Abstract
Context
Spinal muscular atrophy type 1, an autosomal recessive motor neuron disease, is a leading genetic cause of death in infancy and early childhood.
Objective
To determine whether the early initiation of noninvasive respiratory interventions is associated with longer survival.
Design
Single-institution retrospective cohort study identified children with spinal muscular atrophy type 1 from January 1, 2002 to May 1, 2009 who were followed for 2.3 mean yrs.
Setting
Tertiary care children’s hospital and outpatient clinics in a vertically integrated healthcare system.
Patients or Other Participants
Forty-nine children with spinal muscular atrophy type 1 were grouped according to the level of respiratory support their caregivers chose within the first 3 months after diagnosis: proactive respiratory care (n = 26) and supportive care (n = 23).
Interventions
Proactive respiratory care included bilevel non-invasive ventilation during sleep and twice a day cough assist while supportive respiratory care included suctioning, with or without supplemental oxygen.
Measurements and Main Results
Kaplan–Meier survival curves were assessed based on intention to treat. Children treated with early proactive respiratory support had statistically longer survival compared to supportive care (log rank 0.047); however, the adjusted hazard ratio for survival was not statistically different (2.44 [95% confidence interval 0.84–7.1]). Children in the proactive group were more likely to be hospitalized for respiratory insufficiency (83% vs. 46%) and had shortened time after diagnosis until first hospital admission for respiratory insufficiency (median 118 vs. 979 days).
Conclusion
Longer survival time with spinal muscular atrophy type 1 is associated with early, noninvasive respiratory care interventions after diagnosis.
Keywords: noninvasive ventilation, respiratory failure, spinal muscular atrophy, survival analysis
Spinal muscular atrophy (SMA) is an autosomal recessive motor neuron disease that results in profound functional limitations, and is a leading genetic cause of death in infancy and childhood (1). Infants with SMA type 1, the most severe form, present with generalized limb, trunk, and respiratory muscle weakness in the first few weeks to months of life, resulting in respiratory insufficiency and premature death in the majority of those affected. Prior publications have documented a typical life expectancy of <2 yrs in the absence of invasive supportive care (2–4). However, in the last 2 decades, there has been a paradigm shift toward proactive management with more aggressive respiratory and nutritional interventions, especially in the United States.
Proactive chronic respiratory management includes airway clearance via secretion mobilization with manual or mechanical chest physiotherapy and cough assist, and either: 1) noninvasive ventilatory support consisting of bilevel positive airway pressure support (BiPAP) during sleep; or 2) invasive ventilation via tracheostomy (5, 6). Bulbar insufficiency is nearly universal among SMA type 1 patients, and proactive nutritional management typically includes gastrostomy feeding tubes and antireflux procedures and/or medications.
Clinical use of these interventions is thought to be associated with longer survival. Mannaa et al (7) reported an increase in survival, to 62% at 2 and 4 yrs and 8% at 10 yrs, associated with the change to proactive from supportive respiratory and nutritional care. A 2007 study based on data from a self-reported patient registry in the United States evaluated two cohorts, those born from 1980–1994 and those born during 1995–2006. They found that for patients born before 1995, 80.0% died at a mean age of 19 months while those born after 1995 had a significantly lower early mortality, with only 36% having died at a mean age of 22 months (8). This is in contrast to outcomes reported in recent European studies, where use of proactive respiratory support, including home use of non-invasive ventilation and cough assist, is rare. A prospective study in The Nether-lands demonstrated a median age of death of 176 days, with only three of 34 children (9%) surviving to 3 yrs of age (3). Rudnik-Schöneborn et al (4) reported on a cohort of 66 patients born in Germany between 2000 and 2005, and noted a median/mean age to the defined end point of death and/or tracheostomy or ventilator support >14 days of 6.5/7.8 months of age. Only five of 66 patients survived beyond 30 months of age, and only one of 66 (1.5%) were alive without a tracheostomy (4).
In accordance with the recommendations from the Consensus Statement for Standard of Care in SMA, all patients in our institution are offered both palliative/supportive interventions as well as proactive care for respiratory and nutritional needs, which is determined by parental choice. We evaluated if the initiation of mechanical noninvasive respiratory interventions is associated with longer survival compared to children who received only supportive care based on early parental preferences.
METHODS
Study Design, Patient Population, and Setting
This is a retrospective cohort study from January 1, 2002 to May 1, 2009 of patients with SMA type 1 followed by the University of Utah Pediatric Motor Disorders Research Program, who were symptomatic at the time of diagnosis. All children presenting with a clinical diagnosis of SMA were consented to participate in a clinical and genetic study of SMA and followed prospectively throughout the study period. Subjects formally enrolled in clinical trials of medication directed toward treatment of SMA were excluded. Hospital care was provided at the university-affiliated children’s hospital: Primary Children’s Medical Center, which is a 271-bed children’s hospital owned and operated by Intermountain Health-care (Intermountain) (a not-for-profit vertically integrated managed care organization) in the Intermountain West, serving as both the primary pediatric hospital for Salt Lake County and as the tertiary care hospital for five states (UT, MT, WY, ID, and NV) (9).
Diagnosis was based on clinical history, electromyography, and genetic confirmation. The classification of SMA adhered to the criteria of the International SMA consortium (1992) with modifications according to the 59th European Neuromuscular Center International Workshop (10). Disease onset was before 6 months of age in all patients, and none achieved the ability to sit unsupported. Children were categorized into groups according to the level of respiratory support their parents/caregivers chose within the first 3 months after diagnosis.
Study Variables and Data Sources
Patients were identified through the Pediatric Motor Disorders Research Program database and data were collected from electronic medical records through the Intermountain system to supplement both paper and electronic medical data from the database. Caregivers of children during the study period were routinely contacted at 3-month intervals by a clinical research coordinator or nurse for updates, which included information on intercurrent illnesses, receipt of emergency department or hospital care, and changes in respiratory support. Costs were derived from Intermountain Healthcare’s cost accounting program, the Standard Cost Master, which is a transaction-based microcosting accounting system that contains detailed data about the cost of providing health care (11–13).
Clinical Data
Baseline data variables were collected, including age and weight at diagnosis, maximum ulnar compound muscle action potential (CMAP) amplitude at diagnosis, and subsequent emergency department care and hospital care for acute life-threatening events, respiratory insufficiency, or surgical procedures (including gastrostomy feeding tube placement) throughout the study period. An acute life-threatening event was defined as apnea, color change, and marked change in muscle tone, choking, or gagging requiring interventions, including: cardiopulmonary resuscitation, rescue breaths, urgent BiPAP, cough assist, or suctioning.
Respiratory Care
Respiratory care was categorized as proactive respiratory care or supportive care. Early proactive respiratory care was defined as use of noninvasive BiPAP at night and daytime sleep, and cough-assist device use at least twice daily (Respironics, Millersville, PA) initiated in the first 3 months after diagnosis. Supportive care was defined as other respiratory support, such as supplemental oxygen and suctioning. Although initial study group allocation into either care category was established within 3 months of diagnosis by parental choice, respiratory care could be escalated as the disease progressed by parental discretion. We further categorized study groups into early proactive respiratory care, supportive care, and change from supportive to proactive care.
Cost Data
For the 22 children who had care provided exclusively through Intermountain, we derived the cost of inpatient, outpatient, and emergency department care across the tertiary care hospital and the other 20 hospitals or outpatient clinics from the time of diagnosis to time of either death or study period conclusion. All costs were standardized to 2009 U.S. dollars by applying a yearly consumer price index for hospital services (14).
Outcomes
The primary outcome was time to death, comparing children who initially received full proactive respiratory care vs. supportive care. Because parents could redirect care as their child’s disease progressed, a second analysis compared time to death for those who changed groups. Cost of care was determined for the children who received all their care exclusively within Intermountain.
Analyses
The primary analyses were done according to intention to treat. Factors between groups were compared using the chi-squared test and the Mann-Whitney U test. Summary results are expressed as medians (with 25th and 75th quartiles) or percentages. Kaplan-Meier survival curves were generated for the initial two treatment groups (early proactive respiratory care and supportive care). The Kaplan-Meier curves for estimated survival were compared with the log-rank test. A Cox-proportional hazard regression model was used to evaluate whether demographic and clinical variables that differed by treatment groups confounded the association with time to death. Potential confounders were considered in the multivariable model if they differed between groups by p < .15 in the bivariate comparison. To examine the effect of later BiPAP institution, another model with a time-dependent covariate for starting before or after 90 days was developed. All analyses were computed with SPSS 15.0 for Windows (SPSS, Chicago, IL). Statistical significance was defined as p <.05.
RESULTS
Patient Characteristics
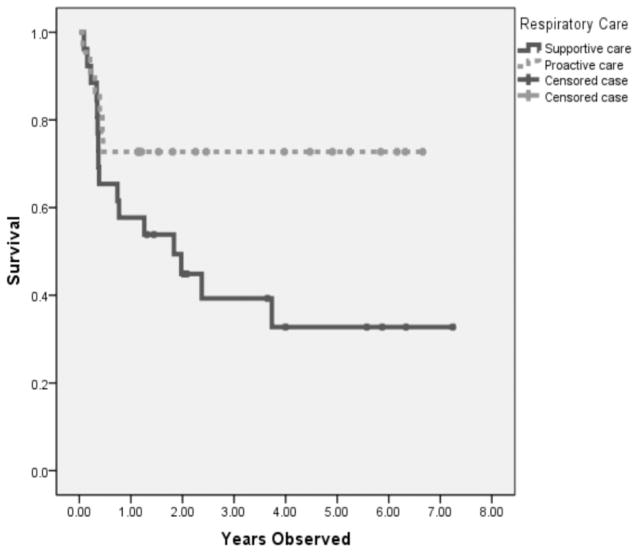
Fifty-four children with SMA type 1 were identified and five were excluded because they were diagnosed before onset of clinical symptoms (due to a sibling with SMA type 1), leaving 49 children in the study group. Demographic and clinical features comparing children who received early proactive respiratory care (n = 26) vs. supportive care (n = 23) are presented in Table 1. Forty-eight children in the study group were identified as having two survival of motor neuron 2 copies, except for one patient who was unable to have testing completed. The patients in the early proactive respiratory care group tended to be older and weaker, assessed by CMAP amplitude at the time of diagnosis (p = .06). Use of nutritional surgical procedures did not differ between the study groups. Children in the proactive respiratory group had significantly fewer days to the first episode of respiratory insufficiency (median 118 vs. 979 days). Three children in both groups received a tracheostomy. Acute life-threatening events were similar in each group (43% vs. 33%). In addition, the proactive respiratory care patients were more likely to receive inpatient care for respiratory insufficiency (83% vs. 46%). However, children treated with early pro-active respiratory support had statistically longer survival compared to supportive care (log rank 0.047) (Fig. 1). Of note, all observed mortality occurred within the first 12 months after initiation of early proactive respiratory care.
Table 1.
Demographic and clinical features of type 1 spinal muscle atrophy children by respiratory support groups
| Variable | Full Proactive Respiratory Care n = 23 | Supportive Care n = 26 | p |
|---|---|---|---|
| Demographics | n (%) | n (%) | |
| Age of diagnosis (days)a | 136 (54, 196) | 69 (38, 145) | .06 |
| Gender: female | 8 (35%) | 12 (46%) | .41 |
| Weight (kg)a | 6.7 (4.9, 7.8) | 5.6 (4.7, 6.8) | .27 |
| Compound muscle action potential amplitudea | 0.19 (0.10, 0.64) | 0.43 (0.22, 1.20) | .06 |
| Survival of motor neuron 2 copiesb | 23 (100%) | 25 (96%) | .47 |
| Nutritional interventions/information | |||
| G tube | 20 (90%) | 19 (73%) | .11 |
| Nissen + G tube | 15 (66%) | 11 (42%) | .17 |
| Age at G tube (days)a,c | 57 (29, 97) | 105 (57, 182) | .12 |
| Respiratory interventions/information | |||
| Initiate bilevel positive airway pressure support (day after diagnosis)a | 44 (22, 93) | 175 (127, 414) | <.0001 |
| Tracheostomy | 3 (13%) | 3 (12%) | .87 |
| Days to first respiratory failure (days after diagnosis)a,d | 118 (63, 458) | 979 (464, 2091) | <.0001 |
| Days to tracheostomya | 89 (62, 1492) | 244 (240, 1235) | .53 |
| Complications | |||
| Acute life-threatening events | |||
| Yes | 10 (43%) | 9 (35%) | .53 |
| number of acute life-threatening eventsa | 0 (0, 1) | 0 (0, 1) | .61 |
| Emergency department care | |||
| Any | 6 (26%) | 7 (27%) | .56 |
| Visit numbera | 0 (0, 1) | 0 (0, 0.25) | .58 |
| Admission for respiratory failure | |||
| Any | 19 (83%) | 12 (46%) | .021 |
| Visit numbera | 2 (1, 4) | 0 (0, 2) | .014 |
| Death | 6 (26) | 16 (62) | .018 |
| Age of death (months)a | 7.6 (6.5, 10.5) | 8.8 (4.7, 23.7) | .85 |
G tube, gastronomy tube.
Continuous data reported as median with interquartile ranges unless otherwise specified;
one child not tested;
excludes 4 children who presented in respiratory failure prior to diagnosis;
among those who received a G tube.
Figure 1.
Early respiratory care and survival in spinal muscular atrophy type 1.
Cox-proportional hazard models demonstrated a trend for longer survival between treatment groups when adjusting for initial CMAP amplitude and placement of a gastrostomy feeding tube as potential confounding variables. Based on the intention-to-treat group, the adjusted hazard ratio for survival among children treated with proactive respiratory care was not statistically different (2.44 [95% confidence interval 0.84–7.1]). When evaluating the three groups to account for later changes in care, institution of BiPAP within 90 days of diagnosis compared to never, the hazard ratio for survival was 3.41 (95% confidence interval 0.79–14.70), while institution of proactive respiratory care later had a hazard ratio of 1.33 (95% confidence interval 0.38–4.59) compared to children treated with supportive respiratory care. All three models were also adjusted for initial CMAP amplitude and placement of a gastrostomy feeding tube and none were statistically significant, with large confidence intervals reflecting small sample size.
Total cost of care for the 22 children followed within Intermountain was $2.93 million, with a median cost of $98,504 (interquartile range $21,477–$169,576). These costs were accrued during the study period for a median time of 686 days (interquartile range 232–1245 days). Children in the supportive care group tended to have a lower cost of care, with a median cost of $76,746 (interquartile range $17,272–$173,900), compared to the early proactive care group with median costs of $116,988 (interquartile range $75,478–$169,576), p = .39.
DISCUSSION
We found that SMA type 1 children treated with early proactive noninvasive respiratory care after diagnosis tended to live longer than children treated with supportive respiratory care. Over half were alive at age 4 yrs in the early pro-active care group and only three (12%) were “rescued” by invasive respiratory support via tracheostomy. Our results suggest that children who transitioned to proactive respiratory care later in the disease course also experienced a trend for longer survival.
This study demonstrates that the survival time of children with SMA type 1 is longer than traditionally expected, and this change is ecologically associated with noninvasive respiratory care interventions. Our survival data confirms other recent reports. In 2004, a study by Chung et al (15) reported survival at ages 2, 4, and 10 yrs as 40%, 30%, and 30%, respectively. Similarly, Oskoui et al (8) reported increased survival in SMA patients comparing cohorts of children born between 1980 and 1994 to those born later (1995–2006) (8, 16). Our study demonstrates that the increased survival time of children with SMA is associated with noninvasive respiratory care interventions among children treated during the same study time with similar nutritional care and other supportive care. However, the children were not randomized to a respiratory treatment group; rather, parents were provided information on available options and presented with choices. Weaker children tended to be more common in the proactive care group, which may reflect parental appreciation of their child’s limited respiratory reserve.
Although this is one of the larger series of type 1 subjects reported to date, it represents a small sample size. The unadjusted analyses demonstrated a difference in survival between the proactive and supportive groups. However, potential confounders were identified based on previous literature that describes their association with survival. Disease severity was estimated using a surrogate end point previously demonstrated to correlate with gross motor function, the maximum ulnar CMAP amplitude (17). Need for nutritional support was indicated by gastrostomy tube placement (18). Survival rates among children with severe neurologic disabilities and demographic features were thus evaluated. When adjusting for these potential confounding factors, statistical significance was not reached. The impact of increased weakness in the early respiratory proactive group may have decreased the survival benefit; however, because of limitations specific to difficulties in quantitating the degree of respiratory, skeletal muscle, and bulbar weakness, and since all patients were already quite symptomatic at the time of presentation, we cannot more fully evaluate the optimal time to institute noninvasive respiratory care. Children who had delayed initiation of noninvasive respiratory care also experienced a trend in survival benefit; however, it was less than the children with the earliest initiation of care. Our data indicate, not surprisingly, that earlier institution of respiratory proactive care in already symptomatic type 1 infants will likely increase healthcare costs overall, since children who survive longer are more costly.
The observation that proactive respiratory care appears to stabilize patients after the first year of life is not unique to this study (19). We have previously demonstrated that the greatest rate of denervation among infants with SMA type 1 occurs within the first 6 months, corresponding with the acute phase of disease progression (17). SMA is unique as compared to other neurodegenerative conditions in that the acute phase of disease progression is subsequently followed by a more chronic plateau phase characterized by months to years of relative stability of motor function (20). Another potential factor that may affect survival in the first year of life is that BiPAP mask fit is more difficult for infants, and the delivery of noninvasive ventilation and cough pressures are less efficient.
An important study limitation is that care was determined by parental preference. This was associated with a somewhat more severely affected cohort of infants receiving more early aggressive care, but may have been associated with other undocumented differences in care, such as greater parental use of other forms of medical care, different thresholds to seek care, and/or parental vigilance. We did not report caregiver or patient quality of life given the retrospective nature of the study and the proxy nature of this potential information given the child’s young age and severe motor impairments. However, this clearly is a critical area for focus in future studies, since an increasing percentage of infants with SMA type 1 are now surviving into childhood.
Improved survival is not associated with improved motor outcomes, since severe denervation occurs early in infants with SMA type 1. However, early identification of such infants via newborn screening in the presymptomatic period would allow early nutritional and respiratory interventions, which could theoretically result in improved strength and respiratory function, reducing overall hospital care and ultimately saving healthcare costs. Newborn screening for SMA may allow for earlier diagnosis, so this question of optimal timing is an important focus for further research to improve outcomes for this common and devastating motor neuron disease (21).
It is important to counsel families carefully regarding their choices and the anticipated range of serious long-term complications should the parents choose proactive care (19). In our experience, in the majority of these long-term survivors, proactive respiratory care becomes increasingly burdensome with regard to daily hours committed to airway clearance and time on BiPAP support.
Adherence to the proactive treatment protocols used in this study was associated with a trend for longer adjusted survival in SMA type 1 infants (22). When counseling parents, we believe it is important to discuss that prolonged survival for children with SMA type 1 in the full proactive respiratory care group involves both daily therapy and parental vigilance. Presently, these children remain at high risk for recurrent hospitalizations and acute life-threatening events. In addition, the cost of care for these children is fairly substantial, and can negatively impact family finances. However, in spite of these obstacles, many families embrace such care willingly, fiercely protect their choices, and consider their quality of life good in spite of the many challenges involved. Our data help inform the larger context in which clinicians and families of children with SMA type 1 should consider options for optimal health-related quality of life for their children and family. In addition, they emphasize the need for further studies to determine whether earlier detection and intervention in SMA type 1 infants can result in meaningful improvements in motor and respiratory function. Finally, this study highlights the need to consider combined end points for clinical studies that incorporate a time requirement for ventilator use and need for urgent respiratory interventions, and not just survival, for infants with SMA type 1.
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Lunn MR, Wang CH. Spinal muscular atrophy. Lancet. 2008;371:2120–2133. doi: 10.1016/S0140-6736(08)60921-6. [DOI] [PubMed] [Google Scholar]
- 2.Zerres K, Wirth B, Rudnik-Schöneborn S. Spinal muscular atrophy – clinical and genetic correlations. Neuromuscul Disord. 1997;7:202–207. doi: 10.1016/s0960-8966(97)00459-8. [DOI] [PubMed] [Google Scholar]
- 3.Cobben JM, Lemmink HH, Snoeck I, et al. Survival in SMA type I: A prospective analysis of 34 consecutive cases. Neuromuscul Disord. 2008;18:541–544. doi: 10.1016/j.nmd.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Rudnik-Schöneborn S, Berg C, Zerres K, et al. Genotype-phenotype studies in infantile spinal muscular atrophy (SMA) type I in Germany: Implications for clinical trials and genetic counselling. Clin Genet. 2009;76:168–178. doi: 10.1111/j.1399-0004.2009.01200.x. [DOI] [PubMed] [Google Scholar]
- 5.Bach JR, Niranjan V, Weaver B. Spinal muscular atrophy type 1: A noninvasive respiratory management approach. Chest. 2000;117:1100–1105. doi: 10.1378/chest.117.4.1100. [DOI] [PubMed] [Google Scholar]
- 6.Schroth MK. Special considerations in the respiratory management of spinal muscular atrophy. Pediatrics. 2009;123:S245–S249. doi: 10.1542/peds.2008-2952K. [DOI] [PubMed] [Google Scholar]
- 7.Mannaa MM, Kalra M, Wong B, et al. Survival probabilities of patients with childhood spinal muscle atrophy. J Clin Neuromuscul Dis. 2009;10:85–89. doi: 10.1097/CND.0b013e318190310f. [DOI] [PubMed] [Google Scholar]
- 8.Oskoui M, Levy G, Garland CJ, et al. The changing natural history of spinal muscular atrophy type 1. Neurology. 2007;69:1931–1936. doi: 10.1212/01.wnl.0000290830.40544.b9. [DOI] [PubMed] [Google Scholar]
- 9.Norlin C, Osborn LM. Organizational responses to managed care: Issues for academic health centers and implications for pediatric programs. Pediatrics. 1998;101:805–811. discussion 811–812. [PubMed] [Google Scholar]
- 10.Munsat TL, Davies KE. Neuromuscul Disord; International SMA consortium meeting; 26–28 June 1992; Bonn, Germany. 1992. pp. 423–428. [DOI] [PubMed] [Google Scholar]
- 11.Evans RS, Classen DC, Stevens LE, et al. Using a hospital information system to assess the effects of adverse drug events. Proc Annu Symp Comput Appl Med Care. 1993:161–165. [PMC free article] [PubMed] [Google Scholar]
- 12.Harbarth S, Burke JP, Lloyd JF, et al. Clinical and economic outcomes of conventional amphotericin B-associated nephrotoxicity. Clin Infect Dis. 2002;35:e120–e127. doi: 10.1086/344468. [DOI] [PubMed] [Google Scholar]
- 13.Ampofo K, Gesteland PH, Bender J, et al. Epidemiology, complications, and cost of hospitalization in children with laboratory-confirmed influenza infection. Pediatrics. 2006;118:2409–2417. doi: 10.1542/peds.2006-1475. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Census Bureau. [Accessed April 12, 2010];2000 Census of Population and Housing: Summary File 3. Available at: http://factfinder.census.gov.
- 15.Chung BH, Wong VC, Ip P. Spinal muscular atrophy: Survival pattern and functional status. Pediatrics. 2004;114:e548–e553. doi: 10.1542/peds.2004-0668. [DOI] [PubMed] [Google Scholar]
- 16.Bach JR, Baird JS, Plosky D, et al. Spinal muscular atrophy type 1: Management and outcomes. Pediatr Pulmonol. 2002;34:16–22. doi: 10.1002/ppul.10110. [DOI] [PubMed] [Google Scholar]
- 17.Swoboda KJ, Prior TW, Scott CB, et al. Natural history of denervation in SMA: Relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57:704–712. doi: 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plioplys AV, Kasnicka I, Lewis S, et al. Survival rates among children with severe neurologic disabilities. South Med J. 1998;91:161–172. doi: 10.1097/00007611-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Bach JR, Saltstein K, Sinquee D, et al. Long-term survival in Werdnig-Hoffmann disease. Am J Phys Med Rehabil. 2007;86:339–345. doi: 10.1097/PHM.0b013e31804a8505. quiz 346–348, 379. [DOI] [PubMed] [Google Scholar]
- 20.Crawford TO. Concerns about the design of clinical trials for spinal muscular atrophy. Neuromuscul Disord. 2004;14:456–460. doi: 10.1016/j.nmd.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Swoboda KJ. Seize the day: Newborn screening for SMA. Am J Med Genet A. 2010;152A:1605–1607. doi: 10.1002/ajmg.a.33519. [DOI] [PubMed] [Google Scholar]
- 22.Wang CH, Finkel RS, Bertini ES, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22:1027–1049. doi: 10.1177/0883073807305788. [DOI] [PubMed] [Google Scholar]