Abstract
In the developing heart, cardiomyocytes undergo terminal differentiation during a critical window around birth. Hypoxia is a major stress to preterm infants, yet its effect on the development and maturation of the heart remains unknown. We tested the hypothesis in a rat model that newborn anoxia accelerates cardiomyocyte terminal differentiation and results in reduced cardiomyocyte endowment in the developing heart via an endothelin-1-dependent mechanism. Newborn rats were exposed to anoxia twice daily from postnatal day 1 to 3, and hearts were isolated and studied at postnatal day 4 (P4), 7 (P7), and 14 (P14). Anoxia significantly increased HIF-1α protein expression and pre-proET-1 mRNA abundance in P4 neonatal hearts. Cardiomyocyte proliferation was significantly decreased by anoxia in P4 and P7, resulting in a significant reduction of cardiomyocyte number per heart weight in the P14 neonates. Furthermore, the expression of cyclin D2 was significantly decreased due to anoxia, while p27 expression was increased. Anoxia has no significant effect on cardiomyocyte binucleation or myocyte size. Consistently, prenatal hypoxia significantly decreased cardiomyocyte proliferation but had no effect on binucleation in the fetal heart. Newborn administration of PD156707, an ETA-receptor antagonist, significantly increased cardiomyocyte proliferation at P4 and cell size at P7, resulting in an increase in the heart to body weight ratio in P7 neonates. In addition, PD156707 abrogated the anoxia-mediated effects. The results suggest that hypoxia and anoxia via activation of endothelin-1 at the critical window of heart development inhibits cardiomyocyte proliferation and decreases myocyte endowment in the developing heart, which may negatively impact cardiac function later in life.
Introduction
The intrauterine environment plays a well-established role in predisposition to cardiovascular disease later in life [1]. Environmental factors during the critical period of heart development may alter the maturation of the heart and thus potentially its life-long function. Cardiomyocytes are the functional contractile units of the heart that undergo a normal maturation process in which terminal differentiation is the final outcome. As the cardiomyocytes terminally differentiate and exit the cell cycle, they lose their proliferative capacity [2]. Cardiomyocyte growth then transitions from hyperplastic to hypertrophic, in which the cells can only increase in size rather than number [3, 4]. Ultimately the proliferative capacity of cardiomyocytes is lost and the adult heart is known to exhibit negligible increases in cell number [5]. Therefore the timing of this transition is pivotal in determining cardiomyocyte endowment in the heart for the rest of the animal’s life.
Hypoxia is a major stress to preterm infants, yet its effect on the development and maturation of the heart remains unknown. Given that the transition of cardiomyocyte terminal differentiation occurs in rodents during the first two weeks of neonatal life [3, 6], which is an equivalent timeframe to the late fetal stage in third trimester of human gestation [2], they provide a reasonable animal model to study the effect of anoxia on preterm infants at the critical window of the heart development. This process of terminal differentiation begins in the rat heart around postnatal day 4 [3] and progresses until day 14 when the heart is essentially mature, thus three time-points within this period were evaluated in this study. Previous studies in rats have shown that maternal hypoxia (10.5% O2) leads to a premature exit from the cell cycle in fetal cardiomyocytes [7–9]. Additionally, neonatal cardiomyocytes have been shown to decrease proliferation when exposed to hypoxic conditions [10]. Studies have also been performed in sheep in which placental restriction is induced, resulting in reduced cardiomyocyte maturation [11] and proliferation [12], increased proportion of mononucleate cardiomyocytes [13], and decreased cardiomyocyte endowment [14]. However, the in vivo effects of anoxia, as a preterm model, on cardiomyocyte proliferation and endowment in the developing rat heart are, as of yet, not known. Additionally, the downstream regulators of cardiomyocyte proliferation and maturation are unknown.
Endothelin-1 (ET-1) expression is induced by hypoxia [15–18]. Studies performed in endothelial cells [19, 20] and cardiomyocytes [21] have identified a HIF-1α binding site in the prepro-ET-1 gene. Furthermore, the cardiomyocyte is both a site of synthesis and action for ET-1 [22, 23], as it acts mainly at the paracrine or autocrine level [24, 25]. Our recent work showed that ex vivo ET-1 treatment promoted terminal differentiation of fetal cardiomyocytes, via an increase in DNA methylation [26]. The predominant ET-1 receptor subtype in cardiomyocytes is the ETA-receptor [23], which is thought to be involved in regulating proliferation [24, 27, 28]. Currently, little is known about the role that basal ET-1 plays in the terminal differentiation of cardiomyocytes, as well as the effect of hypoxia/anoxia-induced ET-1 production on this process.
Therefore, in the present study we tested the hypothesis that in vivo neonatal anoxia decreases proliferation of cardiomyocytes via the ETA-receptor-dependent mechanisms, resulting in reduced cardiomyocyte endowment in the developing heart. Herein, we provide evidence that the ETA-receptor mediates the anoxia-induced decrease in cardiomyocyte proliferation. Furthermore, cardiomyocyte endowment in the developing heart was decreased by anoxia and restored with PD156707, a selective ETA-receptor antagonist.
Methods
Experimental animals
Time-dated pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Portage, MI) and allowed to give birth. Neonatal pups from 7 litters were used and divided into the treatment groups. Data from pups of multiple litters were pooled. Starting at postnatal day 1, newborn rats were placed in a temperature-controlled (37°C) anoxia chamber. Nitrogen was infused into the chamber for 10 minutes and an oxygen sensor was used to verify the level of oxygen in the chamber being < 0.2%. Control animals were placed in a chamber with oxygen maintained at 21%. Anoxia treatments were performed twice a day with 8 hours in between, from postnatal day 1 until postnatal day 3. A group of animals was treated with intraperitoneal injections of an ETA-receptor antagonist, PD156707 (2 mg/kg), prior to each episode of anoxia, twice a day for the first 3 postnatal days. Neonatal pups were anesthetized with isoflurane and hearts isolated for studies on postnatal day 4, 7, and 14. To investigate the comparative effect of prenatal hypoxia, some of the time-dated pregnant Sprague-Dawley rats were treated with either normoxic control or 10.5% O2 from gestational day 15 to 21, as previously described [29, 30]. Following the hypoxia treatment, pregnant rats were allowed to give birth. Hearts were isolated from postnatal day 4 and 7 neonatal rats. All procedures and protocols were approved by the Loma Linda University Institutional Animal Care and Use Committee (IACUC) and all procedure adhered to the guidelines by US National Institutes of Health Guide for the Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/Guide-for-the-care-and-use-of-laboratory-animals.pdf).
Measurement of cardiomyocyte number
Hearts from day 4, 7, and 14 neonatal pups were isolated and the atria excised. The hearts were then completely enzymatically digested to yield primary cardiomyocytes, as previously described [26, 31]. A pre-plate step was performed to enrich the cardiomyocyte population. This is a commonly used method [32] that is based on the differential attachment of cardiomyocytes and non-myocyte cells of the heart. Cardiomyocytes take approximately 24 hours to fully attach to the plate while non-myocytes attach within a couple hours. After a 2-hour pre-plate step to remove attached non-myocytes, cardiomyocytes in the media were collected and used for counting cardiomyocyte number via a hemacytometer. Briefly, an aliquot of cardiomyocytes was counted using a hemacytometer and the counts were multiplied by the total volume of cell suspension and normalized according to the heart weight, to yield the number of cardiomyocytes per heart weight.
Immunocytochemistry
To perform immunocytochemical staining, cardiomyocytes isolated from day 4 and 7 hearts were allowed to attach to plates in Hyclone Medium 199 (Thermo Scientific) supplemented with 10% fetal bovine serum (Gemini Bio-Products) and 1% antibiotics (10,000 I.U./mL penicillin, 10,000 μg/mL streptomycin) at 37°C in 95% air/5% CO2. After 24 hours, cardiomyocytes were fully attached and were double stained with alpha-actinin, a cardiomyocyte marker, and Ki-67, a proliferation marker as described previously [8, 26]. Cardiomyocytes were plated on coverslips and fixed with 4% paraformaldehyde (ThermoScientific) for 15 minutes followed by permeabilization with Triton X-100 (Fisher) for 10 minutes. The cells were blocked with 1% bovine serum albumin for 1 hour at room temperature before incubation with the primary antibodies: mouse anti-α-sarcomeric actinin (A7811, Sigma) (1:200) and rabbit anti-Ki-67 (ab16667, Abcam) (1:100) at room temperature for 1 hour. The samples were incubated with the secondary antibodies: anti-mouse Alexa Fluor 488 (A21202, Life Technologies) and anti-rabbit Alexa Fluor 647 antibodies (A21244, Life Technologies) for 1 hour at room temperature. Nuclei were stained with Hoescht (Sigma) for less than 1 minute. The immunofluorescence staining was assessed using a Zeiss Axio Imager.A1 microscope and quantitative analysis was carried out using ImageJ software (http://imagej.nih.gov/ij/). Ki-67 expression, binucleation, and cell size were measured.
Flow cytometry
Primary cardiomyocytes isolated from day 14 neonatal rats were stained for analysis by flow cytometry. Cells were washed in staining buffer (PBS + 5% FBS), spun down, and re-suspended in 4% paraformaldehyde for 20 minutes at room temperature in the dark. The fixed cells were then washed in permeabilization wash buffer (eBioscience) and supernatant discarded. Cells were stained with antibodies for the cardiomyocyte marker, Troponin T (ab10214, Abcam) (1:200), and proliferation marker, Ki-67-conjugated to allophycocyanin (APC) (eBioscience) (50–5698, 1:200). After incubation and washing, cells were incubated with the secondary antibody for Troponin T, fluorescein isothiocyanate (FITC) (555988, BD Pharmingen) (1:100). Finally cells were washed and resuspended in 1% paraformaldehyde to be run on a FACSAria (BD Biosciences) and analyzed via FACSDiva software (BD Biosciences) for percentage of Ki-67 expressing cardiomyocytes.
Quantitative real-time PCR
RNA was isolated from the postnatal day 4 (P4) hearts and prepro-ET-1 mRNA abundance was determined by real-time RT-PCR using Icycler Thermal cycler (Bio-Rad), as described previously [30]. Reverse transcription and cDNA synthesis was performed using SuperScript III First-Strand Synthesis Supermix for RT-PCR (Life Technologies). The primers are 5’-CTAGGTCTAAGCGATCCTTGAA-3’ (forward) and 5’-CTTGATGCTGTTGCTGATGG-3’ (reverse). PCR was performed in triplicate, and threshold cycle numbers were averaged.
Western immunoblotting
HIF-1α, ETA-receptor (ETAR), and ETB-receptor (ETBR) protein abundance in the P4 heart was measured from control and anoxia groups. The protein abundance of cyclin D2 and p27 was measured in P4 hearts from control and anoxia groups as well as in the presence and absence of PD156707. Tissues were homogenized and protein isolated using the RIPA lysis buffer system (Santa Cruz Biotechnology). Protein concentrations were quantified using the BCA protein assay (ThermoScientific) and all samples were loaded with equal protein onto 7.5% (HIF-1α) or 10% (ETAR, and ETBR, cyclin D2, and p27) polyacrylamide gel with 0.1% sodium dodecyl sulfate (SDS). Proteins were then separated by electrophoresis and transferred onto nitrocellulose membranes. Non-specific binding sites were blocked with Tris-buffered saline solution (TBS) containing 5% dry milk. The membranes were incubated with primary antibodies against HIF-1α (sc10790, Santa Cruz Biotechnology; 1:500), ETAR (sc33536, Santa Cruz Biotechnology; 1:500), ETBR (sc33538, Santa Cruz Biotechnology; 1:500), cyclin D2 (ab3085, Abcam; 1:1000), and p27 (ab7961, Abcam; 1:1000). After washing, membranes were incubated with secondary antibodies. Proteins were visualized with enhanced chemiluminescence reagents and western blots were exposed to Hyper film. Kodak image software was used to quantify all results.
Statistical analysis
Data are expressed as means ± SEM. Statistical analysis (p < 0.05) was determined by two-way analysis of variance (ANOVA) followed by Neuman-Keuls post hoc test or Student’s t test, where appropriate, using GraphPad Prism software. The two-way ANOVA was performed to evaluate the effects of two factors, within each age group: (1) control versus anoxia, and (2) in the presence and absence of PD156707.
Results
Newborn anoxia treatment increased pre-proET-1 mRNA in the heart of P4 neonate
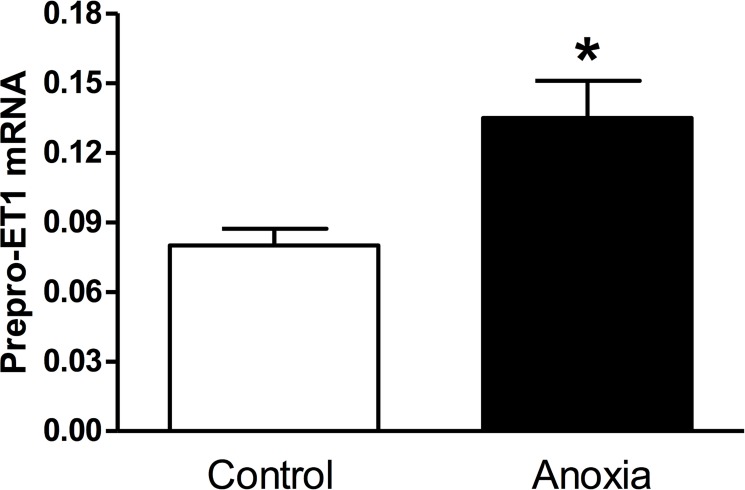
Neonatal rats were exposed to anoxia twice a day from postnatal day 1 to 3, and hearts were isolated at P4. As seen in Fig. 1, there was a significant increase in prepro-ET-1 mRNA abundance in neonatal hearts exposed to anoxia (< 0.2% O2), as compared to the normoxic control (21% O2).
Fig 1. Effect of newborn anoxia on prepro-ET-1 mRNA in the neonatal heart.
Hearts were isolated from day 4 neonatal rats treated with control or anoxia. mRNA abundance of prepro-ET-1 was determined by real-time RT-PCR. Data are means ± SEM. * P < 0.05, anoxia vs. control. n = 3–5.
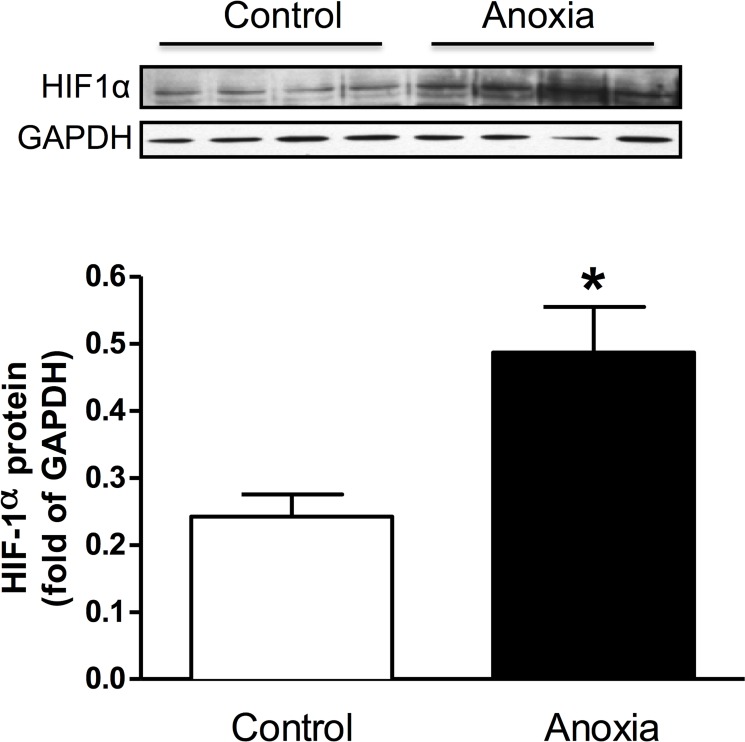
Newborn anoxia treatment increased HIF-1α protein abundance in the heart of the P4 neonate
Hearts from P4 rats treated with anoxia were collected and protein isolated. Neonatal hearts exposed to anoxia had significantly increased levels of the HIF-1α protein (Fig. 2).
Fig 2. Effect of newborn anoxia on HIF-1α protein abundance in the neonatal heart.
Hearts were isolated from day 4 neonatal rats treated with control or anoxia. Protein abundance of HIF-1α was determined by Western immunoblotting. Data are means ± SEM. * P < 0.05, anoxia vs. control. n = 4.
Newborn anoxia treatment decreased cardiomyocyte proliferation
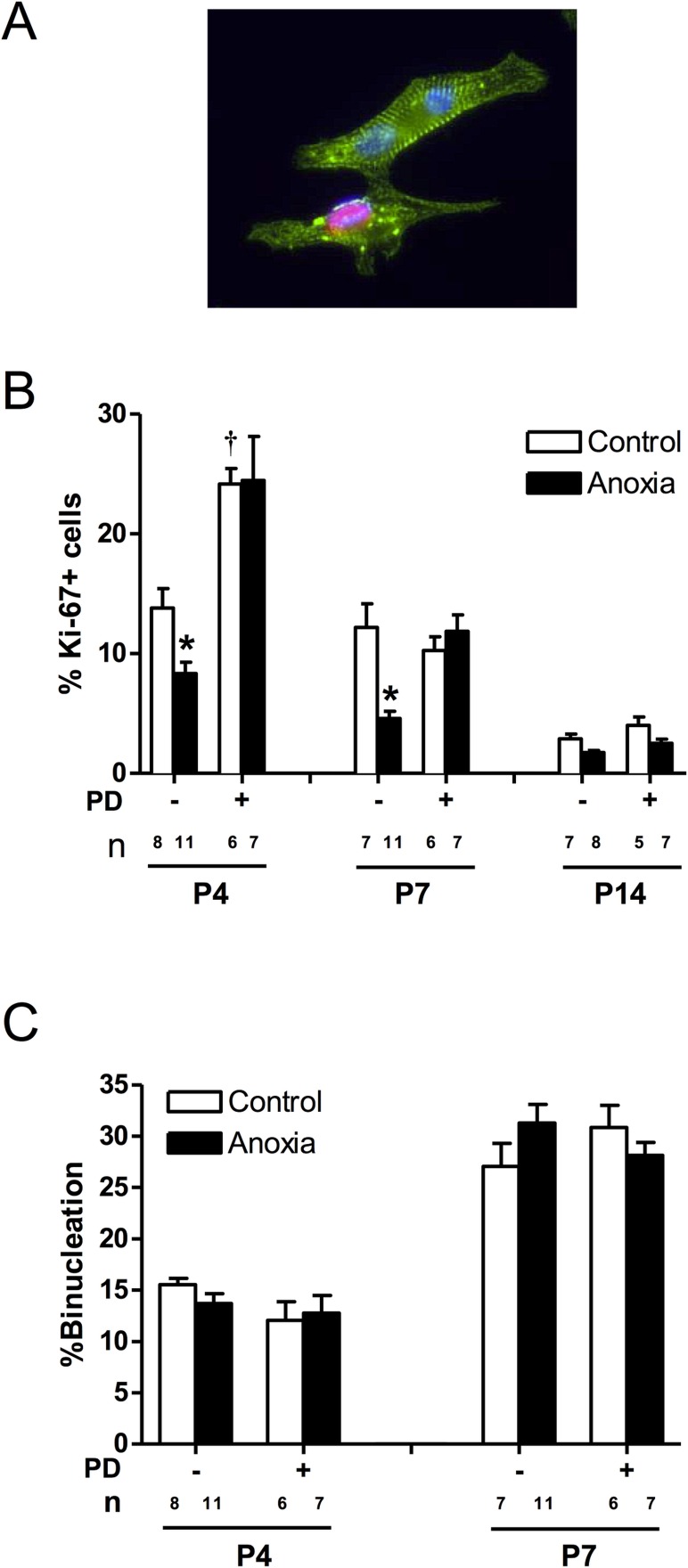
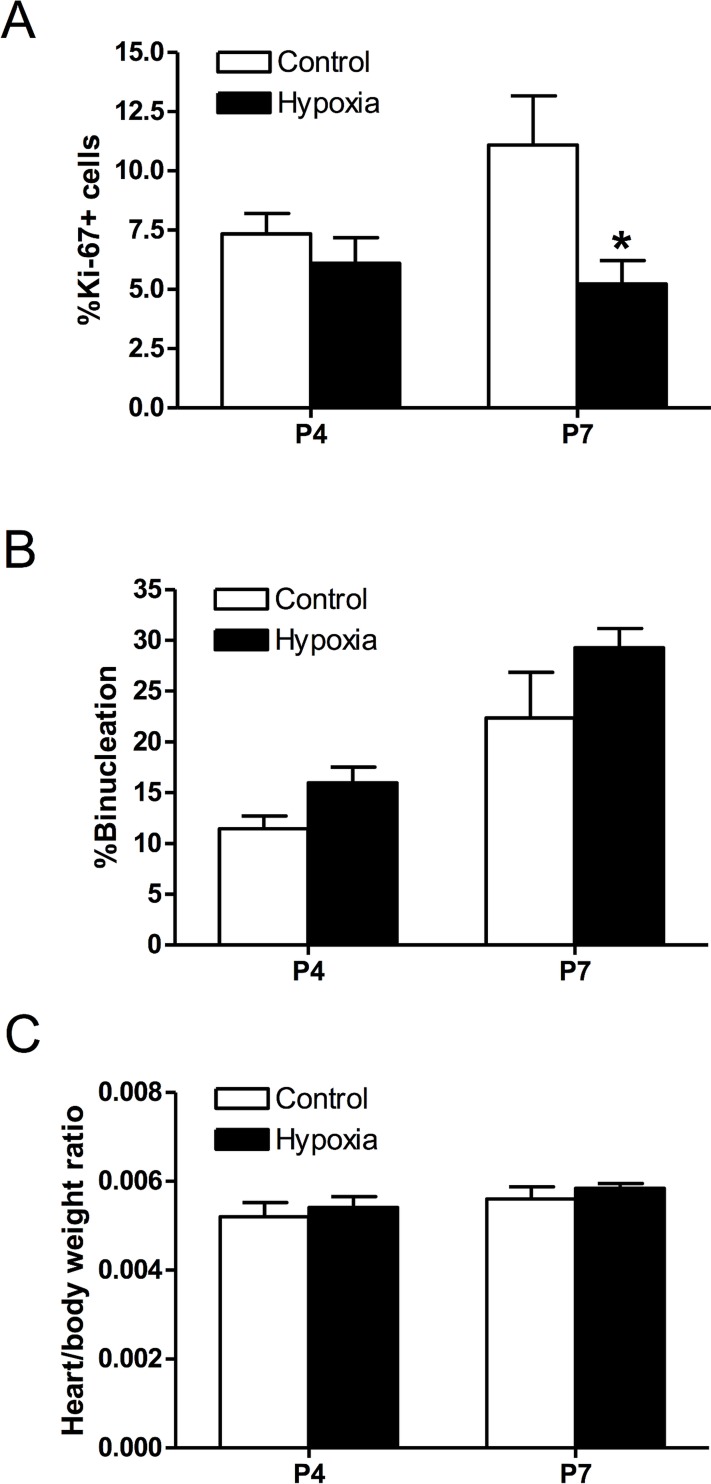
As shown in Fig. 3, there is a development-dependent decrease in cardiomyocyte proliferation at the critical window of the heart development during the first two weeks of life in rodents, and myocyte proliferation reduces to minimal levels at postnatal day 14. Anoxia treatment of newborns caused a significant decrease in the proliferation of neonatal cardiomyocytes at both postnatal days 4 and 7 (Fig. 3B). Treatment of newborn rats with a selective ETA-receptor antagonist, PD156707, caused a significant increase in cardiomyocyte proliferation in P4 neonatal rats (Fig. 3B). In addition, PD156707 abrogated the anoxia-induced effects in the developing hearts (Fig. 3B). In contrast to proliferation, there is a development-dependent increase in cardiomyocyte binucleation in the developing heart (Fig. 3C). Neither anoxia nor PD156707 treatments had significant effects on cardiomyocyte binucleation (Fig. 3C).
Fig 3. Effect of newborn anoxia and PD156707 on proliferation and binucleation of neonatal cardiomyocytes.
Cardiomyocytes were isolated from P4, P7, and P14 neonatal rats that were treated with control or anoxia, in the absence or presence of PD156707. Cells from P4 and P7 rats were stained with α-actinin and Ki-67, and nuclei were stained using Hoechst staining. P14 cardiomyocytes were stained with Ki-67 and analyzed via FACS. Panel A shows a representative image of cardiomyocytes stained with α-actinin (green), Ki-67 (red), and Hoescht (blue). Panel B shows percent of Ki-67 expressing cells. Panel C shows percent of binucleate cells. Data are means ± SEM. * P < 0.05, anoxia vs. control. † P < 0.05, -PD156707 vs. +PD156707. PD: PD156707; n: animal numbers.
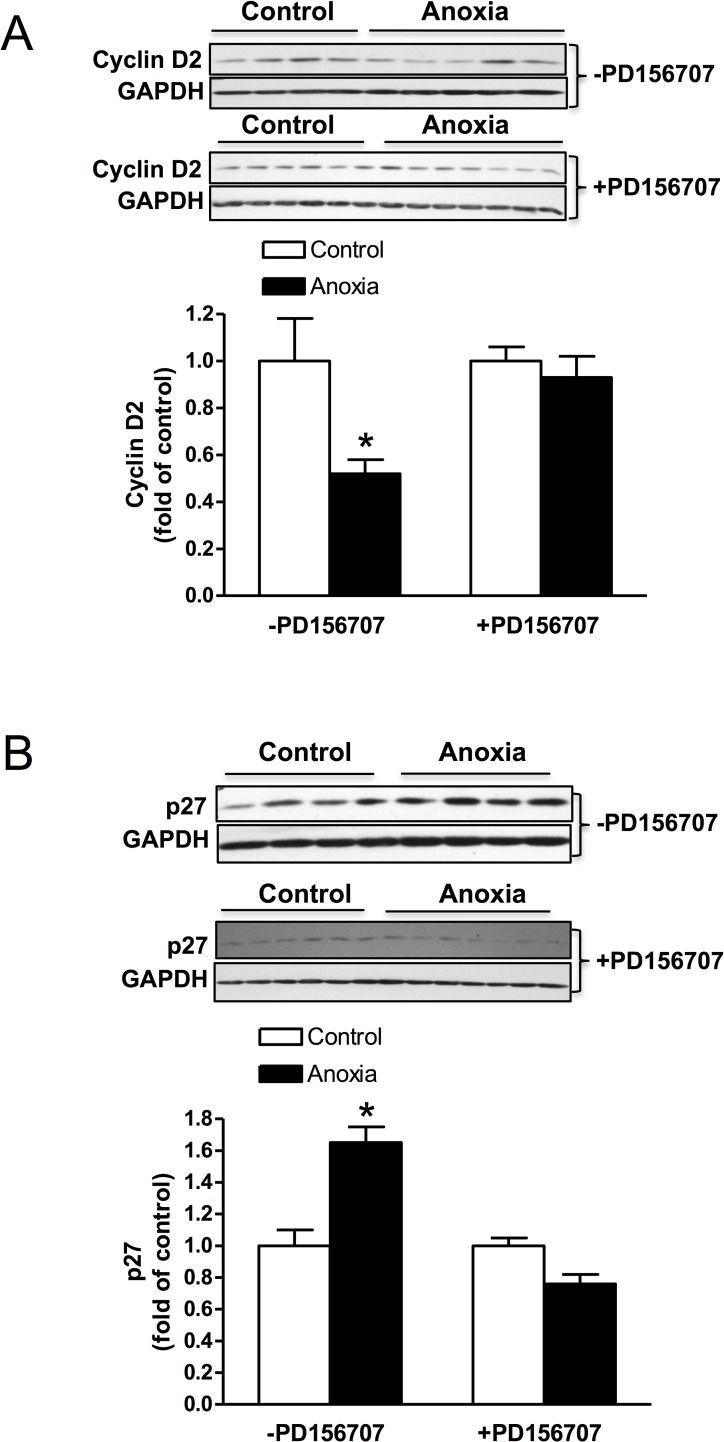
Newborn anoxia treatment decreased cyclin D2 and increased p27 expression in the heart of the P4 neonate
The protein expression of cyclin D2 was decreased due to anoxia treatment and this effect was abolished in the presence of PD156707 (Fig. 4A). On the contrary, p27 expression in the neonatal heart was significantly increased due to anoxia treatment, and PD156707 blocked the effect of anoxia (Fig. 4B).
Fig 4. Effect of newborn anoxia on cyclin D2 and p27 protein expression in the cardiomyocyte.
Hearts were isolated from day 4 neonatal rats treated with control or anoxia in the presence (n = 6–7) or absence (n = 4) of PD156707. Protein abundance of cyclin D2 in the absence and presence of PD156707 (A), and p27 in the absence and presence of PD156707 (B) was determined by Western immunoblotting. Data are means ± SEM. * P < 0.05, anoxia vs. control.
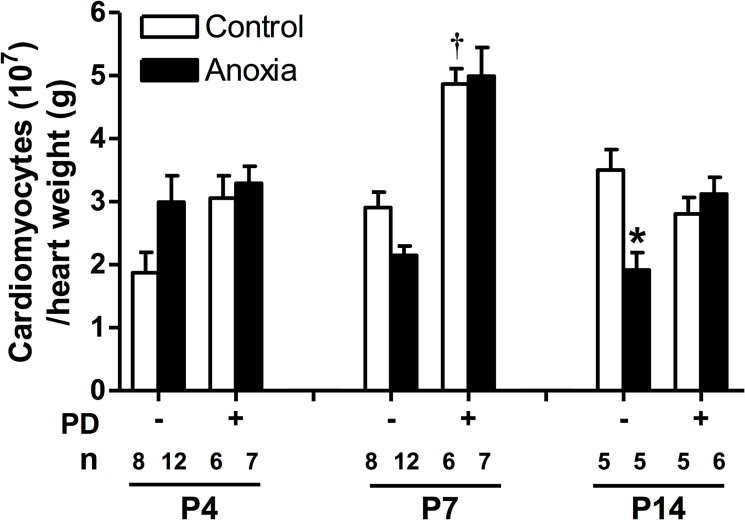
Newborn anoxia treatment decreased cardiomyocyte number by day 14
There was no significant change in cardiomyocyte number due to newborn anoxia treatment at day 4 and 7. However, results for day 14 show that anoxia leads to a significant decrease in cardiomyocyte number per heart weight (Fig. 5). PD156707 alone caused a significant increase in cardiomyocyte number in the day 7 neonate (Fig. 5). In the presence of PD156707, the anoxia-mediated effects at day 14 were blocked (Fig. 5).
Fig 5. Effect of newborn anoxia and PD156707 on number of cardiomyocytes per heart weight.
Cardiomyocytes were isolated from day 4, 7, and 14 neonatal rats that were treated with control or anoxia, in the absence or presence of PD156707. Hearts were weighed and cardiomyocytes counted by hemacytometer. Data are expressed as cardiomyocyte number/g heart weight, and are means ± SEM. * P < 0.05, anoxia vs. control. † P < 0.05, -PD156707 vs. +PD156707. PD: PD156707; n: animal numbers.
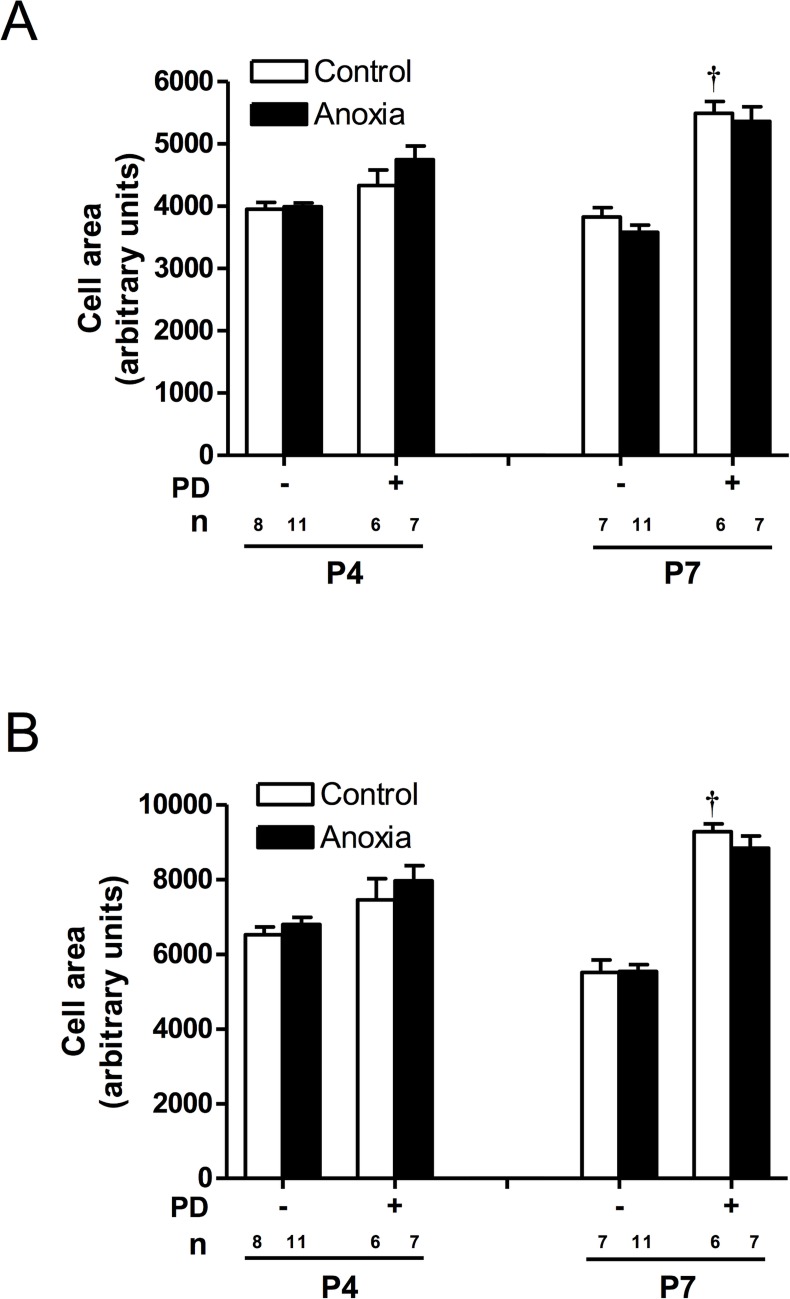
Cell size was increased in the presence of PD156707
Anoxia had no effect on mononucleate or binucleate cell size at either day 4 or 7 (Fig. 6). However, PD156707 treatment was able to increase both mononucleate and binucleate cell size at postnatal day 7 (Fig. 6).
Fig 6. Effect of newborn anoxia and PD156707 on cardiomyocyte size in the neonatal heart.
Cardiomyocytes were isolated from day 4, 7, and 14 neonatal rats that were treated with control or anoxia, in the absence or presence of PD156707. Mononucleate (A) and binucleate (B) cell size was measured using ImageJ. Data are means ± SEM. † P < 0.05, -PD156707 vs. +PD156707. PD: PD156707; n: animal numbers.
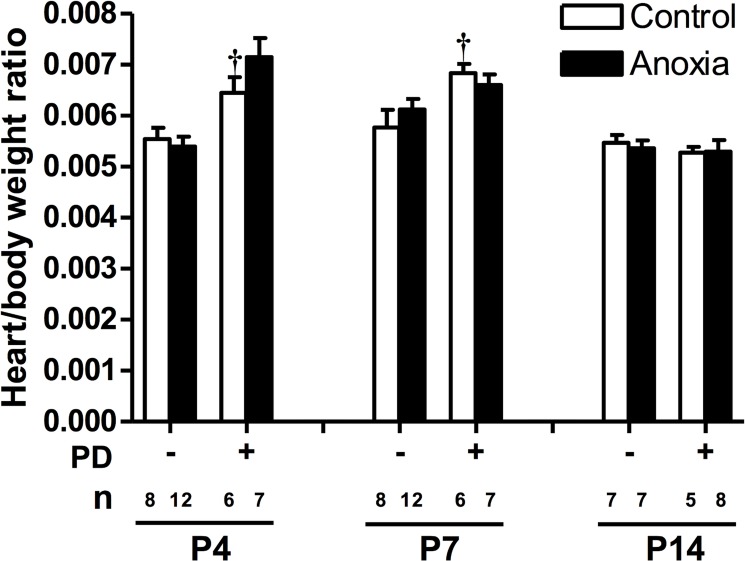
PD156707 increased heart to body weight ratio
There was no significant effect of anoxia on the heart to body weight ratio for any day (Fig. 7). However, PD156707 treatment significantly increased the heart to body weight ratio in day 4 and 7 neonates (Fig. 7). Heart and body weight averages in the presence and absence of anoxia and PD156707 are listed in Table 1.
Fig 7. Effect of newborn anoxia and PD156707 on heart to body weight ratio of neonatal rats.
Body and heart weights were taken from day 4, 7, and 14 neonatal rats that were treated with control or anoxia, in the absence or presence of PD156707. Data are means ± SEM. † P < 0.05, -PD156707 vs. +PD156707. PD: PD156707; n: animal numbers.
Table 1. Effect of newborn anoxia and PD156707 on body and heart weight of neonatal rats.
| Body weight (grams) | Heart weight (grams) | Heart to Body weight ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P4 | P7 | P14 | P4 | P7 | P14 | P4 | P7 | P14 | ||
| -PD156707 | Control | 8.51 ± 0.374 (8) | 14.44 ± 0.550 (8) | 22.45 ± 1.090 (7) | 0.047 ± 0.0023 (8) | 0.083 ± 0.0046 (8) | 0.123 ± 0.0076 (7) | 0.0055 ± 0.0002 (8) | 0.0058 ± 0.0003 (8) | 0.0055 ± 0.0002 (7) |
| Anoxia | 8.06 ± 0.317 (12) | 13.65 ± 0.472 (12) | 22.28 ± 0.879 (7) | 0.044 ± 0.0023 (12) | 0.083 ± 0.0025 (12) | 0.120 ± 0.0072 (7) | 0.0054 ± 0.0002 (12) | 0.0061 ± 0.0002 (12) | 0.0054 ± 0.0002 (7) | |
| +PD156707 | Control | 9.57 ± 0.186 (6) | 15.05 ± 0.289 (6) | 21.68 ± 0.960 (5) | 0.062 ± 0.0029 (6) | 0.103 ± 0.0024* (6) | 0.114 ± 0.0053 (5) | 0.0065 ± 0.0003 (6) | 0.0068 ± 0.0002* (6) | 0.0053 ± 0.0001 (5) |
| Anoxia | 7.57 ± 0.498 (7) | 12.75 ± 0.302 (7) | 23.90 ± 0.779 (8) | 0.054 ± 0.0042 (7) | 0.084 ± 0.0034 (7) | 0.127 ± 0.0081 (8) | 0.0072 ± 0.0004 (7) | 0.0066 ± 0.0002 (7) | 0.0053 ± 0.0002 (8) | |
Body and isolated hearts were weighed from day 4, 7, and 14 neonatal rats that were treated with control or anoxia, in the absence or presence of PD156707. Heart to body weight ratio values are also represented. Data are means ± SEM.
Number of animals is represented in parentheses.
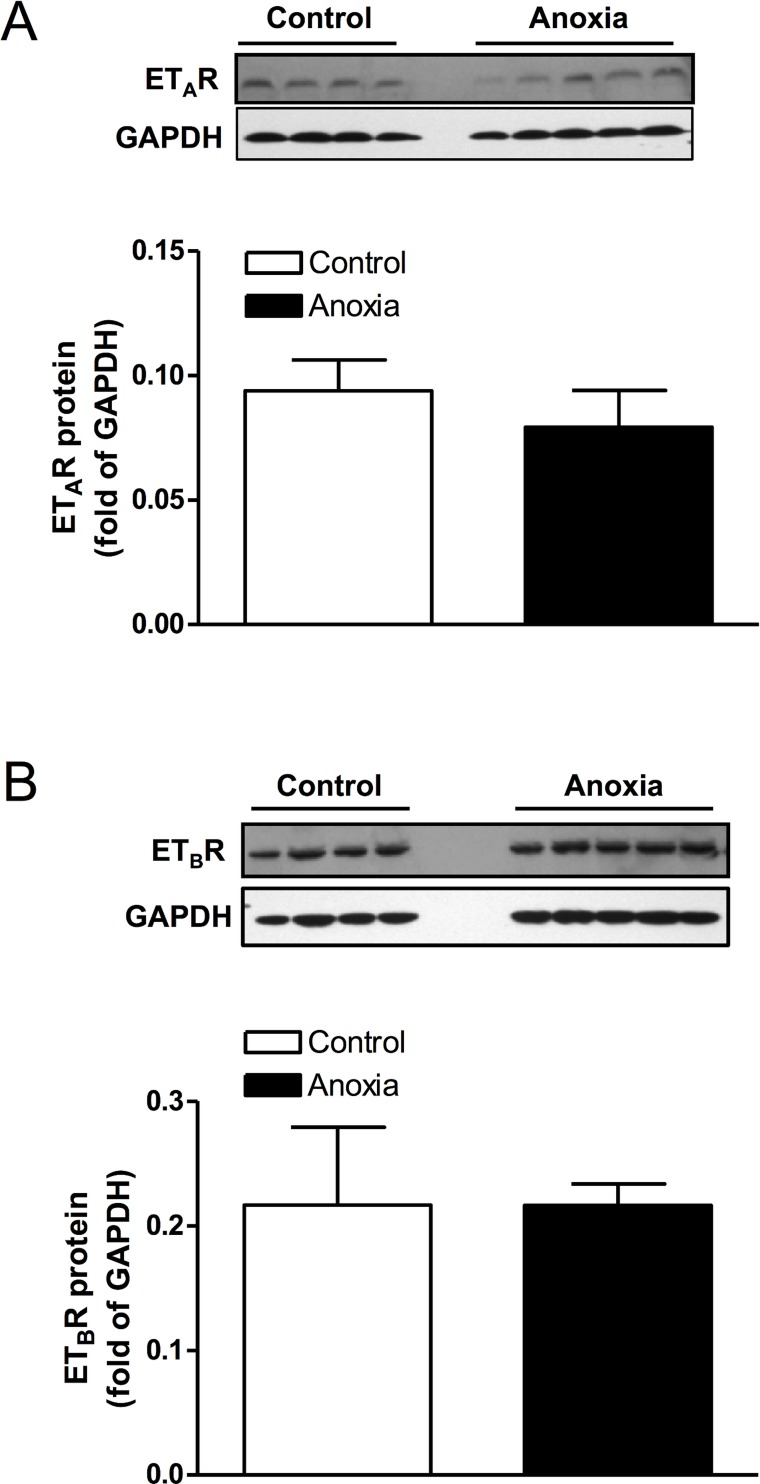
Neonatal anoxia treatment had no effect on ET-receptor density
Hearts from postnatal day 4 rats that were treated with anoxia were collected and protein isolated. There was no significant change in protein abundance of either ETAR or ETBR, due to anoxia treatment (Fig. 8).
Fig 8. Effect of newborn anoxia on ETA- and ETB-receptor protein abundance in the neonatal heart.
Hearts were isolated from day 4 neonatal rats treated with control or anoxia. Protein abundance of ETAR (A) and ETBR (B) was determined by Western immunoblotting. Data are means ± SEM. n = 4–5.
Prenatal hypoxia decreased cardiomyocyte proliferation in the fetal heart
To investigate the comparative effect of prenatal hypoxia, pregnant rats were treated with either normoxic control or 10.5% O2 from gestational day 15 to 21, and hearts were isolated from postnatal day 4 and 7 neonatal rats. Similar to the findings in newborn anoxia treatment, prenatal hypoxia resulted in a significant decrease in the proliferation of cardiomyocytes at postnatal day 7 (Fig. 9A), but had no significant effects on percent binucleation (Fig. 9B) or the heart to body weight ratio (Fig. 9C).
Fig 9. Effect of prenatal hypoxia on neonatal cardiomyocyte proliferation, binucleation, and heart to body weight ratio.
Cardiomyocytes were isolated from day 4 and 7 neonatal rats that were treated with control or maternal hypoxia. Cells were stained with α-actinin and Ki-67, and nuclei stained with Hoechst. Panel A shows percent of Ki-67 expressing cells (n = 3–4). Panel B shows percent of binucleate cells (n = 4). Panel C shows the heart to body weight ratio (n = 8–9). Data are means ± SEM. * P < 0.05, hypoxia vs. control.
Discussion
In the present study, we provide evidence showing that in vivo newborn anoxia leads to a decrease in the proliferation of cardiomyocytes in the developing heart. Furthermore, our results suggest that anoxia treatment leads to a significant reduction in number of cardiomyocytes per heart weight of the day 14 neonate, which is terminally differentiated. The findings that anoxia increased ET-1 production in the heart and the anoxia-induced changes in proliferation and cardiomyocyte number were reversed with PD156707, suggest a mechanism mediated by the ETA-receptor. In addition, basal ET-1 was also found to play a role in cardiomyocyte proliferation, as well as the heart to body weight ratio.
Cardiomyocytes undergo a terminal differentiation process that reaches completion by the first two weeks of neonatal life in rats [2, 6]. After this, cardiomyocytes in the heart have negligible proliferative capacity and further growth is mainly via hypertrophy. Thus the number of cardiomyocytes that will reside in the adult heart is determined during this early stage and if altered may result in life-long consequences. Hypoxic stress during perinatal development has been shown by previous studies to diminish the proliferation of cardiomyocytes [8, 9, 26]. Furthermore, fetal hearts exposed to hypoxia have fewer [7, 14] and larger cardiomyocytes [7], and adult male rats that were exposed to hypoxia in utero were more susceptible to ischemic injury as seen by increased myocardial infarction and reduced recovery [33].
Preterm birth is a complex clinical problem that is highly associated with episodes of severe hypoxia and even anoxia, which can be so severe that the infant must be mechanically ventilated [34]. Preterm infants have an immature respiratory system [35] that is unable to provide adequate oxygen at times and thus ventilatory support is frequently needed. However, several studies have shown that episodic airway obstruction and hypoxemia commonly occur in these infants [36, 37]. Given that the rodent heart is relatively immature at birth, the present study with episodic anoxia treatments of newborn rats provides a reasonable animal model to study the effects of anoxia/hypoxia on the heart development in preterm infants. Anoxia itself has been shown to alter proliferation, and, in rat fibroblasts, leads to arrest of the cell cycle at the G1 and S phase [38].
To confirm the extent of hypoxic exposure to the neonatal hearts used in our study, the protein levels of hypoxia-inducible factor 1 alpha (HIF-1α) were evaluated. The results show that HIF-1α protein abundance is significantly increased in neonatal hearts exposed to in vivo anoxia; furthermore increased levels of HIF-1α in the heart have also been observed in the prenatal hypoxia model [7]. In agreement with previous work, we showed that cardiomyocyte proliferation was decreased following in vivo neonatal anoxia treatment at postnatal day 4 and 7. The cardiomyocytes take approximately 24 hours for them to fully attach to the plate before the immunocytochemical staining of Ki-67 can be performed. While the potential effect of this attachment process on the rate of proliferation may not be excluded in the present study, the same procedure applied to all treatment groups. By postnatal day 14, there was a trend for anoxia to decrease proliferation however this trend was not significant in our data. The heart is thought to be fully mature and essentially an adult phenotype of cardiomyocytes by day 14 in rats, therefore the rate of myocyte proliferation is normally very low at this point and anoxia had no significant effect on lowering it further. Similarly, our results from the prenatal hypoxia model showed a decrease in the proliferation of neonatal cardiomyocytes at postnatal day 7. Interestingly, newborn anoxia had no significant effect on the binucleation of cardiomyocytes. Previous work has shown that maternal hypoxia leads to an increase in the amount of binucleate cardiomyocytes in the fetal heart [7], thus indicating a development stage-specific effect.
Furthermore, we investigated two proteins that are closely involved in the regulation of the cell cycle: cyclin D2 and p27. These proteins have previously been studied and found to be differentially expressed in the hypoxia-treated fetal heart [8]. Cyclin D2 is associated with other cell cycle regulators that work to promote cell cycle activity, while p27 is a cyclin-dependent kinase inhibitor and thus inhibits cell cycle activity. Therefore the expression of these two proteins should be inversely related, as our results indicate. Cyclin D2, a cell cycle promoter, is significantly decreased during anoxia treatment, while the cell cycle inhibitor, p27, is upregulated under these conditions. These results are consistent with our finding that anoxia treatment decreases cardiomyocyte proliferation. In addition, we tested the role of ET-1 acting through its ETAR on the expression of cyclin D2 and p27. In the presence of PD156707, an ETAR antagonist, anoxia had no effect on cyclin D2 expression. However, p27 expression was significantly decreased in the presence of PD156707 compared to control conditions. These findings suggest that ET-1 and the ETAR are key mediators in the anoxia-induced effects on cyclin D2 and p27. Ultimately, these results may help to explain the overall decrease in cardiomyocyte proliferation due to anoxia treatment.
Although a gradual decrease in proliferation in the critical window of the heart development is a normal developmental process, hypoxia and anoxia appear to accelerate this progression, particularly during the early development. The endpoint of cardiomyocyte number is a metric to measure the consequence of altering the proliferative capacity. Our results suggest that anoxia reduces cardiomyocyte endowment at postnatal day 14, when the heart is presumed to be fully mature and cardiomyocytes have terminally differentiated. Anoxia reduced proliferation at days 4 and 7, resulting in fewer cardiomyocytes in the differentiated heart seen at day 14. Given that cardiomyocytes are the functional contractile units of the heart, this decreased cardiomyocyte endowment in the heart may have negative impact in cardiac function and become more susceptible to injury later in life. While our results suggest a significant reduction in cardiomyocyte endowment due to anoxia at the critical window of the heart development, future studies using unbiased and random stereology will be needed to provide conclusive evidence of this effect.
Previous studies from our laboratory and others have shown that hypoxia regulates proliferation of cardiomyocytes and vascular muscle [8, 10, 26, 39]. However the downstream regulators of this response have yet to be identified. Our previous work in an ex vivo model showed that primary fetal cardiomyocytes exhibited a similar decrease in proliferation when treated with endothelin-1 (ET-1) [26]. It is known that ET-1 expression is induced under hypoxic conditions via a HIF-binding site on its promoter [15–20], specifically in cardiomyocytes [21]. ET-1 itself has also been shown to regulate proliferation, having a mitogenic effect on vascular smooth muscle [24, 27, 28]. Moreover our results showed an increase in prepro-ET-1 mRNA in the P4 neonatal heart when exposed to anoxia. Previous work has also shown an increase in prepro-ET-1 mRNA in the fetal heart exposed to maternal hypoxia [26]. These studies taken together implicate a role for ET-1 in mediating the hypoxia- and anoxia-induced decrease in cardiomyocyte proliferation.
A selective ET-receptor antagonist was used to study the role of both basal and anoxia-induced ET-1 in the present study. ET-1 can activate two receptor subtypes: the ETA- and ETB-receptor. Activation of the ETA-receptor leads to vasoconstriction and is primarily found in vascular muscle [40]. In contrast, the ETB-receptor can provide a vasodilation effect as well as vasoconstriction depending on the receptor location, in endothelial cells [41] or vascular muscle [41–44], respectively. The ETB-receptor also plays a role in the clearance of endothelin from tissues [45]. In cardiomyocytes, the ETA-receptor is the predominant subtype [23], and has been implicated in regulating proliferation [24, 27, 28]. Therefore, our study evaluated the effects of PD156707, a selective antagonist for the ETA-receptor [46], on cardiomyocyte proliferation. Due to the short half-life of PD156707 of about one hour [47], it was given twice a day just prior to anoxia exposure in the present study. We also evaluated the protein expression of the ET-receptors, both ETAR and ETBR. Interestingly, the results showed no change in the expression of either receptors due to anoxia treatment, suggesting that a change in receptor density is not contributing to the effects of anoxia or ET-1. The finding that PD156707 ameliorated the anoxia-induced decrease in proliferation of cardiomyocytes at day 4 and 7 implicates the ETA-receptor as a key mediator. Furthermore, the addition of PD156707 alone elicited an increase in proliferation at day 4 beyond that of the control. This observation was not seen at day 7 or day 14, suggesting that the regulation of basal ET-1 function in the heart is dependent on the stage of development. At an earlier stage, basal ET-1 levels play a key role in regulating cardiomyocyte proliferation. The effect of basal ET-1 in regulating cardiomyocyte endowment in the developing heart is intriguing. The treatment of newborn rats with ETA-receptor antagonist led to an increase in cardiomyocyte number per heart weight at day 7, suggesting that an appropriate level of basal ET-1 is necessary to optimize cardiomyocyte endowment in the heart.
Anoxia treatment had no significant effect on mononucleate and binucleate cell size, however inhibition of ETAR by PD156707 caused an increase in cell size at day 7. This may suggest that basal ET-1 plays a role in maintaining cell size and, and if activation of the ETA-receptor is blocked, the cell undergoes hypertrophy. The change in binucleate cell size is likely more relevant because the mononucleate cells still have the capacity to divide and are not yet terminally differentiated.
The heart to body weight ratio was unchanged with anoxia treatment for all age groups. However by blocking basal ET-1 with PD156707, the heart to body weight ratio was increased at postnatal day 7. These results suggest that the heart is increasing in size, which agrees with the results of increased cell size, proliferation, and cardiomyocyte number in the presence of PD156707. In the present study, the cardiomyocyte number were counted in freshly isolated myocytes, and the in vivo PD156707 treatment increased the cardiomyocyte number by about 65% in day 7 hearts. The heart is made up by cardiac fibroblasts, myocytes, endothelial cells, and vascular smooth muscle cells, with the majority being fibroblasts and myocytes. There are significant differences in cell populations of the heart among various species. In rats, the heart is composed of about 60–70% nonmyocytes and 30–40% cardiac myocytes [48–53]. In neonatal rats, cardiac fibroblasts made up about 64% of the total heart, whereas the myocyte population was 30%, with the nonmyocyte and nonfibroblast cell populations comprising the remainder of the heart [54]. In the same study [54], it was found that neonatal and adult mouse hearts contained around 60% cardiac myocytes. However, in a more recent study, 20–30% cardiac myocytes were demonstrated in the mouse heart [55]. Because of the variability of the myocyte maturity in near-term fetuses and neonates among different species, there is also a dramatic difference in the myocyte volume density of the heart between various species. The near-term heart myocyte volume density, for example, was 53–55% in sheep [56] with highly matured heart at birth, but was much lower in rats of 21–30% [57] and rabbits of 22% [58], the hearts of which were much immature at birth and continued the maturity in the first two weeks of postnatal life. However, a study reported that the myocyte volume density in fetal and neonatal rats was around 80–94% [59]. This was a somewhat surprising finding and it is unlikely that rats would have much higher myocyte volume density than lambs given that matured myocytes in lambs should have larger volume than those of immature myocytes in neonatal rats. In the present study, if the PD156707 treatment induced proportional changes in the nonmyocyte composition of the heart, it would increase the cardiomyocyte composition in the heart to around 50%, albeit the proliferation of nonmyocyte cells in the heart could be differentially regulated. It is important to note that although changes in cardiomyocyte size measured in cells that were attached to plates suggest a physiological difference due to the PD156707 treatment, they are not necessarily representative of what’s happening in vivo. The possibility that the PD156707 treatment may cause more rapidly flatten out of myocytes, giving a larger area reading in the 24 hour-period of culture, may not be excluded in the present study. It is likely that the increases of both cardiomyocyte number and cell size may contribute to significantly increased heart weight observed in the PD156707-treated animals. The finding that the heart to body weight ratio is unchanged at day 14 even though anoxia treatment decreases cardiomyocyte endowment implies that the cardiomyocytes may increase their size to compensate for the loss of cells and maintain the size of the heart. However, we were unable to measure cardiomyocyte size from the day 14 hearts due to technical limitations and the difficulty of cardiomyocytes in the attachment to the plate at this stage. Another possibility includes an increase in non-cardiomyocyte cell number and size in the heart after anoxia treatment.
The present study evaluated not only the effects of newborn anoxia treatment on the terminal differentiation of neonatal cardiomyocytes but also the role of basal ET-1 on this process. We identified a mechanism through which neonatal anoxia exposure induces an accelerated loss of cardiomyocyte proliferation via the ETA-receptor, which subsequently results in reduced cardiomyocyte endowment in the fully differentiated heart. Our study also demonstrated the role that basal ET-1 plays in regulating cardiomyocyte size, proliferation, and number in the developing heart. Given the clinical implications of these findings in understanding the effects of hypoxia on the heart development in preterm infants, further investigation into the mechanisms involved is needed.
Acknowledgments
The authors would like to acknowledge Dr. David Baylink and Dr. Xiaobing Zhang for the use of the FACSAria, as well as Amanda Neises for her technical assistance.
A portion of this research used the Loma Linda University School of Medicine Advanced Imaging and Microscopy Core, a facility supported in part by the National Science Foundation through the Major Research Instrumentation program of the Division of Biological Infrastructure Grant No. 0923559 and the Loma Linda University School of Medicine.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by the National Institutes of Health grant HL118861 (to L. Zhang). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Barker DJ (1995) Fetal origins of coronary heart disease. BMJ (Clinical research ed) 311: 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahuja P, Sdek P, MacLellan WR (2007) Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiological reviews 87: 521–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li F, Wang X, Capasso JM, Gerdes AM (1996) Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. Journal of molecular and cellular cardiology 28: 1737–1746. [DOI] [PubMed] [Google Scholar]
- 4. Bugaisky L, Zak R (1979) Cellular growth of cardiac muscle after birth. Texas reports on biology and medicine 39: 123–138. [PubMed] [Google Scholar]
- 5. Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, et al. (2009) Evidence for cardiomyocyte renewal in humans. Science (New York, NY) 324: 98–102. 10.1126/science.1164680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clubb FJ Jr, Bishop SP (1984) Formation of binucleated myocardial cells in the neonatal rat. An index for growth hypertrophy. Laboratory investigation; a journal of technical methods and pathology 50: 571–577. [PubMed] [Google Scholar]
- 7. Bae S, Xiao Y, Li G, Casiano CA, Zhang L (2003) Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. American journal of physiology Heart and circulatory physiology 285: H983–990. [DOI] [PubMed] [Google Scholar]
- 8. Tong W, Xiong F, Li Y, Zhang L (2013) Hypoxia inhibits cardiomyocyte proliferation in fetal rat hearts via upregulating TIMP-4 . American journal of physiology Regulatory, integrative and comparative physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tong W, Xue Q, Li Y, Zhang L (2011) Maternal hypoxia alters matrix metalloproteinase expression patterns and causes cardiac remodeling in fetal and neonatal rats. American journal of physiology Heart and circulatory physiology 301: H2113–2121. 10.1152/ajpheart.00356.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang FX, Chen ML, Shan QJ, Zou JG, Chen C, et al. (2007) Hypoxia reoxygenation induces premature senescence in neonatal SD rat cardiomyocytes. Acta pharmacologica Sinica 28: 44–51. [DOI] [PubMed] [Google Scholar]
- 11. Bubb KJ, Cock ML, Black MJ, Dodic M, Boon WM, et al. (2007) Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. The Journal of physiology 578: 871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Louey S, Jonker SS, Giraud GD, Thornburg KL (2007) Placental insufficiency decreases cell cycle activity and terminal maturation in fetal sheep cardiomyocytes. The Journal of physiology 580: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, et al. (2007) Restriction of placental function alters heart development in the sheep fetus . American journal of physiology Regulatory, integrative and comparative physiology 293: R306–313. [DOI] [PubMed] [Google Scholar]
- 14. Botting KJ, McMillen IC, Forbes H, Nyengaard JR, Morrison JL (2014) Chronic hypoxemia in late gestation decreases cardiomyocyte number but does not change expression of hypoxia-responsive genes. Journal of the American Heart Association 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hashiguchi K, Takagi K, Nakabayashi M, Takeda Y, Sakamoto S, et al. (1991) Relationship between fetal hypoxia and endothelin-1 in fetal circulation. Journal of cardiovascular pharmacology 17 Suppl 7: S509–510. [DOI] [PubMed] [Google Scholar]
- 16. Ostlund E, Lindholm H, Hemsen A, Fried G (2000) Fetal erythropoietin and endothelin-1: relation to hypoxia and intrauterine growth retardation. Acta obstetricia et gynecologica Scandinavica 79: 276–282. [PubMed] [Google Scholar]
- 17. Yamada J, Fujimori K, Ishida T, Sanpei M, Honda S, et al. (2001) Plasma endothelin-1 and atrial natriuretic peptide levels during prolonged (24-h) non-acidemic hypoxemia in fetal goats. The Journal of maternal-fetal medicine 10: 409–413. [DOI] [PubMed] [Google Scholar]
- 18. Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA (2001) Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP. The Journal of biological chemistry 276: 12645–12653. [DOI] [PubMed] [Google Scholar]
- 19. Hu J, Discher DJ, Bishopric NH, Webster KA (1998) Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochemical and biophysical research communications 245: 894–899. [DOI] [PubMed] [Google Scholar]
- 20. Minchenko A, Caro J (2000) Regulation of endothelin-1 gene expression in human microvascular endothelial cells by hypoxia and cobalt: role of hypoxia responsive element. Molecular and cellular biochemistry 208: 53–62. [DOI] [PubMed] [Google Scholar]
- 21. Kakinuma Y, Miyauchi T, Yuki K, Murakoshi N, Goto K, et al. (2001) Novel molecular mechanism of increased myocardial endothelin-1 expression in the failing heart involving the transcriptional factor hypoxia-inducible factor-1alpha induced for impaired myocardial energy metabolism. Circulation 103: 2387–2394. [DOI] [PubMed] [Google Scholar]
- 22. Kedzierski RM and Yanagisawa M (2001) Endothelin system: the double-edged sword in health and disease. Annual review of pharmacology and toxicology 41: 851–876. [DOI] [PubMed] [Google Scholar]
- 23. Kohan DE, Rossi NF, Inscho EW, Pollock DM (2011) Regulation of blood pressure and salt homeostasis by endothelin. Physiological reviews 91: 1–77. 10.1152/physrev.00060.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agapitov AV, Haynes WG (2002) Role of endothelin in cardiovascular disease . Journal of the renin-angiotensin-aldosterone system: JRAAS 3: 1–15. [DOI] [PubMed] [Google Scholar]
- 25. Rubanyi GM, Polokoff MA (1994) Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacological reviews 46: 325–415. [PubMed] [Google Scholar]
- 26. Paradis A, Xiao D, Zhou J, Zhang L (2014) Endothelin-1 Promotes Cardiomyocyte Terminal Differentiation in the Developing Heart via Heightened DNA Methylation. International journal of medical sciences 11: 373–380. 10.7150/ijms.7802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldie RG (1999) Endothelins in health and disease: an overview. Clinical and experimental pharmacology & physiology 26: 145–148. [DOI] [PubMed] [Google Scholar]
- 28. Komuro I, Kurihara H, Sugiyama T, Yoshizumi M, Takaku F, et al. (1988) Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS letters 238: 249–252. [DOI] [PubMed] [Google Scholar]
- 29. Patterson AJ, Xiao D, Xiong F, Dixon B, Zhang L (2012) Hypoxia-derived oxidative stress mediates epigenetic repression of PKCepsilon gene in foetal rat hearts. Cardiovascular research 93: 302–310. 10.1093/cvr/cvr322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xue Q, Dasgupta C, Chen M, Zhang L (2011) Foetal hypoxia increases cardiac AT(2)R expression and subsequent vulnerability to adult ischaemic injury. Cardiovascular research 89: 300–308. 10.1093/cvr/cvq303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xiao Y, He J, Gilbert RD, Zhang L (2000) Cocaine induces apoptosis in fetal myocardial cells through a mitochondria-dependent pathway. The Journal of pharmacology and experimental therapeutics 292: 8–14. [PubMed] [Google Scholar]
- 32. Chlopcikova S, Psotova J, Miketova P (2001) Neonatal rat cardiomyocytes—a model for the study of morphological, biochemical and electrophysiological characteristics of the heart. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia 145: 49–55. [PubMed] [Google Scholar]
- 33. Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, et al. (2003) Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. Journal of the Society for Gynecologic Investigation 10: 265–274. [DOI] [PubMed] [Google Scholar]
- 34. Bolivar JM, Gerhardt T, Gonzalez A, Hummler H, Claure N, et al. (1995) Mechanisms for episodes of hypoxemia in preterm infants undergoing mechanical ventilation. The Journal of pediatrics 127: 767–773. [DOI] [PubMed] [Google Scholar]
- 35. Martin RJ, Wang K, Koroglu O, Di Fiore J, Kc P (2011) Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology 100: 303–310. 10.1159/000329922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dimaguila MA, Di Fiore JM, Martin RJ, Miller MJ (1997) Characteristics of hypoxemic episodes in very low birth weight infants on ventilatory support. The Journal of pediatrics 130: 577–583. [DOI] [PubMed] [Google Scholar]
- 37. Dransfield DA, Spitzer AR, Fox WW (1983) Episodic airway obstruction in premature infants. American journal of diseases of children (1911) 137: 441–443. [DOI] [PubMed] [Google Scholar]
- 38. Gardner LB, Li F, Yang X, Dang CV (2003) Anoxic fibroblasts activate a replication checkpoint that is bypassed by E1a. Molecular and cellular biology 23: 9032–9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cooper AL, Beasley D (1999) Hypoxia stimulates proliferation and interleukin-1alpha production in human vascular smooth muscle cells. The American journal of physiology 277: H1326–1337. [DOI] [PubMed] [Google Scholar]
- 40. Hosoda K, Nakao K, Hiroshi A, Suga S, Ogawa Y, et al. (1991) Cloning and expression of human endothelin-1 receptor cDNA. FEBS letters 287: 23–26. [DOI] [PubMed] [Google Scholar]
- 41. Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, et al. (1990) Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature 348: 732–735. [DOI] [PubMed] [Google Scholar]
- 42. Kawanabe Y, Nauli SM (2011) Endothelin. Cellular and molecular life sciences: CMLS 68: 195–203. 10.1007/s00018-010-0518-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S (1990) Cloning and expression of a cDNA encoding an endothelin receptor. Nature 348: 730–732. [DOI] [PubMed] [Google Scholar]
- 44. Yanagisawa M (1994) The endothelin system. A new target for therapeutic intervention. Circulation 89: 1320–1322. [DOI] [PubMed] [Google Scholar]
- 45. Wilkes BM, Susin M, Mento PF (1993) Localization of endothelin-1-like immunoreactivity in human placenta. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society 41: 535–541. [DOI] [PubMed] [Google Scholar]
- 46. Reynolds EE, Keiser JA, Haleen SJ, Walker DM, Olszewski B, et al. (1995) Pharmacological characterization of PD 156707, an orally active ETA receptor antagonist. The Journal of pharmacology and experimental therapeutics 273: 1410–1417. [PubMed] [Google Scholar]
- 47. Coe Y, Haleen SJ, Welch KM, Liu YA, Coceani F (2002) The endothelin A receptor antagonists PD 156707 (CI-1020) and PD 180988 (CI-1034) reverse the hypoxic pulmonary vasoconstriction in the perinatal lamb. The Journal of pharmacology and experimental therapeutics 302: 672–680. [DOI] [PubMed] [Google Scholar]
- 48. Baudino TA, Carver W, Giles W, Borg TK (2006) Cardiac fibroblasts: friend or foe? American journal of physiology Heart and circulatory physiology 291: H1015–1026. [DOI] [PubMed] [Google Scholar]
- 49. Camelliti P, Borg TK, Kohl P (2005) Structural and functional characterisation of cardiac fibroblasts. Cardiovascular research 65: 40–51. [DOI] [PubMed] [Google Scholar]
- 50. Harvey PR RN (1999) Heart Development. New York: Academic. [Google Scholar]
- 51. Nag AC (1980) Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios 28: 41–61. [PubMed] [Google Scholar]
- 52. Rubart M, Field LJ (2006) Cardiac regeneration: repopulating the heart. Annual review of physiology 68: 29–49. [DOI] [PubMed] [Google Scholar]
- 53. Zak R (1974) Development and proliferative capacity of cardiac muscle cells. Circulation research 35: suppl II:17–26. [PubMed] [Google Scholar]
- 54. Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA (2007) Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. American journal of physiology Heart and circulatory physiology 293: H1883–1891. [DOI] [PubMed] [Google Scholar]
- 55. Walsh S, Ponten A, Fleischmann BK, Jovinge S (2010) Cardiomyocyte cell cycle control and growth estimation in vivo—an analysis based on cardiomyocyte nuclei. Cardiovascular research 86: 365–373. 10.1093/cvr/cvq005 [DOI] [PubMed] [Google Scholar]
- 56. Smolich JJ, Walker AM, Campbell GR, Adamson TM (1989) Left and right ventricular myocardial morphometry in fetal, neonatal, and adult sheep. The American journal of physiology 257: H1–9. [DOI] [PubMed] [Google Scholar]
- 57. Anversa P, Vitali-Mazza L, Loud AV (1975) Morphometric and autoradiographic study of developing ventricular and atrial myocardium in fetal rats. Laboratory investigation; a journal of technical methods and pathology 33: 696–705. [PubMed] [Google Scholar]
- 58. Smith HE, Page E (1977) Ultrastructural changes in rabbit heart mitochondria during the perinatal period. Neonatal transition to aerobic metabolism. Developmental biology 57: 109–117. [DOI] [PubMed] [Google Scholar]
- 59. Mandarim-de-Lacerda CA, Pessanha MG (1995) Stereology of the myocardium in embryos, fetuses and neonates of the rat. Acta anatomica 154: 261–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.