Abstract
Premature ectopic beats are frequently detected on routine 12-lead screening-electrocardiogram (ECG). However, their prognostic importance in individuals without known cardiovascular disease (CVD) is not well established. We evaluated prognostic value of atrial premature complexes (APC’s) and ventricular premature complexes (VPC’s) detected by a single 12-lead-ECG. A prospective cohort of 7504 participants selected from nationally-representative, community-dwelling individuals living in United States, enrolled in the Third Health and Nutrition Examination Survey (NHANES-III) from 1988 – 94 with follow up through December 2006 without known CVD. The main outcomes were all – cause mortality, CVD related mortality and IHD related mortality. Out of 7504 participants (mean age 60 ± 14 years, 47% women, 49% whites), 89 (1.2%) had APC’s and 110 (1.5%) had VPC’s on 12 – lead ECG. During a follow up of up to 18 years, 2386 deaths occurred, of which 963 were due to CVD and 511 were due to IHD. In a multivariable adjusted for demographics, clinical variables and ECG measures, APC’s were significantly associated with all-cause mortality [HR, 1.41 (95% CI, 1.08–1.80)], CVD death [HR, 1.78 (95% CI, 1.26–2.44)] and IHD death [HR, 2.40 (95% CI, 1.59–3.47)]. For VPCs, however, there were no significant associations with all – cause mortality [HR, 1.05 (95% CI, 0.80–1.36)], CVD death [HR, 0.96 (95% CI, 0.62–1.43)] and IHD death [HR, 0.89 (95% CI, 0.47–1.52)]. In conclusion, APC’s, but not VPC’s, on routine screening ECG are predictive of adverse events in community-dwelling individuals without known CVD.
Keywords: Ventricular premature complex, atrial premature complex, mortality, cardiovascular disease mortality, ischemic heart disease mortality
INTRODUCTION
Physicians frequently perform electrocardiographic (ECG) screening based on pertinent historical and objective evaluation of individuals at risk for cardiovascular events. Atrial premature complexes (APC’s) are commonly encountered during these electrocardiographic evaluations and are known to be present in 10–20% of the general population1. More serious forms of cardiac ectopy are ventricular premature complexes (VPC’s), found to be present in 5–10 % of general population2, 3. However, these data are from the studies that have used 24 hours of ambulatory ECG4–6. Prognosis of APC’s and VPC’s on a 12 – lead ECG has been conflicting in the absence of ischemic heart disease and is not well studied. We examined the prognostic significance of APC’s and VPC’s in a representative sample of the United States population without known cardiovascular disease.
METHODS
The National Health and Nutrition Examination Survey (NHANES) III was a survey administered to a representative sample of the noninstitutionalized United States (US) population. NHANES III baseline data were collected from participants during an in home interview and a subsequent visit to a mobile examination center from 1988 to 1994.
The survey design and data are available at the web site of the US Centers for Disease Control and Prevention (http://www.cdc.gov/nchs/nhanes/nh3data.htm). For the purpose of this study, we included all available NHANES III participants with ≥20 year of age, who had good-quality ECGs showing sinus rhythm without major intraventricular conduction delay (including complete bundle branch blocks and/or QRS duration >120 ms) who also had medical, anthropometric measurement, and mortality data available by 2006. Individuals with known cardiovascular disease, ECG evidence of myocardial infarction, paced rhythms or atrial fibrillation were excluded.
Covariate data were obtained by in-home interview and included demographics, medical history including smoking status, and use of medications. Body mass index was calculated as the weight in kilograms divided by the height in meters squared. Blood pressure was measured 3 times during the in-home interview and 3 additional times during the visit to the mobile examination center. All blood pressure measurements for each participant were averaged for the purpose of this study. Medical conditions and medication use were assessed by self-report. The total serum cholesterol was measured enzymatically.
Standard 12-lead ECG was recorded on a (Marquette Medical Systems, Milwaukee, Wisconsin) by trained technicians during the participant’s visit to a mobile examination center. Computerized automated analysis of the electrocardiographic data was performed which included classification of ECG abnormalities using Minnesota ECG Code Classification7. APC’s and VPC’s were initially detected by software then visually confirmed by an ECG coder.
The NHANES III participants were followed up for mortality through December 31, 2006. The method of probabilistic matching was used to link the NHANES III participants with the National Death Index to identify vital status and, for those who died; the cause of death. These participants were matched on 12 identifiers, including Social Security number, gender, and date of birth. Follow up duration was defined as time period between NHANES III examination and date of death or December 31, 2006, whichever occurred first. The cause of death was determined using the underlying cause listed on the death certificates. The “International Classification of Disease” (ICD), ninth revision, was used for deaths occurring from 1988 to 1998 and ICD9/ICD-10 for deaths occurring from 1999 to 2006.
Descriptive statistics are used to summarize the data. Continuous variables are expressed in mean ± standard deviation and categorical variables are given as percentages. For examination of differences, analysis of variance (ANOVA) is used for continuous variables and Fisher Exact test or Chi-square for categorical variables, wherever appropriate. Incidence rates for all cause, cardiovascular disease and ischemic heart disease mortalities were calculated for APC’s and VPC’s which were expressed as 100-person years. Rate ratios were calculated and 95% confidence intervals were expressed. Assumptions for Cox proportional hazard model were tested. Adjusted Cox proportional hazard models were uses to evaluate the association between APC’s and VPC’s (compared to no APC’s or VPC’s) with all-cause, cardiovascular disease, and ischemic heart disease mortality. Two adjusted models were evaluated; Model 1 adjusted for demographics (age, gender and race/ethnicity) while Model 2 adjusted for model 1 smoking status, systolic blood pressure, body mass index, blood pressure medications, total cholesterol, diabetes mellitus, cancer and pulmonary disease (bronchial asthma and chronic obstructive pulmonary disease), electrocardiographic left ventricular hypertrophy (ECG – LVH), and corrected QT (QTc) interval. Consistency of the results were examined across subgroups of the participants stratified by age, sex and race/ethnicity using Cox proportional hazard adjusted in a similar fashion to model 2. Survival analysis was performed by Kaplan-Meier’s method to compare APC vs. VPC vs. controls, and significance was tested by log rank test. P value of <0.05 was considered significant. Analysis was performed on SAS v. 9.3 (SAS Ins. Cary, NC USA) and PASW v. 18.0 (IBM, Chicago, IL USA).
RESULTS
Of the 7504 participants (mean age 60±14, women 47%, 49% whites) in this study, there were 89 (1.2%) individuals that were found to have APC’s and 110 (1.5%) were found to have VPC’s. Table 1 shows baseline characteristics of individuals stratified by presence and absence of APC’s and VPC’s. Participants with premature complexes were more likely to be older, males, of white race, with higher blood systolic blood pressure, and more prevalence of pulmonary disease and ECG evidence of left ventricular hypertrophy. On the other hand they were less likely to be Mexicans. Participants with APC’s were more likely to have cancer while participants with VPC’s were more likely to be females.
Table 1.
Baseline characteristics of the study sample
| Variable | No Ectopic Beats (n = 7305) | Ventricular Premature Complexes (VPC’s) (n = 110) | Atrial Premature Complexes (APC’s) (n = 89) | p |
|---|---|---|---|---|
| Age (years) | 59±13 | 69±12 | 72±11 | <0.0001 |
| Women | 3413 (47%) | 74 (67%) | 54 (61%) | <0.0001 |
| White | 3545 (48%) | 67 (61%) | 58 (65%) | <0.0001 |
| Black | 1706 (23%) | 25 (23%) | 21 (24%) | 0.66 |
| Mexican | 1691 (23%) | 15 (13%) | 8 (9%) | <0.0001 |
| Other races | 295 (8%) | 3 (4%) | 2 (3%) | 0.003 |
| Smoking Status | ||||
| Ever Smoker | 3970 (54%) | 70 (64%) | 55 (62%) | 0.81 |
| Never | 3335 (46%) | 40 (36%) | 39 (44%) | 0.31 |
| Corrected QT duration (ms) | 431±24 | 432±25 | 430±27 | 0.84 |
| Left Ventricular Hypertrophy | 723 (10%) | 15 (14%) | 16 (18%) | 0.02 |
| Antihypertensive Medication | 1599 (22%) | 32 (29%) | 27 (30%) | 0.03 |
| Cancer | 372 (5%) | 4 (4%) | 11 (12%) | 0.02 |
| Pulmonary Disease | 754 (10%) | 17 (15%) | 23 (26%) | <0.0001 |
| Systolic Blood Pressure (mmHg) | 132±20 | 136±18 | 146±27 | <0.0001 |
| Diabetes mellitus | 775 (11%) | 12 (11%) | 8 (9%) | 0.88 |
| Total Cholesterol (mg/dL) | 217±44 | 225±45 | 223±44 | 0.10 |
| Body mass index (kg/m2) | 27.6±5.5 | 27.5±5.4 | 26.2±4.8 | 0.05 |
Values are expressed as mean ± standard deviation or n (%); p-value using t - test for continuous variables and Chi2 for categorical variables
During a mean follow up period of 13 ± 4 years, 2386 deaths occurred (incidence 2.5 per 100 person years). Of these, 983 were due to cardiovascular disease (incidence 1.03 per 100 person years) and 511 deaths were due to ischemic heart disease (incidence 0.55 per 100 person years). There was increased incidence of all-cause mortality, cardiovascular disease mortality and ischemic heart disease mortality in both APC’s and VPC’s groups (Table 2). The highest incidence rate ratio was for APC’s and ischemic heart disease mortality (IRR 5.84; 95% CI 3.97 – 8.61). In Cox proportional hazard analysis adjusted for demographics, comorbid conditions and ECG indices, the presence of APC’s was associated with 41% increased hazard of all-cause mortality, 64% increased hazard of cardiovascular disease mortality, and 106% increased hazard of ischemic heart disease mortality. Even though incidence rates for all types of mortalities were significantly elevated for VPC’s, the Cox proportional adjusted analyses did not show VPC’s as a significant independent risk factor for any type of mortality (Table 3). Survival analyses by Kaplan Meier’s method demonstrate increased all-cause mortality (Figure 1), cardiovascular disease mortality (Figure 2), and ischemic heart disease mortality (Figure 3) in individuals with APC’s and VPC’s. The probability of all-cause mortality, cardiovascular disease mortality and ischemic heart disease mortality for individuals with APC’s is the highest compared to individuals with VPC’s and individuals without any ectopic beats. The above results were consistent across several subgroups of participants stratified by age, sex, and race/ethnicity with no observed significant interactions between the components of each subgroup (Table 4).
Table 2.
Incidence rates of All-Cause, Cardiovascular Disease and Ischemic Heart Disease Mortality Stratified by Presence or Absence of Ectopic Beats
| No Ectopic Beats (n = 7305) | Atrial Premature Complexes (n = 89 ) | Ventricular Premature Complexes (n = 110) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Events | Event rate* | Events | Event rate* | Rate Ratio (CI 95%) | Events | Event rate* | Rate Ratio (CI 95%) | |
|
| ||||||||
| All-Cause Mortality | 2386 | 2.54 | 72 | 8.58 | 3.38 (2.67 – 4.27) | 72 | 5.63 | 2.21 (1.88 – 2.62) |
| CVD Mortality | 963 | 1.03 | 37 | 4.41 | 4.27 (3.08 – 5.94) | 32 | 2.50 | 2.43 (1.9 – 3.09) |
| IHD Mortality | 511 | 0.55 | 27 | 3.21 | 5.84 (3.97 – 8.61) | 16 | 1.25 | 2.27 (1.66 – 3.12) |
per 100 person years CVD, cardiovascular disease; IHD, ischemic heart disease
Table 3.
Risk of mortality in Individuals with Atrial and Ventricular Premature contractions compared to those without ectopic beats
| Atrial Premature Complexes | Ventricular Premature Complexes | |||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | HR (95% CI) | |||
| Model 1 | Model 2 | Model 1 | Model 2 | |
|
| ||||
| All-cause mortality | 1.50 (1.18–1.89) | 1.41 (1.08–1.80) | 1.11 (0.87–1.39) | 1.05 (0.80–1.36) |
| CVD mortality | 1.78 (1.26–2.44) | 1.64 (1.13–2.36) | 1.17 (0.80–1.64) | 0.96 (0.62–1.43) |
| IHD mortality | 2.40 (1.59–3.47) | 2.06 (1.28–3.13) | 1.07 (0.62–1.71) | 0.89 (0.47–1.52) |
CVD, cardiovascular disease; IHD, ischemic heart disease
Model 1: Adjusted for age, sex and race/ethnicity
Model 2: Adjusted for model 1 variables plus smoking status, systolic blood pressure, body mass index, blood pressure medications, total cholesterol, diabetes mellitus, cancer and pulmonary disease (bronchial asthma and chronic obstructive pulmonary disease), electrocardiographic left ventricular hypertrophy, and corrected QT interval
Figure 1.
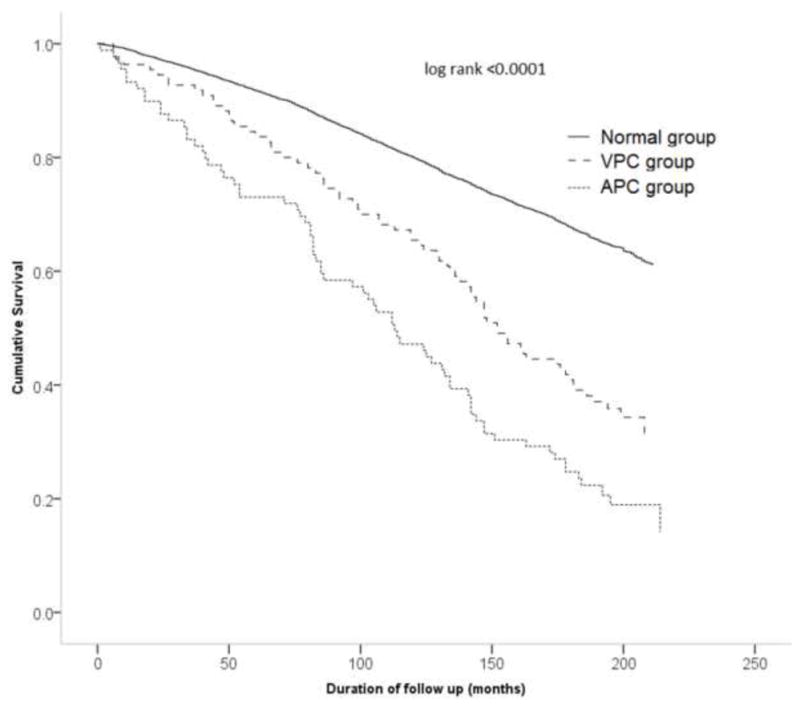
Survival curves demonstrating all-cause mortality in individuals with atrial premature complexes (APC’s) (dotted line), ventricular premature complexes (VPC’s) (broken line) and without any premature complexes (solid line)
Figure 2.
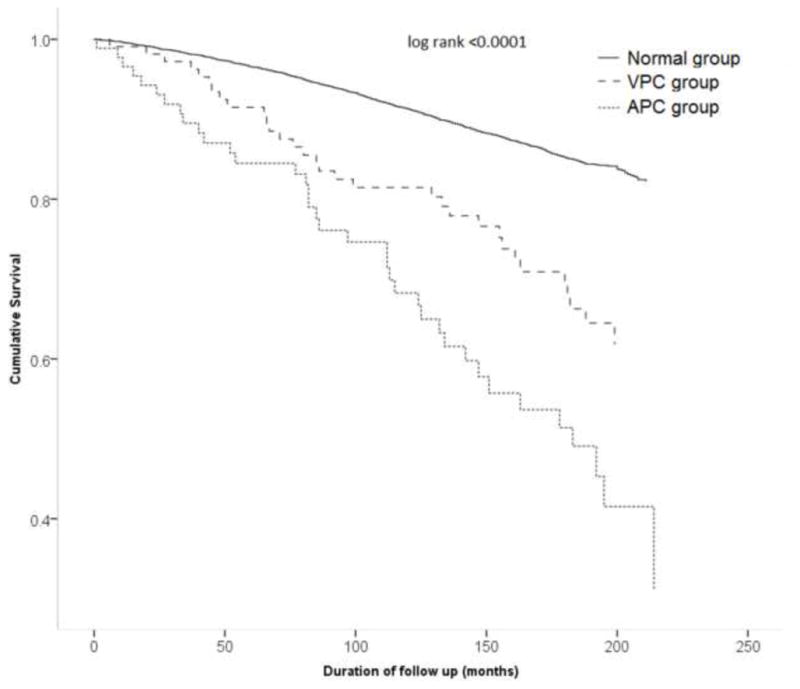
Survival curves demonstrating cardiovascular disease mortality in individuals with atrial premature complexes (APC’s) (dotted line), ventricular premature complexes (VPC’s) (broken line) and without any premature complexes (solid line)
Figure 3.
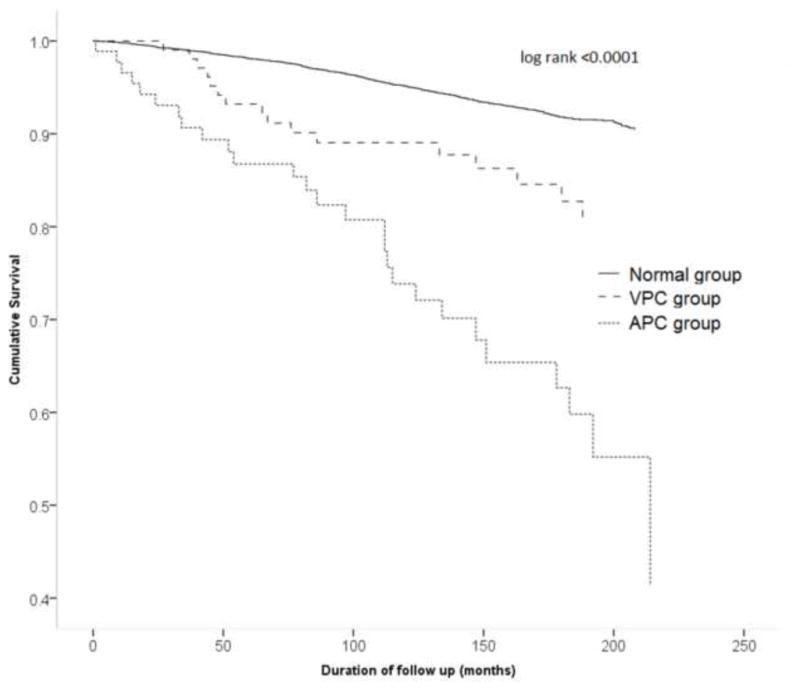
Survival curves demonstrating ischemic heart disease mortality in individuals with atrial premature complexes (APC’s) (dotted line), ventricular premature complexes (VPC’s) (broken line) and without any premature complexes (solid line)
Table 4.
Atrial Premature Complexes and Ventricular Premature Complexes and Mortality in subgroup analysis
| All – Cause Mortality | CVD Mortality | IHD Mortality | ||
|---|---|---|---|---|
|
| ||||
| Hazard Ratio (95%CI) | Hazard Ratio (95%CI) | Hazard Ratio (95%CI) | ||
|
|
||||
| Atrial Premature Complexes | Male | 1.47(0.98–2.11) | 1.52(0.83–2.52) | 1.83(0.83–3.46) |
| Female | 1.53(1.12–2.04) | 2.00(1.29–2.96) | 2.79(1.68–4.34) | |
| Age ≥ 65 years | 1.57(1.20–2.01) | 1.70(1.17–2.46) | 2.05(1.30–3.22) | |
| Age < 65 years | 2.05(0.12–9.10) | 1.43(1.06–1.95) | 1.45(1.10–1.92) | |
| White | 1.36 (0.98 – 1.85) | 1.57(0.96 – 2.41) | 2.03 (1.14 –3.34) | |
| Non-White | 1.54 (0.96 – 2.32) | 1.90 (0.94–3.41) | 2.23 (0.87 – 4.69) | |
| Ventricular Premature Complexes | Male | 1.30(0.80–1.96) | 1.14(0.52–2.13) | 0.56 (0.09 – 1.75) |
| Female | 1.05(0.79–1.38) | 1.21(0.78–1.78) | 1.25(0.69–2.07) | |
| Age ≥ 65 years | 1.36 (1.04–1.76) | 0.83(0.53–1.30) | 0.79(0.43–1.46) | |
| Age < 65 years | 0.48(0.03–2.13) | 0.75(0.56–1.01) | 0.75(0.57–1.00) | |
| White | 1.00(0.72–1.35) | 0.87 (0.51–1.39) | 0.85(0.40–1.57) | |
| Non-White | 1.07(0.64–1.67) | 1.01 (0.42–2.01) | 0.83 (0.20–2.23) | |
CVD, cardiovascular disease; IHD, ischemic heart disease; Models adjusted for age, sex and race/ethnicity, smoking status, systolic blood pressure, body mass index, blood pressure medications, total cholesterol, diabetes mellitus, cancer and pulmonary disease (bronchial asthma and chronic obstructive pulmonary disease), electrocardiographic left ventricular hypertrophy, and corrected QT interval; No significant interactions observed between the components of each subgroups and the outcome
DISCUSSION
This study aimed to evaluate the association of APC’s and VPC’s with risk of mortality in a sample from the general population of the United States free from known CVD. We found that APC’s but not VPC’s were independently associated with risk of all-cause mortality, cardiovascular disease mortality and ischemic heart disease mortality.
A recent study of a Japanese cohort of individuals found that APC’s on 12 – lead ECG double the CVD death which is consistent with our study8. On the other hand, data from atherosclerosis in communities (ARIC) cohort showed conflicting results regarding APC’s on a 2 minute rhythm strip and mortality9. We speculate that the difference might be because of increased sensitivity of 2 minute versus 10 seconds (ordinary ECG) in picking up APC’s. On the other hand, a meta-analysis of 8 prospective cohort studies showed that VPC’s conferred overall higher risk of all-cause mortality, cardiovascular mortality, sudden cardiac death and ischemic heart disease10. However, these studies included individuals with known coronary artery disease which might have biased the results. Our study also found an increased rate ratio of all – cause, CVD and IHD mortality for VPC’s however, the association was not retained after adjustment of age. This is in agreement with studies that did not show association of VPC’s with mortality11, 12.
APC’s are a result of increased automaticity and triggered activity likely secondary to fibrosis of atria13, 14. Additionally, various other potential triggers such as ischemic artery disease15, myocardial infarction16, mitral valvular stenosis17, smoking18, alcohol use19, caffeine use20, pulmonary disease21, and renal failure22 have also been associated with the development of APC’s. Despite low prevalence of the majority of these risk factors in the present cohort, our study still showed a strong association of the presence of APC’s with cardiovascular and all-cause mortality. There are several possible mechanisms for the contribution of APC’s to increased mortality. APC’s may depolarize the sinus node and thereby cause a longer post APC interval, which may trigger Virchow’s triad23. This would lead to increased coagulability of blood with a subsequent increase in cardiovascular events. Also, APC’s have been associated with atrial fibrillation24, which in turn are associated with increased risk of stroke25, myocardial infarction 26 and mortality27. It is quite possible that ectopic beats are an early marker of myocardial fibrosis28 and resultant disease process that manifests itself in the form of elevated filling pressure and associated diastolic dysfunction8. It has been reported that there is increased frequency of APC’s in patients with elevated levels of N-terminal prohormone B-type natriuretic peptide as well as with other risk factors1. Further mechanistic studies are required to assess these and other possible mechanisms for increased mortality with the presence of APC’s.
Currently, there are no formal guidelines for treating asymptomatic premature cardiac beats. Further prospective studies will be needed to confirm the strong independent association of APC’s with mortality that we reported in the present study. Similarly to atrial fibrillation where risk of thromboembolism is increased, it is quite possible that these individuals might have increased periods of ineffective contractions and hence activation of Virchow’s triad29. Though evidence in a recent meta-analysis does not support the role of aspirin in primary prevention of cardiovascular events in low risk individuals due to a small increase in risk of gastrointestinal hemorrhage, it is possible that benefits of aspirin use may outweigh risks in individuals with APC’s30. Investigating the utility of aspirin therapy in patients with APC’s to prevent the associated poor outcomes may be warranted.
Similar to positive association of atrial fibrillation with CVD, it is quite possible that APC’s might also be markers of subclinical CVD26. Hence, their visualization on a screening ECG may direct clinician to have higher suspicion of evaluation of possible underlying CVD. Many of these screening ECG’s are performed at the time of perioperative clearance for non-cardiac surgery where these findings might have an important clinical impact. However, these are mere speculations and further studies are warranted in the light of this study.
This study has several strengths. Given the nature and large sample size of the national survey of this cohort, there is better generalizability of the study to the healthy population of United States. Additionally, the centralized interpretation of ECGs and long term follow up increases the reliability of results from this cohort. The study also has several limitations. The study used classification of outcomes based on ICD 9 and 10 codes, which presents the potential of misclassification of diagnoses and missing information bias due to errors in coding. However, all-cause mortality is a definite outcome and was found to be significant in our study. For other outcomes, the direction of this bias is most likely towards null and hence, associations might have been underestimated. Also, many of the confounding risk factors are by self-report and potentially have interviewer or recall related bias.
In this study, we found that APC’s, but not VPC’s, were a significant predictor of all-cause mortality, cardiovascular mortality and ischemic heart disease mortality in individuals free of CVD. These findings highlight the potential use of ECG as a screening tool in asymptomatic individuals to detect subclinical cardiac disease. Further studies are needed to validate our findings and evaluate the mechanisms by which APC’s are associated with poor outcomes.
Acknowledgments
W Qureshi is funded by Ruth L. Kirsch stein NRSA Institutional Training Grant 5T32HL076132-10. AJ Shah was funded by the NIH grant UL1TR000454 (National Center for Advancing Translational Sciences of the National Institutes of Health) and KL2TR000455 (KL2 scholarship).
Footnotes
FINANCIAL DISCLOSURES:
None reported.
References
- 1.Conen D, Adam M, Roche F, Barthelemy JC, Felber Dietrich D, Imboden M, Kunzli N, von Eckardstein A, Regenass S, Hornemann T, Rochat T, Gaspoz JM, Probst-Hensch N, Carballo D. Premature atrial contractions in the general population: Frequency and risk factors. Circulation. 2012;126:2302–2308. doi: 10.1161/CIRCULATIONAHA.112.112300. [DOI] [PubMed] [Google Scholar]
- 2.Massing MW, Simpson RJ, Jr, Rautaharju PM, Schreiner PJ, Crow R, Heiss G. Usefulness of ventricular premature complexes to predict coronary heart disease events and mortality (from the atherosclerosis risk in communities cohort) Am J Cardiol. 2006;98:1609–1612. doi: 10.1016/j.amjcard.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 3.Simpson RJ, Jr, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of african american and white men and women: The atherosclerosis risk in communities (ARIC) study. Am Heart J. 2002;143:535–540. doi: 10.1067/mhj.2002.120298. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy HL, Whitlock JA, Sprague MK, Kennedy LJ, Buckingham TA, Goldberg RJ. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. New Engl J Med. 1985;312:193–197. doi: 10.1056/NEJM198501243120401. [DOI] [PubMed] [Google Scholar]
- 5.Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Atrial ectopy as a predictor of incident atrial fibrillation: A cohort study. Ann Intern Med. 2013;159:721–728. doi: 10.7326/0003-4819-159-11-201312030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolb C, Nurnberger S, Ndrepepa G, Zrenner B, Schomig A, Schmitt C. Modes of initiation of paroxysmal atrial fibrillation from analysis of spontaneously occurring episodes using a 12-lead holter monitoring system. Am J Cardiol. 2001;88:853–857. doi: 10.1016/s0002-9149(01)01891-4. [DOI] [PubMed] [Google Scholar]
- 7.Prineas RJ, Crow R, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Boston MA: John Wright PSB; 1982. [Google Scholar]
- 8.Inohara T, Kohsaka S, Okamura T, Watanabe M, Nakamura Y, Higashiyama A, Kadota A, Okuda N, Ohkubo T, Miura K, Okayama A, Ueshima H. Long-term outcome of healthy participants with atrial premature complex: A 15-year follow-up of the nippon data 90 cohort. PloS one. 2013;8:e80853. doi: 10.1371/journal.pone.0080853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheriyath P, He F, Peters I, Li X, Alagona P, Jr, Wu C, Pu M, Cascio WE, Liao D. Relation of atrial and/or ventricular premature complexes on a two-minute rhythm strip to the risk of sudden cardiac death (the atherosclerosis risk in communities [ARIC] study) Am J Cardiol. 2011;107:151–155. doi: 10.1016/j.amjcard.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Lee V, Hemingway H, Harb R, Crake T, Lambiase P. The prognostic significance of premature ventricular complexes in adults without clinically apparent heart disease: A meta-analysis and systematic review. Heart. 2012;98:1290–1298. doi: 10.1136/heartjnl-2012-302005. [DOI] [PubMed] [Google Scholar]
- 11.Elkon KB, Swerdlow TA, Myburgh DP. Persistent ventricular ectopic beats: A long-term study. S Afr Med J. 1977;52:564–566. [PubMed] [Google Scholar]
- 12.Kennedy HL, Underhill SJ. Frequent or complex ventricular ectopy in apparently healthy subjects: A clinical study of 25 cases. Am J Cardiol. 1976;38:141–148. doi: 10.1016/0002-9149(76)90140-5. [DOI] [PubMed] [Google Scholar]
- 13.De Carvalho AP, De Mello WC, Hoffman BF. Electrophysiological evidence for specialized fiber types in rabbit atrium. Am J Physiol. 1959;196:483–488. doi: 10.1152/ajplegacy.1959.196.3.483. [DOI] [PubMed] [Google Scholar]
- 14.Wit AL, Cranefield PF. Triggered and automatic activity in the canine coronary sinus. Circ Res. 1977;41:434–445. doi: 10.1161/01.res.41.4.434. [DOI] [PubMed] [Google Scholar]
- 15.Rechavia E, Strasberg B, Mager A, Zafrir N, Kusniec J, Sagie A, Sclarovsky S. The incidence of atrial arrhythmias during inferior wall myocardial infarction with and without right ventricular involvement. Am Heart J. 1992;124:387–391. doi: 10.1016/0002-8703(92)90602-r. [DOI] [PubMed] [Google Scholar]
- 16.Zoni Berisso M, Ferroni A, De Caro E, Carratino L, Mela GS, Vecchio C. Clinical significance of supraventricular tachyarrhythmias after acute myocardial infarction. Eur Heart J. 1986;7:743–748. doi: 10.1093/oxfordjournals.eurheartj.a062135. [DOI] [PubMed] [Google Scholar]
- 17.Ramsdale DR, Arumugam N, Singh SS, Pearson J, Charles RG. Holter monitoring in patients with mitral stenosis and sinus rhythm. Eur Heart J. 1987;8:164–170. doi: 10.1093/oxfordjournals.eurheartj.a062244. [DOI] [PubMed] [Google Scholar]
- 18.Davis MJ, Hockings BE, el Dessouky MA, Hajar HA, Taylor RR. Cigarette smoking and ventricular arrhythmia in coronary heart disease. Am J Cardiol. 1984;54:282–285. doi: 10.1016/0002-9149(84)90183-8. [DOI] [PubMed] [Google Scholar]
- 19.Ettinger PO, Wu CF, De La Cruz C, Jr, Weisse AB, Ahmed SS, Regan TJ. Arrhythmias and the “holiday heart”: Alcohol-associated cardiac rhythm disorders. Am Heart J. 1978;95:555–562. doi: 10.1016/0002-8703(78)90296-x. [DOI] [PubMed] [Google Scholar]
- 20.Dobmeyer DJ, Stine RA, Leier CV, Greenberg R, Schaal SF. The arrhythmogenic effects of caffeine in human beings. New Engl J Med. 1983;308:814–816. doi: 10.1056/NEJM198304073081405. [DOI] [PubMed] [Google Scholar]
- 21.Kleiger RE, Senior RM. Longterm electrocardiographic monitoring of ambulatory patients with chronic airway obstruction. Chest. 1974;65:483–487. doi: 10.1378/chest.65.5.483. [DOI] [PubMed] [Google Scholar]
- 22.Edson J, Avram MM, Gan A, Edson JN. Cardiac arrhythmias in hemodialysis patients. Proc Clin Dial Tarnsplant Forum. 1977;7:82–85. [PubMed] [Google Scholar]
- 23.Sosin MD, Bhatia G, Davis RC, Lip GY. Congestive heart failure and virchow’s triad: A neglected association. Wien Med Wochenschr. 2003;153:411–416. doi: 10.1007/s10354-003-0027-y. [DOI] [PubMed] [Google Scholar]
- 24.Chong BH, Pong V, Lam KF, Liu S, Zuo ML, Lau YF, Lau CP, Tse HF, Siu CW. Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace. 2012;14:942–947. doi: 10.1093/europace/eur389. [DOI] [PubMed] [Google Scholar]
- 25.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 26.Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, Herrington DM, Cushman M. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–114. doi: 10.1001/jamainternmed.2013.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 28.Ozawa K, Funabashi N, Takaoka H, Uehara M, Ueda M, Murakawa Y, Kobayashi Y. Various morphological-types of all and fragmented ventricular premature beats on a 12-lead holter-ecg had positive-relationship with occurrence of lv fibrosis on ct in hcm subjects. Int J Cardiol. 2014;171:450–456. doi: 10.1016/j.ijcard.2013.11.064. [DOI] [PubMed] [Google Scholar]
- 29.Bagot CN, Arya R. Virchow and his triad: A question of attribution. Br J Haematol. 2008;143:180–190. doi: 10.1111/j.1365-2141.2008.07323.x. [DOI] [PubMed] [Google Scholar]
- 30.Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Erqou S, Sattar N, Ray KK. Effect of aspirin on vascular and nonvascular outcomes: Meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:209–216. doi: 10.1001/archinternmed.2011.628. [DOI] [PubMed] [Google Scholar]


