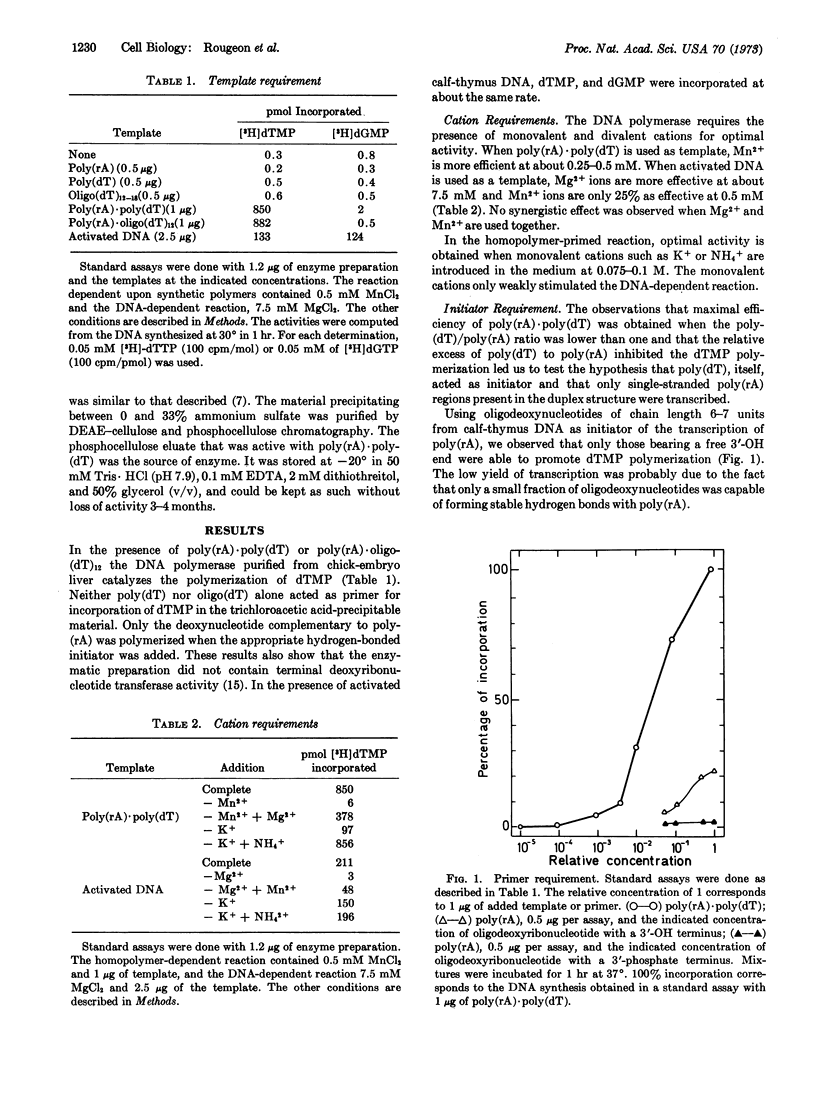
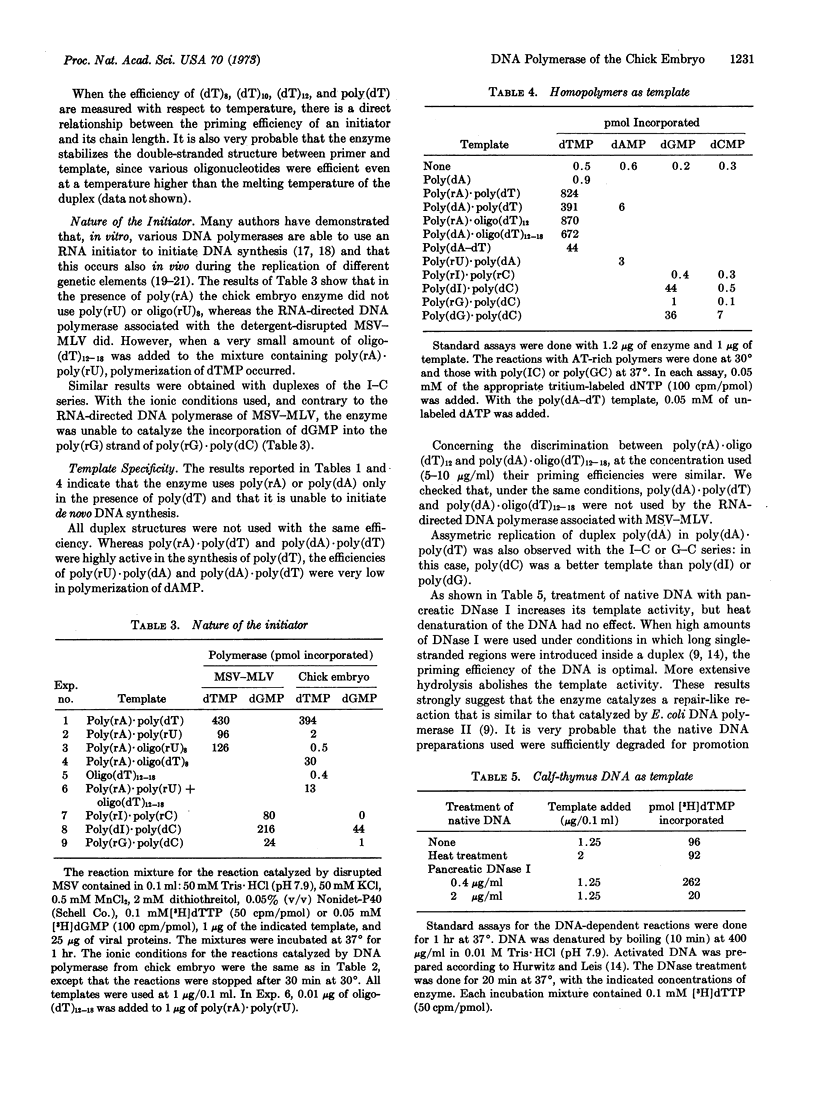
Abstract
A DNA polymerase purified from extracts of chick embryo catalyzes the synthesis of poly(dT) in the presence of poly(rA) or poly(dA) as template and poly(dT) or oligo(dT) as primer. Oligo- or polyribonucleotides are relatively ineffective initiators of polydeoxynucleotide polymerization. Using activated DNA as template, the enzyme catalyzes a repair-like reaction that resembles that catalyzed by RNA-directed DNA polymerase of RNA tumor viruses and Escherichia coli DNA polymerase II. With natural templates containing poly(rA) sequences, such as avian myeloblastosis virus RNA or rabbit globin mRNA, and oligo(dT) as initiator, principally poly(dT) is synthesized. Consequently, the enzyme differs from the RNA-directed DNA polymerase associated with RNA tumor viruses by its incapacity to transcribe heteropolymeric regions of RNA templates. The possible role of the enzyme in repair of DNA during transcription is discussed.
Keywords: homopolynucleotides, oligonucleotides, globin mRNA, 60-70S avian myeloblastosis virus RNA
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Smoler D. Primer requirement and template specificity of the DNA polymerase of RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1507–1511. doi: 10.1073/pnas.68.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr H., Lingrel J. B. Poly A sequences at the 3' termini of rabbit globin mRNAs. Nat New Biol. 1971 Sep 8;233(36):41–43. doi: 10.1038/newbio233041a0. [DOI] [PubMed] [Google Scholar]
- Cavalieri L. G., Carroll E. RNA-dependent DNA polymerase from E. coli: an effect produced by traces of added DNA. Nature. 1971 Jul 23;232(5308):254–255. doi: 10.1038/232254a0. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. A chemical model for transcriptional initiation of DNA replication. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1354–1360. doi: 10.1016/s0006-291x(72)80124-4. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Low molecular weight deoxyribonucleic acid polymerase from rabbit bone marrow. Biochemistry. 1972 Mar 28;11(7):1264–1272. doi: 10.1021/bi00757a023. [DOI] [PubMed] [Google Scholar]
- Fridlender B., Fry M., Bolden A., Weissbach A. A new synthetic RNA-dependent DNA polymerase from human tissue culture cells (HeLa-fibroblast-synthetic oligonucleotides-template-purified enzymes). Proc Natl Acad Sci U S A. 1972 Feb;69(2):452–455. doi: 10.1073/pnas.69.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Cartas M. The genome of RNA tumor viruses contains polyadenylic acid sequences. Proc Natl Acad Sci U S A. 1972 Apr;69(4):791–794. doi: 10.1073/pnas.69.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz J., Leis J. P. RNA-dependent DNA polymerase activity of RNA tumor viruses. I. Directing influence of DNA in the reaction. J Virol. 1972 Jan;9(1):116–129. doi: 10.1128/jvi.9.1.116-129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INMAN R. B., BALDWIN R. L. HELIX--RANDOM COIL TRANSITIONS IN DNA HOMOPOLYMER PAIRS. J Mol Biol. 1964 Apr;8:452–469. doi: 10.1016/s0022-2836(64)80003-6. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Endogenous RNA-directed DNA polymerase activity in uninfected chicken embryos. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1550–1554. doi: 10.1073/pnas.69.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K. I., Gonçalves J. M., Houts G. E., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. II. Properties of the terminal deoxynucleotidyltransferase. J Biol Chem. 1967 Jun 10;242(11):2780–2789. [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Hurwitz J. Isolation and characterization of a protein that stimulates DNA synthesis from avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2331–2335. doi: 10.1073/pnas.69.8.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Hurwitz J. RNA-dependent DNA polymerase activity of RNA tumor viruses. II. Directing influence of RNA in the reaction. J Virol. 1972 Jan;9(1):130–142. doi: 10.1128/jvi.9.1.130-142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Canellakis E. S. Adenine-rich polymer associated with rabbit reticulocyte messenger RNA. Nature. 1970 Aug 15;227(5259):710–712. doi: 10.1038/227710a0. [DOI] [PubMed] [Google Scholar]
- Maïa J. C.C., Rougeon F., Chapeville F. Chick embryo poly (rA:dT)-dependent DNA polymerase. FEBS Lett. 1971 Oct 15;18(1):130–134. doi: 10.1016/0014-5793(71)80427-1. [DOI] [PubMed] [Google Scholar]
- Riley M., Maling B. Physical and chemical characterization of two- and three-stranded adenine-thymine and adenine-uracil homopolymer complexes. J Mol Biol. 1966 Sep;20(2):359–389. doi: 10.1016/0022-2836(66)90069-6. [DOI] [PubMed] [Google Scholar]
- Ross J., Aviv H., Scolnick E., Leder P. In vitro synthesis of DNA complementary to purified rabbit globin mRNA (RNA-dependent DNA polymerase-reticulocyte-hemoglobin-density gradient centrifugation-oligo(dT) primer). Proc Natl Acad Sci U S A. 1972 Jan;69(1):264–268. doi: 10.1073/pnas.69.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Scolnick E. M., Todaro G. J., Aaronson S. A. Separation of murine cellular and murine leukaemia virus DNA polymerases. Nat New Biol. 1971 Jun 9;231(23):163–167. doi: 10.1038/newbio231163a0. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Aaronson S. A., Todaro G. J., Parks W. P. RNA dependent DNA polymerase activity in mammalian cells. Nature. 1971 Jan 29;229(5283):318–321. doi: 10.1038/229318a0. [DOI] [PubMed] [Google Scholar]
- Smith R. G., Gallo R. C. DNA-dependent DNA polymerases I and II from normal human-blood lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2879–2884. doi: 10.1073/pnas.69.10.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Trávnícek M., Watson K. Synthetic DNA-RNA hybrids and RNA-RNA duplexes as templates for the polymerases of the oncogenic RNA viruses. Nature. 1970 Oct 31;228(5270):430–432. doi: 10.1038/228430a0. [DOI] [PubMed] [Google Scholar]
- Stavrianopoulos J. G., Karkas J. D., Chargaff E. DNA polymerase of chicken embryo: purification and properties. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1781–1785. doi: 10.1073/pnas.69.7.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrianopoulos J. G., Karkas J. D., Chargaff E. Mechanism of DNA replication by highly purified DNA polymerase of chicken embryo. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2609–2613. doi: 10.1073/pnas.69.9.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrianopoulos J. G., Karkas J. D., Chargaff E. Nucleic acid polymerases of the developing chicken embryo: a DNA polymerase preferring a hybrid template. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2207–2211. doi: 10.1073/pnas.68.9.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Hirose S., Okazaki R. RNA-linked nascent DNA fragments in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1863–1867. doi: 10.1073/pnas.69.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Bromfeld E., Manly K. F., Baltimore D. Covalently linked RNA-DNA molecule as initial product of RNA tumour virus DNA polymerase. Nat New Biol. 1971 Sep 29;233(39):131–134. doi: 10.1038/newbio233131a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Ginsberg B., Hurwitz J. Deoxyribonucleic acid polymerase II of Escherichia coli. II. Studies of the requirements and the structure of the deoxyribonucleic acid product. J Biol Chem. 1972 Jan 25;247(2):498–504. [PubMed] [Google Scholar]



