Abstract
Proactive nutritional management for children with spinal muscular atrophy type I can provide insight into improved spinal muscular atrophy care. This observational study consisted of a nutritional and medical history survey of children with spinal muscular atrophy type I collected in 2009-2011. Forty-four caregiver survey responses were evaluated using descriptive statistics. Average age of spinal muscular atrophy type I subjects was 5 years (5 mo-16 y). The subject cohort was composed of 22 males, 21 females, and 1 unreported. Nutrition support via feeding tube was utilized by 43 of 44 subjects. A majority of respondents reported using elemental or semi-elemental formula for subjects’ essential caloric intake (34 of 44). Formula intolerance issues were reported by many caregivers (27 of 44). Half of caregivers implemented dietary changes on their own or with guidance from other families; 15 caregivers consulted a registered dietitian. Survey responses and comments indicate need for evidence-based nutritional guidelines for spinal muscular atrophy.
Keywords: SMA nutrition, spinal muscular atrophy type I, elemental diet
Spinal muscular atrophy is an autosomal recessive genetic disorder that affects the anterior horn cells of the spinal cord and results in progressive muscular atrophy and weakness.1
Spinal muscular atrophy type I, also known as Werdnig-Hoffman disease, is the most severe and common form of the disorder. Most children with spinal muscular atrophy type I present with significant weakness by 6 months of age, with longstanding functional physical limitations including lack of head control, hypotonia, and inability to ever sit unassisted.1,2
Historically, the majority of children with spinal muscular atrophy type I did not survive past their second birthday. However, with advances in respiratory care and proactive nutritional management, life expectancy now extends beyond 2 years in an increasing percentage of children with spinal muscular atrophy type I.3-6
Nutrition is of primary concern for patients with spinal muscular atrophy type I because muscle atrophy and disease progression often results in decreased lean body mass and increased fat mass,7,8 gastrointestinal dysmotility, bulbar dysfunction and dysphagia,9,10 and osteoporosis.8,11-13 Many patients with spinal muscular atrophy also exhibit metabolic abnormalities consistent with a secondary fatty acid oxidation disorder.14,15 Weaker patients with spinal muscular atrophy exhibit increased levels of dodecanoic (C12) fatty acid in plasma as well as dicarboxylic aciduria and ketonuria during times of fasting. In normal fat metabolism of healthy individuals, longer chain fats are transported into the mitochondria for beta-oxidation. Increased levels of dicarboxylic acids indicate oxidation in peroxisomes instead of the mitochondria. In contrast to known genetic disorders of mitochondrial fatty acid oxidation, acylcarnitine profiles in spinal muscular atrophy patients are normal and they do not exhibit reduced ketone production under catabolic conditions.15 The exact mechanism of this fatty acid metabolism abnormality in spinal muscular atrophy is unknown, but it is suspected to be related to loss of survival motor neuron function, correlates with severity of spinal muscular atrophy, and is not directly related to a known genetic disorder of mitochondrial fatty acid oxidation.15 Recent research has indicated a severe decrease in mitochondrial DNA relative to nuclear DNA, but not number of mitochondria in spinal muscular atrophy, which may be related to mitochondrial dysfunction.16 Further study is needed to determine whether dietary treatment such as a high-carbohydrate/low-fat diet or use of medium-chain triglycerides, used in mitochondrial long-chain fatty acid oxidation disorders, can ameliorate effects of this abnormality. Prior reports have emphasized the importance of regular monitoring for nutritional compromise in infants with spinal muscular atrophy, particularly regarding need for nutritional support interventions including nasogastric feeding, gastrostomy, and/or fundoplication17,18; others have identified fatty acid oxidation abnormalities indicating a potential need for closer attention to nutritional intake.14,15 The latter observations have important implications for acute illness management. However, a consensus within the broader spinal muscular atrophy community has not yet been achieved regarding the benefit of specific dietary modifications, including restriction of dietary fat intake. Studies in spinal muscular atrophy mouse models have indicated that nutritional supplementation provided in addition to treatment with trichostatin A prolongs survival almost twice as long as drug alone19; in addition, the type of chow that dams receive during pregnancy significantly affects weight and survival in spinal muscular atrophy–affected pups.20 Unfortunately, there is a lack of any other spinal muscular atrophy–specific evidence-based research, aside from natural history data, to guide nutritional management of spinal muscular atrophy. Thus, families are often left to deal with nutrition on their own. Further research and guidance is necessary for children with spinal muscular atrophy type I because improved nutrition and published nutritional management guidelines can improve clinical care and enhance quality of life for children with spinal muscular atrophy type I. As such, the aims of this survey were to bring attention to the role of nutrition in the management of children with spinal muscular atrophy type I, provide a current snapshot of the nutritional practices of these children based on their caregiver responses, and provide direction for future research into nutritional management of spinal muscular atrophy type I.
Methods
Participants were recruited via spinal muscular atrophy social media websites, through flyers handed out at the 2010 Families of SMA national meeting, and via referring dietitians from 3 key referral centers, University of Utah, University of Wisconsin, and Cincinnati Children’s Hospital, from late 2009 to June 2011. This study was performed with the support of the Pediatric Motor Disorders Research Program at the University of Utah. The University of Utah’s institutional review board approved this study as an amendment to the broader outcomes study, “Clinical and Genetic Studies in SMA.” Written informed consent was obtained from all participants. Primary caregivers of children with spinal muscular atrophy type I (respondents) provided responses to the SMA Nutrition Survey online on behalf of their children (subjects). Eligibility requirements included child’s diagnosis of spinal muscular atrophy type I. Responses for subjects older than 18 years were not included in this analysis nor were those for subjects whom survey responses indicated had ever sat unsupported.1 In most cases, the subject was a research or clinical patient at one of the collaborating institutions for this survey.
Respondents were not compensated for survey participation; however, the survey included an opportunity to submit a 3-day food record for nutrient analysis from a registered dietitian with expertise in the area of nutrition and spinal muscular atrophy.
Survey
The SMA Nutrition Survey was given online via the University of Utah’s SMA and Nutrition website (http://smaandnutrition.org/home.php). The survey was built into a spinal muscular atrophy nutrition database that requires a password and login from participants. Login and password information were given to the participants after the IRB-approved institution received signed consent forms. The survey consisted of 29 medical and nutrition questions related to spinal muscular atrophy. Survey questions were reviewed and suggestions were made by a family representative of the spinal muscular atrophy community. Questions included one regarding the highest physical functional status achieved in order to validate spinal muscular atrophy type. Gender, ethnicity, and child’s age were reported in the parental consent form for each subject.
Statistical Methods
Survey data were exported to Microsoft Excel (2007, Redmond, WA), where descriptive statistics were calculated for all survey variables.
Results and Discussion
Forty-four respondents and subjects with spinal muscular atrophy type I were included in the survey results. The subjects ranged in age from 5 months to 16 years. The mean age was 5 years. Subjects consisted of 21 males, 22 females, and 1 unreported. Forty-three of the subjects were white, with 1 unreported. Eight of the subjects had a tracheostomy tube.
Forty-three subjects were clinically classified as spinal muscular atrophy type I because they could not sit unsupported; at the time of the survey, 21 of the subjects could sit supported for 30 minutes a day and 22 subjects could not tolerate supported sitting. One subject was classified as having spinal muscular atrophy type I but was enrolled in the SMA CARNI-VAL type I study (www.clinicaltrials.gov) and was able to achieve sitting unsupported for a short period while on study drug.
Feeding Modality and Diet
The majority of subjects, 43 of 44, depended on a feeding tube for essential energy intake. Of those, 35 were fed via a gastrostomy tube, 7 via a gastrojejunal tube, and 1 with a nasogastric tube. Four subjects were using both feeding tubes and oral feeding strategies. Only 1 subject (age 6 mo) did not have a feeding tube and was still eating orally, but with restricted consistencies and textures. This subject later received feeding tube nutrition support at 6.5 months of age. The average age of feeding tube placement was 11 months.
The types of formulas respondents reported using to meet nutritional needs are summarized in Table 1. Formula types were classified by type of protein content: elemental formulas (100% synthetic free amino acids), semi-elemental formulas (hydrolyzed protein), and milk- and soy-based formulas (intact protein). Half of the respondents (23 of 44) reported subjects’ having formula tolerance issues. Intolerance comments included mentions of gastrointestinal pain, elevated heart rate, increased secretions, thicker secretions, decreased strength, increased reflux, emesis, and poor gastric emptying. Thirteen respondents reported confirmed cow’s milk intolerance and 4 reported confirmed food allergies.
Table 1.
Type of Formula Used to Meet Nutrient Needs for Children With Spinal Muscular Atrophy Type I.
| Type of formula | n (44) |
|---|---|
| Elemental formula | 30 |
| Semi-elemental formula | 4 |
| Human milka | 8 |
| Cow’s milk or soy-based formula | 4 |
| Unreported | 2 |
Several subjects used human milk in combination with other formulas, which accounts for numbers greater than the total number of subjects, but number may not include all subjects using human milk.
Gastroesophageal reflux was reported in 21 subjects. However, 23 of 44 were on acid reducing medication for reflux and 8 subjects were taking medication to improve gut motility. A majority of the subjects (31 of 44) had a Nissen fundoplication, a procedure used to prevent reflux. Bowel-regulating agents were utilized by 28 subjects. Bowel agent usage is summarized in Table 2.
Table 2.
Bowel-Regulating Agent Usage Reported for Children With Spinal Muscular Atrophy Type I.
| Bowel-regulating agent | n (44) |
|---|---|
| Fiber supplement | 9 |
| Irritant laxative | 2 |
| Suppository | 12 |
| Osmotic laxative | 15 |
| Probiotics | 28 |
Thirty-three subjects were breastfed at some point during infancy. Of those, 2 stopped nursing at an age of less than 3 months, 6 stopped between 3 and 6 months, 19 stopped between 7 and 12 months, and 6 stopped nursing at an age greater than 12 months. Several comments indicated continued use of human milk, but this was not captured in the survey results.
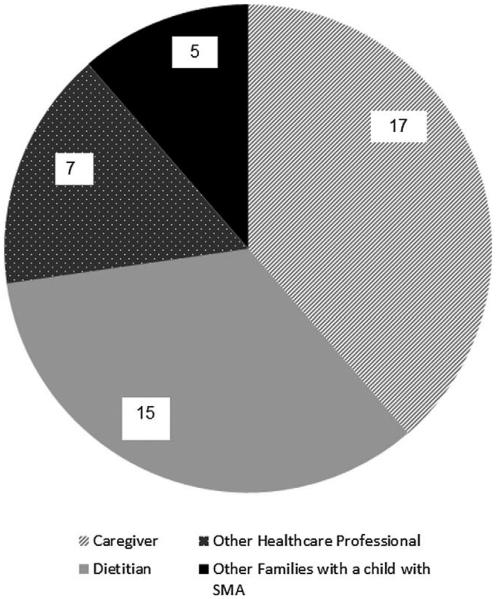
Dietary changes were guided most often by the respondent (17 of 44) or dietitian (15 of 44), as summarized in Figure 1. Most subjects’ diets were reviewed every 6 months (29 of 44), while 8 reported quarterly review, 3 more than once annually, 3 annually, and 1 unreported.
Figure 1.
Persons responsible for guiding dietary changes for child with spinal muscular atrophy type I.
Routine laboratory testing was reported in 28 subjects. Nine reported issues with low blood glucose, and 14 reported issues with low electrolytes. Recurrent yeast infections were reported by 11 subjects.
Respiratory Health
Respiratory care employed by the majority of subjects included cough assist, BiPap, and suction use to aid with oral secretions. The frequency/use of care in this cohort is summarized in Table 3. Nineteen subjects also reported taking medication to reduce oral secretions.
Table 3.
Respiratory Care Use and Frequency.
| Suction use | n (44) |
BiPap use | n (44) |
Cough assist use |
n (44) |
|---|---|---|---|---|---|
| Never | 1 | Never | 0 | Never | 3 |
| Only during URI |
2 | Only during URI |
0 | Only during URI |
1 |
| 1-2 times daily | 4 | <12 hours daily | 17 | 1-2 times daily | 25 |
| ≥3 times daily | 8 | 12-16 hours daily |
12 | ≥3 times daily | 15 |
| Hourly + daily | 28 | >16 hours daily | 5 | ||
| Not reported | 1 | >20 hours daily | 10 |
Abbreviation: URI, upper respiratory tract infection.
Dental Health
Twenty-one subjects used dental sponges for dental health. Daily toothbrush use was reported in 9 subjects and twice daily in 16 subjects. Only 1 subject reported daily tooth scraping.
Discussion
Every subject with spinal muscular atrophy type I in this pediatric cohort eventually relied on essential nutrition support via feeding tube. A majority also reported using elemental formulas, probiotics, and bowel-regulating agents. Formula intolerance issues were prevalent among this population. The average age of our subjects was 5 years, which is much older than the historically suggested life span of a child with spinal muscular atrophy type I, suggesting that respondents to our survey were more likely to have an interest in more aggressive, proactive interventions and in nutritional interventions in particular. This is indicated by the greater use of proactive respiratory interventions in conjunction with early nutrition intervention in this cohort, likely contributing to the increase in mean age of children whose caregivers chose to respond to the survey. Survey responses indicate that nutritional management guidelines are needed for the treatment for spinal muscular atrophy and can help to provide direction for future research.
Because the SMA Nutrition Survey results could be more reflective of the practices of caregivers who are more proactive and attentive toward nutrition in particular, this survey might not reflect the practices of the entire spinal muscular atrophy type I community. In our experience, caregivers in the first few months post diagnosis are more focused on respiratory issues, and might not begin to ask nutritional questions until the respiratory issues have been addressed. Additional comments included in the survey suggest that subjects are using human milk in addition to formula and this was not captured in the survey. Early feedback from a family representative strengthened the survey questions. Because subjects with spinal muscular atrophy type I are so fragile and require many hours of care, this study’s online access was very convenient for family participation. The intensive parental consent form could have been a deterrent for many families because it included information for a broader spinal muscular atrophy study. Some families reported fear of being judged by the researchers and chose not to participate.
Proactive respiratory intervention was employed by the majority of survey respondents. All subjects used BiPap daily and most used cough assist at least 1-2 times daily. The frequency of respiratory care reported in this survey is not representative of that in other recently published spinal muscular atrophy cohorts.4,21 This survey included very few responses from caregivers who reported supportive (vs proactive) respiratory care. These responses suggest a likely bias toward more proactive respiratory care, both invasive and noninvasive, in this survey. In part, the proactive care reported in this survey could be related to the increased average age of the subjects because respiratory support needs often increase with advancing age and disease progression. The majority of subjects in this survey were using elemental formulas. In clinical practice, a predominance of longer-surviving children with spinal muscular atrophy type I are receiving elemental formula as a primary source of nutrition. However, some longer-surviving children with spinal muscular atrophy type I clearly tolerate intact or hydrolyzed protein formulas. The use of elemental formula can be clinically indicated as spinal muscular atrophy and gut dysmotility issues progress. The easier to digest protein source and low fat content of elemental formulas can help with gastric motility, reduce reflux, and can reduce distress sometimes experienced with feeds in this fragile population. The lower fat content of these formulas may be beneficial from a metabolic perspective, given the observed fatty acid oxidation abnormalities, although this remains unproven. As a result of both anecdotal reports as well as benefits seen in symptomatic children in response to a formula change, an increasing number of caregivers initiate use of elemental formulas earlier in their child’s clinical course. Whether this early transition to elemental formula is beneficial warrants further study.
Nutrition studies to date in spinal muscular atrophy and other neuromuscular diseases, such as muscular dystrophy, have focused on the nutritional impact of gastrostomy placement, but not formula type.17,22 Children with other neuromuscular diseases also manifest symptoms related to slowed gastrointestinal motility.23,24 For instance, in Duchenne muscular dystrophy, impaired gastric and colonic motility, but not small intestinal dysmotility, are hypothesized to be caused by impairment of smooth muscle.25-27
Reports of formula intolerance among spinal muscular atrophy patients are common. Possible factors contributing to intolerance might include food allergy/intolerance, the amount or type of fat, specific ingredients in the formula, or feeding rate or volume.
Clinically, we hear frequent anecdotal reports of children with spinal muscular atrophy type I better able to tolerate human milk than any other formula. As such, we often see diet records of patients with spinal muscular atrophy on elemental formulas with the use of supplemental human milk at increased ages. Several respondents indicated the use of human milk in survey comments, and unfortunately, this practice was not adequately captured by the survey. The average age of discontinued breastfeeding (7-12 mo) appears to be roughly associated with the average age of g-tube surgery (11 mo), which warrants further study.
The number of patients with spinal muscular atrophy using elemental and elemental low-fat diets is growing by word of mouth from families. In this cohort, 30 subjects were on an elemental formula, whereas only 15 subjects reported using a registered dietitian to guide their child’s diet. Given the fragile nature of this population and inherent nutrition concerns (including application of enteral feedings, whether or not it is elemental), we strongly suggest that patients with spinal muscular atrophy be referred to a dietitian knowledgeable about spinal muscular atrophy at diagnosis.
Although a majority of subjects had undergone a Nissen procedure, many were still taking medicines intended to treat reflux symptoms. Use of adjunct therapies in conjunction with Nissen might require further review because chronic and prolonged use of H2 blockers can increase risk for gastrointestinal illness. However, although Nissen is intended to reduce reflux symptoms, it is not always fully protective, and progressive gastrointestinal dysmotility is common in spinal muscular atrophy.
Conclusions
This study documents the prominent use of elemental formulas in the diets of longer-surviving children with spinal muscular atrophy type I. To date, no published studies have evaluated the impact on clinical or metabolic outcomes with the use of elemental formulas in either spinal muscular atrophy type I patients or animal models. Although formal studies are not available to date to assess the potential benefits and risks of using such formulas, caregivers responding to this survey list a number of potential benefits and observed improvements in their child with spinal muscular atrophy after switching to an elemental or semi-elemental formula. However, of note, in the food records that we received as part of the broader outcomes study, many families were not using a single formula alone; rather, formulas were extensively supplemented with other nutritional supplements and food, which makes conclusions about beneficial aspects of the diet very challenging to interpret. Future research is needed to evaluate the use of elemental and semielemental formulas in the management of spinal muscular atrophy including optimal macronutrient and micronutrient intakes for nutritional management, the ideal time to transition to these formulas, ideal fat content and composition, and benefits, if any, of early transition to these formulas. Survey comments indicated use of supplemental human breast milk; the use and outcomes of supplemental human milk in patients with spinal muscular atrophy type I also warrants further study. Many families make dietary changes for their child without guidance from a registered dietitian. We suspect that creating evidence-based nutrition guidelines will increase utilization of registered dietitians, and can allow us to gain additional information about the benefits and risks involved in the use of these formulas. Future nutrition and formula studies in spinal muscular atrophy can help expand our understanding of factors contributing to gastrointestinal dysmotility in spinal muscular atrophy patients as well as other neuromuscular populations. Such studies could help to guide better management of associated symptoms that contribute to impaired quality of life and compromised nutrition in these populations.
Acknowledgments
The authors wish to acknowledge Anne Meguiar of SMA Angels Charity Inc for bringing researchers together through her strong commitment to nutrition research in spinal muscular atrophy. We also wish to acknowledge Benjamin Chisum for his contributions to the survey framework and technological support.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by SMA Angels Charity Inc. Additional support was provided by Families of SMA.
Footnotes
Author Contributions
RHD prepared and analyzed data and prepared the draft and final manuscript. BJG, ES, MM, BW, MKS contributed to survey development and manuscript edits. BAL contributed to survey development and provided key data management support and structure. KJS was principal investigator and provided key support, including funding procurement, data collection, analysis, and manuscript editing.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
The study was approved by the Institutional Review Board at the University of Utah. Written informed parental consent (children <18 years) and assent (children >7 years) were obtained for all participants.
References
- 1.Pearn J. Classification of spinal muscular atrophies. Lancet. 1980;1:919–922. doi: 10.1016/s0140-6736(80)90847-8. [DOI] [PubMed] [Google Scholar]
- 2.Wang CH, Finkel RS, Bertini ES, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22:1027–1049. doi: 10.1177/0883073807305788. [DOI] [PubMed] [Google Scholar]
- 3.Oskoui M, Levy G, Garland CJ, et al. The changing natural history of spinal muscular atrophy type 1. Neurology. 2007;69:1931–1936. doi: 10.1212/01.wnl.0000290830.40544.b9. [DOI] [PubMed] [Google Scholar]
- 4.Lemoine TJ, Swoboda KJ, Bratton SL, et al. Spinal muscular atrophy type 1: are proactive respiratory interventions associated with longer survival? Pediatr Crit Care Med. 2012;13:e161–e165. doi: 10.1097/PCC.0b013e3182388ad1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannaa MM, Kalra M, Wong B, Cohen AP, Amin RS. Survival probabilities of patients with childhood spinal muscle atrophy. J Clin Neuromuscul Dis. 2009;10:85–89. doi: 10.1097/CND.0b013e318190310f. [DOI] [PubMed] [Google Scholar]
- 6.Rao K, Wong BL. Early intervention with a semi-elemental, limited protein, low fat enteral formula (SF) improves survival and growth parameters in children with spinal muscular atrophy (SMA) 1 and severe SMA 2 [Abstract] Neuromuscul Disord. 2008;18:761–762. [Google Scholar]
- 7.Sproule DM, Montes J, Montgomery M, et al. Increased fat mass and high incidence of overweight despite low body mass index in patients with spinal muscular atrophy. Neuromuscul Disord. 2009;19:391–396. doi: 10.1016/j.nmd.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poruk KE, Hurst Davis R, Smart AL, et al. Observational study of caloric and nutrient intake, bone density, and body composition in infants and children with spinal muscular atrophy type I. Neuromuscul Disord. 2012;22:966–973. doi: 10.1016/j.nmd.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iannaccone ST. Modern management of spinal muscular atrophy. J Child Neurol. 2007;22:974–978. doi: 10.1177/0883073807305670. [DOI] [PubMed] [Google Scholar]
- 10.Tilton AH, Miller MD, Khoshoo V. Nutrition and swallowing in pediatric neuromuscular patients. Semin Pediatr Neurol. 1998;5:106–115. doi: 10.1016/s1071-9091(98)80026-0. [DOI] [PubMed] [Google Scholar]
- 11.Khatri IA, Chaudhry US, Seikaly MG, Browne RH, Iannaccone ST. Low bone mineral density in spinal muscular atrophy. J Clin Neuromuscul Dis. 2008;10:11–17. doi: 10.1097/CND.0b013e318183e0fa. [DOI] [PubMed] [Google Scholar]
- 12.Shanmugarajan S, Tsuraga E, Swoboda KJ, et al. Bone loss in survival motor neuron (SMN-/-SMN2) genetic mouse model of spinal muscular atrophy. J Pathol. 2009;219:52–60. doi: 10.1002/path.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aton J, Hurst Davis R, Jordan K, et al. Vitamin D intake is inadequate in spinal muscular atrophy type I cohort: correlations with bone health. J Child Neurol. 2013 Jan 17; doi: 10.1177/0883073812471857. Published online. DOI: 10.1177/0883073812471857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tein I, Sloane AE, Donner EJ, et al. Fatty acid oxidation abnormalities in childhood-onset spinal muscular atrophy: primary or secondary defect(s)? Pediatr Neurol. 1995;12:21–30. doi: 10.1016/0887-8994(94)00100-g. [DOI] [PubMed] [Google Scholar]
- 15.Crawford TO, Sladkey JT, Hurko O, et al. Abnormal fatty acid metabolism in childhood spinal muscular atrophy. Ann Neurol. 1999;45:337–343. doi: 10.1002/1531-8249(199903)45:3<337::aid-ana9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Berger A, Mayr JA, Meierhofer D, Fotschl U, Bittner R, Budka H, Grethen C, Huemer M, Kofler B, Sperl W. Severe depletion of mitochondrial DNA in spinal muscular atrophy. Acta Neuropathologica. 2003;105:245–251. doi: 10.1007/s00401-002-0638-1. [DOI] [PubMed] [Google Scholar]
- 17.Durkin ET, Schroth MK, Helin M, et al. Early laparoscopic fundoplication and gastrostomy in infants with spinal muscular atrophy type I. J Pediatr Surg. 2008;43:2031–2037. doi: 10.1016/j.jpedsurg.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 18.Ramelli GP, Aloysius A, King C, et al. Gastrostomy placement in paediatric patients with neuromuscular disorders: indications and outcome. Dev Med Child Neurol. 2007;49:367–371. doi: 10.1111/j.1469-8749.2007.00367.x. [DOI] [PubMed] [Google Scholar]
- 19.Narver HL, Kong L, Burnett BG, et al. Sustained improvement of spinal muscular atrophy mice treated with trichostatin A plus nutrition. Ann Neurol. 2008;64:465–470. doi: 10.1002/ana.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butchbach MER, Rose FF, Jr, Rhoades S, et al. Effect of diet on the survival and phenotype of a mouse model for spinal muscular atrophy. Biochem Biophys Res Commun. 2010;391:835–840. doi: 10.1016/j.bbrc.2009.11.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregoretti C, Ottonello G, Chiarini Testa MB, et al. Survival of patients with spinal muscular atrophy type 1. Pediatrics. 2013;131:1509–1514. doi: 10.1542/peds.2012-2278. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno T, Komaki H, Sasaki M, et al. Efficacy and tolerance of gastrostomy feeding in Japanese muscular dystrophy patients. Brain & Development. 2012;34:756–762. doi: 10.1016/j.braindev.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Wang CH, Bonneman CG, Rutkowski A, et al. Consensus statement on standard of care for congenital muscular dystrophies. J Child Neurol. 2010;25:1559–1581. doi: 10.1177/0883073810381924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bushby K, Finkel R, Birnkrant, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9:177–189. doi: 10.1016/S1474-4422(09)70272-8. [DOI] [PubMed] [Google Scholar]
- 25.Barohn RJ, Levine EJ, Olson JO, Mendell JR. Gastric hypomotility in Duchenne’s muscular dystrophy. N Engl J Med. 1988;319:15–18. doi: 10.1056/NEJM198807073190103. [DOI] [PubMed] [Google Scholar]
- 26.Korman SH, Bar-Oz B, Granot E, Meyer S. Orocaecal transit time in Duchenne muscular dystrophy. Arch Dis Child. 1991;66:143–144. doi: 10.1136/adc.66.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottrand F, Guillonneau I, Carpentier A. Segmental colonic transit time in Duchenne muscular dystrophy. Arch Dis Child. 1991;66:1262. doi: 10.1136/adc.66.10.1262-b. [DOI] [PMC free article] [PubMed] [Google Scholar]