Abstract
Both fetal-calf serum and insulin cause cell elongation in explanted chick-lens epithelia from 6-day-old embryos. We show that 1 μg/ml of insulin, like serum, stimulates a doubling of cell length and an assembly of longitudinally oriented microtubules; colchicine treatment inhibits this cell elongation. In contrast to serum, insulin neither promotes further lens-cell elongation nor appreciably stimulates the synthesis of bulk proteins or of delta crystallin under the present conditions. These data indicate that the early morphological events of lens fiber differentiation can be initiated by insulin in a chemically defined, serum-free medium without significant affects upon protein synthesis.
Keywords: cell culture, colchicine, serum, crystallin synthesis, lens fiber
Full text
PDF

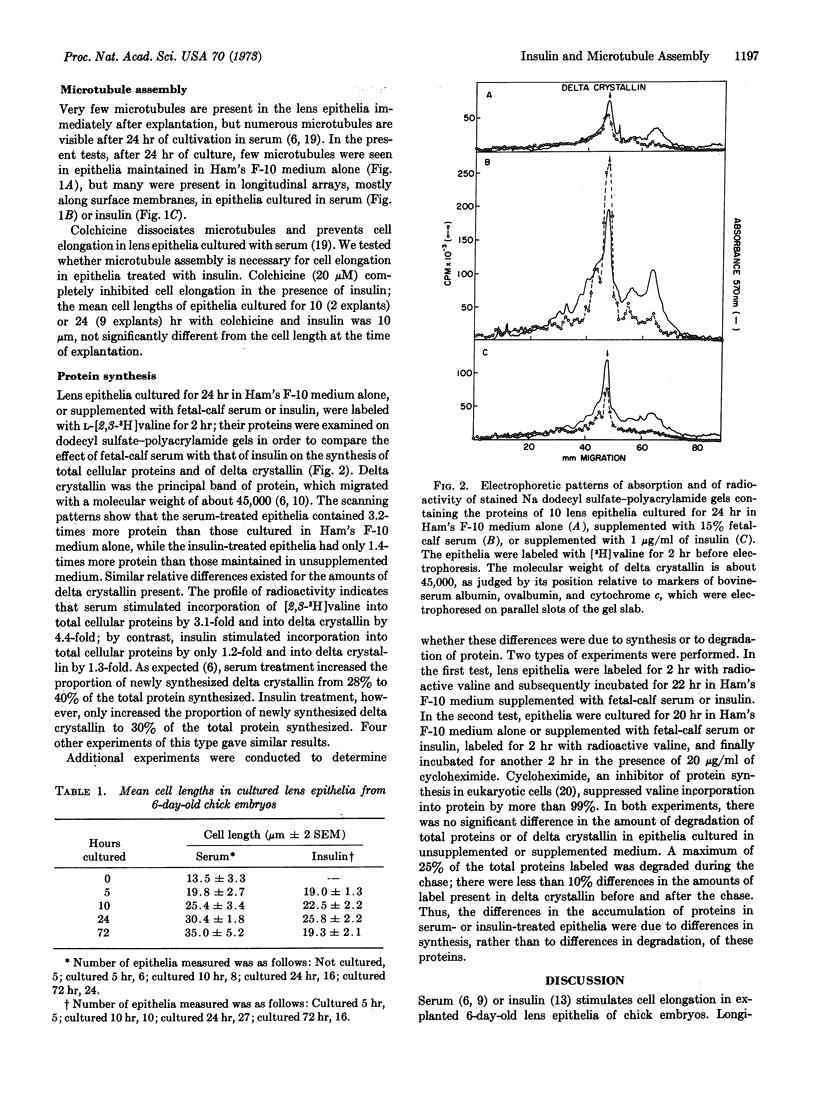

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BYERS B., PORTER K. R. ORIENTED MICROTUBULES IN ELONGATING CELLS OF THE DEVELOPING LENS RUDIMENT AFTER INDUCTION. Proc Natl Acad Sci U S A. 1964 Oct;52:1091–1099. doi: 10.1073/pnas.52.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaker G. J., Birch J. R., Pirt S. J. The glucose, insulin and glutamine requirements of suspension cultures of HeLa cells in a defined culture medium. J Cell Sci. 1971 Sep;9(2):529–537. doi: 10.1242/jcs.9.2.529. [DOI] [PubMed] [Google Scholar]
- ENNIS H. L., LUBIN M. CYCLOHEXIMIDE: ASPECTS OF INHIBITION OF PROTEIN SYNTHESIS IN MAMMALIAN CELLS. Science. 1964 Dec 11;146(3650):1474–1476. doi: 10.1126/science.146.3650.1474. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Buell D. N., Roth J. Water-soluble insulin receptors from human lymphocytes. Science. 1972 Oct 13;178(4057):168–169. doi: 10.1126/science.178.4057.168. [DOI] [PubMed] [Google Scholar]
- Gerschenson L. E., Okigaki T., Andersson M., Molson J., Davidson M. B. Fine structural and growth characteristics of cultured rat liver cells. Insulin effects. Exp Cell Res. 1972 Mar;71(1):49–58. doi: 10.1016/0014-4827(72)90262-5. [DOI] [PubMed] [Google Scholar]
- HAM R. G. An improved nutrient solution for diploid Chinese hamster and human cell lines. Exp Cell Res. 1963 Feb;29:515–526. doi: 10.1016/s0014-4827(63)80014-2. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Reddan J. R., Unakar N. J., Bagchi M. The control of cell division in the ocular lens. Int Rev Cytol. 1971;31:215–300. doi: 10.1016/s0074-7696(08)60060-1. [DOI] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Kuwabara T. Microtubules in the lens. Arch Ophthalmol. 1968 Feb;79(2):189–195. doi: 10.1001/archopht.1968.03850040191017. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN I., OVE P. Growth factors for mammalian cells in culture. J Biol Chem. 1959 Oct;234:2754–2758. [PubMed] [Google Scholar]
- PHILPOTT G. W., COULOMBRE A. J. LENS DEVELOPMENT. II. THE DIFFERENTIATION OF EMBRYONIC CHICK LENS EPITHELIAL CELLS IN VITRO AND IN VIVO. Exp Cell Res. 1965 Jun;38:635–644. doi: 10.1016/0014-4827(65)90387-3. [DOI] [PubMed] [Google Scholar]
- Papaconstantinou J. Molecular aspects of lens cell differentiation. Science. 1967 Apr 21;156(3773):338–346. doi: 10.1126/science.156.3773.338. [DOI] [PubMed] [Google Scholar]
- Pearce T. L., Zwaan J. A light and electron microscopic study of cell behavior and microtubules in the embryonic chicken lens using Colcemid. J Embryol Exp Morphol. 1970 Apr;23(2):491–507. [PubMed] [Google Scholar]
- Piatigorsky J. Insulin initiation of lens fiber differentiation in culture: elongation of embryonic lens epithelial cells. Dev Biol. 1973 Jan;30(1):214–216. doi: 10.1016/0012-1606(73)90060-2. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Rothschild S. S. Loss during development of the ability of chick embryonic lens cells to elongate in culture: inverse relationship between cell division and elongation. Dev Biol. 1972 Jun;28(2):382–389. doi: 10.1016/0012-1606(72)90021-8. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Webster H. D., Craig S. P. Protein synthesis and ultrastructure during the formation of embryonic chick lens fibers in vivo and in vitro. Dev Biol. 1972 Feb;27(2):176–189. doi: 10.1016/0012-1606(72)90096-6. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Webster H. de F., Wollberg M. Cell elongation in the cultured embryonic chick lens epithelium with and without protein synthesis. Involvement of microtubules. J Cell Biol. 1972 Oct;55(1):82–92. doi: 10.1083/jcb.55.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte A., Stoeckel M. E., Brini A. Formation de l'ébauche oculaire et différenciation du cristallin chez l'embryon de poulet. Etude au microscope électronique. Arch Ophtalmol Rev Gen Ophtalmol. 1968 Oct-Nov;28(7):681–706. [PubMed] [Google Scholar]
- Schwartz A. G., Amos H. Insulin dependence of cells in primary culture: influence on ribo-some integrity. Nature. 1968 Sep 28;219(5161):1366–1367. doi: 10.1038/2191366a0. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Soifer D., Braun T., Hechter O. Insulin and microtubules in rat adipocytes. Science. 1971 Apr 16;172(3980):269–271. doi: 10.1126/science.172.3980.269. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Studies on carcinogenesis by avian sarcoma viruses. VI. Differential multiplication of uninfected and of converted cells in response to insulin. J Cell Physiol. 1967 Jun;69(3):377–384. doi: 10.1002/jcp.1040690314. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waymouth C., Reed D. E. A reversible morphological change in mouse cells (strain L, clone NCTC 929) under the influence of insulin. Tex Rep Biol Med. 1965 Jun;23(Suppl):413–419. [PubMed] [Google Scholar]
- Yoshida K., Katoh A. Crystallin synthesis by chick lens. II. Changes in synthetic activities of epithelial and fiber cells during embryonic development. Exp Eye Res. 1971 Mar;11(2):184–194. doi: 10.1016/s0014-4835(71)80022-2. [DOI] [PubMed] [Google Scholar]
- Zwaan J., Ikeda A. Macromolecular events during differentiation of the chicken lens. Exp Eye Res. 1968 Apr;7(2):301–311. doi: 10.1016/s0014-4835(68)80081-8. [DOI] [PubMed] [Google Scholar]



