Abstract
Vascular inflammation plays a significant role in the pathogenesis of atherosclerosis. Luteolin, a naturally-occurring flavanoid, present in many medicinal plants as well as in some commonly consumed fruits and vegetables has received wide attention for its potential to improve vascular function in vitro. However, its effect in vivo and the molecular mechanism of luteolin at physiological concentrations remain unclear. Here, we report that luteolin as low as 0.5 μM significantly inhibited TNF-α-induced adhesion of monocytes to human EA.hy 926 endothelial cells, a key event in triggering vascular inflammation. Luteolin potently suppressed TNF-α-induced expression of the chemokine monocyte chemotactic protein-1 (MCP-1) and adhesion molecules ICAM-1 and VCAM-1, key mediators involved in enhancing endothelial cell-monocyte interaction. Furthermore, luteolin inhibited TNF-α-induced NF-κB transcriptional activity, IκBα degradation, expression of IκB kinase ß (IKKß), and subsequent NF-κB p65 nuclear translocation in endothelial cells, suggesting that luteolin can inhibit inflammation by suppressing NF-κB signaling. In an animal study, C57BL/6 mice were fed a diet containing 0% or 0.6% luteolin for three weeks and luteolin supplementation greatly suppressed TNF-α-induced increases in circulating levels of MCP-1/JE, CXCL1/KC, and sICAM-1 in C57BL/6 mice. Consistently, dietary intake of luteolin significantly reduced TNF-α-stimulated adhesion of monocytes to aortic endothelial cells ex vivo. Histology shows that luteolin treatment prevented the eruption of endothelial lining in the intima layer of the aorta and preserved elastin fibers’ delicate organization as shown by Verhoeff-van Gieson staining. Immunohistochemistry studies further show that luteolin treatment also reduced VCAM-1 and monocyte-derived F4/80-positive macrophages in the aorta of TNF-α-treated mice. In conclusion, luteolin protects against TNF-α-induced vascular inflammation, in both in vitro and in vivo models. This anti-inflammatory effect of luteolin may be mediated via inhibition of the NF-κB-mediated pathway.
Keywords: luteolin, vascular inflammation, TNF-α, mice
1. Introduction
Atherosclerosis is one of the major chronic diseases in human. The formation of atherosclerotic vascular disease involves complex pathological processes. Recent basic, clinical and epidemiological studies have demonstrated that chronic inflammation plays a key role in the initiation and progression of atherosclerosis [1]. Indeed, one of the key early events in the pathogenesis of atherosclerosis is inflammation-triggered endothelial activation that leads to the adhesion of monocytes to the endothelium followed by their transmigration into the subendothelial space [2–4] This process is primarily mediated by several intracellular signaling events that lead to the elevated expression of a number of pro-inflammatory chemokines, such as interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1), and several endothelial adhesion molecules, including vascular cell adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1) and E-Selectin. These chemokines and adhesion molecules play key roles in the firm adhesion of monocytes to the activated endothelial cells (ECs) [2–4].
Accumulating evidence suggests that tumor necrosis factor-α (TNF-α), a key cytokine in the inflammatory cascade, is a pro-atherosclerotic factor that triggers vascular inflammation and the subsequent development of atherosclerosis [5]. TNF-α has been found to mediate interaction of invading monocytes with vascular ECs, thereby triggering extracellular matrix (ECM) deposition in aortic vessels [5]. Consistently human studies have demonstrated that TNF-α is remarkably elevated in the plasma and arteries in subjects with vascular complications [6], and that anti-TNF-alpha therapy improved aortic stiffness and carotid intima media thickness (CIMT) in patients with inflammatory arthropathies [7]. These results indicate that TNF-α is critically involved in the pathogenesis of atherosclerosis. TNF-α can trigger several intracellular signaling events that ultimately up-regulate the expression of chemokines IL-8 and MCP-1 and adhesion molecules VCAM-1, ICAM-1, and E-Selectin. It is well established that activation of NF-κB is essential for the transcriptional regulation of TNF-α-induced IL-8 and MCP-1, as well as adhesion molecules [8, 9]. The p65 heterodimer, which is expressed in vascular cells, is one of the most abundant forms of the NF-κB family members. The increased nuclear translocation of the p65 subunit is detected in the intimal thickening of ECs of human atherosclerotic lesions [10]. Since inflammation-induced endothelial dysfunction is important in the development of atherosclerosis, search for agents that can attenuate TNF-α -induced NF-κB activation in ECs could be an effective strategy to prevent vascular endothelial dysfunction.
In recent years, flavonoids have drawn wide scientific attention because of their diverse health benefits and accumulating epidemiological studies show a positive relationship between flavonoid intake and reduced cardiovascular diseases (CVD) risk [11–14]. Luteolin is a bioflavonoid present in many medicinal plants as well as in some commonly consumed fruits and vegetables including green leafy spices such as parsley, sweet peppers and celery [11–13]. Previous studies showed that luteolin possess numerous beneficial medicinal properties such as antioxidant, antiinflammatory, and anti-allergic actions [15–19]. Data from vitro studies also suggest a protective role of luteolin in the vascular vasculature and the beneficial effect of luteolin on inflammatory process and inflammatory associated CVD [15–19]. In this context, luteolin at higher doses (≥ 25 μM) inhibited oxidized LDL and TNF-α-induced VCAM-1 expression [15–19]. Luteolin at pharmacological concentrations shows lowering plasma lipids [20], inhibiting cholesterol biosynthesis [21], and increasing eNOS gene expression [22]. Luteolin also protected against Fe(2+)-induced lipid peroxidation and dose dependently showed potent radical scavenging ability and Fe(2+)-chelating ability [15], but those effects also require higher doses that are unachievable by dietary intake of this compound.
While those previous studies provide evidence for a protective effect of luteolin against vascular dysfunction, most of the results from reported studies reflected a pharmacological, rather than physiological effect of luteolin because the effective concentrations used in most of the studies are well above achievable plasma luteolin levels (≤ 2μM) in both rodents and humans following consumption of various natural bioflavonoid products containing luteolin [14–19]. Indeed, the average plasma concentrations of luteolin can reach 0.99 μM after the consumption of a complex meal rich in flavonoids (equivalence of average dietary of luteolin 8.08 mg/day per person) in 92 students with a range of 20–28 years [14]. Although the levels of conjugated luteolin can temporarily reach about 10 μM in plasma just after oral ingestion of milligram of luteolin [15–19], under the healthy condition, the majority of the conjugates cannot be entered into the cells because they are excreted via the kidney excretion within 25 h after ingestion [15–19]. Previous studies reported that the intracellular physiological concentrations of luteolin were found to be less than 2 μM [15–19]. As mentioned previously, the concentrations (>10 μM) used in most of previous studies are far greater than the physiological relevant concentrations of luteolin (< 2 μM). Therefore the biological relevance of previous findings is largely unclear and the cellular or molecular action of luteolin at physiologically relevant concentrations needs to be further defined. In addition, the effect of luteolin on proinflammatory mediator-induced vascular inflammation in vivo is largely unknown. We thus investigated whether luteolin at physiologically-achievable concentrations (≤ 2μM) prevents TNF-α-induced endothelial inflammation in endothelial cells (ECs) by examining monocyte-endothelial cells interaction, the production of chemokines and adhesion molecules, as well as the NF-κB pathway in endothelial cells. We hypothesize that luteolin can protect against vascular inflammation. We further examined the effect of dietary intake of luteolin on TNF-α-induced vascular inflammation in mice.
2. Materials and methods
2.1. Reagents
Calcein O, where O = −diacetate tetrakis (acetoxymethyl) ester (calcein-AM), RPMI-1640 and DMEM medium, recombinant human TNF-α, and Lipofectamine transfection reagent were purchased from Life Technologies (Grand Island, NY). Recombinant murine TNF-α was from PeproTech Inc. (Rocky Hill, NJ). Endothelial growth supplements EGM2 and M199 medium were purchased from Lonza (Walkersville, MD). Enzyme-linked immunosorbent assay (ELISA) kits for human and mouse soluble adhesion molecules ICAM-1 (sICAM-1), VCAM-1 (sVCAM-1) and mouse chemokines MCP-1/JE and KC ELISA kits were from R&D Systems (Minneapolis, MN). Goat anti-rabbit IgG, DyLight™-488 conjugated secondary antibody and goat anti-rabbit horseradish peroxidase (HRP)-IgG secondary antibody were purchased from Thermo Fisher Scientific Inc. (Waltham, MA). Anti-p65 and anti-IκB-α were from Cell Signaling Technology, Inc. (Danvers, MA). VCAM-1 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). F4/80 antibody was from (Bachem Americas, Inc., Torrance, CA). Vectastain Elite Rabbit IgG kit and rabbit or rat Vectastain ABC-AP kit were from Vector Laboratories (Burlingame, CA). 3,3′-diaminobenzidine was from Dako North America (Carpinteria, CA). Dual luciferase assay system was from Promega (Wisconsin, USA). Luteolin (≥98%, HPLC) was from Stanford Chemicals Company (Irvine, CA). Goat serum, 4′,6-diamidino-2-phenylindole (DAPI), mounting medium, and all other reagents and chemicals were from Sigma-Aldrich (St. Louis, MO).
2.2. Cell culture
EA.hy926 cells (ATCC, Manassas, VA) were cultured in DMEM supplemented with 10% FBS, 100U/ml penicillin, and 100ug/ml streptomycin in 75cm2 tissue culture flasks at 37°C in a humidified atmosphere of 5% CO2. Primary human umbilical vein endothelial cells (HUVECs, (Walkersville, MD) were cultured in M199 medium containing 2% FBS and endothelial growth supplement EGM-2 and maintained at 37°C in a 5% CO2/95% air environment. THP-1 cells (ATCC, Manassas, VA) were cultured in RPMI-1640 medium containing 10% FBS. WEHI 78/24 monocytic cells (originally provided by Dr. Judith A Berliner, UCLA) were cultured in DMEM medium plus 10% FBS.
2.3. Monocyte adhesion assay
The determination of monocyte adhesion to EA.hy926 endothelial cells was conducted using THP-1 cells as described by us previously [23, 24]. In brief, ECs were grown to confluence, followed by pretreatment with 0.5 μM – 20 μM luteolin for 1 h before addition of 10 ng/mL human recombinant TNF-α. Cells were then incubated with medium containing TNF-α in the continued presence or absence of luteolin for 24 h. ECs were then gently washed with serum-free medium and calcein-AM labeled THP-1 cells (1×106/mL RPMI1640 medium containing 1% FBS) were then added to ECs. After 1 h incubation, EC monolayer was gently washed with EC medium to remove unbound monocytes. The adhered monocytes were determined by measuring the fluorescence using a BioTek Synergy 2 Multi-Mode Microplate Reader (Winooski, VT) at excitation and emission wavelengths of 496 nm and 520 nm.
2.4 Real-time RT-PCR
To study the effects of luteolin on TNF-α-induced ICAM-1, VCAM-1 and IκB kinase (IKKß) mRNA expressions, ECs cells were preincubated with various concentrations of luteolin for 1 h before addition of 10 ng/ mL TNF-α for another 1 h. Total RNA from ECs were isolated using Trizol reagent. 1 μg of RNA from each sample was reverse transcribed to cDNA. The reaction mixture in each well contained 5 μl of distill autoclaved H2O, 10 μl of cyber green, 2 μl of forward and reverse primer. The primers used in quantitative real-time PCR were VCAM-1 (forward, 5′-GGC TGG AGC TGT TTG AGA AC 3′; reverse, 5′- GGT GCT GCA AGT CAA TGA GA -3′), ICAM-1 (forward, 5′-CTCCCTCTCGGGTCTCTCTC-3′; reverse, 5′- ACT GTG GGG TTC AAC CTC TG -3′), MCP-1 (forward, 5′- CCC CAG TCA CCT GCT GTT AT -3′; reverse, 5′- TGG AAT CCT GAA CCC ACT TC -3′), and IKKß (forward, 5′- AACCAGCATCCAGATTGACC 3′; reverse, 5′- CTCTAGGTCGTCCAGCGTTC -3′). The gene expression SYBR® Green Real-Time PCR Master Mixes (Life Technologies, Grand Island, NY) were used for real-time quantitative PCR to measure ICAM-1, VCAM-1, and GAPDH using an ABI 7900HT Fast Real-Time PCR System. The thermal profile consisted of 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 94°C for 15 sec and 60°C for 1 min. The mean qualities of VCAM-1, ICAM-1, MCP-1 and IKKß were normalized based on the mean of control gene GAPDH [50].
2.5. NF-κB transcriptional activity assay
ECs were transfected as described by us recently [24–26]. ECs plated in 96-well plates were co-transfected with 10 μg of NF-κB promoter-luciferase vector and pRL reporter control plasmid using Lipofectamine transfection reagent. Twenty-four hours after transfection, ECs were treated with luteolin for 1 h before addition of 10 ng/mL TNF-α for 6 h. Treated cells were then washed once in phosphate-buffered saline and disrupted in lysis buffer (0.1 MKH2PO4, pH 7.6, 1 mM dithiothreitol and 0.05% Triton X-100). Luciferase activity in the cell extracts was determined by using the dual luciferase assay system and was normalized to pRL activity as described previously [27].
2.6. Immunoblotting analysis of IκB-α
After the treatment, ECs were harvested by scraping into ice-cold lysis buffer (20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM Na4P2O7, 1 mM β-glycerolphosphate, 1 mM Na3VO4) supplemented with protease inhibitor cocktail (1:500) and phosphatase inhibitor cocktail I (1:100). The extracts were sonicated and centrifuged at 10,000 × g for 5 min. Protein levels were measured using a Bio-Rad assay kit. Samples were mixed with Laemmli sample buffer and heated for 5 min at 95°C. Protein levels were measured using a Bio-Rad assay kit. The immunoblot procedure will be followed as we recently described [24–26]. Brieftly, samples were mixed with Laemmli sample buffer and heated for 5 min at 95°C. The gels were blotted onto nitrocellulose membranes, probed with rabbit anti- IκB-α primary antibodies overnight at 4°C, and incubated with secondary antibody conjugated to horseradish peroxidase for 1 h at room temperature. The immunoreactive proteins were detected by superSignal chemiluminescence and digitally imaged using the ChemiDoc™ XRS System (Biorad, Hercules, CA). The intensities of protein bands were quantified with the Image J software (National Institutes of Health, Bethesda, MD).
2.7. Confocal immunofluorescence study of NF-κB p65 nuclear translocation
HUVECs were pre-treated with various concentrations of luteolin for 1 h before addition of 10ng/mL in the continued presence or absence of luteolin for another 2 h on 8-well chamber slides. The cells were then washed with PBS and fixed with 100% ice-cold methanol and blocked with 10% normal goat serum (Sigma, St. Louis, MO) for 30 min at room temperature. The cells were then incubated with rabbit anti-NF-κB p65 antibodyfor 2 h at 4°C and washed with PBS 3 times followed by incubation with goat anti-rabbit IgG DyLight™-488 conjugated secondary antibody for 1 h. The chamber slides were then washed with PBS and mounted with Fluroshield with DAPI mounting medium. NF-κB p65 was visualized with an Olympus Fluoview FV5OO/IX81 Confocal microscope (Waltham, MA).
2.8. Animal and experimental design
Male C57BL/6 mice (10 week old) were obtained from Jackson Laboratory. Mice were housed in micro-isolator cages in a pathogen-free facility. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Virginia polytechnic Institute and State University in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. After an initial acclimation period, the mice were randomly divided into 3 groups, 12 mice per group (control, TNF-α, TNF-α + luteolin). Mice were fed AIN-93G rodent diet or basal modified AIN-93G rodent diet (Dyet, Inc., Bethlehem, PA) containing 0.6% luteolin. After 1 week, the mice were administered an intra-peritoneal injection (i.p.) of TNF-α at 25 μg/kg daily for 7 consecutive days. Administration of the TNF-α to mice at such a dosage regimen has been found to significantly increase intercellular adhesion molecule expression and vascular barrier dysfunction [24]. Control mice received i.p. of PBS for the same period. During the TNF-α administration, mice were continually fed the control or luteolin diet. Body weight and feed intake were recorded weekly during the entire study period. At the end of the experiment, all mice were euthanized 2 h after the last TNF-α injection. Blood samples were collected and serum was frozen at −80°C for ELISA analysis.
2.9. Ex vivo monocyte adhesion assay
Mice were euthanized and aortas from the mice were rapidly excised and washed twice with ice-cold PBS followed by placed in DMEM for 10 min at 37°C. The aortas were opened longitudinally to expose the endothelium and pinned onto 4% agar in 35-mm plates with 1 mL of DMEM containing 1% heat-inactivated FBS. WEHI monocytes were fluorescence labeled with Calcein–AM as described by us recently [24]. The aortas were incubated for 30 min with 1×106 fluorescence-labeled WEHI 78/24 mouse monocytes. After incubation, unbound monocytes were rinsed away and the number of monocytes firmly bound to aorta was captured by using confocal microscopy. Data are expressed as mean ± SEM of 3 areas of the aorta.
2.10. Measurements of chemokines and adhesion molecules
MCP-1/JE, KC and soluble forms of ICAM-1 (sICAM-1) in the serum were measured using ELISA kits following the manufacturer’s instructions. Samples were plotted against standard curves for determination of concentrations in the serum as described by us recently [24].
2.11. Histology
Mice were euthanized and the thoracic aorta was dissected from the heart and surrounding tissues and the adventitial fat tissue was cleaned. The aorta was then placed in the buffered 10% formalin solution overnight for fixation. A 5 μm section of the proximal artery was cut off and placed in 200-proof ethyl alcohol for 24 h followed by paraffin embedment. The sectioned samples were stained with Verhoeff-van Gieson for elastin and hematoxylin-eosin. All stains were performed at the AML Labs (Baltimore, MD) using standard protocol. Sections were examined under bright field of EVOS XL microscope (AMG, Bothell, WA).
2.12. Immunohistochemical localization of VCAM-1 and F4/80 in mice aorta
Paraffin embedded tissue sections were deparaffinized with xylene and rehydrated with graded concentrations of ethanol. Sections were boiled with 10 mM sodium citrate buffer at pH 6.0 followed by incubation with 3% peroxide solution for 10 min for antigen unmasking. The sections were then incubated in 5% normal goat serum in TBST for 30 min and immunohistochemistry for VCAM-1 was performed with a rabbit anti-VCAM-1 antibody (1:1000 dilution) using the Vectastain Elite Rabbit IgG kit. Immunohistochemistry for F4/80 was performed with a rat monoclonal F4/80 antibody diluted 1:50 (Bachem Americas, Inc., Torrance, CA) using the Vectastain Elite Rat IgG kit. The sections were incubated overnight with primary antibodies at 4°C. The appropriate secondary antibodies from the Vectastain ABC-AP kit were used according to manufacturer’s instructions. Visualization was performed using 3,3′-diaminobenzidine and nuclei were counterstained with Harris hematoxylin. Photographs of immuno-stained mouse aorta were digitized and captured using an AMG EVOS XL digital inverted bright field and phase contrast microscope (Bothell, WA). Quantitative analysis of VCAM-1 and F4/80 expressions in aorta was performed with the Image J software.
2.13. Statistical analysis
All data were subjected to analysis of variance (ANOVA) using GraphPad Prism software (La Jolla, CA). Data are expressed as means ± SEM. Significant treatment differences were subjected to Tukey’s multiple comparison tests. A value of p<0.05 was considered different.
3. Results
3.1. Luteolin inhibits TNF-α-induced binding of monocytes to endothelial cells (ECs)
Since inflammation-induced mononuclear cell adhesion to endothelial cells (ECs) is an important step in the development of atherosclerosis, we determined if luteolin can block inflammation-induced adhesion of monocytes to EA.Hy 926 cells, a permanent human umbilical vein endothelial cell (HUVEC) line, which is often used as a model of endothelium for studies of various physiological and pathological processes, especially in vascular inflammation research. Exposure of ECs to 10 ng/mL TNF-α for 24 h significantly increased adhesion of monocytes to ECs (Fig. 1). Pretreatment with luteolin at a concentration as low as 0.5 μM significantly inhibited TNF-α-induced binding of monocytes to ECs, and 20 μM luteolin completely blocked monocyte adhesion to ECs (Fig. 1).
Fig. 1. Luteolin inhibited TNF-α-stimulated monocyte adhesion to EA.Hy926 endothelial cells (ECs).
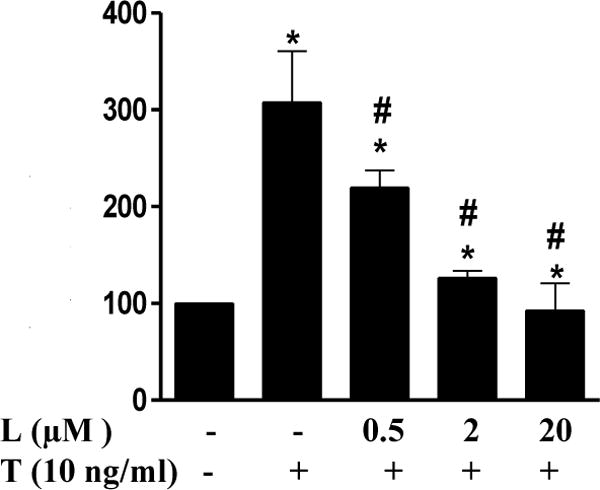
ECs were pre-treated with various concentrations of luteolin (L) for 1 h before addition of TNF-α (T 10 ng/mL) in the continued presence or absence of luteolin for 24 h. THP-1 cells were labeled with a fluorescence probe and the adhesion was determined using a Microplate Reader at excitation and emission wavelengths of 496 nm and 520 nm. Values are mean ± SEM, n=3. *, p<0.05 vs. control; #, p<0.05 vs. TNF-α alone-treated cells.
3.2. Luteolin inhibits TNF-α-stimulated expression of chemokines and adhesion molecule in ECs
Adhesion of monocytes to ECs is critically regulated by both chemotactic cytokines and vascular adhesion molecules. As detected by real-time PCR (Fig. 2), exposure of ECs to TNF-α for 1 h significantly increased expression of MCP-1, VCAM-1, and ICAM-1. Treatment of the cells with luteolin at a concentration as low as 0.1 μM significantly inhibited TNF-α-induced gene expression of these adhesion molecules and chemokine (Fig. 2), with 2 μM of luteolin almost completely preventing the stimulated expression of these proinflammatory molecules by TNF-α (Fig. 2).
Fig. 2. Luteolin suppressed the expression of MCP-1 (A), ICAM-1 (B) and VCAM-1 (C) in ECs.
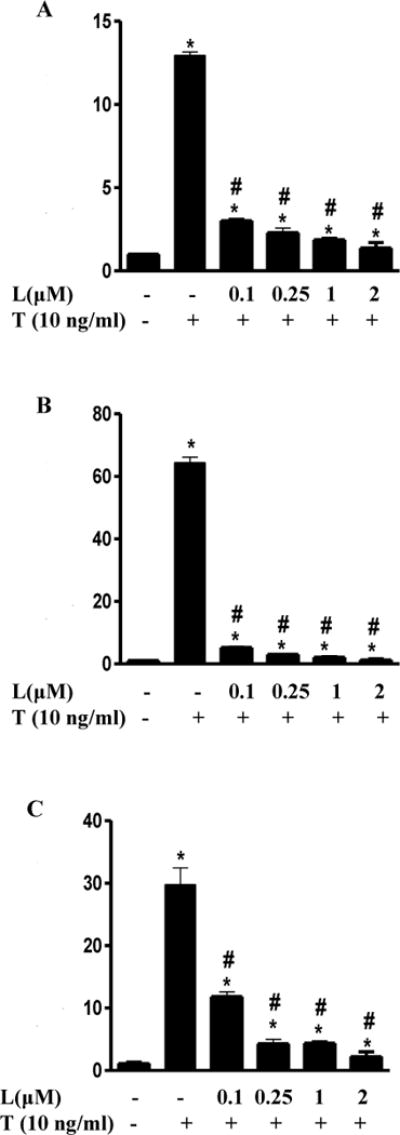
The cells were pre-treated with 0.1 μM, 0.25 μM, 1 μM and 2 μM of luteolin (L) for 1 h before addition of TNF-α (T 10ng/mL) in the continued presence or absence of luteolin for 1 h. The relative mRNA abundance in the same set of samples was evaluated by real-time PCR and changes in transcript abundance were normalized based on the mean of control gene GAPDH (housekeeping gene). Data are expressed as mean ± SEM, n = 3. *, p<0.05 vs. control; #, p<0.05 vs. TNF-α alone-treated cells. MCP-1, monocyte chemoattractant protein -1; VCAM-1, vascular adhesion molecule-1; ICAM-1, soluble intercellular adhesion molecule-1.
3.3. Luteolin prevents TNF-α-induced activation of NF-κB signaling
Activation of NF-κB plays a pivotal role for the transcriptional regulation of chemotactic cytokines and vascular adhesion molecules that are critically involved in leukocyte adhesion to the endothelium [8, 9]. Thus, we determined the effect of luteolin on TNF-α-induced activation of NF-κB signaling. Exposure of ECs to TNF-α for 6 h potently increased NF-κB transcriptional activity indicating the induction of NF-κB-regulated gene expression (Fig. 3A). Consistent with its effect on monocyte-EC interaction, luteolin at 2 μM completely blocking the elevated NF-κB activity by TNF-α (Fig. 3A). Furthermore, Western blot analysis showed that TNF-α-induced IκBα degradation was inhibited in luteolin-treated cells (Fig 3B). The inhibitory effect of luteolin was further confirmed by confocal microscopic examination of NF-κB p65 nuclear translocation, which showed significant decrease in the number of positive fluorescences compared to the control cells (Fig. 3C). Accumulating evidence suggests that inhibitor of kappa B (IκB) kinase (IKK), especially IKKß, is the essential upstream of NF-κB activation [28, 29]. IKKß can phosphorylate the inhibitory IκBα protein (subsequent ubiquitination and degradation) resulting in the dissociation of IκBα from NF-κB [28, 29]. In order to further understand how the nuclear translocation of NF-κB was inhibited by luteolin, we analyzed the express of IKKß in endothelial cells As shown in Fig. 3D, exposure of ECs to TNF-α for 1 h significantly increased IKKß expression in ECs as measure by real-time PCR. However, pretreatment with luteolin potently inhibited TNF-α-induced expression of IKKß (Fig. 3D). These results suggest that luteolin may inhibit inflammation via suppressing the NF-κB signaling pathway.
Fig.3. Luteolin inhibited TNF-α-induced NF-κB signaling in ECs.
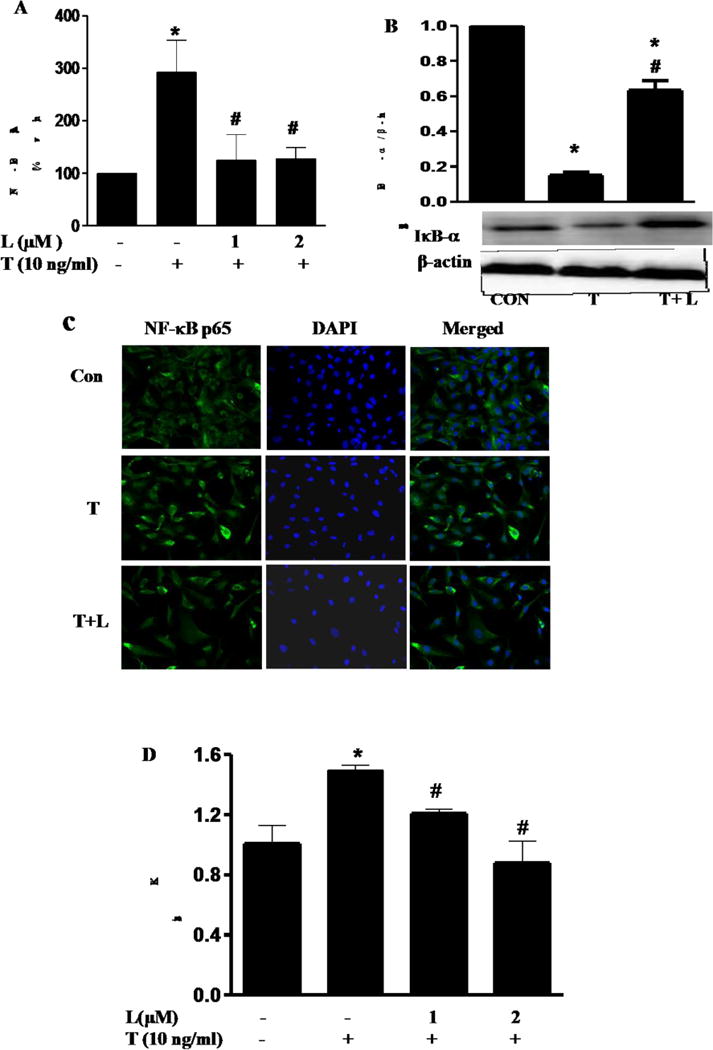
(A) The effect of luteolin on TNF-α-induced NF-κB transcriptional activity in ECs. ECs were co-transfected with NF-κB promoter-luciferase vector and pRL reporter control plasmid. 24 h after transfection, ECs were treated with luteolin for 1 h before addition of TNF-α (10 ng/mL) for 6 h. Luciferase activity, normalized to pRL activity in the cell extracts, was determined. Values are mean ± SEM, n=3. (B) ECs s were pre-treated with 0.25 μM of luteolin (T) for 1 h before addition of TNF-α (T, 10 ng/mL) in the continued presence or absence of luteolin for 15 min. IκBα protein levels in ECs were determined by Western blot analysis. β-actin was used as a loading control. Values are mean ± SEM, n=3. (C) NF-κB p65 nuclear translocation in HUVECs was visualized using immunofluorescence staining. (D), Quantitative real-time PCR was performed to determine IKKβ mRNA expression. Experimental conditions for Figure 3–4 are described in the material and methods section. Representative sections are shown NF-κB p65, overlay and DAPI. *, p<0.05 vs. control; #, p<0.05 vs. TNF-α alone-treated cells.
3.4. Dietary supplementation of luteolin reduces TNF-α-induced vascular inflammation in C57BL/6 mice
We further assessed whether luteolin has the potential to prevent TNF-α-induced-vascular inflammation in vivo. In that regard, ex vivo monocyte adhesion to the endothelium of isolated mouse aortic vessels was examined by using mouse WEHI 78/24 monocytes. As shown in Fig. 4A–B, WEHI 78/24 cells had significantly higher binding to vessel wall of mouse aortas isolated from TNF-α-treated mice than those from control mice, indicating that blood vessels in TNF-α treated mice are activated and inflammatory [30]. However, dietary supplementation of luteolin effectively blocked adhesion of monocytes to the endothelium (Fig. 4A–B). Dietary intake of luteolin had no effect on animal body weight and food intake (data not shown).
Fig.4. Dietary supplementation of luteolin reduced monocyte binding to aortic endothelium (A–B), the secretion of serum chemokines (C–D) and adhesion molecules (E) in TNF-α treated mice.
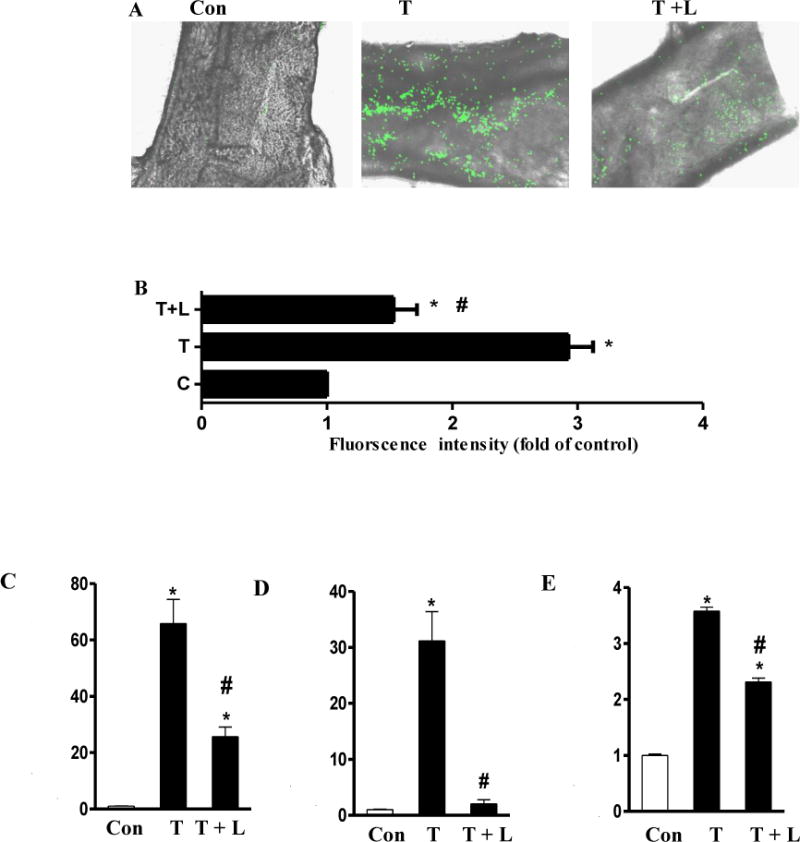
Values are means ± SEM. *, p<0.05 vs. control; #, p<0.05 vs. TNF-α alone-treated mice. TNF-α, Tumor necrosis factor-α; MCP-1/JE, mouse monocyte chemotactic protein 1/JE; CXCL1/KC, Chemokine (C-X-C motif) ligand 1; sICAM-1, soluble intercellular adhesion molecule-1.
MCP-1, IL-8, and VCAM-1 are essential for firm adhesion of monocyte to ECs and subsequent transmigration into vascular tissue [1, 6, 31, 32]. As shown in Fig. 4C–E, the serum concentrations of MCP-1/JE, KC (the mouse homolog of human MCP-1 and IL-8, respectively), and sICAM-1 were greatly elevated in TNF-α-treated mice than those in control mice. Dietary ingestion of luteolin significantly reduced the increased circulating levels of MCP-1/JE (Fig. 4C), KC (Fig. 4D), and sICAM-1(Fig. 4E) in mice by 61%, 93%, and 35%, respectively. These results suggest that luteolin indeed has a potent anti-inflammatory effect in vivo via inhibition of chemokines and adhesion molecules.
Previous studies showed that monocytes can be recruited into the vessel wall and subsequently differentiate into macrophages that ultimately become lipid-rich foam cells during inflammation [33–35]. To further confirm the anti-inflammatory effect of luteolin in vivo, immunohistochemistry was employed to evaluate the expressions of adhesion molecule VCAM-1 and F4/80, a commonly used marker of mouse vascular monocyte-derived macrophages in mouse aorta. As shown in Fig. 5, strong VCAM-1 staining (Fig. 5A) and abundance of F4/80-positive macrophages (Fig. 5B) were present in the mouse aorta in TNF-α-treated group, indicating that blood vessels are activated and inflamed. Dietary ingestion of luteolin almost completely blocked F4/80-positive monocytes-derived macrophages (Figs. 5 B and D) and also significantly reduced the intensity of VCAM-1 staining (Figs. 5A and C) in mouse aorta.
Fig 5. Immunohistochemical staining for adhesion molecule VCAM-1 and F4/80-positive monocytes-derived macrophages in aortic cross-sections. Representative photomicrographs of immunohistochemical staining for VCAM-1 (A) and F4/80-positive monocytes-derived macrophages (B).
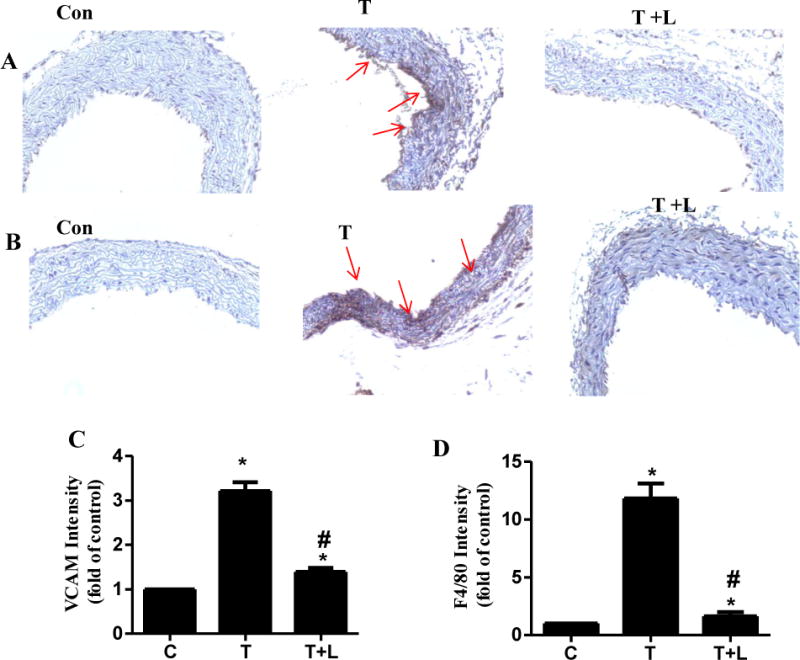
Quantitative analysis of VCAM-1 (C) and F4/80 (D). Arrows indicate typical positive-stained regions at the magnification of 40×. T, TNF-α; T+L, TNF-α + luteolin. Data are expressed as mean ± SEM (n=3), *, p<0.05 vs. control; #, p<0.05 vs. TNF-α alone-treated mice.
3.5. Luteolin prevents TNF-α-induced aortic structure change in the intima layer of artery and disruption of aortic elastin fiber in mouse aortic cross sections
Histopathological analysis of the aorta stained with hematoxylin and eosin revealed that TNF-α treatment caused significant changes in aortic layer structure with inner intima layer also erupted through the endothelial lining (Fig. 6A). In addition, tunica media of artery from TNF-α treated mice showed disorderly arrangement of muscle and collagen fibers. Verhoeff-Van Gieson staining displayed an apparent discontinuity and disruption in the elastin fibers (Fig. 6B), indicating the structural abnormalities in vessels. Dietary luteolin largely prevented these pathologies and maintained the normal aortic structure (Fig. 6).
Fig.6. Dietary luteolin prevented TNF-α-induced aortic endothelial injury (A) and disruption of aortic elastin fiber (B) in aortic cross sections of TNF-α treated mice.
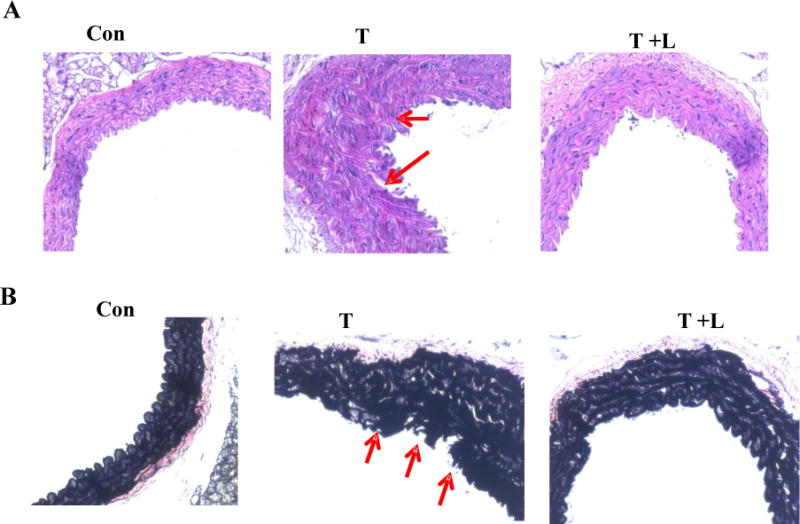
Aortic sections were stained with hematoxylin and eosin or with Verhoeff-Van Gieson as described in the Materials and Methods section. Representative aortic sections stained with hematoxylin and eosin (A) and Verhoeff-Van Gieson staining (B) are shown.
4. Discussion
Chronic inflammation of ECs and subsequent recruitment of monocytes into the arterial wall play an important role in initiating atherogenesis. Monocytes in the sub-endothelial space then develop into macrophages, which ultimately become lipid-rich foam cells, a hallmark of the atherosclerotic plaque [36]. Thus, protection of monocytes from adhesion to the endothelium could be a useful strategy for the prevention of atherosclerosis. Indeed, atherosclerosis fails to develop in animal models where monocytes are depleted [37]. Previous epidemiological studies demonstrate that increased flavonoid intake rich in luteolin may be protective against CVD underlying atherosclerosis [11–19], but the underlying mechanism remains elusive. In the present study, we demonstrated for the first time that luteolin at doses encompassing physiologically achievable concentrations (0.5 μM – 2 μM) suppresses TNF-α-triggered EC-monocyte interaction (Fig. 1). Recent studies reported that luteolin appeared in plasma and urine, both in its free and several conjugates forms in humans consuming a complex fruits and vegetables rich in flavonoid luteolin [15–19]. Intracellular physiological concentrations of luteolin were reported to be less than 2μM after dietary supplementation of various dosages of luteolin via consumption of a complex of fruits and vegetables rich in flavonoid luteolin [15–19]. Adhesion molecules and chemokines, such as VCAM-1, MCP-1/JE and KC, are the key mediators in the regulation of enhanced EC-monocyte interaction and subsequent inflammatory response in ECs [38]. We showed that luteolin also attenuated TNF-α-stimulated expression of chemokines and adhesion molecules in ECs. Furthermore, luteolin at physiologically-relevant concentrations inhibited TNF-α-induced NF-κB transcriptional activity IκBα degradation and subsequent NF-κB p65 nuclear translocation in ECs, suggesting that luteolin inhibits inflammation by suppressing NF-κB signaling. Mice treated with a diet containing 0.6% luteolin also abolished TNF-α-induced increases in circulating adhesion molecules and chemokines and reduced the expression of VCAM-1 and F4/80-positive macrophages in the vasculature (Fig. 4–5). Our findings suggest that luteolin may be a naturally-occurring, low-cost agent for prevention of vascular inflammation and atherosclerosis.
Numerous studies have reported the critical role of chemokines and adhesion molecules in the initiation of complex inflammatory process and pathogenesis of atherosclerosis [38, 39]. The chemokines MCP-1 and IL-8 are essential for monocyte rolling, firm adhesion to ECs and their subsequent transmigration into vascular tissue [40]. Indeed, MCP-1 and IL-8 are found in human atheroma, and mice lacking receptors for these chemokines are less susceptible to atherosclerosis and have fewer monocytes in vascular lesions [40]. In addition to chemokines, adhesion molecules such as ICAM-1, VCAM-1 and E-selectin also play a pivotal role in attracting, binding, and transmigrating monocytes into sites of inflammation [41]. In fact, these adhesion molecules are considered atherosclerotic inflammatory markers [42, 43], which are increased in advanced human coronary atherosclerotic plaques as well as in experimental models of atherosclerosis [42, 43].
In this study, our data showed that luteolin treatment remarkably suppressed TNF-α-induced expression of MCP-1, VCAM-1, and ICAM-1 in cultured ECs. Consistent to this in vitro result, we observed that circulating levels of MCP-1/JE, KC, and sICAM-1 were significantly increased in TNF-α treated-mice compared with normal mice. However, dietary intake of luteolin greatly suppressed the elevated secretion of these chemokines and adhesion molecule in TNF-α-treated mice. Mice do not express IL-8, but KC is considered to be the chemokine that is most closely related to human IL-8 [30]. KC triggers monocyte arrest in carotid arteries with early atherosclerotic lesions in mice [44]. These results suggest that ECs are activated in TNF-α-treated mice and that luteolin may, at least in part, target vascular ECs for exerting the anti-inflammatory action, which may be mediated by inhibiting the release of chemokines and adhesion molecules. In addition to ECs, MCP/JE, KC, as well as ICAM-1 are produced by a variety of cell types such as fibroblasts, macrophages, monocytes, epithelial, smooth muscle, monocytic, and microglial cells [45, 46], suggesting that the circulating chemokines and adhesion molecules could be produced largely from sources other than ECs. Therefore, there is possibility that luteolin may also modulate the production of these inflammatory molecules from other types of cells. Nevertheless, these data suggest that the protective effect of luteolin against vascular inflammation in vivo is likely mediated by inhibition of chemokines and adhesion molecules.
Activation of NF-κB plays a central role in the regulation of inflammatory responses by governing the expression of chemokines and leukocyte adhesion molecules [3, 8, 9]. As discussed above, leukocyte adhesion to the endothelium is mediated through pro-inflammatory chemokines and adhesion molecules, such as MCP-1, VCAM-1, E-selectin and ICAM-1, on ECs. The expression of these chemokines and adhesion molecules is critically up-regulated by the activation of NF-κB [3, 8, 9]. Consistently, NF-κB activation was found to be involved in the pathogenesis of atherosclerosis in patients [47]. Cellular activation by a multitude of extracellular signals leads to nuclear translocation of NF-κB p65. In the nucleus, the p50/p65 dimer can bind to the promoter regions of NF-κB-dependent proinflammatory genes, such as MCP-1 and IL-6, to induce their expression [48]. It was reported that TNF-α is a potent activator of NF-κB [49], which is critical for the transcriptional regulation of TNF-α-induced expression of E-selection, VCAM-1 and ICAM-1 [8, 9]. Normally, NF-κB is associated with the cytoplasmic inhibitory protein IκBα in its inactive form [50]. Cellular stimulation with TNF-α results in the phosphorylation and degradation of IκBα, allowing the p50/65 heterodimer of NF-κB to translocate to the nucleus and initiate expression of target genes [51–53]. The p65 homodimers are the most common dimers in NF-κB signaling. It was suggested that augmented NF-κB activation may arise from the increased nuclear translocation of the p65 subunit [10]. Therefore NF-κB is an interesting target for pharmaceutical interference in the establishment and progression of the pathologic state. In this study, we showed that TNF-α significantly increased NF-κB transcriptional activity, indicating that activation of NF-κB might be critical for the TNF-α-induced inflammatory response. Transfection study of NF-κB promoter construct and confocal microscopic examination of NF-κB p65 nuclear translocation suggested that luteolin inhibited both NF-κB-mediated gene expression and NF-κB nuclear translocation induced by TNF-α in ECs. Luteolin also inhibited TNF-α-mediated phosphorylation of IκBα, thereby blocking its degradation. To our knowledge, we are the first to show that luteolin at physiological concentrations potently suppresses IκBα degradation and NF-κB nuclear translocation in ECs. However, it is presently unclear how luteolin ablates the IκBα/NF-κB signaling pathway.
Our immunohistochemical analyses further showed abundance of F4/80-positive macrophages and the increased expression of VCAM-1 in the mouse aorta of TNF-α-treated group, which were greatly reduced by dietary provision of luteolin, confirming that this compound has a profound protective effect against TNF-α caused vascular inflammation via suppression of TNF-α increased leukocyte-vessel wall interactions and subsequent recruitment of leukocytes into vascular tissues. These data suggest that luteolin may directly target the endothelium for exerting this antiinflammatory action, which is consistent with our observations from in vitro culture of ECs. Our histological examination further indicated that dietary supplementation of luteolin also prevented endothelial structural damage and lining disruption of tunica media muscle fibers in arteries caused by TNF-α. Verhoeff-van Gieson staining has been suggested to be specific for elastic fibers in aortic vessel. In this study the mouse aorta of TNF-α-treated group was shown to have a significant aortic structure change in the intima layer and disruption of aortic elastin fibers, which were improved by dietary supplementation of luteolin. This vascular protective effect of luteolin could be the secondary action whereby luteolin suppressed TNF-α-stimulated production of inflammatory chemokines and vascular adhesion molecules. Various mediators have been shown to be closely associated with disruption of aortic elastin fibers, resulting in the development and progression of atherosclerosis. These mediators includes chemokines, MCP-1, IL-8, adhesion molecules such as ICAM-1, VCAM-1 and E-selectin, NF-κB, and matrix metalloproteinases (MMPs) [54–57]. Our results showed that luteolin reduced TNF-α-stimulated expression of chemokines and adhesion molecules in ECs and suppressed TNF-α-mediated activation of NF-κ. NF-κB is known to regulate inflammatory responses by overexpression of chemokines and leukocyte adhesion molecules and subsequently recruit monocyte-derived macrophages into the arterial wall to from of lipid-rich foam cells [3, 8, 9]. In addition, NF-κB action has been shown to upregulate the proliferation of vascular smooth muscle cells (VCMS) and expressions of MMPs in VCMS [58–60]. Nuclear localization of p65 has been detected in ECs, VCMS and macrophage [58–60]. Both the proliferation of VCMS and recruited macrophage are reported to be the major sources of MMPs [58–60]. Increased expressions of MMP-2 and MMP-9 are known to disrupt aortic elastin fibers resulting in loss of structural integrity and have been suggested to be involved in the pathological vascular remodeling [58–60]. In this study, we found that luteolin significantly decreased TNF-α-mediated activation of NF-κB and IKK-ß expression. This results may explain, at least in part, that the protective effect of luteolin against disruption of aortic elastin fibers is associated with inhibition of the NF-κB-mediated pathway. Since MMPs has been shown to be crucial involvement in change of structure and function of the aorta [58–60], further studies are essential in order to investigate whether luteolin can directly reduce TNF-α-mediated- activities of MMPs in the aortic wall.
In summary, the present study shows that dietary supplementation of luteolin improves vascular endothelial inflammation in vivo via reducing circulation of chemokines and adhesion molecules in plasma and suppressing the expression of VCAM-1 and F4/80 in the aorta of TNF-α-treated C57BL/6 mice. Luteolin at physiologically-relevant concentrations also significantly inhibits TNF-α-mediated adhesion of monocytes to ECs and suppressed TNF-α-induced expression of chemokines and adhesion molecules in ECs. The protective effect of luteolin against vascular inflammation is likely mediated via suppressing the IκBα/NF-κB pathway. These findings provide cellular and molecular evidence that luteolin may be a novel agent to protect against inflammation of the vasculature.
Acknowledgments
This work was supported by grants from National Center for Complementary and Alternative Medicine in the National Institutes of Health (1R15AT005372 to Z. Jia and 1R01AT007077- 01 to D. Liu)
Abbreviations
- CXCL1/KC
Chemokine (C-X-C motif) ligand 1
- ECs
endothelial cells
- FBS
fetal bovine serum
- HUVECs
human umbilical vein endothelial cells
- IκB kinase ß (IKKß)
ICAM-1, intercellular adhesion molecule-1
- IL-8
interleukin-8
- MCP-1/JE
mouse/monocyte chemotactic protein-1/JE
- MMPs
matrix metalloproteinases
- sICAM-1
soluble intercellular adhesion molecule-1
- sVCAM-1
soluble vascular adhesion molecule-1
- TNF-α
Tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statements
The authors have nothing to disclose.
References
- 1.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 2.Mayer K, Merfels M, Muhly-Reinholz M, Gokorsch S, Rosseau S, Lohmeyer J, et al. Omega-3 fatty acids suppress monocyte adhesion to human endothelial cells: role of endothelial PAF generation. Am J Physiol Heart Circ Physiol. 2002;283:H811–8. doi: 10.1152/ajpheart.00235.2002. [DOI] [PubMed] [Google Scholar]
- 3.Chen JW, Chen YH, Lin FY, Chen YL, Lin SJ. Ginkgo biloba extract inhibits tumor necrosis factor-alpha-induced reactive oxygen species generation, transcription factor activation, and cell adhesion molecule expression in human aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:1559–66. doi: 10.1161/01.ATV.0000089012.73180.63. [DOI] [PubMed] [Google Scholar]
- 4.Carluccio MA, Siculella L, Ancora MA, Massaro M, Scoditti E, Storelli C, et al. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler Thromb Vasc Biol. 2003;23:622–9. doi: 10.1161/01.ATV.0000062884.69432.A0. [DOI] [PubMed] [Google Scholar]
- 5.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circulation research. 2007;100:607–21. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 7.Angel K, Provan SA, Fagerhol MK, Mowinckel P, Kvien TK, Atar D. Effect of 1-year anti-TNF-alpha therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: a controlled study. American journal of hypertension. 2012;25:644–50. doi: 10.1038/ajh.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee TK, Nathan L, Dinh H, Reddy ST, Chaudhuri G. 17-epiestriol, an estrogen metabolite, is more potent than estradiol in inhibiting vascular cell adhesion molecule 1 (VCAM-1) mRNA expression. J Biol Chem. 2003;278:11746–52. doi: 10.1074/jbc.M207800200. [DOI] [PubMed] [Google Scholar]
- 9.Boyle EM, Jr, Kovacich JC, Canty TG, Jr, Morgan EN, Chi E, Verrier ED, et al. Inhibition of nuclear factor-kappa B nuclear localization reduces human E-selectin expression and the systemic inflammatory response. Circulation. 1998;98:II282–8. [PubMed] [Google Scholar]
- 10.Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R. Hyperglycemia-induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes. 1999;48:855–64. doi: 10.2337/diabetes.48.4.855. [DOI] [PubMed] [Google Scholar]
- 11.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317S–25S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 12.Maron DJ. Flavonoids for reduction of atherosclerotic risk. Curr Atheroscler Rep. 2004;6:73–8. doi: 10.1007/s11883-004-0119-1. [DOI] [PubMed] [Google Scholar]
- 13.Mennen LI, Sapinho D, de Bree A, Arnault N, Bertrais S, Galan P, et al. Consumption of foods rich in flavonoids is related to a decreased cardiovascular risk in apparently healthy French women. J Nutr. 2004;134:923–6. doi: 10.1093/jn/134.4.923. [DOI] [PubMed] [Google Scholar]
- 14.Cao J, Zhang Y, Chen W, Zhao X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br J Nutr. 2010;103:249–55. doi: 10.1017/S000711450999170X. [DOI] [PubMed] [Google Scholar]
- 15.Nazari QA, Kume T, Takada-Takatori Y, Izumi Y, Akaike A. Protective effect of luteolin on an oxidative-stress model induced by microinjection of sodium nitroprusside in mice. J Pharmacol Sci. 2013;122:109–17. doi: 10.1254/jphs.13019fp. [DOI] [PubMed] [Google Scholar]
- 16.Kanazawa K, Uehara M, Yanagitani H, Hashimoto T. Bioavailable flavonoids to suppress the formation of 8-OHdG in HepG2 cells. Arch Biochem Biophys. 2006;455:197–203. doi: 10.1016/j.abb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Si H, Wyeth RP, Liu D. The flavonoid luteolin induces nitric oxide production and arterial relaxation. Eur J Nutr. 2014;53:269–75. doi: 10.1007/s00394-013-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang S, Dilger RN, Johnson RW. Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J Nutr. 2010;140:1892–8. doi: 10.3945/jn.110.123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimoi K, Okada H, Furugori M, Goda T, Takase S, Suzuki M, et al. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett. 1998;438:220–4. doi: 10.1016/s0014-5793(98)01304-0. [DOI] [PubMed] [Google Scholar]
- 20.Englisch W, Beckers C, Unkauf M, Ruepp M, Zinserling V. Efficacy of Artichoke dry extract in patients with hyperlipoproteinemia. Arzneimittelforschung. 2000;50:260–5. doi: 10.1055/s-0031-1300196. [DOI] [PubMed] [Google Scholar]
- 21.Gebhardt R. Prevention of taurolithocholate-induced hepatic bile canalicular distortions by HPLC-characterized extracts of artichoke (Cynara scolymus) leaves. Planta Med. 2002;68:776–9. doi: 10.1055/s-2002-34417. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Xia N, Brausch I, Yao Y, Forstermann U. Flavonoids from artichoke (Cynara scolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J Pharmacol Exp Ther. 2004;310:926–32. doi: 10.1124/jpet.104.066639. [DOI] [PubMed] [Google Scholar]
- 23.Babu PV, Si H, Fu Z, Zhen W, Liu D. Genistein prevents hyperglycemia-induced monocyte adhesion to human aortic endothelial cells through preservation of the cAMP signaling pathway and ameliorates vascular inflammation in obese diabetic mice. J Nutr. 2012;142:724–30. doi: 10.3945/jn.111.152322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia Z, Babu PV, Si H, Nallasamy P, Zhu H, Zhen W, et al. Genistein inhibits TNF-alpha-induced endothelial inflammation through the protein kinase pathway A and improves vascular inflammation in C57BL/6 mice. Int J Cardiol. 2013;168:2637–45. doi: 10.1016/j.ijcard.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia Z, Zhen W, Velayutham Anandh Babu P, Liu D. Phytoestrogen genistein protects against endothelial barrier dysfunction in vascular endothelial cells through PKA-mediated suppression of RhoA signaling. Endocrinology. 2013;154:727–37. doi: 10.1210/en.2012-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nallasamy P, Si H, Babu PV, Pan D, Fu Y, Brooke EA, et al. Sulforaphane reduces vascular inflammation in mice and prevents TNF-alpha-induced monocyte adhesion to primary endothelial cells through interfering with the NF-kappaB pathway. J Nutr Biochem. 2014 doi: 10.1016/j.jnutbio.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babu PV, Si H, Liu D. Epigallocatechin gallate reduces vascular inflammation in db/db mice possibly through an NF-kappaB-mediated mechanism. Mol Nutr Food Res. 2012;56:1424–32. doi: 10.1002/mnfr.201200040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–13. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 29.Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF, Antonetti DA. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59:2872–82. doi: 10.2337/db09-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivasan S, Bolick DT, Hatley ME, Natarajan R, Reilly KB, Yeh M, et al. Glucose regulates interleukin-8 production in aortic endothelial cells through activation of the p38 mitogen-activated protein kinase pathway in diabetes. J Biol Chem. 2004;279:31930–6. doi: 10.1074/jbc.M400753200. [DOI] [PubMed] [Google Scholar]
- 31.Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Engl J Med. 1995;332:635–41. doi: 10.1056/NEJM199503093321003. [DOI] [PubMed] [Google Scholar]
- 32.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 33.Deckert-Schluter M, Bluethmann H, Kaefer N, Rang A, Schluter D. Interferon-gamma receptor-mediated but not tumor necrosis factor receptor type 1- or type 2-mediated signaling is crucial for the activation of cerebral blood vessel endothelial cells and microglia in murine Toxoplasma encephalitis. Am J Pathol. 1999;154:1549–61. doi: 10.1016/s0002-9440(10)65408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaposhnik Z, Wang X, Lusis AJ. Arterial colony stimulating factor-1 influences atherosclerotic lesions by regulating monocyte migration and apoptosis. J Lipid Res. 2010;51:1962–70. doi: 10.1194/jlr.M005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu D, Nishimura N, Kuo V, Fiehn O, Shahbaz S, Van Winkle L, et al. Activation of aryl hydrocarbon receptor induces vascular inflammation and promotes atherosclerosis in apolipoprotein E−/− mice. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1260–7. doi: 10.1161/ATVBAHA.110.220202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nature reviews Immunology. 2013;13:709–21. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pittet MJ, Swirski FK. Monocytes link atherosclerosis and cancer. European journal of immunology. 2011;41:2519–22. doi: 10.1002/eji.201141727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norata GD, Cattaneo P, Poletti A, Catapano AL. The androgen derivative 5alpha-androstane-3beta,17beta-diol inhibits tumor necrosis factor alpha and lipopolysaccharide induced inflammatory response in human endothelial cells and in mice aorta. Atherosclerosis. 2010;212:100–6. doi: 10.1016/j.atherosclerosis.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 40.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–23. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 41.Martin J, Collot-Teixeira S, McGregor L, McGregor JL. The dialogue between endothelial cells and monocytes/macrophages in vascular syndromes. Current pharmaceutical design. 2007;13:1751–9. doi: 10.2174/138161207780831248. [DOI] [PubMed] [Google Scholar]
- 42.Hong JJ, Jeong TS, Choi JH, Park JH, Lee KY, Seo YJ, et al. Hematein inhibits tumor necrotic factor-alpha-induced vascular cell adhesion molecule-1 and NF-kappaB-dependent gene expression in human vascular endothelial cells. Biochem Biophys Res Commun. 2001;281:1127–33. doi: 10.1006/bbrc.2001.4480. [DOI] [PubMed] [Google Scholar]
- 43.O’Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–51. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huo Y, Weber C, Forlow SB, Sperandio M, Thatte J, Mack M, et al. The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J Clin Invest. 2001;108:1307–14. doi: 10.1172/JCI12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golias C, Tsoutsi E, Matziridis A, Makridis P, Batistatou A, Charalabopoulos K. Review. Leukocyte and endothelial cell adhesion molecules in inflammation focusing on inflammatory heart disease. In Vivo. 2007;21:757–69. [PubMed] [Google Scholar]
- 47.Hegazy DM, O’Reilly DA, Yang BM, Hodgkinson AD, Millward BA, Demaine AG. NFkappaB polymorphisms and susceptibility to type 1 diabetes. Genes and immunity. 2001;2:304–8. doi: 10.1038/sj.gene.6363776. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Reddy MA, Miao F, Shanmugam N, Yee JK, Hawkins D, et al. Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-kappaB-dependent inflammatory genes. Relevance to diabetes and inflammation. J Biol Chem. 2008;283:26771–81. doi: 10.1074/jbc.M802800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paria BC, Malik AB, Kwiatek AM, Rahman A, May MJ, Ghosh S, et al. Tumor necrosis factor-alpha induces nuclear factor-kappaB-dependent TRPC1 expression in endothelial cells. J Biol Chem. 2003;278:37195–203. doi: 10.1074/jbc.M304287200. [DOI] [PubMed] [Google Scholar]
- 50.Gerritsen ME, Williams AJ, Neish AS, Moore S, Shi Y, Collins T. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci U S A. 1997;94:2927–32. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–8. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 52.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 53.Read MA, Whitley MZ, Williams AJ, Collins T. NF-kappa B and I kappa B alpha: an inducible regulatory system in endothelial activation. J Exp Med. 1994;179:503–12. doi: 10.1084/jem.179.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diez J. Arterial stiffness and extracellular matrix. Advances in cardiology. 2007;44:76–95. doi: 10.1159/000096722. [DOI] [PubMed] [Google Scholar]
- 55.Nejjar I, Pieraggi MT, Thiers JC, Bouissou H. Age-related changes in the elastic tissue of the human thoracic aorta. Atherosclerosis. 1990;80:199–208. doi: 10.1016/0021-9150(90)90027-g. [DOI] [PubMed] [Google Scholar]
- 56.Steed MM, Tyagi SC. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxidants & redox signaling. 2011;15:1927–43. doi: 10.1089/ars.2010.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaneko H, Anzai T, Morisawa M, Kohno T, Nagai T, Anzai A, et al. Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis. 2011;217:350–7. doi: 10.1016/j.atherosclerosis.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 58.Bellosta S, Baetta R, Canavesi M, Comparato C, Granata A, Monetti M, et al. Raloxifene inhibits matrix metalloproteinases expression and activity in macrophages and smooth muscle cells. Pharmacological research: the official journal of the Italian Pharmacological Society. 2007;56:160–7. doi: 10.1016/j.phrs.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. The Journal of clinical investigation. 2000;105:1641–9. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. The New England journal of medicine. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]


