Abstract
Behavioral inhibition (BI) is a temperament associated with heightened vigilance and fear of novelty in early childhood, and social reticence and increased risk for anxiety problems later in development. However, not all behaviorally inhibited children develop signs of anxiety. One mechanism that might contribute to the variability in developmental trajectories is the recruitment of cognitive-control resources. The current study measured N2 activation, an ERP (event-related potential) associated with cognitive control, and modeled source-space activation (LORETA; Low Resolution Brain Electromagnetic Tomography) at seven years of age while children performed a go/no-go task. Activation was estimated for the entire cortex and then exported for four regions of interest: ventromedial prefrontal cortex (VMPFC), ventrolateral prefrontal cortex (VLPFC), dorsal anterior cingulate cortex (dorsal ACC), and dorsal lateral prefrontal cortex (DLPFC). BI was measured in early childhood (ages two and three years). Anxiety problems and social reticence were measured at seven years of age to ascertain stability of temperamental style. Results revealed that BI was associated with increased performance accuracy, longer reaction times, greater (more negative) N2 activation, and higher estimated dorsal ACC and DLPFC activation. Furthermore, early BI was only associated with social reticence at age 7 at higher (more negative) levels of N2 activation or higher estimated dorsal ACC or DLPFC activation. Results are discussed in the context of overcontrolled behavior contributing to social reticence and signs of anxiety in middle childhood.
Keywords: Behavioral inhibition, cognitive control, no-go N2, social reticence, dorsal anterior cingulate cortex, dorsolateral prefrontal cortex, children
Behavioral inhibition (BI) is a temperamental style that involves the tendency to exhibit fear and heightened vigilance in response to unfamiliar situations or people (Fox, Henderson, Marshall, Nichols, & Ghera, 2005; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001). Both the stability of BI across childhood and the predictive value of this temperament for the emergence of social reticence and signs of anxiety have been well documented (Bronson, 1970; Chronis-Tuscano et al., 2009; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Gladstone, Parker, Mitchell, Wilhelm, & Malhi, 2005; Hirshfeld et al., 1992; Kagan & Snidman, 1991; Perez-Edgar & Fox, 2005; Schmidt & Fox, 1998). However, not all behaviorally inhibited children display social reticence as older children nor do they develop anxiety problems (Degnan & Fox, 2007; Fox et al., 2001). Thus, a number of studies have examined the moderating factors contributing to the progression of early childhood BI to later anxiety problems (e.g., Fox et al., 2001; McDermott, Perez-Edgar, Henderson, Chronis-Tuscano, Pine, & Fox, 2009; White, McDermott, Degnan, Henderson, & Fox, 2011). Two studies in particular have shown that aspects of cognitive control, including response-monitoring and inhibitory control, moderate the BI/anxiety association (McDermott et al., 2009; White et al., 2011). However, currently little is known about the differential neural activation patterns underlying cognitive control that support the continuity of this temperament. The purpose of the current study was twofold: 1) to ascertain cognitive-control-related neural-activation-pattern differences for high and low BI children; and 2) to investigate which neural patterns moderate the association between early BI and later social reticence and signs of anxiety.
We examined N2 activation, an event-related potential (ERP) associated with aspects of cognitive control, such as inhibitory control and response monitoring/response conflict (e.g., Bekker, Kenemans, & Verbaten, 2004; Dimoska, Johnstone, Barry, & Clarke, 2003; Falkenstein, Hoormann, & Hohnsbein, 1999; Jonkman, Lansbergen, & Stauder, 2003; Nieuwenhuis, Yeung, van den Wildenberg, & Ridderinkhof, 2003). A number of studies, using a range of continuous performance tasks, have shown greater (more negative) cognitive-control-related ERPs for clinically anxious (Hum, Manassis, Lewis, 2013; Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006) and non-clinically anxious (Hajcak, McDonald, & Simons, 2003; Lamm, Granic, Zelazo, & Lewis, 2011; Righi, Mecacci, & Pia Viggiano, 2009) participants compared to non-anxious participants. Thus, high levels of activation may be related to excessive control (i.e., overcontrol). Moreover, two studies have shown that ERN—another cognitive-control-related ERP—and N2 activation moderate the association between withdrawn temperaments and anxiety problems (Henderson, 2010; McDermott et al., 2009). Specifically, Henderson (2010) found that N2 activation moderated the association between aspects of social reticence, specifically shyness, and anxiety in 9–13 year olds (mean age 11.12). Similarly,McDermott et al. (2009) found that ERN activation moderated the association between early childhood BI and anxiety in adolescents (mean age 15.1 years). In both cases, the association between BI and signs of anxiety was strongest for children with larger ERP responses.
These studies suggest that high levels of cognitive-control-related brain activation contribute to the continuity of BI and to signs of anxiety in adolescence. However, these ERP studies were not able to ascertain the cortical regions contributing to this overcontrolled regulation. The present study investigated the cortical specificity underlying the impact of cognitive-control-related brain activation on the continuity of withdrawn behavior by measuring N2 activation and source-space activation (LORETA; Pascual-Marqui, Esslen, Kochi, & Lehmann, 2002). The modeling of source-space activation provides estimated activation for the entire cortex, which subsequently can be used to conduct region specific comparisons of activation levels across conditions or groups for cortical generators underlying ERPs, while still maintaining the excellent temporal resolution that ERPs are known for. Previous studies that utilized only ERP measures of scalp activation were not able to provide this spatial specificity. In the current study, brain activation was measured while 7-year-old children performed a go/no-go task and source-space activation was estimated for the entire cortex. Activation values were exported for four regions of interest (ROIs): dorsal anterior cingulate cortex (dorsal ACC), the ventral lateral prefrontal cortex (VLPFC), the dorsolateral prefrontal cortex (DLPFC), and the ventromedial/orbitofrontal cortex (VMPFC), as these regions have been associated with N2 activation (Bekker et al., 2005; Bokura, Yamaguchi, Kobayashi, 2001; Lavric, Pizzagalli, & Forstmeier, 2004; Lamm, Zelazo, & Lewis, 2006; Lewis et al., 2006; Nieuwenhuis et al., 2003; Stieben et al., 2007).
The current study incorporates a longitudinal design in which temperament was evaluated in toddlerhood (2–3 years of age), while social reticence with peers and neural responses were evaluated several years later in childhood, at age 7. Henderson (2010) assessed social and affective outcomes concurrently with neural responses, and McDermott et al. (2009) assessed both neural responses and anxiety diagnoses in adolescence. The current study builds on the extant literature by evaluating the impact of cognitive-control-related neural activation on the continuity of social reticence in childhood rather than adolescence, and thus helps clarify the developmental emergence of this mechanism.
Given that previous studies have shown greater (more negative) N2 and ERN activation for anxious participants than non-anxious participants (e.g., Hum et al., 2013; Ladouceur, et al., 2006; Lamm et al., 2011; Righi, et al., 2009), we predicted that BI would be associated with both greater N2 activation and more source-space activation. Furthermore, given that greater ERN activation moderated the BI-anxiety association in prior work (McDermott et al., 2009), we also predicted the same pattern of results in the current study. That is, we expected that higher BI scores combined with greater N2/source space activation would be associated with greater observed social reticence and parent report of signs of anxiety.
Method
Participants
Participants were part of a larger cohort of children (N = 291) participating in a longitudinal study of temperament since infancy (see Hane, Fox, Henderson & Marshall, 2008). One hundred and six (48 males) typically-developing children participated in this study at two (M = 2.24 years, SD= .25, range = 1.94–2.80 years), three (M = 3.05 years, SD= .13, range = 2.89–3.60 years), and seven years of age (EEG visit: M = 7.64 years, SD= .23, range = 7.28–8.47 years; behavioral observation visit: M = 7.64 years, SD= .21, range = 7.25–8.59 years). Participants were 68% Caucasian, 15% African American, 2% Asian, 2% Hispanic, and 14% reported more than one ethnicity. Maternal education at the onset of the study was reported as 16% high school graduates, 47% college graduates, 36% graduate school graduates, and 6% reported graduating from other programs. Participants (n = 185) were excluded due to attrition, ERP low trial count, and various technical problems, such as computer problems. Children included in the current study (n=106) were no different from children with missing data (n=185) on sex, χ2 (1, N = 291) = .08, p = .77, ethnicity, χ2 (1, N = 291) = .97, p = .32, maternal education, χ2 (3, N = 291) = .35, p = .95, or infant temperament, χ2 (2, N = 291) = .56, p = .77.
Procedure
Maternal reports of temperament were collected when children were 2 and 3 years of age. Observations of children’s behavioral inhibition were also collected at these ages during various tasks in the laboratory where children were presented with novel stimuli.
EEG data were collected at 7 years of age. Children were seated in a chair 38 inches from the computer screen. The electrode sensor net was applied and the go/no-go task (Zoo Game) was administered. While children performed the Zoo Game, parents completed a number of questionnaires. Additionally, at seven years of age during a separate visit, children were paired with unfamiliar peers recruited from the community to observe their social behavior during various laboratory tasks. Specifically, dyads composed of one child participating in the longitudinal study and one age- and sex-matched peer from the community participated in a six-minute unstructured free play task, a five-minute clean-up task, and a five-minute special toy task. This study received IRB approval from the University of Maryland. Parents provided consent at each time point and children assented to participate in this study at 7 years of age.
Measures
Behavioral Inhibition Measures (ages 2–3 years)
Toddler Behavior Assessment Questionnaire (TBAQ; Goldsmith, 1996)
was collected at 2 and 3 years of age. The TBAQ is a 108-item parent report questionnaire that measures five dimensions of temperament. Of interest in the current study was the dimension of social fearfulness, which measures children’s reactions to unfamiliar adults and contexts (Goldsmith, 1996). The TBAQ has been found to be a valid and reliable questionnaire for use with 16- to 36-month-old children (Goldsmith, 1996). In the current sample, internal consistency was .85 and .88 for social fearfulness at 2 and 3 years of age, respectively.
Behavioral Inhibition Observations (Calkins, Fox, & Marshall 1996; Fox et al., 2001)
Observations of behavioral inhibition were conducted when children were 2 and 3 years of age. Children were presented with an unfamiliar person and various objects during three episodes (i.e., stranger, robot, tunnel). Children's mothers were in the room during this task, however, they were asked to refrain from initiating interactions with their child, but to interact with them as they normally would if their child initiated contact with them. During the stranger episode, an unfamiliar experimenter sat quietly in the room with the child and their mother, and did not make any eye contact for 1 minute, then played with a toy dump truck for 1 minute, and then invited the child to play with the truck for 1 minute. During the next episode, a toy robot, that made unpredictable movements and sounds, was turned on and left in the room for two minutes. Last, the child was shown a pop-up tunnel and was invited to crawl through the tunnel. Children’s latency to vocalize, latency to touch truck or robot/enter the tunnel, and duration of time spent in proximity to their mother were coded during each episode. Inter-rater reliability was achieved at each age, with 19% overlap at age 2 and 10% overlap at age 3. Specifically, intraclass correlations (ICC) across episodes were .78 for latency to vocalize, .95 for children's proximity to their mother, and .89 for latency to touch the stimuli/enter the tunnel at 2 years of age. At 3 years of age, ICCs were .97 for latency to vocalize, .98 for children's proximity to their mother, and .99 for latency to touch the stimuli/enter the tunnel. Observed behavioral inhibition scores were created at each age by standardizing each variable and taking the average. A behavioral inhibition composite score was created based on age 2 and 3 year behavioral observation scores and TBAQ social fearfulness scores, thus combining information from multiple methods across toddlerhood.
Go/No-go ERP task (age 7 years)
The Zoo Game (McDermott et al. under review; Lamm, White, Martin McDermott, & Fox, 2012)
A modified version of the Zoo Game was used, presenting only affectively-neutral animal pictures. The current task consisted of 75% go trials and 25% no-go trials. This ratio of go to no-go trials ensures a prepotent desire to respond (i.e., requiring enhanced response control in the no-go trials). This ratio of trials occurred within two blocks of 140 trials each. No-go trials, for both blocks, consisted of monkey pictures, while go trials consisted of other animal pictures. To increase children’s motivation to participate (i.e., to make the stimuli more interesting), animal pictures were used in this task rather than the traditional stimuli of white letters on a dark background. Prior to completing the two blocks, children completed 12 practice trials to ensure proficiency. Children were asked to help a zoo keeper recapture escaped animals with the help of a chimpanzee referred to as the ‘monkey’. To recapture the animals, children were told to respond via button-press (as fast and accurate as possible) as soon as they saw an animal on the screen unless it was the ‘monkey’. Animal stimuli were presented on the screen for 500 ms, followed by a black screen for 900 ms or until the child responded (see Figure 1). The inter-trial interval was jittered between 200–300 ms. Images were presented on a 17-in monitor using E-prime Software (Psychology Software Tools, Inc., Pittsburgh, PA; Schneider, Eschman, & Zuccolotto, 2002).
Figure 1.
Image of Zoo Game (go/no-go task) showing go and no-go trials.
Social reticence composite (age 7 years)
Children’s Behavior Questionnaire-Short Form (CBQ; Putnam & Rothbart, 2006; Rothbart, Ahadi, Hershey, & Fisher, 2001)
The CBQ Short Form is a valid and reliable maternal report measure of temperament for reporting on children aged 3–8 years. The 94-item measure gives scores on 15 dimensions of temperament. Of interest to the current study are the shyness (i.e., slow or inhibited approach in novel or uncertain situations) and fear (i.e., negative affect, worry, or nervousness in anticipation of pain, distress, and/or potentially threatening situations) subscales.
Social Reticence Observations
Children participating in the longitudinal study were paired with an unfamiliar peer from the community to participate in freeplay (FP), clean-up (CL), and special toy (ST) tasks. Children were introduced in the hallway and then entered the playroom to begin the social tasks. Social interactions were recorded from behind one-way mirrors for later behavioral coding. During the first task, children participated in a 6-minute unstructured FP session in which various age appropriate toys were scattered around the playroom. Wariness (i.e., hesitance, uneasy behavior, fearfulness) and unfocused behavior (i.e., little focus or engagement in any activity) were coded using 7-point scales in 2-minute epochs (1 = behavior not displayed during the epoch; 7 = behavior consistently displayed throughout the epoch). Average scores were created by adding scores across epochs and dividing by the total number of epochs. During CL, children were given 5-minutes to pick up the toys and place them into a bin. The amount of time (in seconds) children spent uninvolved (i.e., watching peer, unfocused behavior, not actively cleaning or playing with toys) was coded. The proportion of time uninvolved was calculated by dividing the time uninvolved over the total time of the task. During the ST task, children were given a Nintendo DS with a game that elicited independent play. Children were told that there was only one special toy so they must share and take turns. Children's initiation to acquire the toy from their peer was coded by the type of strategy used. Of interest to the current study were passive strategies (i.e., reaching, pointing, hovering). Proportions of passive strategies were created by dividing the total number of passive strategies over the total number of attempts to get the toy. Each task was coded by a separate team of two to three coders. Each coding team overlapped on at least 19% of total coded cases. Inter-rater reliability (intraclass correlations) were .70 for wariness during FP, .74 for unfocused behavior during FP, .95 for uninvolved behavior during CL, and .75 for passive strategies during ST. An observed social reticence score was created for free play by standardizing and averaging wariness and unfocused behavior. Then, free play social reticence was combined with uninvolved behavior during CL and passive strategies during ST by standardizing and averaging these scores to create an overall score of social reticence across the three dyad tasks.
A social reticence composite score was created based on the CBQ fear, CBQ shyness, and observed social reticence to combine information from multiple methods.
Anxiety Measure (age 7 years)
Child Behavior Checklist (CBCL, Achenbach & Rescorla, 2001)
The CBCL is a standardized, highly reliable, and valid measure of children’s emotional and behavioral problems (ages 6–18 years). Parents were asked to indicate whether, and to what degree, their child exhibited a list of symptoms on a scale from 0 (never) to 2 (often). For this study, only the anxiety subscale raw score (7 items) was used because it specifically measured anxiety problems (unlike the internalizing measure which captures both anxiety and depression).
EEG data collection and analyses
EEG was recorded using a 64-channel Geodesic Sensor Net and sampled at 250 Hz, using EGI software (Net Station; Electrical Geodesic, Inc., Eugene, OR [data were also processed using Net Station]). Once the impedance values for all EEG channels were reduced to below 50 kΩ, data acquisition began. During recording, all channels were referenced to Cz and after acquisition, data were re-referenced using an average reference.
Data were filtered using a FIR bandpass filter with a lowpass frequency of 50 Hz and a highpass frequency of .3 Hz. To best capture eye blink artifacts, the threshold was set to 140 µV (peak-to-peak) and all trials in which this threshold was violated were excluded from analyses. Furthermore, signal activation change (peak-to-peak) exceeding 150 µV across the entire segment and fast transits exceeding a difference (peak-to-peak) of 120 µV were marked as bad and interpolated.
Scalp data analyses
Waveforms for correct go and no-go trials were segmented into epochs from 200 ms before to 600 ms after stimulus onset and baseline corrected for the 200 ms preceding stimulus onset. Mediofrontal N2 activation was maximal between 280 and 380 ms after stimulus onset; thus, peak activation was exported for this time window. To eliminate trials characterized by attentional lapses or chronic non-responding, no-go trials that did not have a correct go trial preceding and following them were removed from analyses. Due to this strict criterion the mean number of trials comprising correct no-go ERPs was 25.29 (SD = 8.80; range = 14–49). Mean number of trials comprising go ERPs was 137.91 (SD = 36.81; range = 54–198).
Visualization of the correct go and no-go stimulus-locked waveforms revealed clear N1, P2, and N2 components for all mediofrontal electrodes (see Figure 4). Furthermore, both go and no-go N2 waveforms appeared more negative for high BI children than low BI children (BI groups were generated by dividing BI scores into thirds—high, middle, and low BI—and eliminating the middle group). Thus, scalp N2 activation was exported for 8 mediofrontal electrodes: four midline electrodes (8, 6 [Fz], 4 [FCz], and VREF [Cz]) as well as 4 flanking electrodes (9, 7, 3, 54; two on each side). Because of individual differences in peak N2 activation across electrodes, each participant’s greatest (most negative) activation during go or no-go trials was analyzed.
Figure 4.
A) Go and no-go waveforms showing difference between high and low BI scores (µV; more negative activation is down). B) Scatter plot showing negative correlation between BI and go N2 activation (µV). C) Scatter plot showing negative correlation between BI and no-go N2 activation (µV). The effect is still significant after controlling for go N2 activation.
Source-space data analyses
A distributed inverse model that incorporates the change in activation from one electrode to another (in this case 65 electrodes) was used to calculate the source-space activation. This type of algorithm estimates activation voxel-by-voxel and sample-by-sample and does not require any dipoles to be “fit”, thereby limiting the influence of user bias. The specific algorithm used in the current study was LORETA (Low Resolution Brain Electromagnetic Tomography), which applies a constraint to the minimum-norm solution in order to minimize the discrepancy between values of adjacent voxels (to achieve the most realistic model) within the GeoSource interface (Electrical Geodesic, Inc., Eugene, OR; for a review of these constraints and other minimum norm solutions, see Michel, Murray, Lantz, Gonzalez, Spinelli, & Grave de Peralta, 2004). A regularization constant (indicating how much noise is modeled) of 10-4 was applied. This amount of regularization revealed current flow patterns that matched (via visual inspection) the grand-averaged scalp topography better than other levels.
After the data were modelled (LORETA) for the entire cortex (2447 voxels), morphology-based regions of interest (ROIs) were generated using the Montreal Neurological Institute (MNI) average adult MRI (pediatric head models are not available yet). We were interested in four ROIs: the VLPFC ROI (comprised of 44 voxels; lateral part of BA 11 and 47), the dACC ROI (comprised of 50 voxels; dorsal part of BA 24 and 32), the DLPFC ROI (comprised of 126 voxels; BA 9 and dorsal part of BA 46), and the VMPFC/OFC ROI (comprised of 147 voxels; ventromedial parts of BAs 11, 10, 14, and 13; see Figure 2). Source waveform amplitudes (nA) for all voxels within an ROI were extracted for 200 ms before stimulus onset to 600 ms after stimulus onset and baseline corrected using the 200 ms before stimulus onset. To ensure that each participant’s maximal activation was analysed, we chose the voxel and moment in time (within the 100 ms during which the scalp N2 was maximal) that showed the most activation for each ROI.
Figure 2.
Image of ROIs (regions of interest) superimposed on Montreal Neurological Institute (MNI) average MRI.
Statistical Analyses
All behavioral and EEG data with values larger or smaller than 2 SD from the mean were changed to show values of 2 SD from mean, thus preventing statistical analyses from being skewed by outliers. Two types of regression analyses were conducted in this study in order to: 1) determine the association between early BI and later cognitive control (both behavioral and brain measures) and 2) examine the moderating role of brain activation on the relation between early BI and later social reticence and anxiety. For the first analysis the entire sample was used (106 children). Since some parents did not complete the CBQ and/or CBCL questionnaires, and/or some children did not perform the tasks that were coded for social reticence, the second set of regressions was conducted on slightly smaller samples (CBCL anxiety: n = 100; social reticence: n=104).
Preliminary Analyses
Based on theory and confirmed through factor analysis (eigenvalue = 2.04; loadings = .62 to .81), a BI composite score was created. Specifically, BI observation scores and maternal report of social fearfulness on the TBAQ at 2 and 3 years of age were standardized and averaged (M = −.01, SD = .77). The BI composite variable was unrelated to child age, at the 2, r (92) = −.08, p = .46 and 3 year visit, r(100) = −.08, p = .68). A social reticence composite score was created by standardizing and averaging behaviors observed during FP, CL, and ST with the fear and shyness subscales (also standardized) of the CBQ (M = −.0004, SD = .64).
A priori t-tests revealed sex differences on some of the primary independent and dependent variables (see Table 1) and therefore sex was entered as a covariate in all analyses. Preliminary correlation analyses were conducted to examine associations between social reticence and anxiety measures, zoo task performance measures, and brain measures (see Table 2). Furthermore, because the number of trials comprising an ERP can affect ERP amplitude, and because a priori analysis revealed 1) a trend-level association between trial counts and no-go N2 activation, r(106) = .17, p = .08, and 2) t-tests showed significant differences in trial counts between high and low BI children, nogo: t(69) = 2.15, p = .04; go: t(69) = 3.53, p = .001, trial count was controlled for in all subsequent brain analyses (ERP and source-space).
Table 1.
Sex differences
| Measures | Statistic |
|---|---|
| Temperament and anxiety Measures | |
| BI composite | t(104) = −.80, p = .43 |
| Social reticence composite | t(102) = −2.88, p = .005 |
| CBCL Anxiety | t(98) = −.78, p = .44 |
| Zoo Game Performance Measures | |
| Go performance accuracy | t(104) = −.28, p = .78 |
| No-go performance accuracy | t(104) = −3.46, p = .001 |
| Go reaction times | t(104) = −1.62, p = .11 |
| Brain Measures | |
| No-go N2 | t(104) = 1.05, p = .30 |
| No-go DLPFC | t(104) = −2.06, p = .04 |
| No-go Dorsal ACC | t(104) = −1.73, p = .09 |
| No-go VLPFC | t(104)= 2.34, p = .02 |
| No-go VMPFC | t(104) = .57, p = .57 |
Table 2.
Preliminary Pearson correlation analyses (r-values) between temperament, anxiety, task performance, and brain measures.
| BI | SR | Anx. | Go RT | Go PA | No-go PA | N2 | DLPFC | dACC | |
|---|---|---|---|---|---|---|---|---|---|
| Behavioral Inhibition (BI) | — | .26* | .16 | .24* | −.08 | .23* | −.30** | .24* | .26* |
| Social Reticence (SR) | .26* | — | .23* | .28** | −.08 | .12 | −.20* | .09 | .23 |
| CBCL Anxiety (Anx) | .16 | .23* | — | .28** | .06 | .07 | .004 | −.01 | .03 |
| Go Reaction Times (RT) | .24* | .28** | .28** | — | −.23* | .40** | .01 | .06 | .03 |
| Go Performance Accuracy (PA) | −.08 | −.08 | .06 | −.23* | — | .23* | −.06 | .05 | −.01 |
| No-go Performance Accuracy (PA) | .23* | .12 | .07 | .40** | .23* | — | .00 | −.08 | −.14 |
| No-go N2 | −.30** | −.20* | .004 | .01 | −.06 | .00 | — | −.44** | −.48** |
| Dorsolateral PFC (DLPFC) | .24* | .09 | −.01 | .06 | .05 | −.08 | −.44** | — | .56** |
| Dorsal ACC (dACC) | .26* | .23 | .03 | .03 | −.01 | −.14 | −.48** | 56** | — |
p < .05;
p < .005
Results
Behavioral Analyses
Go/no-go performance accuracy
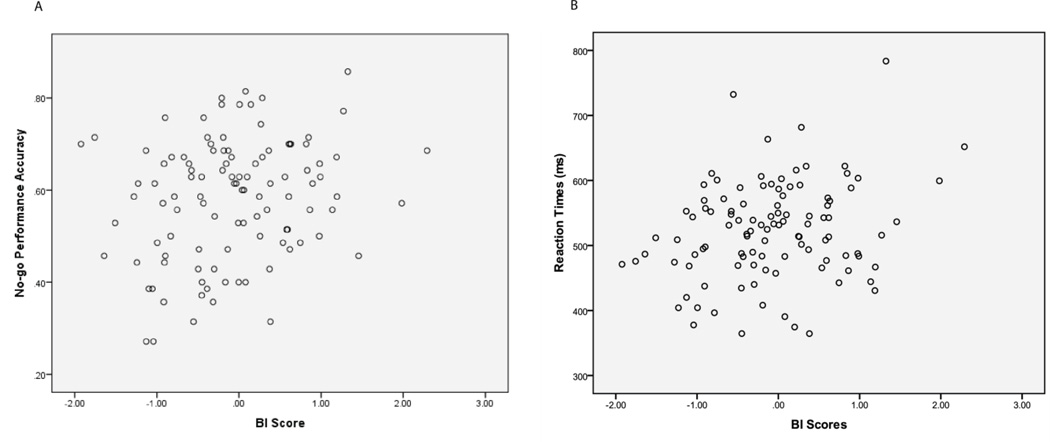
To assess the relations between BI and no-go accuracy, we conducted a step-wise linear regression, with Sex in step one and BI in step two. Results revealed a positive association between BI and no-go accuracy, after controlling for Sex, β = .20, t = 2.20, p = .03, suggesting that as BI increased, no-go accuracy also increased (Figure 3). No effects were found for go accuracy.
Figure 3.
A) Scatter plot showing positive correlation between BI and no-go performance accuracy. B) Scatter plot showing positive correlation between BI and go reaction times.
To assess the relations between performance accuracy and brain measures, we conducted two step-wise linear regressions, with Sex and Trial Count in step one, performance accuracy scores (go or no-go) in step two, and brain data (go or no-go) as dependent measures. Go accuracy was not associated with go brain data (neither N2 nor source space) over-and-above Sex and Trial Count. Similarly, no-go accuracy was not associated with no-go brain data (neither N2 nor source space) over-and-above Sex and Trial Count.
Reaction Time (RT)
To assess the relation between BI and go RT, we conducted a step-wise linear regression, with Sex in step one and BI in step two. Results revealed a positive association between BI and go RT, , β = .22, t = 2.36, p = .02, such that higher levels of BI were associated with slower reaction time. To control for potential speed/accuracy tradeoff, we conducted an additional step-wise linear regression, with Sex and mean go and no-go accuracy in step one and BI in step two. Consistent with the initial analysis, results in this second analysis also revealed a positive association between BI and go RT, β = .19, t = 2.01, p = .05. Thus, as levels of BI increased reaction times also increased even after controlling for accuracy rates (Figure 3).
To assess the relations between RT and brain measures, we conducted two step-wise linear regressions, with Sex and Trial Count in step one, go RT scores in step two, and go or no-go brain data as dependent measures. Go RT was not associated with either go or no-go brain data (neither N2 nor source space) over-and-above Sex and Trial Count. These results indicate that differences in brain data cannot be simply due to differences in task performance (neither RT nor performance accuracy).
ERP Analyses
To assess the relation between BI and N2 activation, we conducted a step-wise linear regression, with Sex and Trial Count (specific to trial type: no-go or go) in step one and BI in step two. For both the no-go and go N2, results revealed a negative association between BI and N2 activation, no-go: β = −.27, t = −2.90, p = .005, go: β = −.29, t = −2.24, p = .03, indicating greater (more negative) N2 activation as BI increased. Because BI was associated with both go and no-go N2 activation, to ascertain the link between BI and cognitive control (i.e., no-go N2), an additional linear regression was conducted with go N2 activation entered in step one (along with Trial Count and Sex). Results revealed a negative association between BI and no-go N2 activation, over-and-above go N2 activation, β = −.13, t = −2.17, p = .03, indicating greater (more negative) activation during no-go trials as BI increased. Additionally, because grand averaged waveforms showed group differences for the P2, we conducted a similar step-wise linear regression with no-go P2 activation entered in step one (along with Trial Count and Sex). Results revealed a negative association between BI and no-go N2 activation, over-and-above no-go P2 activation, β = −.16, t = −1.97, p = .05, again indicating greater (more negative) N2 activation as BI increased.
Next, to determine if no-go N2 activation moderated the BI/anxiety and/or BI/social reticence associations, we conducted moderation analyses with early BI as the independent variable, no-go N2 activation as the moderator, and CBCL anxiety or social reticence as the dependent variable. To avoid multi-collinearity, the predictors were mean centered and interaction variables were calculated as product terms of the mean-centered predictors (Aiken & West, 1991). Step-wise linear regressions were conducted with Sex and go N2 activation (covariates) in step 1, BI first and then no-go N2 activation second in step 2, and the BI-by-N2 interaction term in step 3. The interaction between BI and no-go N2 activation was significant for social reticence, β = −.19, t = −2.00, p = .05, and not significant for CBCL anxiety , β = .05, t = .52, p = .61. Simple slopes were tested to follow-up the significant interaction by re-calculating no-go N2 activation into new variables representing high (more negative) N2 activation and low (less negative) N2 activation and running additional regression analyses using the re-calculated scores, as suggested by Aiken and West (1991). Follow up analyses showed that when N2 activation was high (more negative), early BI was positively related to later social reticence, β = .37, t = 2.85, p = .005. However, when N2 activation was low (less negative), early BI was unrelated to later social reticence, β = −.03, t = −.18, p = .86.
Source Space Analyses
To assess the relation between BI and no-go source-space activation, we conducted a series of step-wise linear regressions, with Sex and Trial Count in step one and BI in step two for each ROI (DLPFC, dorsal ACC, VLPFC, VMPFC). Results revealed a positive association between BI and cortical activation for DLPFC, β = .19, t = 2.00, p = .05, and dorsal ACC, β = .21, t = 2.26, p = .03 (Figure 5).
Figure 5.
A) Scatter plot showing positive correlation between BI and estimated no-go DLPFC activation (nA). B) Scatter plot showing positive correlation between BI and estimated no-go dorsal ACC activation (nA). For both A and B greater source-space activation is up.
Next, to determine if no-go DLPFC and dorsal ACC activation moderated the BI/anxiety and/or BI/social reticence associations, we conducted moderation analyses with early BI as the independent variable, either DLPFC or dorsal ACC as the moderator, and CBCL anxiety or the social reticence composite measure as the dependent variable (see Figure 6). To avoid multi-collinearity, the predictors were mean centered and interaction variables were calculated as product terms of the mean-centered predictors (Aiken & West, 1991). Step-wise linear regressions were conducted with Sex and go source space activation (covariates) in step 1, BI scores first and then source-space activation second in step 2, the BI-by-source space interaction term in step 3. Two of the four regression models yielded significant interactions: one, with DLPFC as the moderator associating early BI with later social reticence, β = .35, t = 3.92, p < .001; and two, with dorsal ACC as the moderator associating early BI with later social reticence, β = .26, t = 2.82, p = .006. No interaction effects (BI-by-source-space activation) were associated with CBCL signs of anxiety (DLPFC: β = −.03, t = −.30, p = .77; dorsal ACC: β = −.10, t = −.98, p = .33).
Figure 6.
A, scatter plot showing association between BI and social reticence. The remainder of the figures show no-go brain activation (ERP and source-space) moderating the relation between early BI and social reticence at age 7 (figures show slope differences); B, no-go N2 activation; C, estimated dorsolateral prefrontal cortex activation; and D, estimated dorsal anterior cingulate activation.
To ensure that our pattern of effects was not simply due to individual differences in arousal or vigilance, additional moderation analyses were conducted. To control for individual differences in arousal, we re-ran the above moderation analyses with mean activation for the time period before stimulus onset (-200), a measure of each participants’ base-rate of arousal, in step 1. We repeated this process with mean RT and mean accuracy in step 1 (separately) as measures of vigilance. All interaction effects outlined above were still significant after controlling for these measures, suggesting that our moderation effects outlined above cannot be simply due to individual differences in arousal or vigilance.
Simple slopes were tested to follow-up the significant interactions by re-calculating source-space activation (DLPFC and dorsal ACC separately) into new variables representing high source-space activation and low source-space activation and running additional regression analyses using the re-calculated scores, as suggested by Aiken and West (1991). Follow-up analyses showed that when DLPFC or dorsal ACC activation was elevated, early BI was positively related to later social reticence (DLPFC: β = .56, t = 4.54, p < .001; dorsal ACC: β = .41, t = 3.47, p = .001). However, when DLPFC or dorsal ACC activation was low, early BI was unrelated to later social reticence (DLPFC: β = −.19, t = −1.36, p = .18; dorsal ACC: β = −.07, t = −.54, p = .59).
Discussion
The goals of the current study were twofold: 1) to examine the relations between BI and cognitive-control-related brain and behavior measures; and 2) to examine whether cognitive-control-related cortical activation moderated the association between early BI and later anxiety problems and/or social reticence at 7 years of age. BI was measured with behavioral observations and maternal-report questionnaires at two and three years of age. Cortical activation was measured using the N2—an ERP associated with cognitive control (e.g., Bekker, et al., 2004; Dimoska, et al., 2003; Falkenstein, et al., 1999; Jonkman, et al., 2003; Nieuwenhuis, et al., 2003)—and estimated source-space activation, captured during a go/no-go task at 7 years of age. Two outcome measures assessed at 7 years of age were used: 1) Maternal report of anxiety on the CBCL; and 2) a composite assessing social reticence, which included maternal-report of shyness and fear (CBQ questionnaire) and detailed behavioral observations of children’s interactions with an unfamiliar peer.
Main effects of BI on neural activation
As predicted, results revealed that BI was associated with greater (more negative) N2 activation and that this effect was evident even after controlling for go N2 activation and no-go P2 activation. These results are consistent with cognitive-control-related ERP studies with a variety of clinical and non-clinical samples of children with heightened anxiety. Specifically, Ladouceur, et al. (2006) found greater ERN activation for clinically anxious children than non-anxious children using a flanker task. Righi, et al. (2009) found greater N2 activation for more compared to less non-clinically anxious college students using a go/no-go task. Hum, et al., (2013) found greater N2 activation for clinically anxious children than non-anxious children using a go/no-go task.
The current data also demonstrated that BI was associated with longer latency RTs and greater performance accuracy. Interestingly, behavioral data did not correlate with brain data, suggesting that activation differences were probably not due to differences in task difficulty (i.e., it is unlikely that BI differences in brain activation were observed simply because the task was more difficult for children higher on BI). Rather, this pattern of behavior and brain results is more likely due to behaviorally inhibited children applying excessive regulatory resources (overcontrolled behavior). Such a hypothesis is in line with a number of developmental theories of emotion regulation (e.g., Caspi, Harrington, Milne, Amell, Theodore, & Moffitt, 2003; Eisenberg et al., 2009; Nigg, 2000). Further support for this hypothesis comes from the fact that overcontrolled behavior has been associated with internalizing behavior problems (for a review see Eisenberg et al., 2009), such as anxiety disorders.
We also found that BI was associated with estimated source-space activation. More specifically, BI was associated with greater DLPFC and dorsal ACC activation. These source-space results are in line with previous N2 source-space activation results. For example, Lamm, Granic, Zelazo, and Lewis (2011) found greater prefrontal cortical activation for a group of anxious aggressive children than control children using a similar method of modeling source-space activation. However, our data are at odds with some of the fMRI data (e.g., Bishop, 2009; Bishop, Duncan, Brett, & Lawrence, 2004; Etkin, Prater, Hoeft, Menon, & Schatzberg, 2010; Etkin & Wager, 2007); this could be due to physiological differences inherent in each method, suggesting the need to examine brain function using both fMRI and ERP measures in the same subjects.
Moderation Effects
The second research question addressed in this study examined if cognitive control moderated the relations between early BI and later maternal reported signs of anxiety and/or later social reticence. As predicted, greater levels of cognitive-control-related brain activation (both N2 and source-space) moderated the association between early BI and later social reticence. This study builds on the extant literature by elucidating which brain regions (modeled activation) specifically moderate this association. Interestingly, only DLPFC and dorsal ACC activation—two brain regions consistently linked with cognitive control (e.g., Braver, Paxton, Locke, & Barch, 2009; Carter & van Veen, 2007)—moderated this association, supporting the notion that excessive cognitive control (i.e., overcontrolled behavior) contributes to the relation between early BI and later social reticence (e.g., Caspi, Harrington, Milne, Amell, Theodore, & Moffitt, 2003; Nigg, 2000). It is also noteworthy that this moderation effect was evident even after controlling for go activation, suggesting that these results cannot be simply due to basic ongoing motor or attentional control.
It is not clear why cognitive-control-related brain activation did not moderate the association between early BI and maternal reported signs of anxiety. It could be that despite oversampling for BI in infancy, our sample of typically developing 7-year-old children did not show enough anxiety problems to reveal any effects. This notion is supported by the fact that McDermott, et al. (2009) and Henderson (2010), two studies showing that cognitive-control-related ERPs moderated the link between withdrawn temperaments and anxiety, assessed samples of children considerably older (mean age 15.1 years and mean age 11.2 years, respectively) than our sample of 7-year-old children. This hypothesis is further supported by Meyer, Weinberg, Klein, and Hajcak (2012) who found that the relation between greater ERN and elevated anxiety problems was only evident in older children (roughly 11–13 years) and not younger children (roughly 8–10 years). Thus, it may be that 7-year-old children show enough variability in social reticence to reveal significant effects but not enough variability in anxiety symptoms, and that with age, anxiety symptoms may increase. Therefore, following up the current sample into adolescence might yield the same pattern of results as outlined by Henderson (2010) and McDermott et al. (2009). Together, these results suggest that inhibited behavior in conjunction with high levels of cognitive-control-related neural activation in childhood might function as a biomarker for difficulties with social behavior (i.e., social reticence) that may develop into later anxiety problems.
Limitations
There are limitations to the current study. First, the use of source-space analyses allowed us to ask region specific questions which scalp ERPs do not. However, activation patterns are estimated effects and therefore should be interpreted with caution. Second, the moderator and outcome variables in the current study were measured concurrently. Therefore, we cannot conclude whether N2 activation predicts social reticence or if greater N2 activation is a result of being higher on social reticence. Future studies should examine N2 activation at earlier ages to examine whether it is associated with later social reticent behavior.
Conclusions
The current study contributes to the extant literature by ascertaining that BI is associated with greater dorsal ACC and DLPFC activation, and that source-space activation moderates the relation between BI and social reticence. This finding supports the notion that high levels of cognitive control (i.e., overcontrol) contributes to the emergence of social reticence amongst young children characterized by behavioral inhibition. This knowledge may allow us greater measurement specificity for cognitive-control training studies, perhaps by training behaviorally inhibited children to apply cognitive-control strategies more efficiently (i.e., show moderate decreases in DLPFC and dorsal ACC activation).
Acknowledgments
The project described was supported by NICHD grant number R37HD17899 to Nathan A. Fox, NIMH grant number R01MH093349 to Nathan A. Fox, and NIMH grant number P50MH078105 to Megan R. Gunnar. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD, NIMH, or NIH.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Aiken LS, West GM. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Bekker EM, Kenemans JL, Verbaten MN. Electrophysiological correlates of attention, inhibition, sensitivity, and bias in a continuous performance task. Clinical Neurophysiology. 2004;115:2001–2013. doi: 10.1016/j.clinph.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;2:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Kobayashi S. Electrophysiological correlates for response inhibition in a go/nogo task. Clinical Neurophysiology. 2001;112:2224–2232. doi: 10.1016/s1388-2457(01)00691-5. [DOI] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. PNAS. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson CW. Fear of visual novelty: Developmental patterns in males and females. Developmental Psychology. 1970;2:33–40. [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of Inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Caspi A, Harrington H, Milne B, Amell JW, Theodore RF, Moffitt TE. Children's behavioral styles at age 3 are linked to their adult personality traits at age 26. Journal of Personality. 2003;71:495–513. doi: 10.1111/1467-6494.7104001. [DOI] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, Raggi VL, Fox NA. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of American Academy of Child and Adolescent Psychiatry. 2009;48:1–8. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Development and Psychopathology. 2007;19:729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- Dimaska A, Johnstone SJ, Barry RJ, Clarke AR. Inhibitory motor control in children with attention-deficit/hyperactivity disorder: Event-related potentials in the stop-signal paradigm. Biological Psychiatry. 2003;54:1345–1354. doi: 10.1016/s0006-3223(03)00703-0. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Valiente C, Spinrad TL, Cumberland A, Liew J, Reiser M, Zhou Q, Losoya SH. Longitudinal relations of children’s effortful control, impulsivity, and negative emotionality to their externalizing, internalizing, and co-occurring behavior problems. Developmental Psychology. 2009;45:988–1008. doi: 10.1037/a0016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. American Journal of Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in go/nogo tasks and their relation to inhibition. Acta Psychologica. 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Fox NA. Dynamic cerebral processes underlying emotion regulation. In: Fox NA, editor. The development of emotion regulation: Biological and behavioral considerations. Monographs of the Society for Research in Child Development 59 (2–3, Serial No. 240) 1994. pp. 152–166. [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Gladstone GL, Parker GB, Mitchell PB, Wilhelm KA, Malhi GS. Relationship between self-reported childhood behavioral inhibition and lifetime anxiety disorders in a clinical sample. Depression and Anxiety. 2005;22:103–113. doi: 10.1002/da.20082. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder. Archives of General Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH. Studying temperament via construction of the Toddler Behavior Assessment Questionnaire. Child Development. 1996;67:218–235. [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biological Psychology. 2003;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hane AA, Fox NA, Henderson HA, Marshall PJ. Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology. 2008;44:1491–1496. doi: 10.1037/a0012855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA. Electrophysiological correlates of cognitive control and the regulation of shyness in children. Developmental Neuropsychology. 2010;35:177–193. doi: 10.1080/87565640903526538. [DOI] [PubMed] [Google Scholar]
- Hirshfeld DR, Rosenbaum Jf, Biederman J, Bolduc EA, Faraone SV, Snidman N, Reznick JlS, Kagan J. Stable behavioral inhibition and its association with anxiety disorder. Journal of American Academy of Child and Adolescent Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Hum KM, Manassis K, Lewis MD. Neural mechanisms of emotion regulation in childhood anxiety. Journal of Child Psychology and Psychiatry. 2013;54:552–564. doi: 10.1111/j.1469-7610.2012.02609.x. [DOI] [PubMed] [Google Scholar]
- Jonkman LM, Lansbergen M, Stauder JEA. Developmental differences in behavioral and event-related brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology. 2003;40:752–761. doi: 10.1111/1469-8986.00075. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. Temperamental factors in human development. American Psychologist. 1991;46:856–862. doi: 10.1037//0003-066x.46.8.856. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47:1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Lamm C, Granic I, Zelazo PD, Lewis MD. Magnitude and chronometry of neural mechanisms of emotion regulation in subtypes of aggressive children. Brain and Cognition. 2011;77:159–169. doi: 10.1016/j.bandc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Lamm C, White LK, Martin McDermott J, Fox NA. Neural activation underlying inhibitory control in the context of neutral and affectively charged pictures in children. Brain and Cognition. 2012;79:181–187. doi: 10.1016/j.bandc.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Zelazo PD, Lewis MD. Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Neuropsychologia. 2006;44:2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Lavric A, Pizzagalli DA, Forstmeier S. When ‘go’ and ‘nogo’ are equally frequent: ERP components and cortical tomography. European Journal of Neuroscience. 2004;20:2483–2488. doi: 10.1111/j.1460-9568.2004.03683.x. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Lamm C, Segalowitz SJ, Stieben J, Zelazo PD. Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience. 2006;18(3):430–443. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- McDermot JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2012;2:152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave d Peralta R. EEG source imaging. Clinical Neurophysiology. 2004;115:2195–2222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg w, Kidderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Esslen M, Kochi K, Lehmann D. Functional imaging with low resolution brain electromagnetic tomography (LORETA): review, new comparisons, and new validation. Japanese Journal of Clinical Neurophysiology. 2002;30:81–94. [PubMed] [Google Scholar]
- Perez-Edgar K, Fox NA. A behavioral and electrophysiological study of children’s selective attention under neutral and affective conditions. Journal of Cognition and Development. 2005;6:89–118. [Google Scholar]
- Putnam SP, Rothbart MK. Development of short and very short forms of the Children's Behavior Questionnaire. Journal of Personality Assessment. 2006;87:103–113. doi: 10.1207/s15327752jpa8701_09. [DOI] [PubMed] [Google Scholar]
- Righi S, Mecacci L, Viggiano MP. Anxiety, cognitive self-evaluation and performance: ERP correlates. Journal of Anxiety Disorders. 2009;23:1132–1138. doi: 10.1016/j.janxdis.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, Fisher P. Investigations of temperament at three to seven years: The children’s behavior questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA. Fear-potentiated startle responses in temperamentally different human infants. Developmental Psychobiology. 1998;2:113–120. doi: 10.1002/(sici)1098-2302(199803)32:2<113::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime user’s guide. Pittsburgh, PA: Psychology Software Tools; 2002. [Google Scholar]
- Stieben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology. 2007;19:455–480. doi: 10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- White LK, McDermott JM, Kegnan KA, Henderson HA, Fox NA. Behavioral inhibition and anxiety: The moderating roles of inhibitory control and attention shifting. Journal of Abnormal Child Psychology. 2011;39:735–747. doi: 10.1007/s10802-011-9490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]