Highlights
-
•
Tamoxifen could increase the risk for venous thromboembolic complications.
-
•
Clinicians should concern the possibility of thrombotic event with tamoxifen.
-
•
In high-risk patients, new aromatase inhibitors might be considered alternative.
Abbreviations: CVT, cerebral venous thrombosis; DVT, deep vein thrombosis
Keywords: Breast cancer, Tamoxifen, Cerebral venous thrombosis
Abstract
Introduction
Tamoxifen reduces breast cancer risk, but can cause thromboembolic complications. Cerebral venous thrombosis is a rare form of stroke in which blood clots occlude the dural sinus or cerebral veins.
Presentation of case
A 46-year old female presented with a severe headache and nausea of subacute onset. She had undergone masectomy for breast cancer 20 months ago and had been taking tamoxifen. Brain CT and MRI confirmed cerebral venous infarction and venous thrombosis in the superior sagittal sinus and straight sinus. She had elevated D-dimer level, decreased levels of protein S activity and antithrombin III. Doppler ultrasound revealed concurrent deep vein thrombosis in her right leg. There was no evidence of breast cancer recurrence. With oral anticoagulation, she discharged without neurological complications. The abnormal laboratory findings of coagulopathy returned to normal after discontinuation of tamoxifen.
Discussion
Considering the abnormal findings in the workup of coagulopathy and no other risk factor for venous thrombosis, this thromboembolic complication can be attributed to the coagulopathy with use of tamoxifen.
Conclusion
Clinicians should warn about the possibility of thromboembolic complications with tamoxifen.
1. Introduction
Tamoxifen is a selective estrogen receptor modulator, which is used for both breast cancer treatment and prevention. However, tamoxifen could induce an acquired hypercoagulable state by reducing the levels of natural anticoagulant proteins.1,2 Despite the clinical benefit of tamoxifen therapy on breast cancer, there was an increased risk of thromboembolic complications including ischemic stroke.3–6 Cerebral venous thrombosis (CVT) is a rather uncommon form of stroke, in which thrombosis of the dural sinus or cerebral veins leads to infarction, hemorrhage, or both. It arises in close association with hypercoagulable states, puerperium, oral contraceptives, and malignancy. We present a case of a breast cancer patient who developed CVT and concurrent deep vein thrombosis (DVT) with the use of tamoxifen, probably through a hypercoagulable state.
2. Case report
A 46-year old female was admitted for severe headache and vomiting that started two weeks ago. The patient was alert, fully oriented, and did not have fever or demonstrated any signs of infection. She did not have a history of migraine and denied the possibility of pregnancy or use of oral contraceptive. Twenty months ago, this patient had been diagnosed with breast cancer for which she underwent breast conserving surgery with axillary lymph node dissection. As she was found to have estrogen-receptor positive invasive ductal carcinoma without metastasis, to prevent recurrence of breast cancer, she had been taking tamoxifen 200 mg daily. During the follow-up period, there was no evidence of breast cancer recurrence; the whole-body positron emission tomography scan taken two months ago did not show evidence of local or distant recurrence.
Upon admission, a non-contrast CT scan revealed a poorly demarcated space-occupying lesion with hypointense signal in the left basal ganglia and thalamus (Fig. 1A). Under the impression of brain metastasis or primary tumor with peritumoral edema, brain MRI scan was performed. T2-weighted brain MRI revealed a heterogeneous signal intensity involving the left basal ganglia, and thalamus (Fig. 1B). Sagittal T1-weighted imaging showed a thrombus occluding the superior sagittal sinus and straight sinus (Fig. 1C arrow), and gradient-echo imaging revealed hemorrhagic change of the lesion (Fig. 1D). Based on the MRI, she was diagnosed as CVT with edematous changes that occurred from thrombosis of cerebral sinus. Even though she did not complain of pain or weakness of leg, her right leg was disproportionately large by inspection and she reported slight tenderness upon palpation. Under the impression of concurrent DVT, she underwent Doppler ultrasound, which confirmed partial obstruction with internal thrombus at the confluent level of posterior tibial vein, just distal to trifurcation of right popliteal vein (Fig. 2, arrow).
Fig. 1.
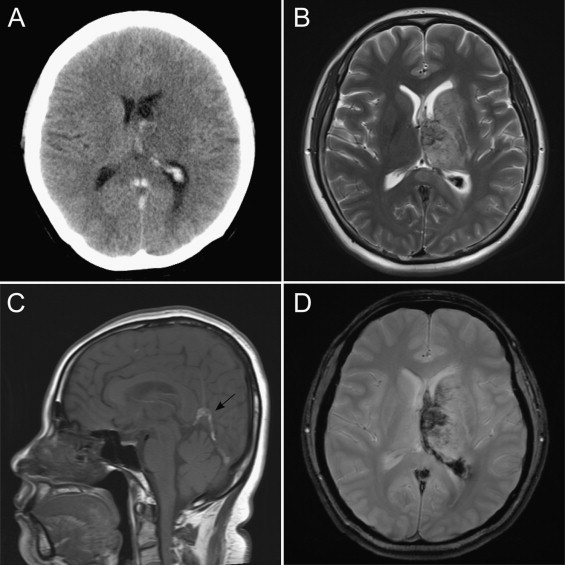
Cerebral venous infarction in left periventricular area. (A) Non-contrast brain CT. (B) T2-weighted MRI. (C) Sagittal T1-weighted MRI shows sinus thrombosis (arrow). (D) Gradient-echo MRI.
Fig. 2.
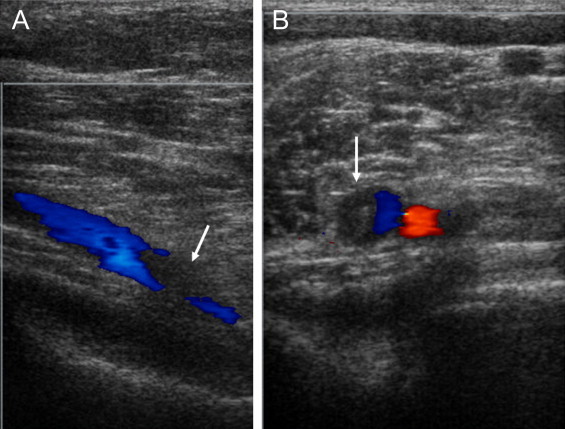
Doppler ultrasonography of right popliteal vein. There is partial obstruction of the posterior tibial vein (blue color) by the thrombus as indicated by the arrows. (A) Transverse image. (B) Axial image.
We performed tests for clotting abnormalities before anticoagulant therapy. Protein C activity was within the normal range while protein S activity was decreased to 47% (normal range of 55–123) and antithrombin III to 73% (normal range of 83–128). Lupus anticoagulant and anticardiolipin antibodies were not detected. Factor V Leiden gene mutation, which is associated with protein S deficiency, was negative. Homocysteine was within the normal range. Both D-dimer and fibrinogen were elevated to 2796.90 ng/mL (normal range of 0–500) and 477 mg/dL (normal range of 231–473), respectively.
After the diagnosis of CVT, she was treated with an oral anticoagulant. Because she was obese with the body mass index of 29.5 kg/m2, we encouraged her to exercise regularly and stressed the importance of reaching her ideal body weight. With the prothrombin time kept within the therapeutic range (2–3 by international normalized ratio), she was discharged home headache-free and without focal neurologic signs. For prevention of breast cancer, tamoxifen is substituted with anastrozole which is another inhibitor of aromatase and without thrombotic complication.5 After one month of oral anticoagulation, follow-up MRI revealed resolution of the space-occupying lesion and cerebral venous thrombus (Fig. 3). After six months from discontinuation of tamoxifen, the laboratory abnormalities of coagulation returned to normal (protein S, antithrombin III, D-dimer, and fibrinogen).
Fig. 3.
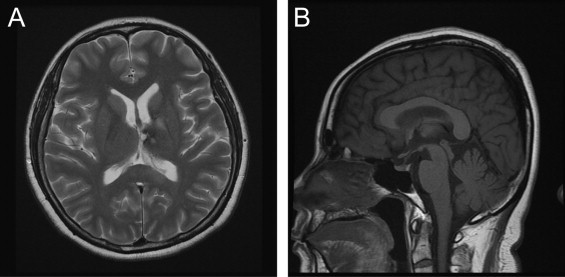
Follow-up brain MRI after one month. The lesion of left basal ganglia and sinus thrombus were disappeared. (A) Axial T2-weighted MRI. (B) Sagittal T1-weighted MRI.
3. Discussion
Tamoxifen, through its tissue-specific antagonist effects on estrogen receptors in mammary tissue, has been shown to decrease mortality and recurrence in all stages of breast cancer and to reduce the incidence of breast cancer in high-risk women.5,6 Despite such benefits rendered by the agent, there has been heightened concern that tamoxifen use increases the risk of thrombotic complications.3 One meta-analysis showed that those treated with tamoxifen had an 82% increased risk of ischemic stroke.7 In randomized trials, the association between the risk of thromboembolic events and the use of tamoxifen was also significant.4,8 The first two years after the initiation of tamoxifen therapy, which was also the case for our patient, was considered to be the most vulnerable time to developing venous thromboembolic complications9 After diagnosis of CVT, the case patient took anastrozole in place of tamoxifen. The anastrozole is a new aromatase inbibitor and known to have fewer thromboembolic complications.5
Even though the mechanisms by which tamoxifen increases the risk of thrombosis have yet to be elucidated, alterations in the concentrations of natural anticoagulants have been implicated.1 Like in this case, a significant decrease in both protein S activity and antithrombin III level were reported in tamoxifen users.2
This patient had a history of breast cancer, and cancer itself is another predisposing condition for thrombotic complications. However, there was no evidence of cancer recurrence during the follow-up period. Therefore, we concluded that tamoxifen-related hypercoagulability was the main cause of venous thrombosis rather than the breast cancer itself. The concurrent DVT and normalization of coagulopathy after discontinuation of tamoxifen likewise attests to the presence of underlying hypercoagulable condition with tamoxifen.
4. Conclusion
This case illustrates concurrent CVT and DVT suspected to have arisen from the use of tamoxifen. Clinicians should warn about the possibility of thromboembolic complications with tamoxifen. Especially for those with an elevated risk of thromboembolism, a physician may need to consider new aromatase inhibitors without thromboembolic risk.
Conflict of interest
No conflict of interest.
Funding
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2014R1A1A1002067).
Ethical approval
Written informed consent was taken from the patient.
Author’s contributions
K.Y. collected the data and wrote the first draft. OJ.K. and J.K. revised the manuscript critically for intellectual content and finalized the manuscript. All authors approved the final version of the manuscript.
Contributor Information
Yoon Kim, Email: yoon924@chamc.co.kr.
Ok Joon Kim, Email: okjun77@cha.ac.kr.
Jinkwon Kim, Email: antithrombus@gmail.com.
References
- 1.Anderson J.A., Weitz J.I. Hypercoagulable states. Clin Chest Med. 2010;31:659–673. doi: 10.1016/j.ccm.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Jones A.L., Powles T.J., Treleaven J.G., Burman J.F., Nicolson M.C., Chung H.I. Haemostatic changes and thromboembolic risk during tamoxifen therapy in normal women. Br J Cancer. 1992;66:744–747. doi: 10.1038/bjc.1992.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braithwaite R.S., Chlebowski R.T., Lau J., George S., Hess R., Col N.F. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003;18:937–947. doi: 10.1046/j.1525-1497.2003.20724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pritchard K.I., Paterson A.H., Paul N.A., Zee B., Fine S., Pater J. Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer. National Cancer Institute of Canada Clinical Trials Group Breast Cancer Site Group. J Clin Oncol. 1996;14:2731–2737. doi: 10.1200/JCO.1996.14.10.2731. [DOI] [PubMed] [Google Scholar]
- 5.Gibson L.J., Dawson C., Lawrence D.H., Bliss J.M. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst Rev. 2009;7:CD003370. doi: 10.1002/14651858.CD003370.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher B., Costantino J., Redmond C., Poisson R., Bowman D., Couture J. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 7.Bushnell C.D., Goldstein L.B. Risk of ischemic stroke with tamoxifen treatment for breast cancer: a meta-analysis. Neurology. 2004;63:1230–1233. doi: 10.1212/01.wnl.0000140491.54664.50. [DOI] [PubMed] [Google Scholar]
- 8.Decensi A., Maisonneuve P., Rotmensz N., Bettega D., Costa A., Sacchini V. Effect of tamoxifen on venous thromboembolic events in a breast cancer prevention trial. Circulation. 2005;111:650–656. doi: 10.1161/01.CIR.0000154545.84124.AC. [DOI] [PubMed] [Google Scholar]
- 9.Onitilo A.A., Doi S.A., Engel J.M., Glurich I., Johnson J., Berg R. Clustering of venous thrombosis events at the start of tamoxifen therapy in breast cancer: a population-based experience. Thromb Res. 2012;130:27–31. doi: 10.1016/j.thromres.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


