Abstract
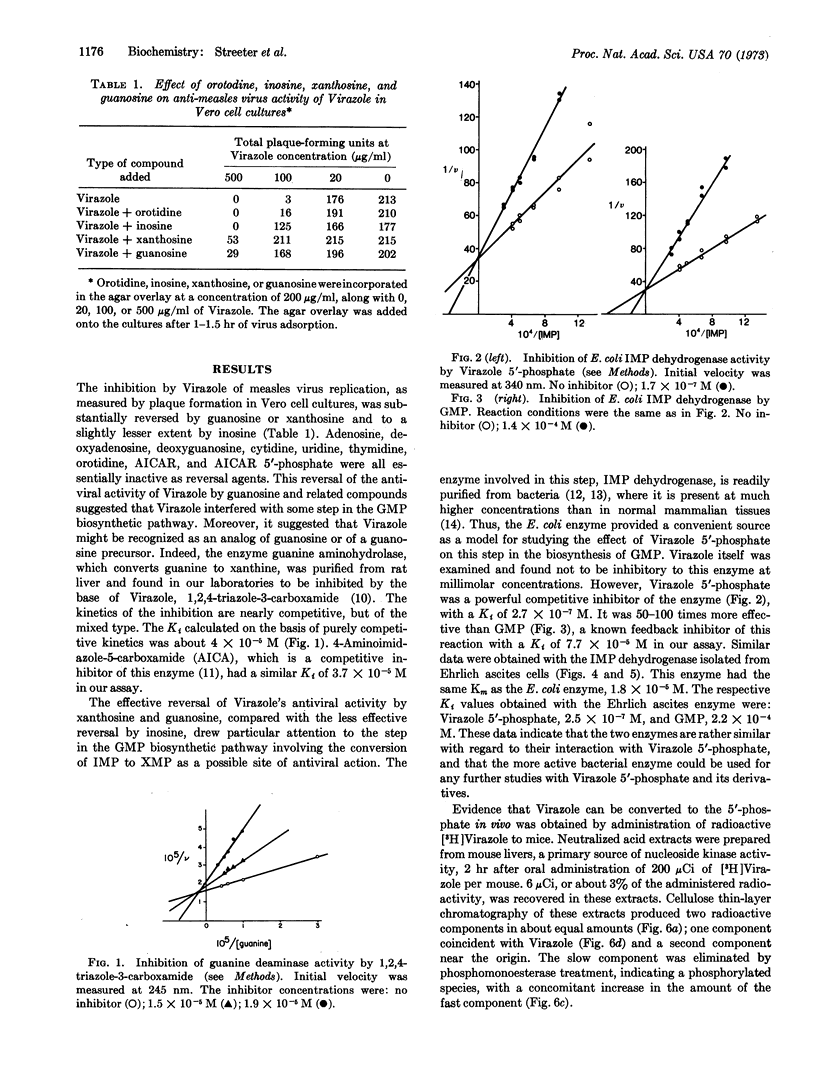
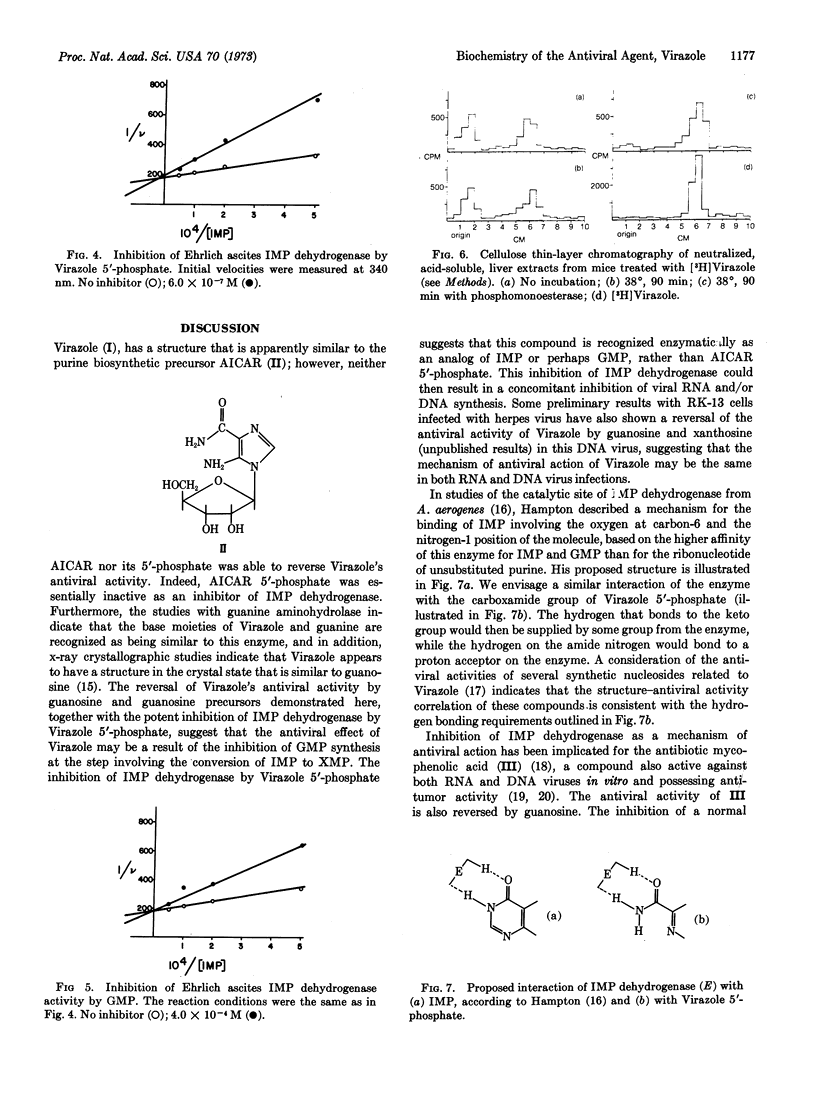
The antiviral activity of the synthetic nucleoside, Virazole (1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide), against measles virus in Vero cell cultures was substantially reversed by xanthosine, guanosine, and to a slightly lesser extent by inosine. Virazole 5′-phosphate was subsequently found to be a potent competitive inhibitor of inosine 5′-phosphate dehydrogenase (IMP:NAD+ oxidoreductase, EC 1.2.1.14) isolated from Escherichia coli (Km = 1.8 × 10-5 M) with a Ki of 2.7 × 10-7 M. Guanosine 5′-phosphate (GMP) was a competitive inhibitor of this enzyme with a Ki of 7.7 × 10-5 M. Virazole 5′-phosphate was similarly active against IMP dehydrogenase isolated from Ehrlich ascites tumor cells, with a Ki of 2.5 × 10-7 M. The Km for this enzyme was 1.8 × 10-5 M, and the Ki for GMP was 2.2 × 10-4 M. These results suggest that the antiviral activity of Virazole might be due to the inhibition of GMP biosynthesis in the infected cell at the step involving the conversion of IMP to xanthosine 5′-phosphate. This inhibition would consequently result in inhibition of the synthesis of vital viral nucleic acid.
Keywords: purine and pyrimidine precursors; IMP dehydrogenase; Virazole 5′-phosphate; guanase; 1,2,4-triazole-3-carboxamide
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. H., Sartorelli A. C. Inosinic acid dehydrogenase of sarcoma 180 cells. J Biol Chem. 1968 Sep 25;243(18):4762–4768. [PubMed] [Google Scholar]
- Cline J. C., Nelson J. D., Gerzon K., Williams R. H., Delong D. C. In vitro antiviral activity of mycophenolic acid and its reversal by guanine-type compounds. Appl Microbiol. 1969 Jul;18(1):14–20. doi: 10.1128/am.18.1.14-20.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J., Cook J. M. The inhibition of nucleic acid synthesis by mycophenolic acid. Biochem J. 1969 Jul;113(3):515–524. doi: 10.1042/bj1130515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPTON A. REACTIONS OF RIBONUCLEOTIDE DERIVATIVES OF PURINE ANALOGUES AT THE CATALYTIC SITE OF INOSINE 5'-PHOSPHATE DEHYDROGENASE. J Biol Chem. 1963 Sep;238:3068–3074. [PubMed] [Google Scholar]
- Hampton A., Nomura A. Inosine 5'-phosphate dehydrogenase. Site of inhibition by guanosine 5'-phosphate and of inactivation by 6-chloro- and 6-mercaptopurine ribonucleoside 5'-phosphates. Biochemistry. 1967 Mar;6(3):679–689. doi: 10.1021/bi00855a006. [DOI] [PubMed] [Google Scholar]
- Kanzawa F., Hoshi A., Kuretani K. Inhibition of guanine deaminase with derivatives of imidazole. Chem Pharm Bull (Tokyo) 1971 Aug;19(8):1737–1738. doi: 10.1248/cpb.19.1737. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B., MOYED H. S., GEHRING L. B. Enzymes essential for the biosynthesis of nucleic acid guanine; inosine 5'-phosphate dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1957 May;226(1):339–350. [PubMed] [Google Scholar]
- ROUSH A., NORRIS E. R. Deamination of 8-azaguanine by guanase. Arch Biochem. 1950 Nov;29(1):124–129. [PubMed] [Google Scholar]
- Saccoccia P. A., Jr, Miech R. P. Inosinic acid dehydrogenase in mammalian tissues. Mol Pharmacol. 1969 Jan;5(1):26–29. [PubMed] [Google Scholar]
- Sidwell R. W., Huffman J. H., Khare G. P., Allen L. B., Witkowski J. T., Robins R. K. Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972 Aug 25;177(4050):705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- Subak-Sharpe H. An animal virus with DNA of high guanine + cytosine content which codes for S-RNA. J Mol Biol. 1965 Jul;12(3):924–928. doi: 10.1016/s0022-2836(65)80339-4. [DOI] [PubMed] [Google Scholar]
- Williams R. H., Lively D. H., DeLong D. C., Cline J. C., Sweeny M. J. Mycophenolic acid: antiviral and antitumor properties. J Antibiot (Tokyo) 1968 Jul;21(7):463–464. doi: 10.7164/antibiotics.21.463. [DOI] [PubMed] [Google Scholar]
- Witkowski J. T., Robins R. K., Sidwell R. W., Simon L. N. Design, synthesis, and broad spectrum antiviral activity of 1- -D-ribofuranosyl-1,2,4-triazole-3-carboxamide and related nucleosides. J Med Chem. 1972 Nov;15(11):1150–1154. doi: 10.1021/jm00281a014. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Kato T., Takenishi T. A novel method for phosphorylation of nucleosides to 5'-nucleotides. Tetrahedron Lett. 1967 Dec;50:5065–5068. doi: 10.1016/s0040-4039(01)89915-9. [DOI] [PubMed] [Google Scholar]




