Abstract
The failure rate of randomized phase III oncology clinical trials is extremely high, even when preceded by encouraging preclinical studies and phase II trial results of the same therapy. Thus there is considerable effort being made to improve the predictive clinical potential of preclinical models, in addition to improving phase II trial design. With respect to the former, preclinical models have historically relied heavily on treatment of primary spontaneous or transplanted tumors rather than the more common and therapeutically challenging clinical trial circumstance of advanced metastatic disease. Here we show that the oral antiangiogenic tyrosine kinase inhibitor (TKI), sunitinib, which failed to meet primary or secondary survival endpoints in four separate phase III metastatic breast cancer (MBC) trials, either alone or with chemotherapy, similarly failed to show monotherapy or combination chemotherapy efficacy in a model of postsurgical advanced MBC, using a metastatic variant of the MDA-MB-231 triple negative human breast cancer. In contrast, the drug was effective when used to treat established orthotopic primary tumors. Similar results were obtained with pazopanib monotherapy, another antiangiogenic oral TKI. However, when an antibody targeting the VEGF pathway (DC101) was tested, it showed a trend in modestly improving the efficacy of paclitaxel therapy, thus resembling to a degree prior phase III clinical results of bevacizumab plus paclitaxel in MBC. Our results suggest the potential value of treating postsurgical advanced metastatic disease as a possible strategy to improve preclinical models for predicting outcomes in patients with metastatic disease.
Introduction
An enduring problem in oncology experimental therapeutics has been the limited value of models involving treatment of tumor-bearing mice to consistently predict outcomes later assessed in clinical trials, particularly at the randomized phase III level1–4. A common scenario observed is positive and sometimes even remarkable preclinical activity which is then followed by complete failure in the clinic1–4. Such failures add substantially to the cost of approved agents as well as exposing cancer patients enrolled in such trials to ineffective therapies. As a result, there is considerable effort to identify potential causes for this discrepancy and develop significantly improved preclinical models1–4 such as genetically engineered mouse models of cancer and patient-derived xenografts (PDXs) as opposed to the historically more common use of transplantation of established cultured tumor cell lines grown as solid primary tumors.
While many factors have been proposed for the discrepant therapeutic outcomes observed between preclinical and clinical studies1–4 one factor which has received scant attention is the failure to duplicate in mice treatment of advanced visceral metastatic disease5, 6. Most phase I and II solid tumor clinical trials and the majority of phase III trials involve patients with such disease. In many or most cases the primary tumor has been surgically resected. The failure rate is extremely high in phase III metastatic therapy trials7 and when therapies succeed, the benefits in survival are frequently incremental8. Therefore we have developed several models of postsurgical advanced metastatic disease using established human tumor cell lines grown in immune deficient mice to mimic the more challenging circumstance of treating patients with metastatic disease5. In most cases, the cell lines used are variants previously selected in vivo for aggressive spontaneous metastatic spread after the primary orthotopic tumor has been surgically resected5. One such variant, called LM2-4, was serially selected in vivo from the commonly employed MDA-MB-231 triple negative human breast cancer cell line9.
Here we report the use of the aforementioned postsurgical model of LM2-4 to evaluate the impact of several antiangiogenic drugs, used alone or in combination with paclitaxel chemotherapy, and compare the results obtained with conventional treatment of established primary tumors. One of the drugs we tested is sunitinib, an oral TKI which targets VEGF and PDGF receptors, among several others10. Based partly on very encouraging preclinical results in three different established primary breast cancer models (a transgenic model, a chemically-induced rat model, and a human tumor xenograft model)10, and a bone colonization experiment10, sunitinib was subsequently evaluated in metastatic breast cancer patients; four independent phase III trials were undertaken11–15, three in combination with chemotherapy (paclitaxel, or docetaxel, or capecitabine). All four trials failed to meet efficacy endpoints of survival11–15. This stands in contrast to a phase III trial involving the anti-VEGF antibody, bevacizumab (Avastin®), when used with chemotherapy, e.g. paclitaxel, which provided a clinical benefit, at least in PFS, though not in OS16. We also tested another antiangiogenic TKI, pazopanib and a monoclonal antibody that targets the mouse VEGF receptor-2 (DC101). Sunitinib and DC101 were also evaluated with concurrent paclitaxel chemotherapy.
The purpose of these studies was to further validate the preclinical strategy of using postsurgical models of advanced metastatic disease to predict clinical outcomes involving treatment of patients with metastatic disease by addressing the following questions and comparing the results to prior phase III trial outcomes: i) is it the case that antiangiogenic drug monotherapy has reduced or no therapeutic benefit when treating mice with advanced metastatic disease in contrast to established primary tumors? ii) what is the impact on outcomes when chemotherapy is used in combination with the antiangiogenic agent? And, iii) is there a difference in outcomes when using TKIs vs antibodies in combination with chemotherapy?
Materials and Methods
Female CB-17 SCID mice were purchased from Charles River Canada, and female YFP SCID mice17 were bred in house from breeding pairs generously provided by Dr. Janusz Rak, McGill University, Montreal. Mice at 6 – 8 weeks of age were used. MDA-MB 231/LM2.4 is a variant cell line of MDA-MB 231 selected in vivo for aggressive spontaneous metastatic spread from established but resected primary tumors and was grown in cell culture as previously described9. Cell line authentication was carried out by genotyping using Illumina mouse linkage panel and confirmed to be human in origin. Routine mycoplasma screening is carried out in-house using commercial kits, which confirmed the cell line is mycoplasma free. Mammary fat pad injection (2x106 cells) was carried out as previously described9. Weekly caliper measurements were carried out to determine tumor growth and tumor volume was calculated using the formula a2b/2 where a is the width and b is the length. Treatment of primary tumors was initiated when average volume was approximately 100 – 150mm3, i.e., 12 – 15 days after cell injection. Surgical resection of the primary tumors was carried out when the average tumor size was 400mm3.
All mice were randomized just prior to initiation of treatment. Antiangiogenic drugs were generously provided by the manufacturers, namely, sunitinib (Pfizer), pazopanib (GSK), and DC101, the monoclonal antibody targeting mouse VEGFR-2 (ImClone/Eli Lilly). All drugs were prepared according to manufacturer’s specifications. Paclitaxel was dispensed by institutional cancer centre pharmacy at 60mg/ml and further diluted with normal saline to the appropriate concentration. Control mice received either vehicle and/or normal saline as appropriate. Sunitinib was administered by gavage at 60mg/kg dose daily for the first 14 days followed by 5 days daily with 2 days’ break thereafter in order to reduce toxicity as measured by weight loss. Pazopanib 150mg/kg was administered by gavage daily without interruption. Paclitaxel was administered intraperitoneally (ip) at 50mg/kg every 3 weeks in the studies which included combination with DC101 but the dose and schedule was changed to 30mg/kg once every 2 weeks in studies which involved combination with sunitinib or pazopanib in order to minimize toxicity observed in SCID mice18.
Results and Discussion
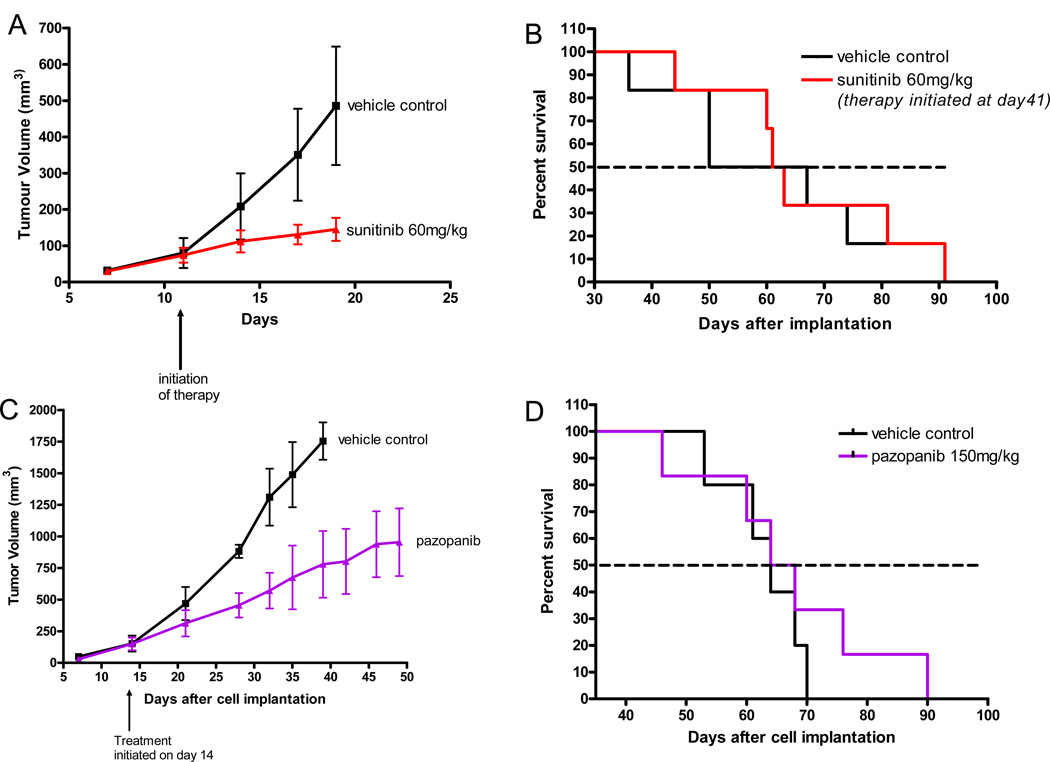
We first tested sunitinib on the growth of primary established orthotopic (mammary fat pad) tumors. Cells from the established variant of MDA-MB-231 called LM2.4, which was selected in vivo for aggressive spontaneous metastatic spread after surgical resection of the primary tumor9 were injected into the mammary fat pad of 6 – 8 week old female SCID mice, as described previously9 in the Materials and Methods. We did not use a luciferase tagged clone of LM2.419 since we have found that these cells have a reduced ability for spontaneous metastasis (unpublished observations), the basis of which is currently unknown. When the primary tumors reached a volume of approximately 100–150mm3, sunitinib was administered daily by gavage at a preclinically effective dose of 60mg/kg, and the treatment continued until endpoint. As shown in Fig. 1A, a robust growth delay was observed, similar to Abrams et al using another human breast cancer xenograft model (called MX-I)10. In the case of Abrams et al. this was paralleled by an increase in overall survival (OS), the extent of which was shown to be further enhanced by combination with chemotherapy, e.g. docetaxel10. In our case, the mice were sacrificed in this preliminary experiment at an earlier defined endpoint, namely, when the control group tumors reached an average size of approximately 500mm3 (Fig 1A). However, in another experiment, shown in Fig. 1B where the primary tumor was surgically resected (at day 20) and the same therapy initiated 3 weeks later (i.e., when the mice have established visceral metastatic disease based on numerous previous studies, e.g. ref. 9 in addition to reproducibility and lack of variability of the short median survival times) no impact of the same treatment on survival was observed. This pattern of discrepancy in outcomes is not specific for sunitinib as we observed a similar pattern using pazopanib (as shown in Fig. 1C and 1D) where primary tumors in control mice were allowed to grow in this case to endpoint of 1700mm3 and the therapy maintained until endpoint.
Figure 1. Differential impact of sunitinib or pazopanib monotherapy on primary tumor growth versus postsurgical advanced metastatic breast cancer.
Sunitinib administered daily inhibits primary tumor growth (Fig. 1A), but has no survival benefit when treating advanced metastatic disease (Fig. 1B). The lower panel shows similar results with pazopanib administered daily (Figs. 1C and 1D). For Figs. 1A and B, 2x106 MDA-MB 231/LM2-4 cells were implanted into the mammary fat pad of 6 CB-17 SCID female mice; in the primary tumor study, treatment with sunitinib was initiated 12 days later when average tumor size was 100mm3; in the advanced metastasis therapy study, primary tumors were surgically resected 20 days after cell injection when average size was approximately 400mm3 and sunitinib treatment was initiated 21 days later.
For Figs. 1C and 1D, 2x106 MDA-MB 231/LM2-4 cells were implanted in the primary tumor study (Fig. 1C) pazopanib treatment was initiated 14 days later when average tumor size was 150mm3; in the advanced metastasis therapy study (Fig. 1D), the primary tumors were surgically resected 20 days after cell injection when average size was approximately 400mm3 and treatment was initiated 19 days later.
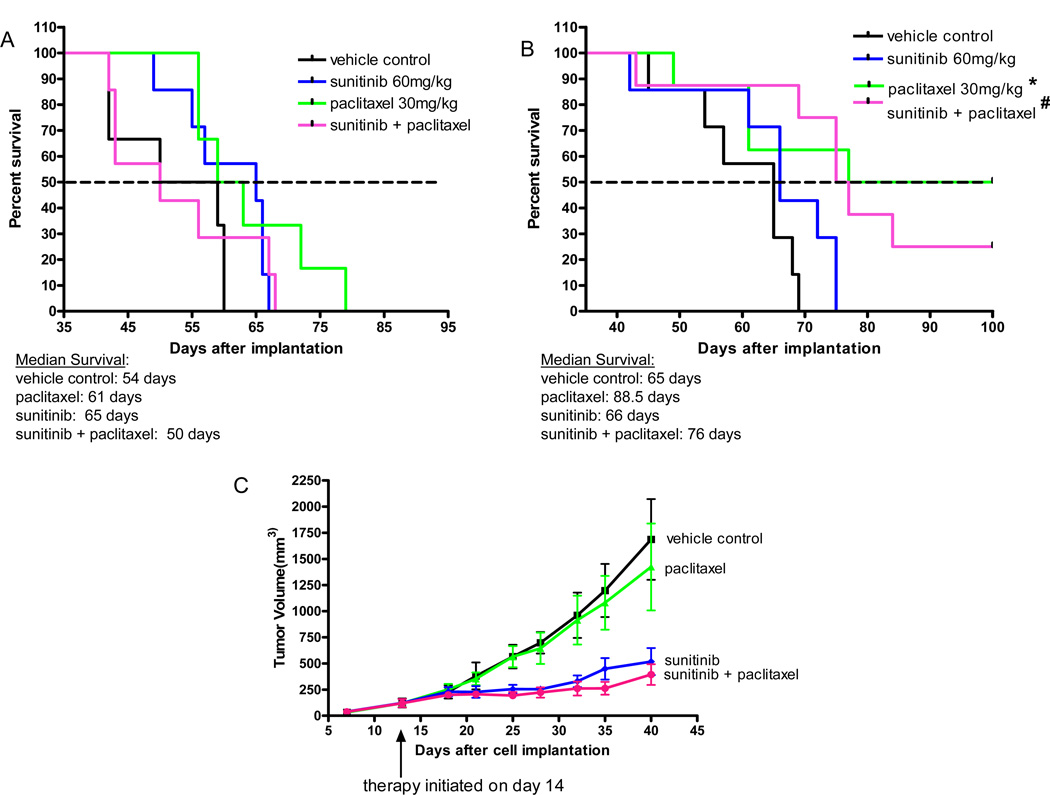
We next assessed the impact of adding paclitaxel to sunitinib. Shown in Fig. 2A and 2B is the impact on survival of mice with advanced metastatic disease when maximum tolerated dose (MTD) paclitaxel (PTX) was combined with sunitinib. Two independent experiments were performed. If anything, there was a trend to reduced survival in the combination treatment group compared to the mice receiving paclitaxel alone, an observation that is consistent with a phase III breast cancer trial of sunitinib plus another taxane, docetaxel12, where no added benefit in PFS was observed and OS was slightly reduced in the combination treatment group12. We would note that in the two experiments shown in Fig. 2A and 2B, there was a difference in the therapeutic impact of paclitaxel alone. In one experiment (shown in Fig. 2A) the paclitaxel monotherapy treatment had no statistically significant benefit in median overall survival whereas it did in the experiment shown in Fig. 2B. This difference may be due to the more aggressive tumor growth we noted in the experiment shown in Fig. 2A where all of the control mice died by day 60 compared to experiment 2B where all control mice died by day 70. Nevertheless in both experiments sunitinib did not improve paclitaxel treatment outcomes. We also tested the effect of paclitaxel plus sunitinib in the established primary tumor model (Fig. 2C); sunitinib had a noticeable anti-tumor effect in contrast to paclitaxel; the two drug combination was not significantly different from the sunitinib treated group.
Figure 2. Differential impact of sunitinib plus paclitaxel chemotherapy when treating established primary tumors versus postsurgical advanced metastatic disease.
Two independent metastatic therapy experiments are shown in Fig. 2A and 2B. Paclitaxel alone administered ip at an MTD of 30mg/kg once every 2 weeks shows extensions of median survival in Fig. 2A and Fig. 2B which was not statistically significant in one case (Fig. 2A). Combination of sunitinib with paclitaxel does not improve the survival advantages over paclitaxel alone (Fig. 2B) and may even worsen outcome (Fig. 2A). In the advanced metastasis studies, median survival for 1) control vehicle treatment was 54 – 65 days, 2) paclitaxel treatment was 61 – 88.5 days, 3) sunitinib treatment was 65 – 66 days, and 4) sunitinib plus paclitaxel was 50 – 76 days; p values were not significant for Fig. 2A; Fig. 2B *paclitaxel vs control p=0.03; # sunitinib + paclitaxel vs control p=0.003. In established primary tumors, MTD paclitaxel alone shows no activity whereas sunitinib alone or in combination with MTD paclitaxel inhibited tumor growth (Fig. 2C). In the advanced metastasis study (Fig. 2B), primary tumors were surgically resected 20 days after injection of 2x106 cells when average size was approximately 400mm3 and treatment was initiated 20 days later; in the primary tumor study (Fig. 2C) treatment was initiated 14 days after cell injection when average tumor size was 150mm3.
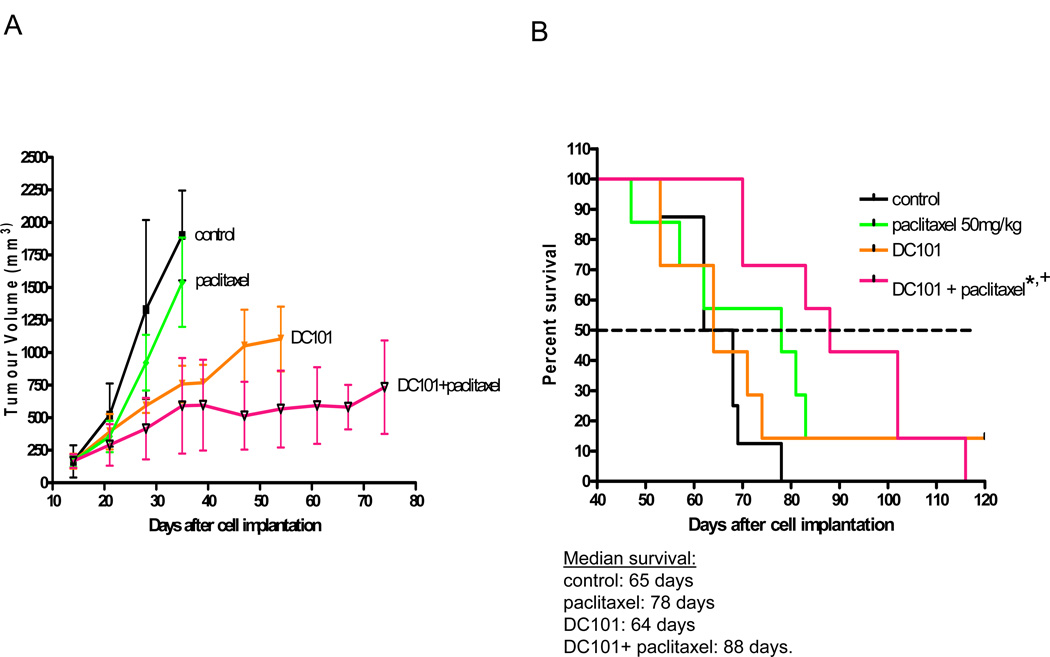
We next tested the antiangiogenic anti-VEGFR-2 antibody known as DC10120. The rationale for doing so was based on the results of the prior E2100 phase III trial evaluating bevacizumab with paclitaxel in metastatic breast cancer patients, which showed a gain in PFS of almost 6 months compared to paclitaxel alone16. As shown in Fig. 3A, DC101 had a robust anti-tumor effect when used to treat established LM2.4 primary tumors. The paclitaxel treatment, once again, did not have an anti-tumor effect when used to treat primary tumors. The two drug combination was the most effective. However, as shown in Fig. 3B the pattern of response as assessed by survival analysis was somewhat different in the postsurgical advanced metastatic setting. Similar to sunitinib or pazopanib, DC101 was seemingly devoid of activity, at least when used as monotherapy and assessed by its impact on survival, since median survival was 64–65 days in both the control and DC101 treatment groups. The paclitaxel monotherapy treatment showed a trend for a survival benefit (from 65 to 78 days) but this did not reach statistical significance. However, median survival was significantly prolonged to 88 days in the combination treatment group compared to control, untreated mice. The difference in median survival between the DC101 + paclitaxel group (88 days) and the paclitaxel group (78 days) was not statistically significant, but would likely have led to a benefit in PFS had we been able to undertake such an assessment, and if so, would mirror the results of the E2100 phase III trial. Taken together, there does seem to be a modest benefit in improving the therapeutic impact of paclitaxel by combination with the VEGF pathway targeting antibody, but not with sunitinib, observations which appear to reflect prior phase III clinical trial results13, 16, including a head-to-head comparison of sunitinib plus paclitaxel versus bevacizumab plus paclitaxel13 as well as the known modest to minimal PFS activity of single agent paclitaxel in metastatic triple negative breast cancer16.
Figure 3. Differential impact of an antibody to VEGF receptor-2 (DC101) plus MTD paclitaxel on established orthotopic primary LM2-4 tumors versus postsurgical advanced metastatic disease.
DC101 800µg/mouse administered ip twice per week showed marked anti-tumor activity when treating established primary tumors (Fig. 3A) but no survival activity in the setting of postsurgical advanced metastasis therapy (Fig. 3B); MTD paclitaxel 50mg/kg ip once every 3 weeks showed no activity in the primary tumor therapy model but showed an increase, although not statistically significant, in median survival in the postsurgical advanced metastasis model; combination of DC101 with MTD paclitaxel increased the extent of inhibition of primary tumor growth (Fig. 3A) and had a significant survival benefit in advanced metastatic model (Fig. 3B). In the advanced metastasis studies (Fig. 3B), median survival for 1) control treatment was 65 days, 2) paclitaxel treatment was 78 days, 3) DC101 treatment was 64 days, and 4) DC101 + paclitaxel was 88 days; * DC101 + paclitaxel vs control p=0.0006; + DC101 + paclitaxel vs paclitaxel p=0.42. In the primary tumor study (Fig. 3A) treatment with DC101 was initiated 15 days later when average tumor size was 150mm3; in the advanced metastasis study (Fig. 3), primary tumors were surgically resected 21 days after cell injection when average size was approximately 400mm3 and treatment was initiated 25 days later.
Several questions are raised by our results. First, what is the basis for the widely divergent effects we have observed when treating primary tumor bearing mice versus mice with postsurgical advanced metastatic disease with antiangiogenic drugs? Some possibilities include reduced expression of VEGF, or VEGFR-2 in the tumor or tumor vasculature of established metastases compared to the primary tumors. The qualitative characteristics of the vasculature in the slightly ‘older’ metastases may be substantially different from the primary tumors such that there is a greater proportion of late/mature vessels that are known to be less responsive to VEGF pathway targeting drugs21. Metastases in vasculature rich organs such as the lung and liver may be more adept at co-opting the existing vasculature than tumors growing in the mammary fat pad22. Second, would a similar pattern of results be observed if using GEMMs or PDXs? With respect to GEMMs, surgical resection of the multiple asynchronously arising primary tumors and the well known observed lack of distant metastases in most such models make this difficult to answer. Nevertheless, some GEMM primary tumor therapy studies have shown a remarkable retrospective correlation with prior phase III clinical trial PFS or OS results of the respective tumor types (lung and pancreatic cancer) in part by using clinically relevant endpoints of tumor response23. As for PDXs, some recent studies have shown the presence of metastases in clinically relevant patterns in non-resected primary tumor bearing patient derived breast cancer xenografts obtained from patients with different breast cancer major subtypes24, thus making it possible to use these models for preclinical adjuvant and metastatic therapy investigations.
Finally, is there any evidence that an investigational therapy previously shown to be highly effective in the postsurgical preclinical metastatic setting shows prospective clinical activity? In this regard, we have reported that doublet oral low-dose metronomic chemotherapy using cyclophosphamide and a 5-FU prodrug (UFT), i.e., tegafur + uracil, had potent activity in the postsurgical metastatic setting using the LM2-4 breast cancer model9, whereas the activity was much less impressive when treating primary tumors in control unresected mice9. A similar version of this metronomic chemotherapy was tested in phase II metastatic breast cancer trial, in combination with bevacizumab, with very encouraging results25. However, as discussed in the Introduction, such phase II trial results have to be confirmed in a larger randomized phase III trial, of which one is underway evaluating metronomic doublet cyclophosphamide and capecitabine with bevacizumab (NCT01131195; www.clinicaltrials.gov). Nevertheless, for now, our results suggest the value of preclinical modeling postsurgical advanced metastatic disease as a potential strategy to improve how they might predict clinical outcomes. While not practical for routine drug screening, use of such models may be useful to confirm prior preclinical studies utilizing conventional primary tumor therapy models, before embarking on expensive phase II or III metastatic therapy clinical trials.
Acknowledgments
This work was supported by grants from the Ontario Institute for Cancer Research, the Canadian Breast Cancer Foundation, and the National Institutes of Health, USA (CA-41233) to RSK. RSK holds a Tier 1 Canada Research Chair. We thank GSK, through Dr. Rakesh Kumar, for pazopanib, Pfizer, through Dr. J. Christensen, for sunitinib, and ImClone Systems, through Dr. Bronek Pytowski, for the DC101 antibody.
Footnotes
Disclosures: Robert S. Kerbel discloses he is currently a paid consultant to Taiho Pharmaceuticals, Japan and was formerly a recipient of sponsored research agreements with GSK and Pfizer.
Authors Contributions
Conception and Design: all authors
Development of Methodology: S. Man and P. Xu
Acquisition of data: E. Guerin, S. Man and P. Xu
Analysis and Interpretation of data: all authors
References
- 1.Kamb A. What's wrong with our cancer models? Nat Rev Drug Discov. 2005;4:161–165. doi: 10.1038/nrd1635. [DOI] [PubMed] [Google Scholar]
- 2.Kung AL. Practices and pitfalls of mouse cancer models in drug discovery. Adv Cancer Res. 2007;96:191–212. doi: 10.1016/S0065-230X(06)96007-2. [DOI] [PubMed] [Google Scholar]
- 3.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170:793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharpless NE, DePinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 5.Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS. Perspective: Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nature Reviews Cancer. 2011;11:135–141. doi: 10.1038/nrc3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steeg PS, Anderson RL, Bar-Eli M, Chambers AF, Eccles SA, Hunter K, et al. Preclinical drug development must consider the impact on metastasis. Clin Cancer Res. 2009;15:4529–4530. doi: 10.1158/1078-0432.CCR-09-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fojo T, Amiri-Kordestani L, Bates SE. Potential pitfalls of crossover and thoughts on iniparib in triple-negative breast cancer. J Natl Cancer Inst. 2011;103:1738–1740. doi: 10.1093/jnci/djr386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma S, McLeod D, Batist G, Robidoux A, Martins IR, Mackey JR. In the end what matters most? A review of clinical endpoints in advanced breast cancer. Oncologist. 2011;16:25–35. doi: 10.1634/theoncologist.2010-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munoz R, Man S, Shaked Y, Lee C, Wong J, Francia G, et al. Highly efficacious non-toxic treatment for advanced metastatic breast cancer using combination UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–3391. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- 10.Abrams TJ, Murray LJ, Pesenti E, Holway VW, Colombo T, Lee LB, et al. Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with "standard of care" therapeutic agents for the treatment of breast cancer. Mol Cancer Ther. 2003;2:1011–1021. [PubMed] [Google Scholar]
- 11.Mackey JR, Kerbel RS, Gelmon KA, McLeod DM, Chia SK, Rayson D, et al. Controlling angiogenesis in breast cancer: a systematic review of anti-angiogenic trials. Cancer Treat Rev. 2012;38:673–688. doi: 10.1016/j.ctrv.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Bergh J, Greil R, Voytko N, Makhson A, Cortes J, Lortholary A, et al. Sunitinib (SU) in combination with docetaxel (D) versus D alone for the first-line treatment of advanced breast cancer (ABC) J Clin Oncol. 2011;28:LBA 1010. doi: 10.1200/JCO.2011.35.7376. [DOI] [PubMed] [Google Scholar]
- 13.Robert NJ, Saleh MN, Paul D, Generali D, Gressot L, Copur MS, et al. Sunitinib plus paclitaxel versus bevacizumab plus paclitaxel for first-line treatment of patients with advanced breast cancer: a phase III, randomized, open-label trial. Clin Breast Cancer. 2011;11:82–92. doi: 10.1016/j.clbc.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crown J, Dieras V, Starosiawska E, Yardley DA, Davidson N, Bachelot TD, et al. Phase III trial of sunitinib (SU) in combination with capecitabine (C) versus C in previously treated advanced breast cancer (ABC) J Clin Oncol. 2010 doi: 10.1200/JCO.2012.43.3391. abstract no. LBA 1011. [DOI] [PubMed] [Google Scholar]
- 15.Barrios CH, Liu MC, Lee SC, Vanlemmens L, Ferrero JM, Tabei T, et al. Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res Treat. 2010;121:121–131. doi: 10.1007/s10549-010-0788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 17.Tait LR, Pauley RJ, Santner SJ, Heppner GH, Heng HH, Rak JW, et al. Dynamic stromal-epithelial interactions during progression of MCF10DCIS.com xenografts. Int J Cancer. 2007;120:2127–2134. doi: 10.1002/ijc.22572. [DOI] [PubMed] [Google Scholar]
- 18.Shaked Y, Henke E, Roodhart J, Mancuso P, Langenberg M, Colleoni M, et al. Rapid chemotherapy-induced surge in endothelial progenitor cells: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebos JML, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin D, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sitohy B, Nagy JA, Jaminet SC, Dvorak HF. Tumor-surrogate blood vessel subtypes exhibit differential susceptibility to anti-VEGF therapy. Cancer Res. 2011;71:7021–7028. doi: 10.1158/0008-5472.CAN-11-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leenders WP, Kusters B, de Waal RM. Vessel co-option: how tumors obtain blood supply in the absence of sprouting angiogenesis. Endothelium. 2002;9:83–87. doi: 10.1080/10623320212006. [DOI] [PubMed] [Google Scholar]
- 23.Singh M, Lima A, Molina R, Hamilton P, Clermont AC, Devasthali V, et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nature Biotechnology. 2010;28:585–593. doi: 10.1038/nbt.1640. [DOI] [PubMed] [Google Scholar]
- 24.DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17:1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer: clinical and biological activity. J Clin Oncol. 2008;26:4899–4905. doi: 10.1200/JCO.2008.17.4789. [DOI] [PubMed] [Google Scholar]