Abstract
Interleukin-35 is a novel inhibition cytokine secreted by CD4+CD25+ regulatory T-cells (Treg) in murine. However, it is disputed whether IL-35 could be secreted by Treg cells in humans. In this study, the levels of IL-35 were detected, and its relationship with regulatory T-cells in chronic hepatitis B (CHB) patients was investigated. It was shown that the levels of IL-35 in CHB patients were higher than those in normal controls, and the levels increased gradually, accompanied with the severe liver inflammation and necrosis and poor synthesis function. Treg cells may secrete IL-35, whose levels would become higher, accompanied by a longer activated time. Thus, IL-35 as a cytokine secreted by Treg cells may accelerate liver inflammation and necrosis, and inhibit the synthesis function.
Introduction
It is estimated that 350 million people are infected with chronic hepatitis B virus (HBV) worldwide, a condition that can be associated with acute or chronic hepatitis B, fulminant hepatitis, liver cirrhosis (LC), or hepatocellular carcinoma (9). However, so far, there are no satisfactory protocols to cure chronic hepatitis B (CHB). Furthermore, its pathogenesis and how HBV maintains chronic persistent infection is not fully understood. Therefore, further study into its pathogenesis and new treatment methods is needed to resolve the issue. It is thought that CD4+CD25+ regulatory T-cells (Treg) could inhibit HBV antigen-specific T-cell responses, accompanied with chronic persistent HBV infection. Although several studies have assessed the mechanism of Treg cells mediated-suppression, many details remain unknown. It is thought that Treg cells could be suppressed through direct contact with target cells or antigen-presenting cells (17,18,19) and secreted cytokines (1,6,16). Membrane molecules (12,14) also play some role in the suppression of Treg cells.
Interleukin-35 (IL-35) was discovered and designated by the end of 2007, and identified as a novel immunosuppressive/anti-inflammatory cytokine of the IL-12 family, which includes IL-12, IL-23, and IL-27 (15). IL-35 is a heterodimeric protein with two subunits, Epstein–Barr virus induced gene 3 (EBI3) and IL-12p35, which shares EBI3 with IL-27. As a result, both IL-35 and IL-27 are immunosuppressive, while the other two cytokines not including EBI3 are not. It is known that EBI3 is the downstream target molecule of Foxp3, the function marker of Treg cells (2).
To date, research on IL-35 has focused mainly on its relationship with auto-immune diseases, inflammation, and infection (10,20,21). Whether HBV, as a type of virus, could activate and improve the secretion of IL-35 is under discussion. A preliminary test discovered that the levels in the serum of HBV infections were higher compared to normal controls (NC). Furthermore, it is reported that CD4+ T-cells express IL-35 in human peripheral blood mononuclear cells (PBMCs) (8).
In this study, the expression levels of IL-35 in chronic severe hepatitis B (CSHB), CHB, liver cirrhosis (LC), and asymptomatic carriers (ASC) were assayed to investigate the role of IL-35 in HBV infection.
Materials and Methods
Patients and donors
Peripheral blood samples were obtained from patients with chronic HBV infection and healthy controls. No patients were seropositive for hepatitis A, C, D, or E virus, or human immunodeficiency virus. Patients with an overt comorbid condition, such as fatty liver, alcoholic liver disease, or autoimmune disease, and patients who received antiviral, immunomodulatory, or immunosuppressive treatments during the past 6 months were all excluded. The study was carried out in accordance with the management guidelines for CHB (2010) of the Hepatology Association Chinese Medical Association, and was approved by the local ethics committee. Informed consent was obtained from the donors before blood donation.
Isolation of plasma and PBMCs
Coagulated peripheral blood from 27 CSHB, 69 CHB, 29 ASC, and 26 NC was collected and centrifuged to obtain plasma. Five milliliters of heparinized aseptic peripheral blood from 20 CSHB, 40 CHB, 15 ASC, and 15 NC were collected from ulnar vein. PBMCs were obtained by separating the blood sample via Ficoll separation (Ficoll-Paque density gradient centrifugation). Then they were washed three times with phosphate-buffered saline (PBS) and collected.
Cell isolation, expansion, and labeling
PBMCs were obtained in aseptic condition, as described in the previous section. CD4+CD25+ Treg and CD4+CD25− effector T-cells (Teff) were isolated from fresh PBMCs using the CD4+CD25+ Treg cell isolation kit. In brief, CD4+ T-cells were first purified by negative selection using a LD column. The enriched CD4+ T-cells were then incubated with CD25 microbeads followed by separation using a MS column. The negative fraction (CD4+CD25− T-cells) flowed down and was collected, while the positive fraction was reserved in the MS column and was washed down with PBS away from the magnetic field. The isolations were performed according to the manufacturer's instructions. Purity was verified by membrane staining of CD4 and CD25 (anti-CD4-FITC, anti-CD25-PE; eBioscience).
Treg and conventional CD4+ T-cells (Tconv) were expanded in RPMI-1640 medium with anti-CD3 and anti-CD28, 20% (v/v) fetal bovine serum (FBS), and either 500 IU/mL rhIL-2 for Treg or 100 IU/mL for Tconv. Treg cells were expanded for 7 days.
RNA extraction and quantitative real-time polymerase chain reaction analysis
RNA was isolated by TRIzol extraction, followed by reverse transcription into cDNA using M-MLV reverse transcriptase and random primer (all reagents from Invitrogen). Quantitative real-time polymerase chain reaction (PCR) was performed using SYBR Green. EBI3 primers were as follows: 5′-GCA GAC GCC AAC GTC CAC-3′ (sense) and 5′-CCA GTC ACT CAG TTC CCC GT-3′ (antisense). IL-12p35 primers were as follows: 5′-TGG CCC TGT GCC TTA GTA GTA T-3′ (sense) and 5′-GGT TCT TCA AGG GAG GAT TTT T-3′ (antisense). Foxp3 primers: 5′-CAA ACA GCC ACA TTC CCA GAG TTC-3′ (sense) and 5′-CAA CCT GAG TCT GCA CAA GTG C-3′ (antisense). IL-10 primers: 5′-GTG AAG ACT TTC TTT CAA A-3′ (sense) and 5′-TTG GAG CTT ATT AAA GG-3′ (antisense). TGF-β primers: 5′-ATC TAC AAC AGC ACC AGG GAC T-3′ (sense) and 5′-TTT GGG TTC TGC AAA CGA AA-3′ (antisense). β-actin primers: 5′-CGA AAC TAC CTT CAA CTC CAT C-3′ (sense) and 5′-AGT GAT CTC CTT CTG CAT CCT-3′ (antisense). The thermal cycle profile was as follows: predegeneration for 3 min at 95°C; degeneration for 30 s at 94°C, renaturation for 30 s at 61°C and 1 min at 72°C for 40 cycles. Analysis was performed as follows. At the end of reaction, the ABI 7500 system automatically analyzed the results and obtained the value of Ct. Results were normalized to β-actin. Relative expression of RNA transcripts was quantified using the formula 2−ΔΔCt. ΔΔCt represents that the minus Ct value between the goal and housekeeping gene (β-actin).
Intracellular staining
PE-conjugated anti-IL-12A and IgG1 (isotype control) were used for intracellular staining according to the manufacturer's instructions (R&D corporation). The Permeabilization Wash Buffer used for intracellular staining of IL-12A was purchased from BioLegend. IL-12A was analyzed according to the manufacturer's instructions.
Detection of the cytokines IL-10, TGF-β, and IL-35
Cytokines IL-35, IL-10, and TGF-β were analyzed in the plasma of peripheral blood and at different time points by analysis of the supernatant with commercially available enzyme-linked immunosorbent assay kits for human TGF-β, IL-10, and IL-35 according to the protocols supplied by the manufacturer.
Statistical analysis
Statistical analysis was performed using SPSS v13.0 software (SPSS, Inc.). All data were indicated by mean±standard deviation (SD). One-way analysis of variance was initially performed to determine whether an overall statistically significant change existed before using the two-tailed paired or unpaired Student's t-test. The Mann–Whitney U-test was used to compare the unpaired data and determine statistically significant differences in mean cell number or mean percentage in flow cytometry (FCM). The correlativity of quantitative data was performed by scatter/dot graph, and the coefficient was indicated by r. Differences with a p-value of <0.05 were considered statistically significant.
Results
Levels of IL-35 in the serum of HBV-infected patients
The serum level of IL-35 in HBV-infected patients was 3,206.90±1,769.89 pg/mL compared to 180.68±61.64 pg/mL in NC, meaning that the levels of IL-35 in HBV-infected patients was significantly higher than those found in NC (p<0.05; Table 1). The levels in CSHB, CHB, and ASC were 7,178.00±1,303.00, 3,371.30±918.53, and 1,292.10±859.12 pg/mL respectively; there were significant differences among each group (Table 2 and Fig. 1). The results indicated that the levels of IL-35 increase in proportion to the severity of chronic liver injury.
Table 1.
Serum Levels of Interleukin-35 in Patients with HBV Infection
| Group | n | IL-35 (pg/mL) |
|---|---|---|
| NC | 26 | 180.68±61.64 |
| HBV infection | 125 | 3,206.90±1,769.89* |
Data shown are mean±standard deviation (SD).
t=4.07 compared with NC (p<0.01).
HBV, hepatitis B virus; IL, interleukin; NC, normal controls.
Table 2.
Comparison of Serum Levels of IL-35 in Different Patients with HBV Infection
| Group | n | IL-35 (pg/mL) |
|---|---|---|
| NC | 26 | 180.68±61.64 |
| ASC | 29 | 1,292.10±859.12 |
| CHB | 69 | 3,371.30±918.53 |
| CSHB | 27 | 7,178.00±1,303.00 |
Comparison of serum levels of IL-35 among different HBV infection groups: F=40.48, p<0.05.
ASC, asymptomatic carriers; CHB, chronic heptatitis B; CSHB, chronic severe hepatitis B.
FIG. 1.
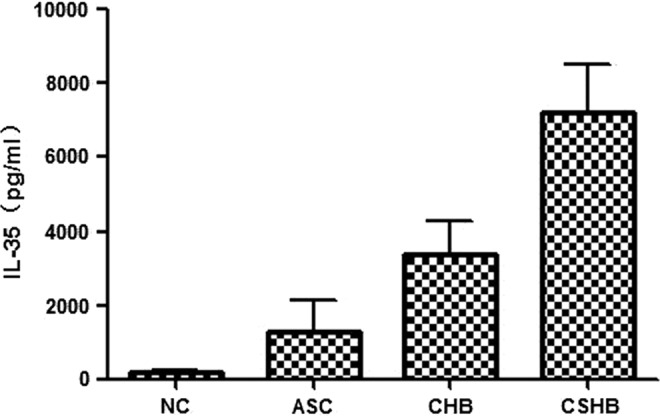
The serum levels of interleukin (IL)-35 in different patients with hepatitis B virus (HBV) infection. Serum levels of IL-35 in asymptomatic carriers (ASC), chronic hepatitis B (CHB), and chronic severe hepatitis B (CSHB) groups were assayed. The serum levels of IL-35 in HBV infection was significantly higher than that in normal controls (NC) (p<0.05; Table 1). The levels in CSHB, CHB, and ASC were 7,178.00±1,303.00, 3,371.30±918.53, and 1,292.10±859.12 pg/mL, respectively. There were significant differences among different groups (Table 2).
Correlativity between the levels of IL-35 and partial liver function indices
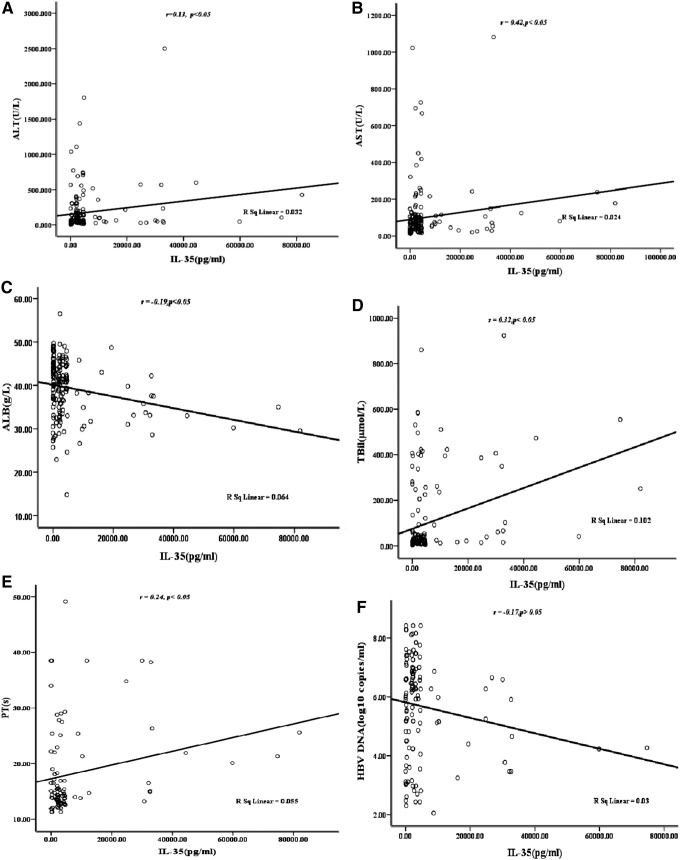
The correlativity between the levels of IL-35 and partial liver function indices were analyzed, and it was found that the levels of IL-35 were positively correlated with ALT, AST, TBIL, and PT, negatively with ALB, and nonsignificantly with HBV DNA (Fig. 2A–F).
FIG. 2.
The correlation between serum level of IL-35 and other indexes in HBV-infected patients. (A) ALT; (B) AST; (C) ALB; (D) TBIL; (E) PT; (F) log HBV DNA. The levels of IL-35 were positively correlated with ALT, AST, TBIL, and PT (p<0.05), negatively with ALB (p<0.05), and was not significantly correlated with HBV DNA.
Levels of IL-35 and Foxp3 mRNA in patients with HBV infection
The levels of IL-35 and Foxp3 mRNA were investigated using quantitative RT-PCR. The results indicated that the values of 2−ΔΔCt relating to EBI3, a subset of IL-35 in different patients with HBV infection, were significantly different (F=47.85, p<0.05; Table 3), and the levels of IL-35 and Foxp3 mRNA increased progressively in CSHB, CHB, ASC, and NC. The tendency in another subset of IL-35, IL-12p35, and Foxp3 was identical (Table 3). According to the formula and normalizing to β-actin, the relative expression was negatively correlated with the value of 2−ΔΔCt. In other words, the relative expression of EBI3, IL-12p35, and Foxp3 in CHSB, CHB, ASC, and NC decreased progressively (Fig. 3).
Table 3.
Comparison of Serum mRNA Levels of EBI3, IL-12p35, and Foxp3 in the Different Patients
| Group | n | EBI3 | IL-12p35 | Foxp3 |
|---|---|---|---|---|
| NC | 15 | 9.24±1.23 | 8.34±1.17 | 9.8±0.52 |
| ASC | 15 | 14.48±1.24 | 17.4±1.24 | 19.0±0.65 |
| CHB | 40 | 22.45±1.21 | 26.8±1.49 | 28.5±0.48 |
| CSHB | 20 | 36.2±0.96 | 45.8±0.67 | 38.4±0.46 |
FIG. 3.
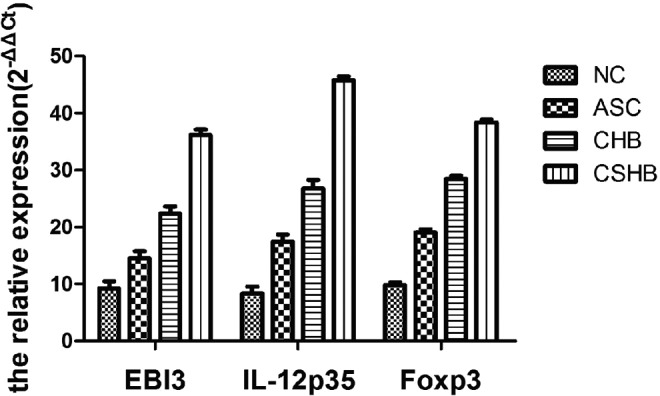
Comparison of serum mRNA levels of EBI3, IL-12p35, and Foxp3 in the different patients. EBI3 levels in different patients with HBV infection was significantly different (F=47.85, p<0.05; Table 3), and that increased progressively in NC>ASC>CHB>CSHB. The tendency in other subsets of IL-35, IL-12p35 (F=32.95, p<0.05), and Foxp3 (F=45.65, p<0.05) was identical (Table 3).
Correlativity of IL-35 and Foxp3 mRNA in patients with HBV infection
The correlativity of IL-35 and Foxp3 as a specific marker of Treg cells was then analyzed. In this process, the data showed that the levels of IL-35 mRNA were positively correlated with those of Foxp3; in other words, the levels of IL-35 mRNA increased in line with the higher levels of Foxp3, and the same tendency was expressed between EBI3 and IL-12p35 (p<0.05; Fig. 4A–C).
FIG. 4.
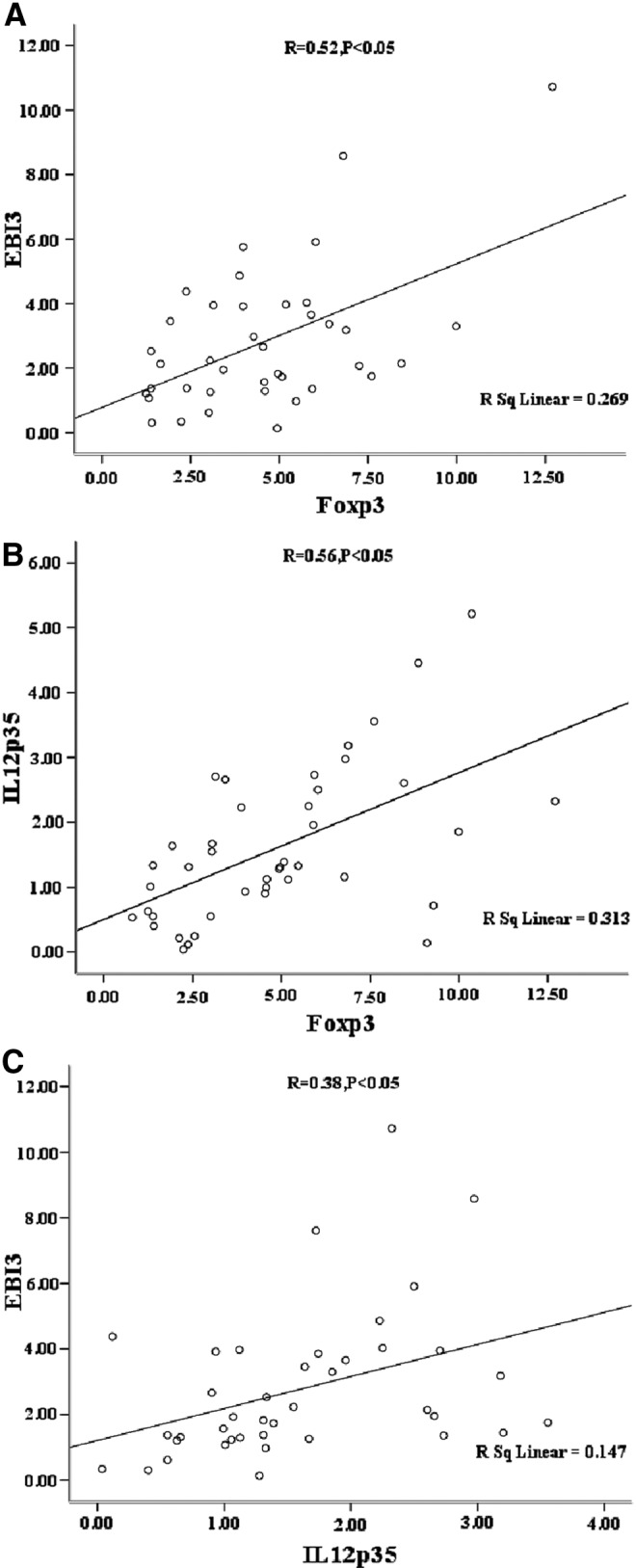
The correlation between two subunits of IL-35 (EBI3 and IL-12p35) and Foxp3 (A) and (B) two subunits (EBI3 and IL-12p35) levels of IL-35 was positively correlated with Foxp3, respectively (p<0.05). (C) EBI3 was also positively correlated with IL-12p35 (p<0.05).
The purity of CD4+CD25+Treg and CD4+CD25− T-cells
The purity of CD4+CD25+ Treg and CD4+CD25− T-cells sorted by MACS were analyzed. The purity of CD4+CD25− T-cells was 74.35±8.66%, while that of CD4+CD25+ Treg cells was 79.11±9.11%, with a survival rate of 92–95%.
Levels of intracellular cytokine IL-12p35 in the unactivated and activated CD4+CD25+ Treg and CD4+CD25− T-cells
To clarify whether Treg cells express and secrete IL-35, the levels of intracellular cytokine IL-12p35 were established by FCM. The results showed that the levels became higher proportionally to the longer activation time in CD4+CD25+ Treg cells. However, little IL-12p35 was detected in unactivated and activated CD4+CD25− T-cells, which were both activated with anti-CD3, anti-CD28, and rhIL-2 (Table 4, Figs. 5 and 6).
Table 4.
Levels of Intracellular IL-12p35 in the Unactivated and Activated CD4+CD25+ Tregs and CD4+CD25− T-cells (n=5)
| Group | 0 days (%) | 3 days (%) | 5 days (%) | 7 days (%) |
|---|---|---|---|---|
| CD4+ CD25+ Treg |
0.81±0.22 | 1.27±0.28 | 2.61±0.42 | 4.54±0.69 |
| CD4+ CD25− T-cells |
0.07±0.002 | 0.08±0.002 | 0.09±0.003 | 0.08±0.006 |
FIG. 5.
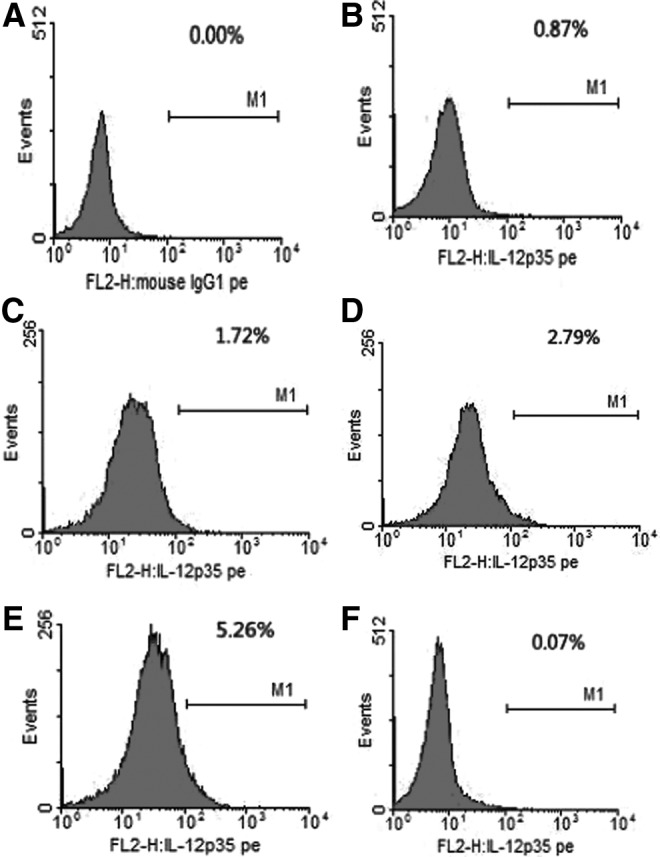
Levels of intracellular IL-12p35 in the unactivated and activated CD4+CD25+ Tregs and CD4+CD25− T-cells. (A) control; (B), (C), (D), and (E) represent the activated levels of CD4+CD25+ Treg in 0, 3, 5, and 7 days; (F) represents the unactivated levels of CD4+CD25− T-cells. CD4+CD25+ Treg and CD4+CD25− T-cells were acquired through magnetic activated cell sorting (MACS) from fresh peripheral blood mononuclear cells (PBMCs).
FIG. 6.
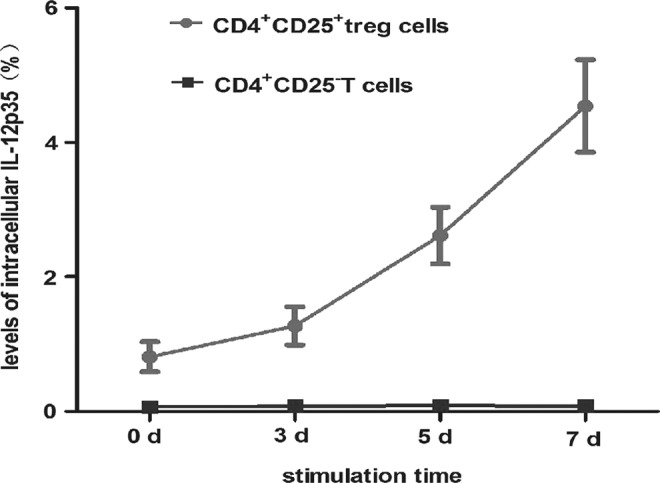
Levels of intracellular IL-12p35 from CD4+CD25+ Treg and CD4+CD25− T-cells. CD4+CD25+ Treg and CD4+CD25− T-cells were both activated with anti-CD3, anti-CD28, and rhIL-2 for 0, 3, 5, and 7 days. Intracellular cytokine IL-12p35 was detected by flow cytometry. The levels of IL-12p35 became higher, accompanied by a longer activation time in CD4+CD25+ Treg cells (F=141.14, p<0.05). However, lower levels of IL-12p35 were detected in unactivated and activated CD4+CD25− T-cells. Levels of intracellular cytokine IL-12p35 in CD4+CD25+ Tregs were higher than in CD4+CD25− Tregs at 0, 3, 5, and 7 days respectively (Table 4 and Fig. 5). CD4+CD25+ Treg and CD4+CD25− T-cells were acquired through MACS from fresh PBMCs.
Levels of intracellular EBI3, IL-12p35, Foxp3, IL-10, and TGF-β in the unactivated and activated CD4+CD25+ Tregs
mRNA levels of IL-35 two subsets EBI3 and IL-12p35 in CD4+ CD25+Tregs at different activation time were detected by RT-PCR. Levels of EBI3 and IL-12p35 increased gradually in accordance with the activation time, which suggested that both activated and unactivated CD4+CD25+ Tregs can express IL-35. IL-10 and TGF-β mRNA, which are known to be secreted by CD4+CD25+ Tregs, were also assayed, and as anticipated, the levels became higher after activation. The levels of Foxp3, the marker of Treg cells, became higher as the activation time increased (Table 5 and Fig. 7).
Table 5.
Levels of Intracellular EBI3, IL-12p35, Foxp3, IL-10, and TGF-β in the Unactivated and Activated CD4+CD25+ Tregs (n=5)
| Group | 0 days | 3 days | 5 days | 7 days |
|---|---|---|---|---|
| EBI3 | 0.13±0.05 | 0.22±0.03 | 0.35±0.09 | 0.82±0.22 |
| IL-12p35 | 0.07±0.03 | 0.14±0.02 | 0.24±0.07 | 0.56±0.19 |
| Foxp3 | 0.05±0.04 | 0.20±0.06 | 0.48±0.15 | 1.00±0.14 |
| IL-10 | 0.05±0.03 | 0.26±0.09 | 0.42±0.06 | 0.68±0.16 |
| TGF-β | 0.06±0.05 | 0.23±0.03 | 0.40±0.11 | 0.75±0.09 |
FIG. 7.
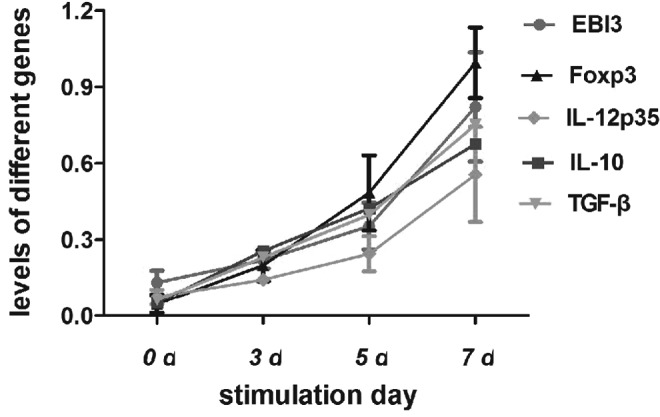
Levels of intracellular EBI3, IL-12p35, Foxp3, IL-10, and TGF-β in the unactivated and activated CD4+CD25+ Tregs. CD4+CD25+ Tregs were activated with anti-CD3, anti-CD28, and rhIL-2 for 0, 3, 5, and 7 days. mRNA levels of IL-35 two subsets, EBI3 and IL-12p35 in CD4+CD25+ Tregs, at different activation times were detected by reverse transcription polymerase chain reaction. Levels of EBI3 and IL-12p35 increased gradually in accordance with the activation time, which suggested that both activated and unactivated CD4+CD25+ Tregs can express IL-35. IL-10 and TGF-β mRNA, which are known to be secreted by CD4+CD25+ Tregs, were also assayed, and as anticipated, the levels were higher after activation. The levels of Foxp3, the marker of Treg cells, became higher as the activation time increased (Table 5). CD4+CD25+ Treg and CD4+CD25− T-cells were acquired through MACS from fresh PBMCs.
Levels of cytokines IL-35, IL-10, and TGF-β in the culture supernatant from CD4+CD25+ Tregs
Meanwhile, the levels of IL-35 mRNA were quantitated, and it was found that both activated and unactivated Treg cells express IL-35, which increased gradually in accordance with the activation time. IL-10 and TGF-β mRNA, which are known to be secreted by Treg cells, were also quantitated, and as anticipated, the levels were higher after activation. The levels of cytokine IL-35, IL-10, and TGF-β in the culture supernatant could be detected in both activated and unactivated Treg cells (Table 6 and Fig. 8).
Table 6.
Levels of Cytokine IL-35, IL-10, and TGF-β in the Culture Supernatant from CD4+CD25+ Tregs (n=5)
| Group | 3 days (pg/mL) | 5 days (pg/mL) | 7 days (pg/mL) |
|---|---|---|---|
| TGF-β | 75.06±7.46 | 149.86±41.24 | 379.48±84.85 |
| IL-10 | 151.56±48.20 | 317.58±35.55 | 478.41±76.06 |
| IL-35 | 74.92±12.61 | 189.26±45.31 | 315.86±36.06 |
FIG. 8.
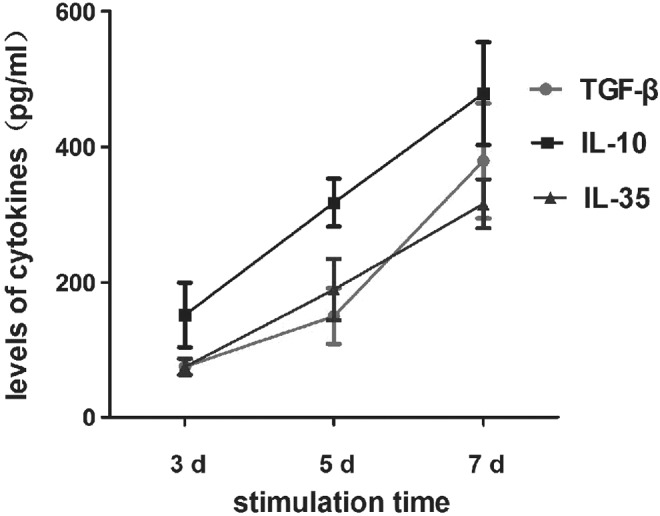
Levels of cytokine IL-35, IL-10, and TGF-β in the culture supernatant from CD4+CD25 + Treg cells. CD4+CD25+ Treg cells were activated with anti-CD3, anti-CD28, and rhIL-2 for 0, 3, 5, and 7 days. Levels of cytokine IL-35, IL-10, and TGF-β in the culture supernatant could be detected and became higher proportionally to the longer activation time in CD4+CD25+ Treg cells (p<0.05). CD4+CD25+ Treg and CD4+CD25− T-cells were acquired through MACS from fresh PBMCs.
Discussion
Treg cells play a key role in maintaining self-tolerance, preventing autoimmunity diseases, limiting chronic inflammatory diseases such as asthma and inflammatory bowel disease, and regulating the homeostasis of lymphocyte expansion (3). However, the mechanism of Treg cells mediated suppression is not completely clear. Several published studies support that Treg cells mediate suppression through direct contact and the secreted cytokines IL-10 and TGF-β. In the transwell experiment, Treg cells co-cultured with neutralized anti-IL-10 and TGF-β antibodies in the upper well could not effectively prevent Teff cells from proliferating in the lower well, which revealed that there still are soluble factors in the mechanism of Treg cells mediating suppression other than IL-10 and TGF-β (4). In recent years, IL-35 was discovered and studied, which may bring new pathways to mediate the function of Treg cells. In acute infection decade, IL-35 expands Th1 cells to clear pathogens, inhibits the differentiation of Th17 cells away from excessive autoimmune reaction, and expands Treg cells, which may inhibit Teff cells to prevent the organism from immune injury in the subsequent chronic infection (11). In vitro studies on CHC and self-limited HCV infection showed that Treg cells co-cultured with anti-IL-35 antibodies could inhibit the proliferation of Teff cells in the co-culture experiments (3). This finding may inspire new approaches to exploring the mechanism of virus persistent chronic infection such as HCV and HBV.
First, the levels of IL-35 in the serum of patients with HBV infection were analyzed, and it was found that the levels found in patients with HBV infection were significantly higher than those found in NC, and the levels decreased progressively in CHSB, CHB, and ASC. The levels were positively correlated with ALT, AST, TBil, and PT, and negatively with ALB. ALT and AST levels indicate inflammation of the liver, while TBil reflex indicates necrosis of the liver tissue. The fact that the levels of AST, ALT, and TBil became higher means that IL-35, as an immunosuppressive cytokine, maintains the homeostasis between HBV and effective T-cells through immune regulation. HBV replicates and injures liver tissue, causing more immune suppression factors to lessen the impairment. In this research, the levels of IL-35 were correlated with liver inflammation, necrosis, and synthesis in patients with HBV infection. The result may indicate that IL-35 could inhibit the immune injury. It suggests that there is no direct correlation between IL-35 and HBV replication, or the HBV loads in peripheral blood could not reflect those in the liver tissue, while no significant correlation was found between IL-35 and HBV DNA.
Next, the levels of IL-35 and Foxp3 mRNA in CHB patients were analyzed. Foxp3 was the specificity and fundamental marker of Treg cells, and it was analyzed to identify the correlation between IL-35 and Treg cells indirectly. In this research, the levels of IL-35 mRNA were found to increase progressively in CSHB, CHB, ASC, and NC, identical to Foxp3 mRNA. The correlation between the levels of IL-35 and Foxp3 mRNA were also analyzed, and the result suggested that there was a significantly positive correlation. Furthermore, in previous research, the percentage of CD4+CD25+ Treg cells was higher than that of NC. It is common knowledge that Foxp3 was the major gene regulating Treg cells to maintain an immunosuppressive function (7). In summary, IL-35 may mediate Treg cells to play an immunosuppressive function, causing HBV to become chronic in vivo.
In the animal model, IL-35 was constitutively expressed and secreted by Treg cells, and mediated the immunosuppressive role of Treg cells (11). It is still unclear whether IL-35 is expressed by Treg cells in vivo. French scientists (11) concluded that neither Treg nor other subsets such as CD4+, CD8+, and γδ T-cells expressed IL-35, while another study reported that activated, not unactivated, Treg cells could express IL-35 (15).
This research shows that the levels of Foxp3 mRNA expressed by CD4+CD25+ Treg cells became higher after activation, which coincides with the idea of Zorn (22). Both unactivated and activated Treg cells were found to express EBI3, IL-12p35 mRNA, and intracellular cytokine IL-12p35, and the levels increased a longer activation time. The levels of IL-10 and TGF-β also increased in line with the longer activation time. IL-35, like IL-10 and TGF-β, was an immunosuppressive cytokine secreted by Treg cells.
These findings suggest that IL-35 was secreted by CD4+CD25+ Treg cells, which could inhibit liver inflammation and necrosis. IL-35 suppression or neutralization may represent a valid immunotherapeutic strategy for the treatment of chronic persistent infection such as HBV and conditions in which immunological homeostasis might exist.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (Grant No. 81371867), National Major Scientific and Technological Special Project during the 12th Five-year Plan Period (2012ZX10002007), Education to Promote Health project of key personnel of Jiangsu Province (RC2011117), and Specialized Foundation for Clinical Medical Science and Technology of Jiangsu Province (No. BL2014033).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Asseman C, Mauze S, Leach MW, et al. . An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 1999;190:995–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan SE, Song-Zhao GX, Abraham T, et al. . Inducible reprogramming of human T cells into Treg cells by a conditionally active form of FOXP3[J]. Eur J Immunol 2008;38:3282–3289 [DOI] [PubMed] [Google Scholar]
- 3.Belkai DY, and Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol 2005;6:353–360 [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi V, Collison LW, Guy CS, et al. . Cutting edge: human regulatory T cells require IL-35 to mediate suppression and infectious tolerance. J Immunol 2011;186:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Collison LW, Workman CJ, Kuo TT, et al. . The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007;450:566–569 [DOI] [PubMed] [Google Scholar]
- 6.Fahlén L, Read S, Gorelik L, et al. . T cells that cannot respond to TGF-β escape control by CD4+CD25+regulatory T cells. J Exp Med 2005;201:737–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hori S, Nomura T, and Sakaguchi S. Control of regulatory T cells development by the transcription factor Foxp3. Science 2003;299:1057–1061 [PubMed] [Google Scholar]
- 8.Liu F, Tong F, He Y, et al. . Detectable expression of IL-35 in CD4+ T cells from peripheral blood of chronic hepatitis B patients. Clin Immunol 2011;139:1–5 [DOI] [PubMed] [Google Scholar]
- 9.Lok AS, and McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50:661–662 [DOI] [PubMed] [Google Scholar]
- 10.Neurath MF. IL-12 family members in experimental colitis. Mucosal Immunol 2008;S28–30 [DOI] [PubMed] [Google Scholar]
- 11.Niedbala W, Wei XQ, Cai B, et al. . IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulation T cells and suppression of Thl7 cells. Eur J Immunol 2007;37:3021–3029 [DOI] [PubMed] [Google Scholar]
- 12.Paust S, Lu L, McCarty N, et al. . Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci U S A 2004;101:10398–10403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy J, Illes Z, Zhang X, et al. . Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 2004;101:15434–15439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu J, Yamazaki S, Takahashi T, et al. . Stimulation of CD25 (+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol 2002;3:135–142 [DOI] [PubMed] [Google Scholar]
- 15.Siemasko KF, Gao J, Calder VL, et al. . In vitro expanded CD4+CD25+Foxp3+ regulatory T cells maintain a normal phenotype and suppress immune-mediated ocular surface inflammation. Invest Ophthalmol Vis Sci 2008;49:5434–5440 [DOI] [PubMed] [Google Scholar]
- 16.Suri-Payer E, and Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4+CD25+ T cells. J Autoimmun 2001;16:115–123 [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Kuniyasu Y, Toda M, et al. . Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol 1998;10:1969–1980 [DOI] [PubMed] [Google Scholar]
- 18.Tang Q, Adams JY, Tooley AJ, et al. . Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol 2006;7:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornton AM, and Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 1998;188:287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Boehmer H. Mechanisms of suppression by suppressor T cells. J Nat Immunol 2005;6:338–344 [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Yang M, Htut TM, et al. . Epstein-Barr virus-induced gene 3 negatively regulates IL-17, IL-22 and RORgamma t. Eur J Immunol 2008;8:1204–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zorn E, Nelson EA, Mohseni M, et al. . IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 2006;108:1571–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]