Limonium stocksii is a potential commercial cut-flower crop for saline areas using brackish water. We therefore were interested to learn about the mechanism of its salinity tolerance. Plants grew well under lower saline conditions (300 mM NaCl) but higher salinities reduced growth. An increase in leaf osmolality and the management of salinity-induced oxidative stress are the key strategies employed. Exogenous AsA application improved the functioning of the AsA-dependent antioxidant system, leading to better growth.
Keywords: Antioxidant, exogenous ascorbic acid application, halophyte, Limonium stocksii, salt tolerance
Abstract
Salinity causes oxidative stress in plants by enhancing production of reactive oxygen species, so that an efficient antioxidant system, of which ascorbic acid (AsA) is a key component, is an essential requirement of tolerance. However, antioxidant responses of plants to salinity vary considerably among species. Limonium stocksii is a sub-tropical halophyte found in the coastal marshes from Gujarat (India) to Karachi (Pakistan) but little information exists on its salt resistance. In order to investigate the role of AsA in tolerance, 2-month-old plants were treated with 0 (control), 300 (moderate) and 600 (high) mM NaCl for 30 days with or without exogenous application of AsA (20 mM) or distilled water. Shoot growth of unsprayed plants at moderate salinity was similar to that of controls while at high salinity growth was inhibited substantially. Sap osmolality, AsA concentrations and activities of AsA-dependant antioxidant enzymes increased with increasing salinity. Water spray resulted in some improvement in growth, indicating that the growth promotion by exogenous treatments could partly be attributed to water. However, exogenous application of AsA on plants grown under saline conditions improved growth and AsA dependent antioxidant enzymes more than the water control treatment. Our data show that AsA-dependent antioxidant enzymes play an important role in salinity tolerance of L. stocksii.
Introduction
In plants soil salinity causes both osmotic stress and ionic toxicity, which can be lethal under prolonged exposure (Zhu 2001; Munns and Tester 2008; Yu et al. 2012). Halophytes survive salinity by sequestering salts in vacuoles and accumulating organic osmolytes in their cytoplasm (Flowers and Colmer 2008; Hameed and Khan 2011; Nedjimi 2014), thus reducing ion toxicity while maintaining osmo-balance. Halophytes may also employ shoot succulence, salt exclusion from roots, salt excretion through specialized salt glands and sequestering excess salt in old leaves to complete their life cycle under salinity (Flowers and Colmer 2008; Shabala and Mackay 2011). High salinity typically compromises carbon fixation, leading to the over reduction of light-harvesting complexes that cause production of reactive oxygen species (ROS) (Ozgur et al. 2013; Hamed et al. 2014). These ROS are managed within a narrow functionally important range by using enzymatic and non-enzymatic antioxidants (Jithesh et al. 2006; Bose et al. 2013). High salt stress, however, can make these systems inadequate causing severe injury that may lead to death (Hameed and Khan 2011).
Ascorbic acid (AsA) plays a key role in salt tolerance of many halophytes (Jithesh et al. 2006; Hameed et al. 2012; Ozgur et al. 2013). It quenches ROS directly as well as through the Asada–Halliwell–Foyer pathway (Noctor and Foyer 1998; Gest et al. 2013). It also recycles the lipid-soluble antioxidant α-tocopherol (Lushchak and Semchuk 2012). It may also contribute to maintaining photosynthesis, cell-cycle progression, cell wall expansion, gene expression, synthesis of many hormones, anthocyanin and flavonoids (Smirnoff and Wheeler 2000; Arrigoni and De Tullio 2002; Pignocchi and Foyer 2003; Gest et al. 2013). AsA is absorbed readily after exogenous application (Younis et al. 2010; Hameed et al. 2012) and moves within the plant (Franceschi and Tarlyn 2002; Tedone et al. 2004; Herschbach et al. 2010). Therefore, foliar application of AsA improves salt tolerance of crop plants in a number of ways (Athar et al. 2008; Dolatabadian et al. 2008; ElHariri et al. 2010; Farahat et al. 2013), but little information is available on its role in halophytes (Hameed et al. 2012).
Limonium stocksii, which is found in the coastal areas of Gujarat (India), Sindh and Balochistan (Pakistan) (Bokhari 1973), is a salt-secreting perennial halophyte in Plumbaginaceae. This species is characterized by beautiful pink-purple flowers and has the potential to become a commercially important cut-flower like many other Limonium (aka Sea Lavender or Statice) species (http://www.teleflora.com/about-flowers/statice.asp). Profit margins could be significantly enhanced if L. stocksii could be grown using seawater and on saline land. Zia et al. (2008) reported that it is a highly salt-tolerant species, but the mechanism of its salt tolerance is not very well understood. We hypothesized that AsA would play a key role in improving salt tolerance; therefore, we investigated: (i) the magnitude of oxidative damage, (ii) levels of enzymatic and non-enzymatic antioxidants and (iii) the role of exogenously applied AsA/water, in response to increasing NaCl concentration.
Methods
Seed collection and study site
Seed-bearing inflorescences of L. stocksii were collected during July 2009 from Hawks Bay, Karachi, Pakistan (24°52′21.87″N, 66°51′24.58″E, 17 ft. altitude, ∼1.5 km away from the sea front). Seeds were separated from the inflorescence and surface sterilized using 1 % sodium hypochlorite for a minute followed by rinsing with distilled water, air-dried and stored at room temperature.
Growth conditions
Seeds were sown in plastic pots (12 cm diameter) containing sandy soil and sub-irrigated with half-strength modified Hoagland's solution (Epstein 1972) soon after seedling emergence. Salinity treatments [0 (Control), 300 and 600 mM NaCl] were applied at a rate of 150 mM NaCl per day to minimize osmotic shock. Tap water was used daily to compensate for evaporative loss and the irrigation medium was replaced every third day to avoid salt build up. One week after the final salinity concentrations were reached, plant shoots were sprayed until dripping with AsA (20 mM) or distilled water (each containing 0.1 % Tween-20); these treatments continued twice a week until harvest. Unsprayed plants were controls. Five plants per pot with four pots per treatment were used and were harvested after 30 days of salinity treatment.
Growth parameters
Shoot fresh weight (FW) was measured soon after harvest while dry weight (DW) was determined after placing plant samples in a forced-draft oven at 60 °C for at least 48 h or until constant weight was achieved.
Leaf water status
Leaf sap osmolality was determined with the help of a vapour pressure osmometer (VAPRO 5520, Wescor Inc., Logan, UT, USA).
Leaf water content, the difference between FW and DW, was calculated on a DW basis using the following formula:
Osmoprotectants and antioxidants
Free proline and total soluble sugars (TSS) were quantified in hot-water extracts, which were prepared by boiling finely ground dry plant material in distilled water at 100 °C for 1 h (Khan et al. 2000). Proline in hot-water extracts was quantified according to Bates et al. (1973). Diluted hot-water extract (1 mL) was added to 1 mL of ninhydrin : glacial acetic acid (1 : 1 v/v) mixture in a test tube, followed by heating at 100 °C for 1 h. After cooling in an ice-bath, proline was estimated from the absorbance (at 520 nm; Beckman DU-530 spectrophotometer, Beckman Coulter Inc., Brea, CA, USA) of the chromophore extracted in toluene using a standard curve with pure proline.
Total soluble sugars were determined using anthrone (Yemm and Willis 1954). Diluted hot-water extract was mixed with anthrone reagent and incubated in a water bath at 100 °C for 30 min. The reaction was stopped in an ice bath and absorbance was noted at 630 nm with Beckman DU-530 spectrophotometer.
Ascorbic acid concentration was estimated by using a slightly modified method of Luwe et al. (1993). Freshly harvested plant sample (0.5 g) was ground in liquid nitrogen mortar and then homogenized in ice-cold trichloroacetic acid (TCA, 1 % w/v). The homogenate was then centrifuged at 12 000 × g for 20 min at 4 °C and the supernatant (50 µL) mixed with potassium phosphate buffer (0.95 mL, 100 mM, pH 7.0) and ascorbate oxidase (1 μL of 1 Unit µL−1). Oxidation of AsA (ε = 14.3 mM−1 cm−1) was then recoded at 265 nm.
Antioxidant enzymes
Antioxidant enzymes were extracted as described by Polle et al. (1994) with some modifications. Fresh plant samples (0.5 g) were powdered in liquid nitrogen mortar and homogenized in potassium phosphate buffer (5 mL of 100 mM, pH 7.0) containing 0.5 % triton X-100, 2 % (w/v) polyvinylpyrrolidone, 5 mM disodium ethylenediaminetetraacetic acid and 1 mM l-AsA. Homogenates were then centrifuged at 12 000 × g for 20 min at 4 °C and the supernatants used in enzyme assays carried out at 25 °C. The activity of superoxide dismutase (SOD; EC 1.15.1.1) was measured by the method of Beauchamp and Fridovich (1971); catalase (CAT; EC 1.11.1.6) by Aebi (1984); guaiacol peroxidase (GPX, EC 1.11.1.7) by Zaharieva et al. (1999); ascorbate peroxidase (APX, EC 1.11.1.11) by Nakano and Asada (1981) and glutathione reductase (GR, EC 1.6.4.2) by Foyer and Halliwell (1976). Enzyme activities were defined as in original methods and expressed as units of enzyme activity per milligram protein, which was estimated according to Bradford (1976).
Hydrogen peroxide content
Endogenous hydrogen peroxide (H2O2) was measured using the method of Loreto and Velikova (2001).
Lipid peroxidation
The extent of lipid peroxidation was estimated by quantifying the malondialdehyde (MDA) content of leaves according to the method of Heath and Packer (1968).
Statistical analysis
Statistical analysis of the data was carried out using SPSS version 11.0 for windows (SPSS Inc. 2001). Two-way analysis of variances (ANOVAs) were used to determine whether salinity, exogenous treatments and their interactions as grouping factors had significant effect on response variables such as biomass and biochemical parameters. A post hoc Bonferroni test was performed to find significant (P < 0.05) differences among individual means of the treatments.
Results
Growth parameters
Two-way ANOVA indicated significant effects of salinity, exogenous treatments and their interaction on shoot FW and DW of L. stocksii (Table 1). Neither FW nor DW of the control (untreated) plants was affected by moderate salinity (300 mM NaCl). However, high salinity (600 mM NaCl) reduced plant growth to ∼50 % in comparison with control (Fig. 1). Exogenous application of water improved FW and DW under saline conditions, while application of exogenous AsA solution ameliorated FW and DW in all salinity treatments. Improvement in plant DW by AsA application was significantly higher than that by water-spray, while FW improvement by AsA and water-sprays were comparable.
Table 1.
Two-way ANOVA of the effects of exogenous treatments (E), salinity (S) and their interaction (E × S) on different parameters of Limonium stocksii. Numbers are the F-values with the level of significance given as superscript. *P < 0.05, **P < 0.01, ***P < 0.001 and ns, non-significant.
| Parameters | E | S | E × S |
|---|---|---|---|
| FW | 13.172*** | 15.684*** | 3.312* |
| DW | 23.557*** | 44.626*** | 3.432* |
| Leaf osmolality | 3.308ns | 1.125* | 1.480ns |
| MDA | 23.069*** | 28.447*** | 5.240*** |
| H2O2 | 8.254** | 1.601* | 2.377* |
| Proline | 30.134*** | 1.593ns | 3.393ns |
| TSS | 970.97*** | 391.98*** | 50.981*** |
| AsA | 531.19*** | 688.07*** | 342.2*** |
| SOD | 4.051* | 54.321*** | 2.506ns |
| CAT | 1.238ns | 3.811ns | 0.920ns |
| GPX | 0.529ns | 23.781*** | 0.109ns |
| APX | 37.139*** | 29.036*** | 5.183** |
| GR | 26.738*** | 19.499*** | 1.499* |
Figure 1.
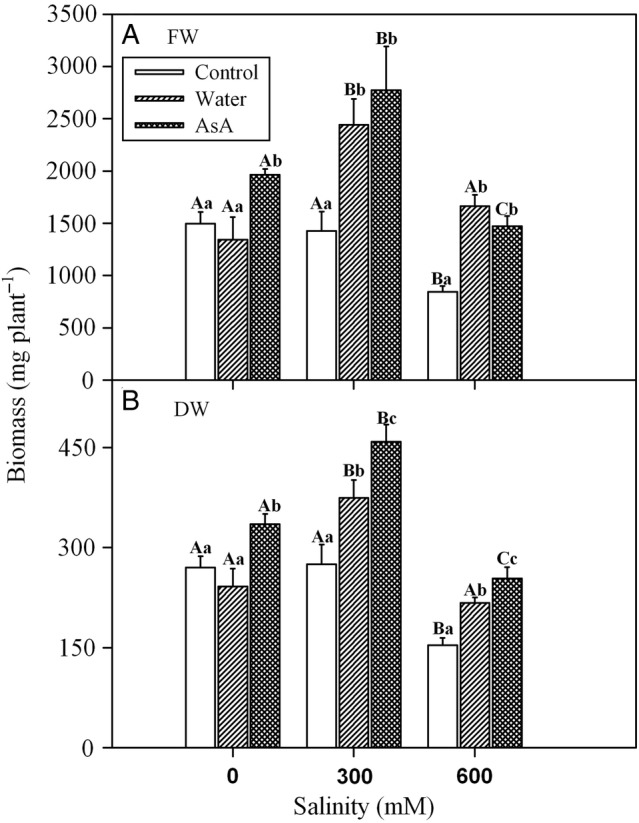
Effect of salinity and exogenous treatments on shoot growth of L. stocksii seedlings. Fresh (A) and dry (B) weight values (in mg plant−1) are given as bars representing mean ± standard error of four plants. Different capital letters across salinity treatments and small letters within each salinity level are significantly different from each other (P < 0.05; Bonferroni test).
Leaf water status
Analysis of variance showed a significant effect of salinity on the osmolality of leaf sap of L. stocksii seedlings (Table 1). Increase in salinity caused a rise in osmolality (Fig. 2A), although exogenous treatments did not affect the values of osmolality. Leaf water content was not affected by increases in salinity (Fig. 2B). Exogenous treatments had no effect on leaf water content, except that water-spray at 600 mM NaCl increased water content compared with other treatments and control.
Figure 2.

Effect of salinity and exogenous treatments on (A) leaf sap osmolality and (B) water content of L. stocksii seedlings. Bars represent mean ± standard error. Different capital letters across salinity treatments and small letters within each salinity level are significantly different from each other (P < 0.05; Bonferroni test).
Osmoprotectants and antioxidants
Salinity did not affect proline concentrations in L. stocksii (Table 1). Exogenous AsA increased proline concentrations of L. stocksii seedlings in all salinity treatments while water-spray enhanced proline concentrations only in 600 mM NaCl treatment (Fig. 3A). TSS increased under saline conditions and exogenous treatments resulted in a further increase in the concentration of TSS, with pronounced effects in the case of exogenous AsA (Table 1 and Fig. 3B). Salinity treatments caused an increase in endogenous AsA concentration and exogenous treatments, in particular exogenous AsA, further increased endogenous AsA levels (Table 1 and Fig. 3C).
Figure 3.
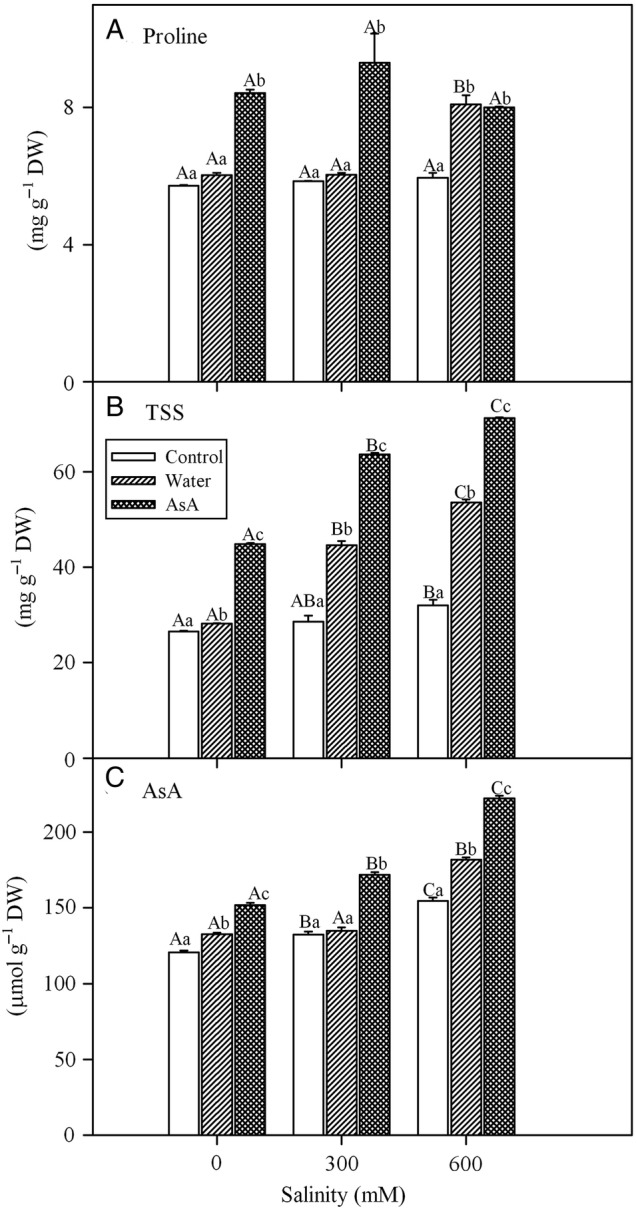
Effect of salinity and exogenous treatments on (A) proline, (B) TSS and (C) reduced ascorbate (AsA) concentrations of L. stocksii leaves. Bars represent mean ± standard error. Different capital letters across salinity treatments and small letters within each salinity level are significantly different from each other (P < 0.05; Bonferroni test).
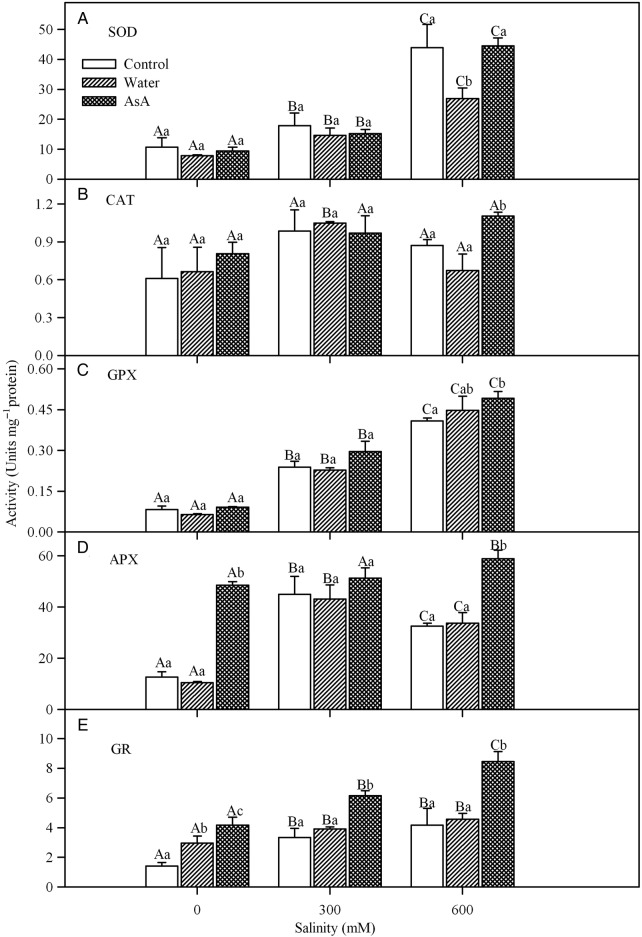
Antioxidant enzymes
Salinity, exogenous treatments and their interaction had no significant effect on CAT activity (Table 1 and Fig. 4B). However, the activity of SOD increased with increases in salinity (Fig. 4A) although exogenous treatments had no significant effect on SOD activity (Table 1). Likewise, the activity of GPX increased significantly with increases in NaCl concentration (Fig. 4C), but it was unaffected by exogenous treatments and their interaction (Table 1). The activity of APX showed a somewhat different response; activity increased under saline conditions with a maximum value in 300 mM NaCl treatment and exogenous treatment with AsA also caused a significant increase in APX activity (Fig. 4D). Salinity, exogenous treatments and their interaction had a significant effect on GR activity (Table 1) and exogenous AsA caused a substantial increase in GR activity (Fig. 4E).
Figure 4.
Effects of salinity and exogenous treatments on SOD (A), CAT (B), GPX (C), APX (D) and GR (E) activities of L. stocksii leaves. Bars represent mean ± standard error. Different capital letters across salinity treatments and small letters within each salinity level are significantly different from each other (P < 0.05; Bonferroni test).
Hydrogen peroxide content
Analysis of variance indicated a significant effect of salinity, exogenous treatments and their interaction on H2O2 concentration (Table 1). The concentration of H2O2 increased with an increase in salinity but exogenous treatments with both water and AsA decreased endogenous H2O2 levels under saline conditions (Fig. 5).
Figure 5.
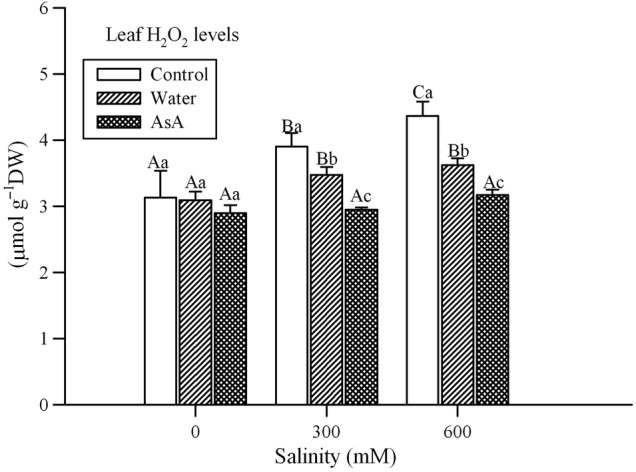
Effect of salinity and exogenous treatments on hydrogen peroxide (H2O2) concentrations of L. stocksii leaves. Bars represent mean ± standard error. Different capital letters across salinity treatments and small letters within each salinity level are significantly different from each other (P < 0.05; Bonferroni test).
Lipid peroxidation
A significant effect of salinity, exogenous treatments and their interaction was found on MDA concentration (indicator of lipid peroxidation) of L. stocksii seedlings (Table 1). An increase in salinity resulted in a rise in MDA concentration and exogenous treatments reduced MDA under saline conditions (Fig. 6).
Figure 6.
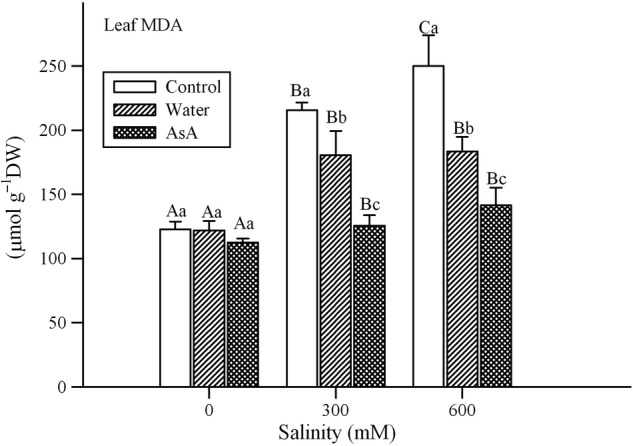
Effect of salinity and exogenous treatments on lipid peroxidation (MDA concentration) in L. stocksii leaves. Bars represent mean ± standard error. Different capital letters across salinity treatments and small letters within each salinity level are significantly different from each other (P < 0.05; Bonferroni test).
Discussion
Limonium stocksii gained similar biomass over 30 days in 300 mM NaCl as in the absence of salt; however, a further increase in salinity markedly inhibited biomass production. Although growth inhibition occurred at high salinity, L. stocksii appears to be more tolerant than other members of the genus: as growth inhibition was observed in L. perezii at 110 mM NaCl (Grieve et al. 2005) and in L. sinense at 300 mM NaCl (Ding et al. 2009); growth of L. pectinatum was promoted at 100 mM NaCl and was similar to control at 200 mM NaCl (Morales et al. 2001). Our results are also similar to growth responses reported for co-occurring halophytes such as Suaeda fruticosa, Arthrocnemum macrostachyum, Avicennia marina, Aeluropus lagopoides and Urochondra setulosa (Gul and Khan 1998; Aziz and Khan 2000; Khan et al. 2000; Gulzar and Khan 2006).
Halophytes utilize internal inorganic ions (Na+ and Cl−) for osmotic adjustment, by sequestering them in vacuoles with associated synthesis and accumulation of organic/compatible solutes in the cytoplasm (Wyn Jones and Gorham 2002; Flowers and Colmer 2008; Hussin et al. 2013). The increase in leaf sap osmolality of L. stocksii seedlings with rise in external salinity could be an indicator of such solute accumulation for osmotic adjustment. The linear increase in shoot Na+ and Cl− ions in L. stocksii seedlings reported in an earlier study (Zia et al. 2008) also supports this assumption. Similar results have been reported for co-occurring species such as S. fruticosa (Hameed et al. 2012), A. marina (Aziz and Khan 2000) and U. setulosa (Gulzar and Khan 2006). Accumulation of compatible solutes such as choline-O-sulfate and betaines of β-alanine, proline and hydroxyl-proline, as reported for taxa within Plumbaginaceae, could also be responsible for osmotic adjustment in our test species (Hanson et al. 1994; Gagneul et al. 2007; Flowers and Colmer 2008).
Salinity is widely reported to enhance production of ROS (Ozgur et al. 2013), which can damage important cell components such as proteins, lipids and nucleic acids (Blokhina et al. 2003; Apel and Hirt 2004; Gill and Tuteja 2010). We also observed an increase in H2O2 concentration (a common ROS) in L. stocksii seedlings in response to increasing salinity, as reported for many other halophytes (Jithesh et al. 2006; Ozgur et al. 2013). Lipid peroxidation, a non-enzymatic autoxidation process due to ROS, is commonly used as a measure of salinity-induced oxidative stress and plant sensitivity (Ozgur et al. 2013). It is generally measured in terms of MDA contents, which are common end-products of lipid peroxidation. Generally, a positive linear relationship between MDA concentration and salinity is reported for halophytes (Jithesh et al. 2006; Ozgur et al. 2013). For instance, MDA concentrations increased with increasing salinity in halophytes such as Cakile maritima (Ksouri et al. 2007), Sesuvium portulacastrum (Lokhande et al. 2011), Gypsophila oblanceolata (Sekmen et al. 2012) and Sphaerophysa kotschyana (Yildiztugay et al. 2013). We also found a linear increase in MDA concentrations with increasing NaCl treatments in L. stocksii. Although the relationship between MDA levels and plant performance is complex (Hameed et al. 2012; Alhdad et al. 2013), some stress signalling roles of MDA are also emerging (Weber et al. 2004). Slightly higher MDA concentrations in S. fruticosa (Hameed et al. 2012) were associated with better growth.
Halophytes employ a number of enzymatic and non-enzymatic antioxidants to prevent oxidative damage and keep ROS concentrations within a narrow functional rage (Jithesh et al. 2006; Ozgur et al. 2013). Superoxide dismutase is considered the first line of defence against ROS under stress conditions (Alscher et al. 2002). Limonium stocksii seedlings under saline conditions showed increased activity of SOD, which was similar to that reported for Bruguiera parviflora (Parida et al. 2004), Atriplex portulacoides (Benzarti et al. 2012) and S. portulacastrum (Lokhande et al. 2011). Catalase activity in L. stocksii remained unchanged with the increase in salinity indicating either constitutive expression or importance of other than CAT-based H2O2 detoxification under salinity stress. Similar CAT activity was also reported for another halophyte A. portulacoides in up to 400 mM NaCl salinity (Benzarti et al. 2012). Guaiacol peroxidase activity increased significantly in L. stocksii seedlings under salinity as in B. parviflora under saline conditions (Parida et al. 2004). Apart from their role in H2O2 detoxification, GPXs are also involved in a number of physio-chemical processes such as growth, auxin metabolism, biosynthesis of ethylene and lignin (Lagrimini and Rothstein 1987; Dionisio-Sese and Tobita 1998; Kim et al. 1999; Matamoros et al. 2003; Jouili et al. 2011). Activities of APX and GR were reported to increase in A. portulacoides (Benzarti et al. 2012), Salicornia brachiata (Parida and Jha 2010) and B. parviflora (Parida et al. 2004) under salinity stress (Jithesh et al. 2006). A similar increase in the activities of these enzymes in L. stocksii was also observed indicating their role in ROS detoxification under salinity stress.
Many low-molecular-weight antioxidant substances such as AsA are also involved in ROS detoxification in halophytes (Jithesh et al. 2006; Hameed and Khan 2011). AsA contents of L. stocksii leaves increased under saline conditions similar to Sphaerophysa kotschyana (Yildiztugay et al. 2013). Increased AsA concetrations in L. stocksii could be due to better recycling via the Asada–Halliwell–Foyer pathway and/or its increased biosynthesis. Soluble sugar concentrations of L. stocksii leaves increased under saline conditions and this supports a direct relationship between sugar (sucrose) concentration and AsA biosynthesis (Nishikawa et al. 2005). Soluble sugars can also act as ROS scavengers (Couée et al. 2006; Nishizawa et al. 2008; Keunen et al. 2013).
Proline accumulation is often used as stress indicator in plants (Kishor and Sreenivasulu 2013; Ben Rejeb et al. 2014). However, proline levels remained unchanged in L. stocksii seedlings under saline conditions. This may indicate adequate stress management and the absence of injury symptoms also supports this assumption.
Experiments for studying effects of exogenous application of different chemicals such as AsA involve spray of aqueous solutions and any improvement in growth and salt tolerance by such treatments may be due to water rather than the chemical. Hameed et al. (2012) showed the importance of such water-spray in salinity tolerance of a coastal halophyte S. fruticosa. Water-spray improved sub-cellular defence in S. fruticosa probably by mitigating osmotic constraint thereby water acquisition (Hameed et al. 2012). Water-spray on L. stocksii seedlings resulted in a significant improvement in growth and levels of antioxidant enzymes. This indicates that the improvement in growth by exogenous AsA treatment could partly be due to water in the solution rather than the AsA itself: an increase in leaf water content and levels of TSS and proline and low oxidative damage support this conclusion. However, the DW was greater in the AsA treatment than the water-spray alone at all salinities (Fig. 1), suggesting specific effects of AsA. At high salinity, the TSS concentration and the activities of the antioxidant enzymes were higher with the AsA-treatment than with the water-spray alone (only for GPX the difference was not significant). The proline concentration was higher in the AsA treatment than with the water-spray at the 300 mM treatment, but not at the higher salinity. Based on our results, it appears that AsA does bring about specific improvements to growth and these were associated with biochemical changes in the leaves. Similar results for the treatment with AsA have been reported for S. fruticosa (Hameed et al. 2012) and in many non-halophytes (Shalata and Neumann 2001; Dolatabadian et al. 2008; Younis et al. 2010). Exogenously applied AsA is easily absorbed, transported and metabolized in plants (Sapers et al. 1991; Franceschi and Tarlyn 2002), where it has a variety of metabolic and physiological functions in antioxidant defence, photosynthesis, transmembrane electron transport, biosynthesis of plant hormones and/or cell expansion (Conklin et al. 1996; Arrigoni and De Tullio 2000, 2002; Khan et al. 2011).
Exogenous application of AsA decreased lipid peroxidation in L. stocksii seedlings under salinity as reported for S. fruticosa (Hameed et al. 2012) and in crops such as Brassica napus (Dolatabadian et al. 2008) and Phaseolus vulgaris (Saeidi-Sar et al. 2013), indicating better antioxidant defence. Shalata and Neumann (2001) pointed out that the protective effects of exogenous AsA appeared to be related to its antioxidant activity rather than its possible use as an organic substrate for respiratory metabolism. Exogenous AsA significantly increased levels of endogenous AsA and Asada–Halliwell–Foyer pathway enzymes in L. stocksii seedlings compared with both un-sprayed and water-sprayed plants. Likewise, exogenous AsA also increased APX and GR activities in Vicia faba seedlings compared with control plants (Younis et al. 2010). These findings show that the exogenous AsA improves salinity tolerance through stimulating antioxidant defence.
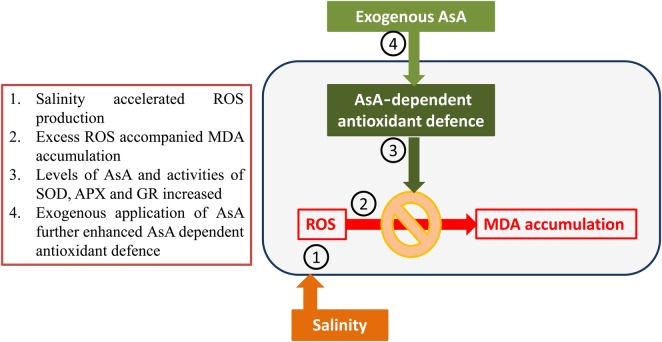
Conclusions
Shoot growth of L. stocksii plants was inhibited at high salinity and coincided with higher levels of MDA. Leaf osmolality progressively increased to maintain osmo-balance. AsA-dependent antioxidant enzymes contributed to the salinity resistance as evident by increasing level of AsA and activities of Asada–Halliwell–Foyer pathway enzymes (Fig. 7). Exogenous AsA improved the functioning of AsA-dependent antioxidant system.
Figure 7.
Pictorial description of key findings.
Sources of Funding
The authors thank Higher Education Commission of Pakistan for provision of funds under a research grant entitled ‘Salt-induced Oxidative Stress: Consequences and Possible Management’.
Contributions by the Authors
Obtaining funds: M.A.K.; experiment designing: A.H., B.G., M.A.K.; execution of experiments: A.H., T.H.; data analyses: A.H., S.G., I.A., B.G.; paper writing: M.A.K., A.H., S.G., I.A.
Conflicts of Interest Statement
None declared.
Acknowledgements
We thank Ms Aysha Rasheed for her help in some biochemical assays. We also thank anonymous reviewers and Associate Editor Tim Flowers for their valuable comments, which were very helpful.
Literature Cited
- Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Alhdad GM, Seal CE, Al-Azzawi MJ, Flowers TJ. The effect of combined salinity and waterlogging on the halophyte Suaeda maritima: the role of antioxidants. Environmental and Experimental Botany. 2013;87:120–125. doi: 10.1016/j.envexpbot.2012.10.010. [DOI] [Google Scholar]
- Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. Journal of Experimental Botany. 2002;53:1331–1341. doi: 10.1093/jexbot/53.372.1331. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Arrigoni O, De Tullio MC. The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. Journal of Plant Physiology. 2000;157:481–488. doi: 10.1016/S0176-1617(00)80102-9. [DOI] [Google Scholar]
- Arrigoni O, De Tullio MC. Ascorbic acid: much more than just an antioxidant. Biochimica et Biophysica Acta. 2002;1569:1–9. doi: 10.1016/S0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- Athar HR, Khan A, Ashraf M. Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environmental and Experimental Botany. 2008;63:224–231. doi: 10.1016/j.envexpbot.2007.10.018. [DOI] [Google Scholar]
- Aziz I, Khan MA. Physiological adaptations to seawater concentration in Avicennia marina from Indus delta, Pakistan. Pakistan Journal of Botany. 2000;32:151–170. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant and Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Ben Rejeb K, Abdelly C, Savouré A. How reactive oxygen species and proline face stress together. Plant Physiology and Biochemistry. 2014;80:278–284. doi: 10.1016/j.plaphy.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Benzarti M, Rejeb KB, Debez A, Messedi D, Abdelly C. Photosynthetic activity and leaf antioxidative responses of Atriplex portulacoides subjected to extreme salinity. Acta Physiologiae Plantarum. 2012;34:1679–1688. doi: 10.1007/s11738-012-0963-5. [DOI] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokhari MH. Pakistan: University of Karachi Press; 1973. Flora of West Pakistan No. 28: Plumbaginaceae. [Google Scholar]
- Bose J, Rodrigo-Moreno A, Shabala S. ROS homeostasis in halophytes in the context of salinity stress tolerance. Journal of Experimental Botany. 2013;65:1241–1257. doi: 10.1093/jxb/ert430. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Conklin PL, Williams EH, Last RL. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proceedings of the National Academy of Sciences of the USA. 1996;93:9970–9974. doi: 10.1073/pnas.93.18.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couée I, Sulmon C, Gouesbet G, El Amrani A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. Journal of Experimental Botany. 2006;57:449–459. doi: 10.1093/jxb/erj027. [DOI] [PubMed] [Google Scholar]
- Ding F, Song J, Ruan Y, Wang B-S. Comparison of the effects of NaCl and KCl at the roots on seedling growth, cell death and the size, frequency and secretion rate of salt glands in leaves of Limonium sinense. Acta Physiologiae Plantarum. 2009;31:343–350. doi: 10.1007/s11738-008-0240-9. [DOI] [Google Scholar]
- Dionisio-Sese ML, Tobita S. Antioxidant responses of rice seedlings to salinity stress. Plant Science. 1998;135:1–9. doi: 10.1016/S0168-9452(98)00025-9. [DOI] [Google Scholar]
- Dolatabadian A, Sanavy SAMM, Chashmi NA. The effects of foliar application of ascorbic acid (Vitamin C) on antioxidant enzymes activities, lipid peroxidation and proline accumulation of Canola (Brassica napus L.) under conditions of salt stress. Journal of Agronomy and Crop Science. 2008;194:206–213. doi: 10.1111/j.1439-037X.2008.00301.x. [DOI] [Google Scholar]
- El Hariri DM, Sadak MS, El-Bassiouny HMS. Response of flax cultivars to ascorbic acid and α-tocopherol under salinity stress conditions. International Journal of Academic Research. 2010;2:101–109. [Google Scholar]
- Epstein E. Mineral nutrition in plants: principles and perspectives. New York: Wiley; 1972. [Google Scholar]
- Farahat MM, Mazhar AAM, Mahgoub MH, Zaghloul SM. Salt tolerance in Grevillea robusta seedlings via foliar application of ascorbic acid. Middle-East Journal of Scientific Research. 2013;14:9–15. [Google Scholar]
- Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytologist. 2008;179:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Tarlyn NM. L-ascorbic acid is accumulated in source leaf phloem and transported to sink tissues in plants. Plant Physiology. 2002;130:649–656. doi: 10.1104/pp.007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneul D, Aïnouche A, Duhazé C, Lugan R, Larher FR, Bouchereau A. A reassessment of the function of the so-called compatible solutes in the halophytic Plumbaginaceae Limonium latifolium. Plant Physiology. 2007;144:1598–1611. doi: 10.1104/pp.107.099820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gest N, Gautier H, Stevens R. Ascorbate as seen through plant evolution: the rise of a successful molecule. Journal of Experimental Botany. 2013;64:33–53. doi: 10.1093/jxb/ers297. [DOI] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Grieve CM, Poss JA, Grattan SR, Shouse PJ, Leith JH, Zeng L. Productivity and mineral nutrition of Limonium species irrigated with saline wastewaters. HortScience. 2005;40:654–665. [Google Scholar]
- Gul B, Khan MA. Population characteristics of a coastal halophyte Arthrocnemum macrostachyum. Pakistan Journal of Botany. 1998;30:189–197. [Google Scholar]
- Gulzar S, Khan MA. Comparative salt tolerance of perennial grasses. In: Khan MA, Weber DJ, editors. Ecophysiology of high salinity tolerant plants. The Netherlands: Springer; 2006. pp. 239–253. [Google Scholar]
- Hamed KB, Chibani F, Abdelly C, Magne C. Growth, sodium uptake and antioxidant responses of coastal plants differing in their ecological status under increasing salinity. Biologia. 2014;69:193–201. doi: 10.2478/s11756-013-0304-1. [DOI] [Google Scholar]
- Hameed A, Khan MA. Halophytes: biology and economic potentials. Karachi University Journal of Science. 2011;39:40–44. [Google Scholar]
- Hameed A, Hussain T, Gulzar S, Aziz I, Gul B, Khan MA. Salt tolerance of a cash crop halophyte Suaeda fruticosa: biochemical responses to salt and exogenous chemical treatments. Acta Physiologiae Plantarum. 2012;34:2331–2340. doi: 10.1007/s11738-012-1035-6. [DOI] [Google Scholar]
- Hanson AD, Rathinasabapathi B, Rivoal J, Burnet M, Dillon MO, Gage DA. Osmoprotective compounds in the Plumbaginaceae: a natural experiment in metabolic engineering of stress tolerance. Proceedings of the National Academy of Sciences of the USA. 1994;91:306–310. doi: 10.1073/pnas.91.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Herschbach C, Scheerer U, Rennenberg H. Redox states of glutathione and ascorbate in root tips of poplar (Populus tremula × P. alba) depend on phloem transport from the shoot to the roots. Journal of Experimental Botany. 2010;61:1065–1074. doi: 10.1093/jxb/erp371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussin S, Geissler N, Koyro H-W. Effect of NaCl salinity on Atriplex nummularia (L.) with special emphasis on carbon and nitrogen metabolism. Acta Physiologiae Plantarum. 2013;35:1025–1038. doi: 10.1007/s11738-012-1141-5. [DOI] [Google Scholar]
- Jithesh MN, Prashanth SR, Sivaprakash KR, Parida AK. Antioxidative response mechanisms in halophytes: their role in stress defence. Journal of Genetics. 2006;85:237–254. doi: 10.1007/BF02935340. [DOI] [PubMed] [Google Scholar]
- Jouili H, Bouazizi H, El Ferjan E. Plant peroxidases: biomarkers of metallic stress. Acta Physiologiae Plantarum. 2011;33:2075–2082. doi: 10.1007/s11738-011-0780-2. [DOI] [Google Scholar]
- Keunen E, Peshev D, Vangronsveld J, Van Den Ende W, Cuypers A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant, Cell and Environment. 2013;36:1242–1255. doi: 10.1111/pce.12061. [DOI] [PubMed] [Google Scholar]
- Khan MA, Ungar IA, Showalter AM. The effect of salinity on the growth, water status, and ion content of a leaf succulent perennial halophyte, Suaeda fruticosa (L.) Forssk. Journal of Arid Environments. 2000;45:73–84. doi: 10.1006/jare.1999.0617. [DOI] [Google Scholar]
- Khan TA, Mazid M, Mohammad F. A review of ascorbic acid potentialities against oxidative stress induced in plants. Journal of Agrobiology. 2011;28:97–111. [Google Scholar]
- Kim K-Y, Huh G-H, Lee H-S, Kwon S-Y, Hur Y, Kwak S-S. Molecular characterization of cDNAs for two anionic peroxidases from suspension cultures of sweet potato. Molecular and General Genetics. 1999;261:941–947. doi: 10.1007/s004380051041. [DOI] [PubMed] [Google Scholar]
- Kishor PBK, Sreenivasulu N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant, Cell and Environment. 2013;37:300–311. doi: 10.1111/pce.12157. [DOI] [PubMed] [Google Scholar]
- Ksouri R, Megdiche W, Debez A, Falleh H, Grignon C, Abdelly C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiology and Biochemistry. 2007;45:244–249. doi: 10.1016/j.plaphy.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Lagrimini ML, Rothstein S. Tissue specificity of tobacco peroxidase isozymes and their induction by wounding and tobacco mosaic virus infection. Plant Physiology. 1987;84:438–442. doi: 10.1104/pp.84.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokhande VH, Nikam TD, Patade VY, Ahire ML, Suprasanna P. Effects of optimal and supra-optimal salinity stress on antioxidative defence, osmolytes and in vitro growth responses in Sesuvium portulacastrum L. Plant Cell, Tissue and Organ Culture. 2011;104:41–49. doi: 10.1007/s11240-010-9802-9. [DOI] [Google Scholar]
- Loreto F, Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiology. 2001;127:1781–1787. doi: 10.1104/pp.010497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak VI, Semchuk NM. Tocopherol biosynthesis: chemistry, regulation and effects of environmental factors. Acta Physiologiae Plantarum. 2012;34:1607–1628. doi: 10.1007/s11738-012-0988-9. [DOI] [Google Scholar]
- Luwe MWF, Takahama U, Heber U. Role of ascorbate in detoxifying ozone in the apoplast of spinach (Spinacia oleracea L.) leaves. Plant Physiology. 1993;101:969–976. doi: 10.1104/pp.101.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoros MA, Dalton DA, Ramos J, Clemente MR, Rubio MC, Becana M. Biochemistry and molecular biology of antioxidants in the rhizobia-legume symbiosis. Plant Physiology. 2003;133:499–509. doi: 10.1104/pp.103.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales MA, Olmos E, Torrecillas A, Sánchez-Blanco MJ, Alarcon JJ. Differences in water relations, leaf ion accumulation and excretion rates between cultivated and wild species of Limonium sp. grown in conditions of saline stress. Flora. 2001;196:345–352. [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology. 1981;22:867–880. [Google Scholar]
- Nedjimi B. Effects of salinity on growth, membrane permeability and root hydraulic conductivity in three saltbush species. Biochemical Systematics and Ecology. 2014;52:4–13. doi: 10.1016/j.bse.2013.10.007. [DOI] [Google Scholar]
- Nishikawa F, Kato M, Hyodo H, Ikoma Y, Sugiura M, Yano M. Effect of sucrose on ascorbate level and expression of genes involved in the ascorbate biosynthesis and recycling pathway in harvested broccoli florets. Journal of Experimental Botany. 2005;56:65–72. doi: 10.1093/jxb/eri007. [DOI] [PubMed] [Google Scholar]
- Nishizawa A, Yabuta Y, Shigeoka S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiology. 2008;147:1251–1263. doi: 10.1104/pp.108.122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Ozgur R, Uzilday B, Sekmen AH, Turkan I. Reactive oxygen species regulation and antioxidant defence in halophytes. Functional Plant Biology. 2013;40:832–847. doi: 10.1071/FP12389. [DOI] [PubMed] [Google Scholar]
- Parida AK, Jha B. Salt tolerance mechanisms in mangroves: a review. Trees — Structure and Function. 2010;24:199–217. doi: 10.1007/s00468-010-0417-x. [DOI] [Google Scholar]
- Parida AK, Das AB, Mohanty P. Defense potentials to NaCl in a mangrove, Bruguiera parviflora: differential changes of isoforms of some antioxidative enzymes. Journal of Plant Physiology. 2004;161:531–542. doi: 10.1078/0176-1617-01084. [DOI] [PubMed] [Google Scholar]
- Pignocchi C, Foyer CH. Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Current Opinion in Plant Biology. 2003;6:379–389. doi: 10.1016/S1369-5266(03)00069-4. [DOI] [PubMed] [Google Scholar]
- Polle A, Otter T, Seifert F. Apoplastic peroxidases and lignification in needles of Norway spruce (Picea abies L.) Plant Physiology. 1994;106:53–56. doi: 10.1104/pp.106.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeidi-Sar S, Abbaspour H, Afshari H, Yaghoobi SR. Effects of ascorbic acid and gibberellin A3 on alleviation of salt stress in common bean (Phaseolus vulgaris L.) seedlings. Acta Physiologiae Plantarum. 2013;35:667–677. doi: 10.1007/s11738-012-1107-7. [DOI] [Google Scholar]
- Sapers GM, Miller RL, Douglas FW, Jr, Hicks KB. Uptake and fate of ascorbic acid-2-phosphate in infiltrated fruit and vegetable tissue. Journal of Food Science. 1991;56:419–422. doi: 10.1111/j.1365-2621.1991.tb05294.x. [DOI] [Google Scholar]
- Sekmen AH, Turkan I, Tanyolac ZO, Ozfidan C, Dinc A. Different antioxidant defense responses to salt stress during germination and vegetative stages of endemic halophyte Gypsophila oblanceolata Bark. Environmental and Experimental Botany. 2012;77:63–76. doi: 10.1016/j.envexpbot.2011.10.012. [DOI] [Google Scholar]
- Shabala S, Mackay A. Ion transport in halophytes. In: Kader JC, Delseny M, editors. Advances in botanical research. Vol. 57. Amsterdam: Elsevier; 2011. pp. 151–199. [Google Scholar]
- Shalata A, Neumann PM. Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. Journal of Experimental Botany. 2001;52:2207–2211. doi: 10.1093/jexbot/52.364.2207. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. Critical Reviews in Biochemistry and Molecular Biology. 2000;35:291–314. doi: 10.1080/10409230008984166. [DOI] [PubMed] [Google Scholar]
- SPSS. SPSS version 11.0 for Windows. SPSS Inc; 2001. [Google Scholar]
- Tedone L, Hancock RD, Alberino S, Haupt S, Viola R. Long-distance transport of L-ascorbic acid in potato. BMC Plant Biology. 2004;4:16. doi: 10.1186/1471-2229-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Chételat A, Reymond P, Farmer EE. Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. The Plant Journal. 2004;37:877–888. doi: 10.1111/j.1365-313X.2003.02013.x. [DOI] [PubMed] [Google Scholar]
- Wyn-Jones RG, Gorham J. Intra- and inter-cellular compartmentation of ions. In: Läuchli A, Lüttge U, editors. Salinity: environment — plants — molecules. Dordrecht: Kluwer Academic Publishers; 2002. pp. 159–180. [Google Scholar]
- Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiztugay E, Ozfidan-Konakci C, Kucukoduk M. Sphaerophysa kotschyana, an endemic species from Central Anatolia: antioxidant system responses under salt stress. Journal of Plant Research. 2013;126:729–742. doi: 10.1007/s10265-013-0573-3. [DOI] [PubMed] [Google Scholar]
- Younis ME, Hasaneen MNA, Kazamel AMS. Exogenously applied ascorbic acid ameliorates detrimental effects of NaCl and mannitol stress in Vicia faba seedlings. Protoplasma. 2010;239:39–48. doi: 10.1007/s00709-009-0080-5. [DOI] [PubMed] [Google Scholar]
- Yu S, Wang W, Wang B. Recent progress of salinity tolerance research in plants. Russian Journal of Genetics. 2012;48:497–505. doi: 10.1134/S1022795412050225. [DOI] [PubMed] [Google Scholar]
- Zaharieva T, Yamashita K, Matsumoto H. Iron deficiency induced changes in ascorbate content and enzyme activities related to ascorbate metabolism in cucumber roots. Plant and Cell Physiology. 1999;40:273–280. doi: 10.1093/oxfordjournals.pcp.a029538. [DOI] [Google Scholar]
- Zhu J-K. Plant salt tolerance. Trends in Plant Science. 2001;6:66–71. doi: 10.1016/S1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- Zia S, Egan T, Khan MA. Growth and selective ion transport of Limonium stocksii under saline conditions. Pakistan Journal of Botany. 2008;40:697–709. [Google Scholar]