared with control 6-4vector (1 out of 10 mice; *, Fisher's exact test P < 0.05). Similar alterations in the frequency of metastatic disease were not noted n other organs such as the kidney and liver.
EDNRB overexpression facilitates intracranial melanoma growth
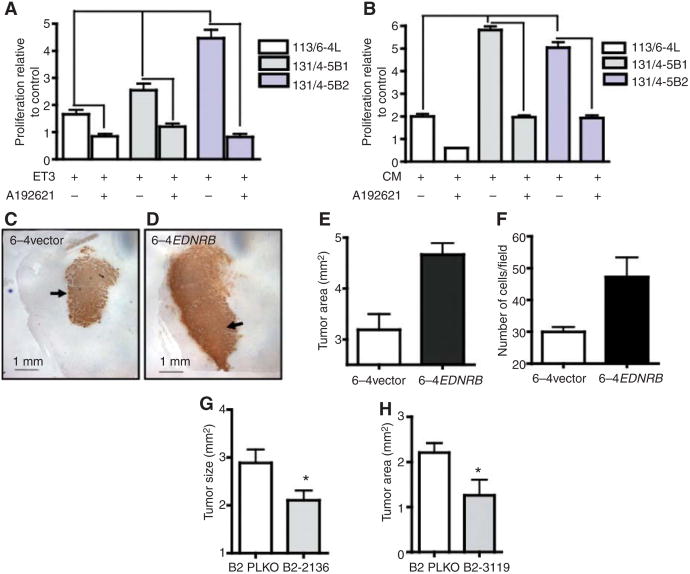
To examine the mechanism that may regulate the enhanced incidence of CNS metastases noted with the EDNRB overexpressing cell line, we examined whether EDNRB could facilitate preferential melanoma proliferation within the CNS microenvironment. We postulated that this effect may be the result of interaction of EDNRB with its ligands (endothelin-1, -2, or -3) to induce melanoma proliferation. In this regard, high levels of expression of endothelin-3 (ET3) have been noted in the brain (14). We also noted expression of ET3 and lower expression of ET1 in severe combined immunodeficient mice (SCID) mice (Supplementary Fig. S6A). In this case, expression of ET3 was primarily associated with neurons in the cortex and cerebellum (Supplementary Fig. S6B–S6D). Thus, it is possible that interaction of EDNRB with ET3 may lead to enhanced tumor growth in organs such as the brain in which these molecules are expressed, and thus lead to an increased incidence of CNS metastases. Indeed, as would be expected by the higher levels of EDNRB expression, both brain metastatic cells lines showed greater proliferation in the presence of ET3 (Fig. 3A) compared with the visceral metastatic variant 113/6-4L cell line. Moreover, this enhanced proliferation could be blocked by the addition of the EDNRB-specific inhibitor A192621. In the absence of ET3, A192621 failed to induce a significant inhibitory effect (Supplementary Fig. S7A).
Figure 3.
Role of EDNRB in melanoma cell proliferation and intracranial melanoma growth. A, both brain metastatic cell lines 131/4-5B1 and B2 show increased proliferation in the presence of EDNRB ligand ET3, which is reversed by the addition of EDNRB-specific inhibitor A192621 (one-way ANOVA, P < 0.05). Values are expressed as proliferation relative to the respective cell line cultured in the absence of ET3. B, brain-metastatic cell lines show enhanced proliferation in the presence of brain-CM compared with the visceral metastatic parental 113/6-4L subline. This enhanced proliferation was inhibited by A192621 (one-way ANOVA, P < 0.05). Values are expressed as proliferation relative to the respective cell line cultured in the absence of ET3. C and D, intracranially implanted 6-4 EDNRB cells formed larger tumors (indicated by arrows) when compared with empty vector control. Tumor area in brain cross-sections was significantly greater in 6-4 EDNRB group (t test P < 0.05; E) and showed higher number of Ki67-positive cells (t test, P < 0.05; F). All values are expressed as mean ±SEM. G and H, knockdown of EDNRB in 131/4-5B2cell line (knockdown lines hp2136 and hp3119, respectively) resulted in a significant decrease in intracranial tumor size (t test, P < 0.05).
We have previously reported that the brain metastatic cell lines 131/4-5B1 and B2, when compared with visceral metastatic parental variants, display enhanced proliferation in the presence of brain-conditioned media (CM; 13). We asked whether this effect could be the result of interaction of EDNRB with endothelins present in the microenvironment of the CNS. Our results show that the enhanced proliferation mediated by brain-CM could be blocked by the EDNRB inhibitor A192621 (Fig. 3B), implicating the endothelins as major drivers of CNS-stimulated proliferation in the brain metastatic melanoma cell lines. However, because the inhibitory effect mediated by A192621 maybe influenced by mechanisms other than EDNRB inhibition (15), we examined whether this effect could also be achieved through genetically mediated downregulation of EDNRB. Stable knockdown of this gene was achieved by shRNA and a number of clones were chosen that showed significant knockdown (Supplementary Fig. S5C). When cultured in the presence of brain-CM, selected knockdown cell lines for B2 showed decreased proliferative potential when compared with control cell lines (respective 131/4-5B2 cell line transduced with empty vector PLKO (plasmid lentiviral cloning vector); Supplementary Fig. S7B and S7C).
Collectively these results implicated the EDNRB axis as a potentially relevant factor that facilitates melanoma cell growth within the CNS. To directly examine whether overexpression of this molecule could indeed lead to preferential growth of EDNRB overexpressing cells once they have reached the CNS, we implanted EDNRB overexpressing cells (6-4EDNRB) intracranially in SCID mice. Our results showed that 6-4EDNRB cells implanted intracranially gave rise to larger tumors than those associated with 6-4 vector cells (Fig. 3C–E, Supplementary Fig. S7D). These larger tumors also showed increased levels of cell proliferation as denoted by the presence of higher levels of Ki67-positive cells (Fig. 3F). In contrast, the overexpression of EDNRB failed to enhance tumor cell proliferation in vitro (Supplementary Fig. S7E) and in the case of orthotopic (subdermal) implantation this overexpression led to a slight delay in subdermal tumor growth (data not shown).
The relevance of EDNRB was further examined by inducing stable knockdown in the brain metastatic variant 131/4-5B2. When implanted intracranially, the knockdown cell lines (B2-hp2136 and B2-hp3119) generated significantly smaller tumors when compared with the empty vector control (B2 PLKO; Fig. 3G and H).
Therapeutic targeting of EDNRB in visceral metastases and intracranial melanoma
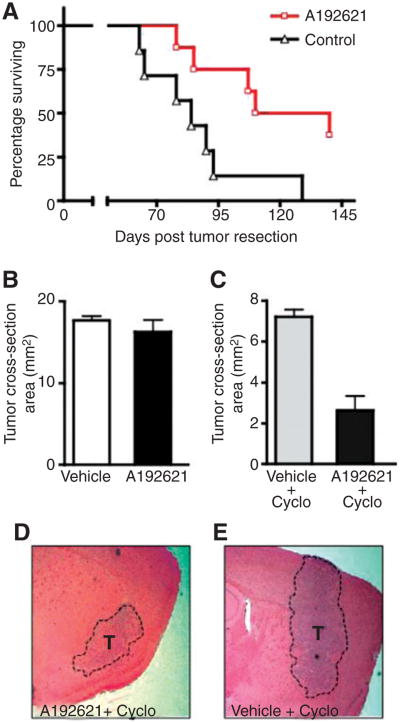
Given the in vitro efficacy of EDNRB-specific inhibitor A192621 against melanoma cell lines shown here and its ability to significantly inhibit primary tumor growth (16), we asked whether this inhibitor could also affect visceral metastases or intracranial melanoma growth in vivo. Using the 131/4-5B2 spontaneous metastasis model described above, we noted that daily treatment with A192621 (60 mg/kg, daily gavage) mediated significant suppression of visceral metastatic disease leading to both a longer median survival (125 days for A192621 treated mice versus 84 days for vehicle control; P < 0.05) as well as an improved HR (0.28; Fig. 4A). Despite the efficacy noted in this setting, A192621 given in the same fashion (60 m/Kg daily gavage for 1 month) failed to induce an inhibitory effect against intracranially implanted 131/4-5B2 melanoma tumors (Fig. 4B). We speculate that this lack of efficacy might be ascribed to the inability of A192621 to cross the BBB (17); a limitation that is common to most chemotherapeutic agents presently available.
Figure 4.
Therapeutic targeting of EDNRB in metastatic disease. A, using the spontaneous brain metastatic 131/4-5B2 model, EDNRB-specific inhibitor A192621 mediated suppression of metastatic disease leading to significant prolongation of median survival (log rank test, P < 0.05) and improved HR when compared with mice treated with vehicle control. B, daily treatment with A192621 failed to inhibit intracranial melanoma tumors. C to E, a combination of A192621 and cyclosporin A resulted in a significant decrease in intracranial tumor size when compared with cyclosporin A + vehicle (Student's t test, P < 0.05).
The impermeability associated with BBB is the result of multiple factors; included among these is the presence of numerous efflux pumps (18). Previous studies have shown that inhibition of the efflux pump P-glycoprotein (PGP) can result in increased levels of drugs within the brain parenchyma (19). Therefore, we examined whether a combination of A192621 with a known PGP-inhibitor, such as cyclosporin A, could mediate improved penetration and permeability of the EDNRB inhibitor and thus result in an improved therapeutic effect against intracranial melanomas. In vitro examination showed that A192621 did not act synergistically with cyclosporin A to induce increased cellular toxicity against 131/4-5B2 melanoma cells (i.e., similar toxicity was noted for cyclosporin A alone and its combination with A192621; Supplementary Fig. S7F). This combination did result in increase levels of toxicity in mice (indicated by loss of weight and lethargy). As such, treatment could only be conducted for 2 weeks. Nevertheless, at the end of this treatment period we noted that mice treated with the combination of A192621 + cyclosporin A had smaller intracranial melanomas compared with mice treated with cyclosporin A alone (Fig. 4C–E; t test P < 0.05). These results highlight both the relevance of EDNRB axis to melanoma brain metastases and the involvement of PGP, likely among other factors, in preventing effective delivery of the EDNRB inhibitor A192621 to the brain parenchyma.
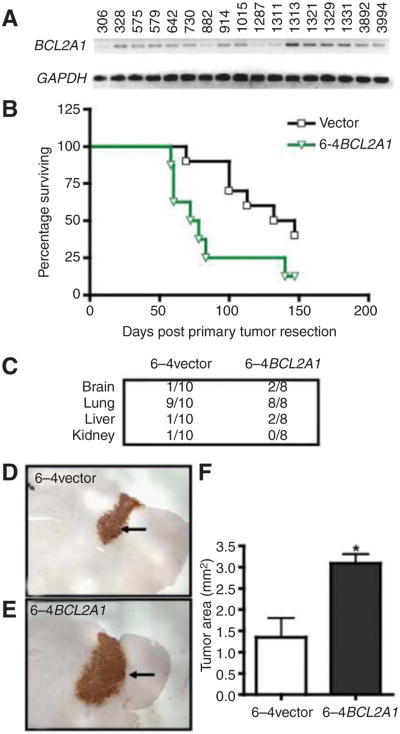
BCL2a1 expression enhances tumor cell survival in CNS leading to intracranial tumor growth
The ability of EDNRB overexpression to mediate increases in spontaneous CNS metastasis is contrasted with that of other genes highlighted by our expression profile analysis. In the case of BCL2A1 for instance, its expression was noted to be significantly higher in the brain metastatic variants (compared with parental 113/6-4L; Supplementary Fig. S2C) and was also noted in a majority of clinical samples of CNS melanoma metastases (13 out of 17 samples showed moderate levels of expression; Fig. 5A). Although a number of studies have noted specific upregulation of BCL2A1 in melanomas (20), no direct correlation has been established between expression and metastatic potential. Our results show that the induced overexpression of BCL2A1 in the 113/6-4L cell line mediates a robust spontaneous visceral prometastatic effect which nevertheless did not translate into a significant increase in incidence of spontaneous CNS metastases or in the distribution of metastases in other organs (Fig. 5B and C). Nevertheless, we found that intracranially implanted 6-4BCL2A1 cells gave rise to larger tumors than those from similarly implanted 6-4vector cells (Fig. 5D–F; Supplementary Fig. S7D). This suggests that the overexpression of BCL2A1 does not influence the dissemination of tumor cells from the primary tumor or from lung metastases, but that it may exert a postextravasation metastatic effect once the melanoma cells have reached the CNS (i.e., colonization of secondary site). Given the antiapoptotic function ascribed to BCL2A1 (21, 22), it is possible that its overexpression may enhance tumor cell survival leading to larger intracranial melanomas (as well as higher visceral metastatic potential). Indeed, we have found that the brain metastatic variants show enhanced resistance to inhibitory/proapoptotic agents such as TNF-α and that this resistance can be attributed in part to the overexpression of BCL2A1 (Supplementary Fig. S8A and S8B). Within the context of the CNS, such an effect could mediate resistance to the inhibitory activity that activated microglial cells (through the release of factors such as TNF-α) appear to play against intracranial tumors (23).
Figure 5.
Examination of the role of BCL2A1 in intracranial tumor growth. A, RT-PCR examination of BCL2A1 in clinical samples of melanoma CNS metastases. B, mice implanted orthotopically with 6-4BCL2A1 cells showed more aggressive spontaneous metastatic disease leading to a shorter median survival. C, the overexpression of BCL2A1 did not lead to a significant increase in incidence of metastatic disease in various organs. D to F, intracranially implanted 6-4BCL2A1 cells gave rise to larger intracranial melanomas when compared with 6-4vector cells.
Discussion
Brain metastases remain a significant and increasingly important therapeutic challenge. Development of effective targeted therapeutic approaches can be facilitated by first improving our understanding of the molecular and cellular mediators, and pathways, that are relevant to the brain metastatic phenotype (24, 25). Thus far, knowledge of the factors that contribute to the prevalence of melanoma CNS metastases is meager. In this regard, it is unclear whether the genes that regulate visceral metastases are also relevant to brain metastatic disease or whether spread to CNS is mediated by brain-specific mechanisms. Resolution of this important issue can have an influential role on the identification of relevant therapeutic targets as well as the design of treatment strategies (preventive vs. interventive; 1). Previous work suggests that in breast cancer, a number of genes previously shown to be relevant to this and various other cancer types can mediate metastatic spread to both CNS and lungs (26). It is possible that in a similar manner the extremely high incidence of CNS metastases in malignant melanoma may be reflective of alterations in gene expression that occur commonly in this cancer type and which can influence visceral and brain metastatic disease. The evidence presented here appears to support such a hypothesis and implicates the upregulation of EDNRB as an important contributor to metastatic colonization and growth/survival in the brain.
Our results provide direct evidence that EDNRB overexpression leads to enhanced metastatic aggressiveness of melanoma and decreased median survival as a result of advanced metastatic disease to lungs. This data correlate well and extends previous studies, which have implicated EDNRB as a marker of melanoma progression affecting processes relevant to metastatic disease (27–29). Beyond this role, evidence obtained from our model now implicates EDNRB also as relevant factor in increasing the incidence of spontaneous CNS metastases and promoting intracranial melanoma growth. This effect appears to be mediated, at least in part, through the interaction EDNRB with its ligands, potentially ET3 which is highly expressed in the brain, so as to enhance melanoma cell proliferation within the CNS. It is possible that the high levels of EDNRB may also contribute to increase the incidence of brain metastases by facilitating other processes such as invasion and angiogenesis. Because ET1 and ET3 are highly expressed in lungs (14), a similar interaction of EDNRB with these ligands may mediate the increased metastatic lung disease associated with EDNRB overexpression. The fact that BCL2A1 overexpression did not affect the incidence of spontaneous CNS metastases and yet translated into enhanced intracranial tumor growth highlights the different results that can be obtained when using spontaneous or experimental metastasis models. It would be interesting to examine whether other genes previously suggested to be relevant to melanoma CNS metastases (e.g., STAT3, TGFβ-2, melanotransferrin; 30–32) can indeed confer enhanced spontaneous CNS metastatic potential or whether their effect is only associated with facilitating growth at the secondary site.
Overall, our results suggest that alterations in EDNRB expression may be an important aspect of the stepwise progression of melanoma to the brain metastatic phenotype. Because EDNRB also appears to influence visceral metastatic disease, targeting of the EDNRB signaling pathway might represent a promising therapeutic approach for anti-CNS melanoma therapy. EDNRB inhibitors have already shown significant preclinical effects with respect to inhibiting the growth of primary melanoma (33), in contrast, their effectiveness in treatment of melanoma CNS metastases has not been examined. This is critical because agents that show efficacy in primary tumor therapy models may lack activity when treating metastatic disease (34). Our identification of the potential relationship of EDNRB with brain metastases lends support for such efforts being conducted and indeed this is being pursued in ongoing studies in our laboratory.
An important aspect in the design of effective therapeutic treatments against CNS metastases is the unique requirement that therapeutic agent show not only activity against the tumor but also the ability to cross the BBB (35, 36). In this respect, as reported here, we have found that despite the in vitro and in vivo antitumor activity ascribed to A192621 EDNRB-specific inhibitor, and its significant efficacy against visceral metastatic disease, it was not able to inhibit the growth of intracranially implanted melanomas, at least when given alone. This lack of efficacy could be a consequence of poor penetration of this agent across the BBB. The improved outcome associated with the combination of A192621 with a PGP inhibitor (cyclosporin A) seems to bolster the hypothesis that the lack of A192621 permeability is, at least in part, because of this efflux pump which is known to be highly expressed by brain endothelial cells. The efficacy of A192621 (at least in combination with cyclosporin A) to inhibit intracranial melanoma suggests that the development of BBB-permeable EDNRB inhibitors could provide an effective approach for treatment of CNS metastases. It is worth noting that the significant efficacy achieved with A192621 against visceral disease in our studies contrasts the minimal effect achieved with other EDNRB inhibitors in clinical trials (i.e., bosentan). It is likely that these differences may be ascribed to a more effective binding and inhibition of EDNRB achieved with A192621 (37, 38). Another important difference is that our studies were conducted in an adjuvant like setting (i.e., against residual disease and aimed at preventing the eventual emergence of overt metastases) rather than in the advanced stages of metastatic disease commonly studied in clinical trials. Given that no effective adjuvant therapies against malignant melanoma are presently available, a reexamination of the use of EDNRB inhibitors in this setting in clinical trials may be warranted.
Examination of other genes highlighted by our microarray studies (which are likely to play a role in brain metastases) is being conducted. This analysis may provide additional targets for therapy. Interestingly, none of the genes highlighted in our analysis of melanoma brain metastatic disease overlap with those previously noted as influential to the brain metastatic scenario in breast cancer (26, 39). This suggests that the brain metastatic phenotype may not have a common genetic signature across various cancer types but rather that each cancer type likely makes use of different mechanisms to effectively colonize the CNS. Our results provide identification of potential prognostic molecular markers of melanoma brain metastases which are critical for identifying patients that are at higher risk of developing CNS metastases who could benefit from early aggressive preventive treatment.
In addition to potentially shedding light on mediators that may be relevant to brain metastasis, our model can also serve as a tool to examine the efficacy of novel antimelanoma therapies. Recent successes achieved with BRAFV600E inhibitors highlight the importance of this mutant protein to melanoma metastasis (4). Our model expresses this predominant mutation, thus it represents an important tool to examine its relevance to brain metastatic disease and the effects of its inhibition in therapy against CNS metastases.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Meenhard Herlyn (The Wistar Institute) for the WM239 melanoma cell line, Abbott Laboratories for the EDNRB-specific inhibitor A192621, and TACMASS (Tissue Acquisition and Cellular/Molecular Analysis Shared Service; supported by the Arizona Cancer Center Support Grant NIH CA023074) for the immunohistochemical staining and scoring of clinical samples.
Grant Support: W. Cruz-Munoz was supported by a Canadian Cancer Society Research Institute of Canada/Terry Fox Foundation fellowship. M.L. Jaramillo, M. Banville, C. Collins, A. Nantel, and M.D. O'Connor-McCourt were supported by the NRC Genome Health Initiative Cancer Program (NRC publication number NRC53130). This work was supported by grants to R.S. Kerbel from the NIH-USA (CA-41233) and also by Ontario Institute for Cancer (OICR). R.S. Kerbel holds a Tier I Canada Research Chair in Tumor Biology, Angiogenesis and Antiangiogenic Therapy.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
Authors' Contributions: Conception and design: W. Cruz-Munoz, M.L. Jaramillo, S. Man, M.D. O'Connor-McCourt, R.S. Kerbel
Development of methodology: W. Cruz-Munoz, M.L. Jaramillo, S. Man, M. Banville, L.D. Cranmer
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): W. Cruz-Munoz, R.S. Kerbel, M.L. Jaramillo, M. Banville, G. Francia, S.S. Morgan, L.D. Cranmer
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): W. Cruz-Munoz, M.L. Jaramillo, A. Nantel, S.S. Morgan, M.D. O'Connor-McCourt
Writing, review, and/or revision of the manuscript: W. Cruz-Munoz, M.L. Jaramillo, S.S. Morgan, L.D. Cranmer, M.D. O'Connor-McCourt, R.S. Kerbel
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): W. Cruz-Munoz, S. Man, P. Xu, M. Banville, C. Collins, M.D. O'Connor-McCourt
Study supervision: W. Cruz-Munoz, S. Man, M.D. O'Connor-McCourt, R.S. Kerbel
References
- 1.Maher EA, Mietz J, Arteaga CL, DePinho RA, Mohla S. Brain metastasis: opportunities in basic and translational research. Cancer Res. 2009;69:6015–20. doi: 10.1158/0008-5472.CAN-08-4347. [DOI] [PubMed] [Google Scholar]
- 2.Tarhini AA, Agarwala SS. Cutaneous melanoma: available therapy for metastatic disease. Dermatol Ther. 2006;19:19–25. doi: 10.1111/j.1529-8019.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- 3.Bafaloukos D, Gogas H. The treatment of brain metastases in melanoma patients. Cancer Treat Rev. 2004;30:515–20. doi: 10.1016/j.ctrv.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vultur A, Villanueva J, Herlyn M. BRAF inhibitor unveils its potential against advanced melanoma. Cancer Cell. 2010;18:301–2. doi: 10.1016/j.ccr.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13:1648–55. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 7.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75:5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 8.Soffietti R, Ruda R, Trevisan E. Brain metastases: current management and new developments. Curr Opin Oncol. 2008;20:676–84. doi: 10.1097/CCO.0b013e32831186fe. [DOI] [PubMed] [Google Scholar]
- 9.Aragon-Ching JB, Zujewski JA. CNS metastasis: an old problem in a new guise. Clin Cancer Res. 2007;13:1644–7. doi: 10.1158/1078-0432.CCR-07-0096. [DOI] [PubMed] [Google Scholar]
- 10.Tarhini AA, Agarwala SS. Management of brain metastases in patients with melanoma. Curr Opin Oncol. 2004;16:161–6. doi: 10.1097/00001622-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Mayer M. A patient perspective on brain metastases in breast cancer. Clin Cancer Res. 2007;13:1623–4. doi: 10.1158/1078-0432.CCR-06-2842. [DOI] [PubMed] [Google Scholar]
- 12.Palmieri D, Chambers AF, Felding- Habermann B, Huang S, Steeg PS. The biology of metastasis to a sanctuary site. Clin Cancer Res. 2007;13:1656–62. doi: 10.1158/1078-0432.CCR-06-2659. [DOI] [PubMed] [Google Scholar]
- 13.Cruz-Munoz W, Man S, Xu P, Kerbel RS. Development of a preclinical model of spontaneous human melanoma central nervous system metastasis. Cancer Res. 2008;68:4500–5. doi: 10.1158/0008-5472.CAN-08-0041. [DOI] [PubMed] [Google Scholar]
- 14.Fagan KA, McMurtry IF, Rodman DM. Role of endothelin-1 in lung disease. Respir Res. 2001;2:90–101. doi: 10.1186/rr44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montgomery JP, Patterson PH. Endothelin receptor B antagonists decrease glioma cell viability independently of their cognate receptor. BMC Cancer. 2008;8:354. doi: 10.1186/1471-2407-8-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosano L, Spinella F, Genovesi G, Di Castro V, Natali PG, Bagnato A. Endothelin-B receptor blockade inhibits molecular effectors of melanoma cell progression. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S136–9. doi: 10.1097/01.fjc.0000166247.35992.dd. [DOI] [PubMed] [Google Scholar]
- 17.Chichorro JG, Zampronio AR, Rae GA. Endothelin ET(B) receptor antagonist reduces mechanical allodynia in rats with trigeminal neuropathic pain. Exp Biol Med (Maywood) 2006;231:1136–40. [PubMed] [Google Scholar]
- 18.Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–74. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 19.Kemper EM, Verheij M, Boogerd W, Beijnen JH, van Tellingen O. Improved penetration of docetaxel into the brain by co-administration of inhibitors of P-glycoprotein. Eur J Cancer. 2004;40:1269–74. doi: 10.1016/j.ejca.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Shankavaram UT, Reinhold WC, Nishizuka S, Major S, Morita D, Chary KK, et al. Transcript and protein expression profiles of the NCI-60 cancer cell panel: an integromic microarray study. Mol Cancer Ther. 2007;6:820–32. doi: 10.1158/1535-7163.MCT-06-0650. [DOI] [PubMed] [Google Scholar]
- 21.Park IC, Lee SH, Whang DY, Hong WS, Choi SS, Shin HS, et al. Expression of a novel Bcl-2 related gene, Bfl-1, in various human cancers and cancer cell lines. Anticancer Res. 1997;17:4619–22. [PubMed] [Google Scholar]
- 22.Karsan A, Yee E, Harlan JM. Endothelial cell death induced by tumor necrosis factor-alpha is inhibited by the Bcl-2 family member, A1. J Biol Chem. 1996;271:27201–4. doi: 10.1074/jbc.271.44.27201. [DOI] [PubMed] [Google Scholar]
- 23.He BP, Wang JJ, Zhang X, Wu Y, Wang M, Bay BH, et al. Differential reactions of microglia to brain metastasis of lung cancer. Mol Med. 2006;12:161–70. doi: 10.2119/2006-00033.He. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruz-Munoz W, Kerbel RS. Preclinical approaches to study the biology and treatment of brain metastases. Semin Cancer Biol. 2011;21:123–30. doi: 10.1016/j.semcancer.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11:352–63. doi: 10.1038/nrc3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–9. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demunter A, De Wolf-Peeters C, Degreef H, Stas M, van den Oord JJ. Expression of the endothelin-B receptor in pigment cell lesions of the skin. Evidence for its role as tumor progression marker in malignant melanoma. Virchows Arch. 2001;438:485–91. doi: 10.1007/s004280000362. [DOI] [PubMed] [Google Scholar]
- 28.Lahav R, Suva ML, Rimoldi D, Patterson PH, Stamenkovic I. Endothelin receptor B inhibition triggers apoptosis and enhances angiogenesis in melanomas. Cancer Res. 2004;64:8945–53. doi: 10.1158/0008-5472.CAN-04-1510. [DOI] [PubMed] [Google Scholar]
- 29.Bagnato A, Rosano L, Spinella F, Di Castro V, Tecce R, Natali PG. Endothelin B receptor blockade inhibits dynamics of cell interactions and communications in melanoma cell progression. Cancer Res. 2004;64:1436–43. doi: 10.1158/0008-5472.can-03-2344. [DOI] [PubMed] [Google Scholar]
- 30.Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, et al. Activation of STAT3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–96. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Zhang F, Tsan R, Fidler IJ. Transforming growth factor-beta2 is a molecular determinant for site-specific melanoma metastasis in the brain. Cancer Res. 2009;69:828–35. doi: 10.1158/0008-5472.CAN-08-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolland Y, Demeule M, Fenart L, Beliveau R. Inhibition of melanoma brain metastasis by targeting melanotransferrin at the cell surface. Pigment Cell Melanoma Res. 2009;22:86–98. doi: 10.1111/j.1755-148X.2008.00525.x. [DOI] [PubMed] [Google Scholar]
- 33.Lahav R, Heffner G, Patterson PH. An endothelin receptor B antagonist inhibits growth and induces cell death in human melanoma cells in vitro and in vivo. Proc Natl Acad Sci U S A. 1999;96:11496–500. doi: 10.1073/pnas.96.20.11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer. 2011;11:135–41. doi: 10.1038/nrc3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong ET, Berkenblit A. The role of topotecan in the treatment of brain metastases. Oncologist. 2004;9:68–79. doi: 10.1634/theoncologist.9-1-68. [DOI] [PubMed] [Google Scholar]
- 36.Cranmer LD, Trevor KT, Bandlamuri S, Hersh EM. Rodent models of brain metastasis in melanoma. Melanoma Res. 2005;15:325–56. doi: 10.1097/00008390-200510000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Ohlstein EH, Nambi P, Hay DW, Gellai M, Brooks DP, Luengo J, et al. Nonpeptide endothelin receptor antagonists. XI. Pharmacological characterization of SB 234551, a high-affinity and selective nonpeptide ETA receptor antagonist. J Pharmacol Exp Ther. 1998;286:650–6. [PubMed] [Google Scholar]
- 38.Wu-Wong JR, Dixon DB, Chiou WJ, Sorensen BK, Liu G, Jae HS, et al. Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: in vitro studies. Clin Sci (Lond) 2002;103(Suppl 48):107S–11S. doi: 10.1042/CS103S107S. [DOI] [PubMed] [Google Scholar]
- 39.Chen EI, Hewel J, Krueger JS, Tiraby C, Weber MR, Kralli A, 3rd, et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 2007;67:1472–86. doi: 10.1158/0008-5472.CAN-06-3137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.