Abstract
Matrilineal overthrows in macaque societies are rare but devastating events, often resulting in severe morbidity, mortality, and loss of individual and group fitness. The handful of documented macaque overthrows provides scant evidence to reveal the severity or longevity of reproductive consequences resulting from such violent events. We analyzed archival records from semi-free ranging rhesus monkeys, Macaca mulatta, across six years [55≤N≤107, from 2007–2012] during which time a matrilineal overthrow occurred [in 2009] to test the hypothesis that extremely violent interactions such as a matrilineal overthrow would significantly reduce reproductive fitness for the involved matrilines and for the troop collectively. The matrilineal overthrow resulted in a significant increase in infant loss for the population from the previous year [χ2=8.117, df=1, P=0.004], as evidenced by the fact that in 2009, but not in other years, the proportion of infants lost was greater than the proportion of viable infants [χ2=4.55, df=1, P=0.03]. Moreover, the deposed matriline suffered 100% infant loss in 2009, a significant change from the previous year [χ2=7.87, df=1, P=0.005] while the attacking matriline suffered 50% infant loss [also a significant change from the previous year; χ2=4.44, df=1, P=0.035], with the uninvolved, lowest-ranking matriline showing no change in infant loss from the previous year [χ2=0.008, df=1, P=0.93]. The deposed matriline did not produce viable offspring again until three years later. We further found that rates of severe fighting [as indicated by the number of fight wounds requiring medical treatment] were positively correlated with infant loss across the six years of the study [r[s]=0.943, P=0.005]. Our data indicate that extreme periods of intra-group conflict, such as the matrilineal overthrow, have marked short-term consequences for individual fitness, and may be extreme examples of the long-term influences that group violence exerts on the mean fitness within a primate troop.
Keywords: Macaque, primate, violence, overthrow, reproduction, fitness
INTRODUCTION
Matrilineal overthrows in rhesus monkey [Macaca mulatta] troops are rare events characterized by severe aggression and violence, in which the highest-ranking matriline is deposed by a lower-ranking matriline. These events are significant in part because rhesus monkeys, like all cercopithecines, typically exhibit nepotistic rank inheritance whereby daughters rank just below their mothers in reverse age order [i.e., youngest daughters outrank older daughters; however, there is variation in the ability of daughters to rank with their mothers and in the ability of the youngest daughters to ascend to the top above their sisters; Chapais 2002; Silk 2001; 2009]. Thus, matrilineal overthrows can be devastating for many individuals at once. In addition to resulting in significant morbidity, mortality, and troop fission [Chance et al., 1977; Eaton et al., 1982; Samuels & Henrickson, 1983], these events can confer costs to individual fitness and mean fitness within the group [Wilson et al., 1978; Ehardt & Bernstein, 1986; Ruiz-Lamibdes et al., 2013]. Only a handful of overthrows have been documented for primates [primarily macaques], most of them in captivity [Marsden, 1968; Koyama et al., 1970; Gouzoules et al., 1980; Eaton, et al., 1982; Samuels & Henrickson, 1983; Ehardt & Bernstein, 1986; Gygax et al., 1997; Hambright & Gust, 2003], and several of these have also reported fetal death [with one reporting prolonged sterility] resulting from these periods of extreme violence [Nash, 1974; Chance, et al., 1977; Ehardt & Bernstein, 1986]. In both human and non-human primate societies, there is evidence that less extreme periods of conflict are also associated with morbidity and mortality [Yunes, 1993; Higley et al., 1996; Goldstone, 2002; Wrangham et al., 2006]. One proposed explanation for this relationship is that larger troops, while successful in inter-group competitions, are more likely to generate intra-group competitions over desirable resources [particularly food], thus resulting in lower reproductive success [Isbell, 1991; Janson & Goldsmith, 1995; van Schaik 1983]. However, primate studies are lacking in objective measures of the reproductive consequences of extremely violent events, as well as less severe rates of conflict.
Our rhesus monkey troop experienced a matrilineal overthrow, the first in its 25-year history, in March 2009. This overthrow was preceded by the removal of the eldest daughter of the ranking alpha female just one month prior, as well as by the increase in population of the challenging matriline in the 1–2 years prior. The immediate consequences of the overthrow were catastrophic, particularly for the deposed matriline, which realized 100% fetal and infant loss in that year. We analyzed archival data in the years leading up to, during, and after the overthrow to determine the effect of the overthrow on reproductive sucess for each of the matrilines and for the troop collectively and to examine the relationship between less severe catastrophic encounters and reproductive success. We predicted that the overthrow would result in significant declines in reproductive success for each matriline and for the troop as a whole and that rates of fighting would correlate with reproductive success, defined as infant survival past 90 days, across the six years of the study.
METHODS
All procedures described in this report adhere to the ASP Principles for the Ethical Treatment of Nonhuman Primates, the NIH Guide for the Care and Use of Laboratory Animals, and the NICHD Animal Care and Use Committee [ACUC] standards.
Subjects and Housing
Archival records from rhesus macaques [age: newborn-29 years] were analyzed between 2007 and 2012 [55≤N≤107; 27–33% male depending on the year]. For the duration of the study, monkeys lived in the field station at the NIH Animal Center at the Laboratory of Comparative Ethology [LCE] in Poolesville, MD, a 2.0-hectare, open-air enclosure that contains natural foliage, climbing structures, corn-crib shelters to provide respite from inclement weather, and a 0.9-hectare pond in the middle of the field station, with a 0.07-hectare island in the middle of the pond easily accessible to the monkeys via a manmade pier [Supplementary Figure 1]. Thus, the total usable land space [total field station area minus pond area] was 1.17-hectares. Monkeys also had continual access to three indoor, floor-to-ceiling pens located in a building ajdacent to the field. Each pen measured 2.7m W × 5.8m L × 4.3m H; in these pens monkeys were provided with commercial lab diet [Purina Monkey Chow #5038, St. Louis, MO] twice daily and were supplemented with fresh fruits/vegetables once daily. Water was provided ad libitum.
The troop consisted of three major families, with all members of Matriline 1 [n=28 at start of study in 2007] occupying the top ranks since the troop was formed in the early 1980s, followed by Matriline 3 [n=37 at start of study], then Matriline 4 [n=20 at start of study; Matriline 2 was removed from the field in 2004 for management reasons]. On February 5, 2009, the eldest daughter of the ranking matriarch was removed from the troop for treatment for kidney failure. On March 10, 2009, Matriline 3 staged an overthrow and attacked the alpha female of Matriline 1 along with her immediate family members, and severe fighting amongst these troop members ensued. This coup resulted in numerous consequences for the entire troop, including fatalities, near-fatal wounds, and spontaneous abortions; most, but not all, of these were realized by Matriline 1. Most importantly, the overthrow resulted in a permanent shift in the hierarchy, such that Matriline 3 immediately assumed dominance, followed by Matriline 4, with the few remaining members of Matriline 1 occupying the lowest ranks. Owing to the severity of injuries and fatalities suffered by Matriline 1, a colony decision was made to replicate as near as possible what would have occurred in a wild troop, where the surviving members of the deposed matriline would have been “exiled”, resulting in group fission [Ruiz-Lambides et al., 2013]: thus, the majority of surviving Matriline 1 members were removed permanently from the field so that by November 2009 only four of the original 31 members remained. Additionally, after the deposition of Matriline 1 by Matriline 3, severe fighting persisted amongst the largest family units of Matriline 3 to determine which family would assume dominance [over the matriline as well as the troop]. In order to ease the transition for a new alpha female after the stressful events of the overthrow and to reduce the likelihood of further trauma, another colony decision was made to remove 13 members of Matriline 3, all of which belonged to a single family/matriarch. Thus, by the end of 2009 the troop had decreased in size from 95 total members in February to 49 total members in November. Table 1 shows the composition of the LCE troop before and after the overthrow.
Table 1.
Composition of the LCE Troop Before and After the Overthrow.
| Pre-Overthrow (Jan 2009) | Post-Overthrow (Jan 2010) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Matriline | Adult Femalesa |
Adult Males |
Juveniles | Infants | TOTAL | Adult Females |
Adult Males |
Juveniles | Infants | TOTAL |
| 1 | 13 (12.1) | 4 (3.7) | 12 (11.2) | 5 (4.7) | 34 (31.8) | 0 (0) | 1 (2.0) | 3 (6.1) | 0 (0) | 4 (8.1) |
| 3 | 17 (15.9) | 1 (0.9) | 26 (24.3) | 11 (10.3) | 55 (51.4) | 11 (22.4) | 0 (0) | 16 (32.6) | 4 (8.1) | 31 (63.3) |
| 4 | 6 (5.6) | 1 (0.9) | 8 (7.5) | 3 (2.8) | 18 (16.8) | 7 (14.3) | 0 (0) | 5 (10.2) | 2 (4.0) | 14 (28.6) |
| TOTAL | 36 (33.6) | 6 (5.6) | 46 (43.0) | 19 (17.8) | 107 | 18 (36.7) | 1 (2.0) | 24 (49.0) | 6 (12.2) | 49 |
For all cells, the total number is displayed, along with the perecentage of the entire population in parentheses.
Fight Wounds
We examined veterinary records for the total incidence of fight wounds received by adult members of the troop requiring medical treatment in each year [infant, juvenile, and adolescent monkeys so rarely received severe fight wounds that they were not included in analyses; Dettmer et al., 2014]. These wounds were reflective of severely aggressive interactions amongst troop members, but were not as catastrophic as the overthrow. The total number of severe fight wounds in a year was used for analysis.
Infant Loss
Records were analyzed each year from 2007–2012 for the incidence of infant loss, defined as the sum of all miscarriages [i.e., spontaneous abortions], stillbirths, infant deaths, and infant removal for medical reasons at ≤ 3 months [which very rarely occurred], divided by the total number of confirmed pregnancies in that year [confirmed via ultrasound at semi-annual health exams each February]. Miscarriages and stillbirths were confirmed by visual observations of confirmed pregnant females by animal care and research staff, which occurred daily. In all cases, the aborted fetuses, which mothers carried [in the case of stillbirths], or extremely bloody vaginal discharge [in the case of miscarriages] were readily visible.
Statistical Analysis
Chi-squared analyses were used to determine whether significant changes in infant loss [coded as 0=viable/survived, 1=infant loss] from year to year, and across all years of the study, both for the troop collectively and for each matriline separately. Spearman correlation was used to analyze the relationship between total number of fight wounds in a year and total rate of infant loss.
RESULTS
Immediate Reproductive Consequences of the Matrilineal Overthrow
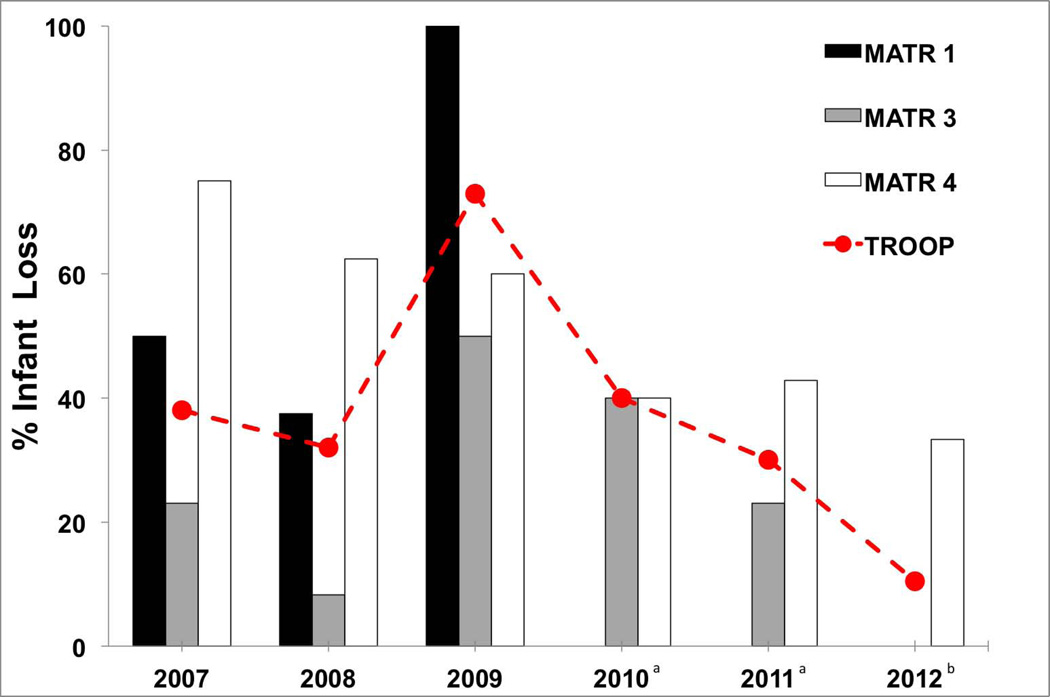
The troop realized its highest-ever infant mortality rate in the year of the overthrow [2009]: 72% [χ2=18.39, df=5, P<0.001; Figure 1, red line]. As a result of the overthrow, Matriline 1 suffered the loss of 100% of pregnancies in 2009, up from 37.5% in 2008 [χ2=7.97, df=1, P<0.01; Figure 1]. Fifty-four percent of the infant loss experienced by Matriline 1 resulted from miscarriages [including a miscarriage by the second-ranking female] in the days following the overthrow, whereas no miscarriages occurred for this matriline in 2008. The other 46% of infant loss for this matriline resulted from the humane euthanasia or removal of mother/infant dyads, or pregnant females, owing to severe injuries to the mother and/or infant after the overthrow. The overthrow also resulted in reproductive consequences for the attacking Matriline 3, which experienced 50% infant loss in 2009, up from 8.3% in 2008 [χ2=4.44, df=1, P<0.05; Figure 1]. Unlike Matriline 1, Matriline 3 experienced no miscarriages; rather, 25% of the loss in 2009 resulted from stillbirth [no stillbirths occurred for this matriline in 2008], 25% from the removal of a mother/infant dyad owing to injuries resulting from the overthrow, 25% from the nursery-rearing of an infant due to inability to cling to the mother, and 25% due to unknown causes. The uninvolved Matriline 4 saw no change in infant loss resulting from the overthrow: 62.5% in 2008 vs. 60% in 2009 [χ2=0.008, df=1, P=0.93; Figure 1].
Figure 1.
Infant mortality rates across the six years of the study for the troop [red line] and for each matriline. aNo females of reproductive age were present for Matriline 1. bReproducing females were present for matriline 1 for the first time since the overthrow in 2009; they experienced no infant loss in 2012.
Long-Term Reproductive Consequences of the Matrilineal Overthrow
The biggest consequence of the overthrow was realized by Matriline 1, which did not reproduce viable offspring again until 2012, three years after the overthrow. The reason for this is that only a few young females from Matriline 1 remained after the overthrow, and they did not reach reproductive age until 2012. However, the attacking Matriline 3 did not realize a significant reduction in infant loss until three years after the overthrow [i.e., until 2012; χ2=6.97, df=1, P<0.01], which underscores the severity of this event.
In 2010, after group stability was once again achieved, the troop as a whole saw a significant decrease in infant loss compared to 2009 [by 32.7%; χ2=3.96, df=1, P<0.05], and this pattern continued for 2011 [χ2=7.67, df=1, P<0.01] and 2012 [χ2=16.02, df=1, P<0.001; Figure 1]. Year-to-year, the troop’s rate of infant loss did not significantly change from 2010 to 2011 [χ2=0.38, df=1, P=0.54] or from 2011 to 2012 [χ2=2.27, df=1, P=0.13].
Separately, Matrilines 3 and 4 saw no differnece in infant loss from 2009–2010 [0.18<χ2<0.40, df=1, Ps>0.53] and 2009–2011 [0.34<χ2<1.6, df=1, Ps>0.20]. Comparing infant loss in 2012 to the year of the overthrow [2009], Matriline 1 saw a significant decrease [χ2=11.00, df=1, P=0.001], as did Matriline 3 [χ2=6.97, df=1, P=0.008], while Matriline 4 showed no change [χ2=0.78, df=1, P=0.38; Figure 1, Tabe 2].
Table 2.
Rates of Infant Loss for Each Matriline, and For the Troop Collectively, From 2007–2012.
| Year | MATR1a | MATR3b | MATR4 | TROOPa |
|---|---|---|---|---|
| 2007 | 50% (4) | 23.1% (13) | 75% (4) | 38% (21) |
| 2008 | 37.5% (8) | 8.3% (12) | 62.5% (8) | 32% (28) |
| 2009 | 100%** (9) | 50%* (8) | 60% (5) | 73%** (22) |
| 2010 | - | 40% (10) | 40% (5) | 40%* (15) |
| 2011 | - | 23.1% (13) | 42.9% (7) | 30% (20) |
| 2012 | 0%** (2) | 0% (11) | 33.3% (6) | 11% (19) |
Note: Numbers in parentheses indicate total number of confirmed pregnancies.
Significant change in infant loss from previous year, P<0.05 (Chi-Square Test)
Significant change in infant loss from previous year, P<0.01 (Chi-Square Test)
Significant change in infant loss across six years of study (P<0.05, Chi-Square Test)
Trend for change in infant loss across six years of study (P<0.10, Chi-Square Test)
Table 2 displays the rate of infant loss per year for each matriline, and for the troop collectively.
Rates of Fighting and Infant Loss
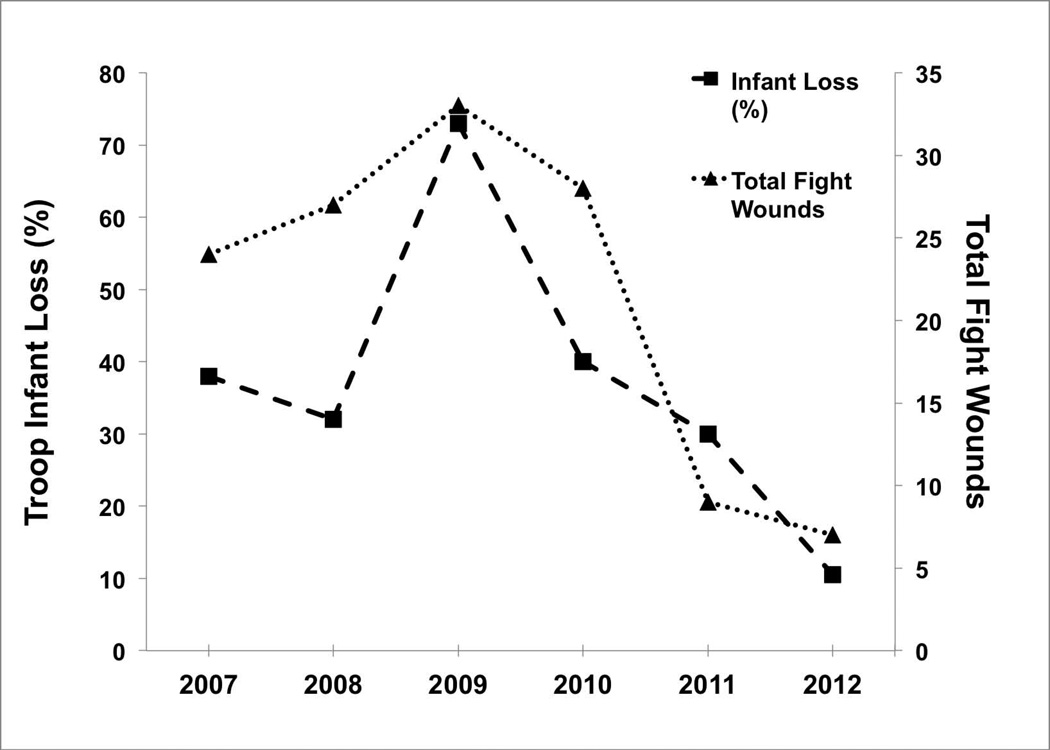
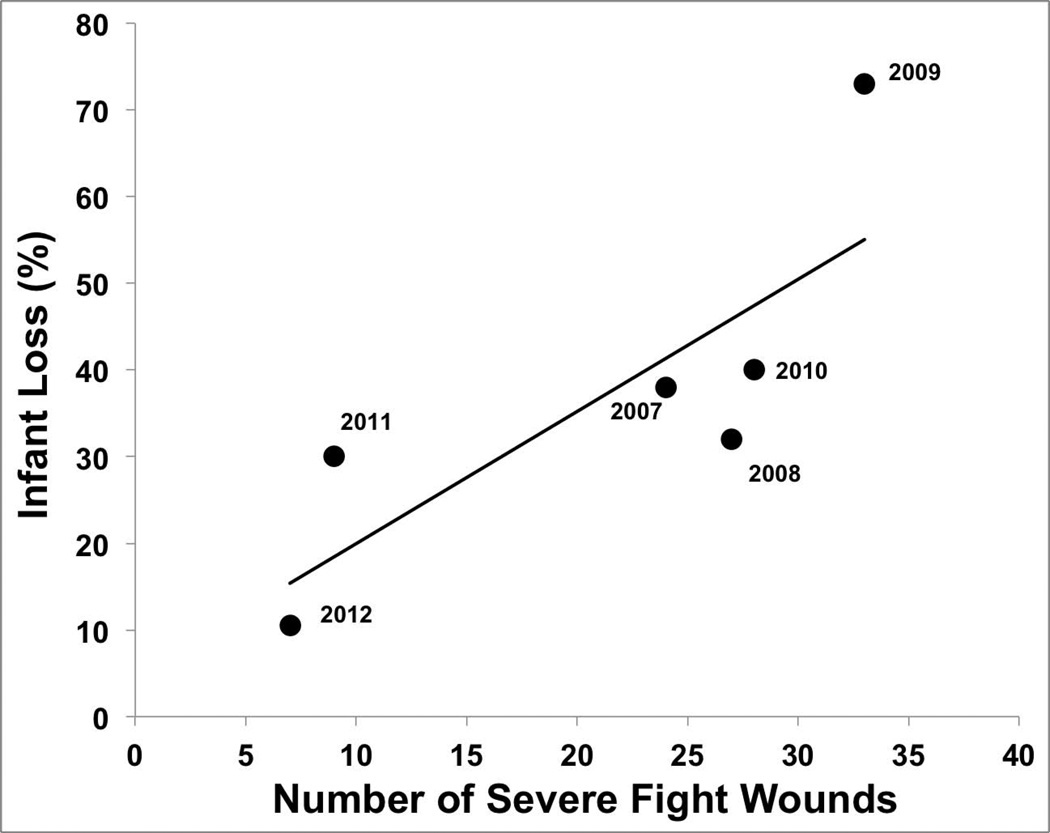
Unsurprisingly, the total number of severe fight wounds peaked during the overthrow [2009], and declined every year after; a similar pattern was observed for infant loss [Figure 2]. Visual inspection of Figure 2 indicates a close relationship between annual rates of severe fight wounds and infant loss across the 6 years of the study; indeed, these two variables were strongly correlated [Spearman correlation: r[s]=0.943, P=0.005, N=6; Figure 3], demonstrating that wounding rate in adult monkeys is linked with contemporaneous infant loss. When examined for each matriline separately, the correlation was significant only for Matriline 3 [r[s]=0.94, P=0.005, N=6; Supplemental Figure 2].
Figure 2.
Total fight wounds and infant mortality rates across the six years of the study.
Figure 3.
Number of severe fight wounds is correlated with infant mortality over the long term.
DISCUSSION
We sought to determine the extent to which extreme intra-group violence impacts mean reproductive success in a semi-free ranging troop of rhesus monkeys by examining demographic data surrounding the matrilineal overthrow at the LCE field station in 2009. We further sought to determine whether any relationship between intra-group conflict and infant loss was isolated to rare, extreme events like the overthrow, or whether an overall pattern of correlation between the two variables emerged over time. Using an analysis of demographic and veterinary records over six years, we found that the matrilineal overthrow in 2009 resulted in immediate reproductive consequences for both matrilines involved, but that these consequences were catastrophic for the deposed matriline, which lost all pregnancies in the days and weeks following the overthrow. However, the violent overthrow also affected the challenging matriline, which realized 50% infant loss in that year and a 41.7% increase in infant loss from the previous year.
Interestingly, the uninvolved matriline saw no change in infant loss due to the overthrow; this is likely because they remained on the periphery of the upheaval and as such sustained only one minor injury [confirmed by veterinary records, data not shown]. The resulting stress of the overthrow very likely affected the mean reproductive success of only the two involved matrilines, while the remaining matriline’s uninvolvement not only preserved their total reproductive success, but also resulted in a collective rise in rank over the deposed matriline. This notion is supported by our data, which showed no significant change in infant loss for the uninvolved Matriline 4 across the years of the study, while Matrilines 1 and 3 suffered significant increases in infant loss in the year of the overthrow and did not show significant recovery for several years. As such, although we were not present to observe the overthrow, we can be confident that Matriline 4’s uninvolvement was beneficial to their mean reproductive success. Additionally, their uninvolvement appeared to mimic that of many adult males during macaque overthrows, who often do not come to the aid of their female counterparts [Ehardt and Bernstein, 1986; 1992]. Even when they do, their efforts are largely unsuccessful [Ehardt and Bernstein, 1986; 1992], as were those of the former alpha male of our troop, who was closely bonded with the ranking alpha female at the time of the coup. This was the only adult male to sustain severe injuries during the overthrow.
Our troop’s rate of infant loss at the beginning of the study was near 40%, which is a rate higher than those reported for the semi-free ranging macaques at Cayo Santiago [Drickamer, 1974; Rawlins & Kessler, 1986] or in India [Southwick & Siddiqi, 1977]. We suspect that this high rate was at least in part a result of the troop’s growing size. We observed the highest ever population in 2008 at 107 individuals, though in 2007 the population was already quite large at 97 individuals. As reduced reproductive success has been observed in larger primate groups [van Noordwijk & van Schaik, 1999; Steenbeek & van Schaik, 2001], it is possible that the reproductive impact of the growing troop was already being realized by the start of this study. Indeed, in earlier years [1998–2002] when the population ranged from 60–90 individuals, the infant mortality rate averaged 13%; similarly, after the overthrow when the population was again reduced [ranging from 65–80 individuals from 2010–2012], the infant mortality rate averaged 19%. These rates are on par with free-ranging populations [Drickamer, 1974; Rawlins & Kessler, 1986; Southwick & Siddiqi, 1977]. Though the Cayo Santiago and Northern India populations are much larger than ours, their home range sizes are also significantly larger. Population density rather than population size per se may influence a troop’s mean reproductive success. This notion is supported by findings from our laboratory showing higher chronic cortisol levels in rhesus monkeys housed at higher densities [Dettmer et al., 2014]; these higher circulating glucocorticoids may negatively impact reproductive processes.
Intriguingly, we found that rates of severe wounding [which are indicative of heightened but non-lethal intra-group violence] were significantly correlated with rates of infant loss across six the years of the study. Other studies have found that both violent and non-violent conflicts result in group fission, which can change the demographics and genetics of the population, resulting in significant consequences for the mean reproductive fitness within a group [Dittus, 1988; Kuester & Paul, 1997; Melnick & Kidd, 1983; Menard & Vallet, 1993; Ruiz-Lambides, 2013]. Some studies have also found that primate troops seem to possess a maximum population threshold; e.g., one troop fissioned once the population exceeded around 120 individuals [Malik, Seth, & Southwick, 1985]. Interestingly, the overthrow in our captive troop occurred just six months after the highest-ever recorded population in 2008 at 107 individuals, suggesting that the population threshold may be innate to cercopithecines [and perhaps other primates]. Together, these studies and our data indicate that an extremely violent event such as the overthrow may be a magnified example of the toxic relationship between group aggression and a group’s mean reproductive fitness. Moreover, for nonhuman primate societies, it appears that certain group dynamics [and group violence in particular] significantly affect individual mothers, a finding that is supported by data from human populations as well [Collins & David, 1996; Masi, Hawkley, Piotrowski, & Pickett, 2007]. In our examination of other demographics [e.g., nubmer of adolescent males and females, number of breeding females, population density] we found no significant correlation between any of these variables and rates of infant loss [data not shown], suggesting that for this captive population, the greatest predictor of the troop’s mean reproductive success is the incidence of severe fighting.
Taken together, findings from the current study underscore the deleterious consequences of excessive intra-group conflict in rhesus monkeys, consequences that drastically affect the mean fitness within the group and counteract the reproductive advantages of a long tenure of dominance for a matriline [Ehardt & Bernstein, 1986]. They further highlight the significance of less severe, but more regularly occurring, forms of group conflict with respect to reproductive success. As such, in our study population, the matrilineal overthrow serves as an extreme example for the long-term pattern we observed intra-group conflict and mean reproductive fitness. Collectively, primate societies may possess a maximum threshold for tolerance of social strife; once this threshold is surpassed warlike episodes may result and the ramifications for the population are severe.
Supplementary Material
Aerial view of the 2.0-hectare field station at the Laboratory of Comparative Ethology [LCE].
Correlation between number of severe fight wounds and infant mortality for each matriline. **p<0.01. Note: numbers next to symbols indacte the year of study, 1=2007; 2=2008; 3=2009; 4=2010; 5=2011; 6=2012.
ACKNOWLEDGEMENTS
The Division of Intramural Research at the Eunice Kennedy Shriver National Institute of Child Health & Human Development supported this research. We thank Samantha Haynie for assistance with compilation of the archival data, and the animal care staff at the NIH Animal Center for their dedicated care and treatment of the monkeys in the time surrounding the overthrow.
REFERENCES
- Chance MRA, Emory GR, Payne RG. Status referents in long-tailed macaques [Macaca fascicularis]: precursors and effects of a female rebellion. Primates. 1977;3:611–632. [Google Scholar]
- Chapais B. The role of alliances in social inheritance of rank among female primates. In: Harcourt AH, de Waal FB, editors. Coalitions and alliances in humans and other animals. Oxford, UK: Oxford Science Publications; 2002. pp. 29–59. [Google Scholar]
- Collins JW, David RJ. Urban violence and African-American pregnancy outcome: an ecologic study. Ethnicity & Disease. 1997;3:184–190. [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Meyer JS, Suomi SJ. Population density-dependent hair cortisol concentrations in rhesus monkeys [Macaca mulatta] Psychoneuroendocrinology. 2014;42:59–67. doi: 10.1016/j.psyneuen.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittus WP. Group fission among wild toque macaques as a consequence of female resource competition and environmental stress. Animal Behaviour. 1988;36:1626–1645. [Google Scholar]
- Drickamer LC. A ten-year summary of reproductive data for free-ranging Macaca mulatta. Folia primatologica. 1974;21:61–80. doi: 10.1159/000155596. [DOI] [PubMed] [Google Scholar]
- Eaton GG, Olson LA, Senner JW. Aggression in captive groups of rhesus macaques: A preliminary report. Primate News. 1982;20:14–17. [Google Scholar]
- Ehardt CL, Bernstein IS. Matrilineal overthrows in rhesus monkey groups. International Journal of Primatology. 1986;2:157–181. [Google Scholar]
- Ehardt CL, Bernstein IS. Conflict intervention behaviour by adult male macaques: structural and functional aspects. Coalitions and alliances in humans and other animals. 1992:83–111. [Google Scholar]
- Goldstone JA. Population and security: how demographic change can lead to violent conflict. Journal of International Affairs. 2002;1:3–21. [Google Scholar]
- Gouzoules H. A description of genealogical rank changes in a troop of Japanese monkeys [Macaca fuscata] Primates. 1980;2:262–267. [Google Scholar]
- Gygax L, Harley N, Kummer H. A matrilineal overthrow with destructive aggression in Macaca fascicularis. Primates. 1997;2:149–158. [Google Scholar]
- Hambright MK, Gust DA. A descriptive analysis of a spontaneous dominance overthrow in a breeding colony of Rhesus Macaques [Macaca mulatta] Laboratory Primate Newsletter. 2003;1:8–10. [Google Scholar]
- Higley JD, Mehlman PT, Higley SB, et al. Excessive mortality in young free-ranging male nonhuman primates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Archives of General Psychiatry. 1996;6:537. doi: 10.1001/archpsyc.1996.01830060083011. [DOI] [PubMed] [Google Scholar]
- Isbell LA. Contest and scramble competition: patterns of female aggression and ranging behvior among primates. Behavioral Ecology. 1999;2:143–155. [Google Scholar]
- Janson CH, Goldsmith ML. Predicting group size in primates: foraging costs and predation risks. Behavioral Ecology. 1995;6:326–336. [Google Scholar]
- Koyama N. Changes in dominance rank and division of a wild Japanese monkey troop in Arashiyama. Primates. 1970;4:335–390. [Google Scholar]
- Kuester J, Paul A. Group fission in Barbary macaques [Macaca sylvanus] at Affenberg Salem. International journal of primatology. 1997;18:941–966. doi: 10.1159/000156354. [DOI] [PubMed] [Google Scholar]
- Malik I, Seth PK, Southwick CH. Group fission in free-ranging rhesus monkeys of Tughlaqabad, Northern India. International journal of primatology. 1985;6:411–422. [Google Scholar]
- Marsden HM. Agonistic behaviour of young rhesus monkeys after changes induced in social rank of their mothers. Animal Behaviour. 1968;1:38–44. doi: 10.1016/0003-3472(68)90106-1. [DOI] [PubMed] [Google Scholar]
- Masi CM, Hawkley LC, Piotrowski ZH, Pickett KE. Neighborhood economic disadvantage, violent crime, group density, and pregnancy outcomes in a diverse, urban population. Social Science & Medicine. 2007;65:2440–2457. doi: 10.1016/j.socscimed.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Melnick DJ, Kidd KK. The genetic consequences of social group fission in a wild population of rhesus monkeys [Macaca mulatta] Behavioral Ecology and Sociobiology. 1983;12:229–236. [Google Scholar]
- Menard N, Vallet D. Dynamics of fission in a wild Barbary macaque group [Macaca sylvanus] International journal of primatology. 1993;14:479–500. [Google Scholar]
- Nash LT. Parturition in a feral baboon [Papio anubis] Primates. 1974;2–3:279–285. [Google Scholar]
- Rawlins RG, Kessler MJ. Demography of the free-ranging Cayo Santiago Macaques [1976–1983] In: Rawlins RG, Kessler MJ, editors. The Cayo Santiago macaques: history, behavior and biology. Albany: SUNY Press; 1986. pp. 47–72. [Google Scholar]
- Ruiz-Lamibdes AV, Aure B, Caraballo G, Platt ML, Brent LJ. Matrilineal overthrow followed by high mortality levels in free-ranging rhesus macaques. American Journal of Primatology. 2013;75:98–99. [Google Scholar]
- Samuels A, Henrickson RV. Brief report: Outbreak of severe aggression in captive Macaca mulatta. American Journal of Primatology. 1983;3:277–281. doi: 10.1002/ajp.1350050314. [DOI] [PubMed] [Google Scholar]
- Silk JB. Nepotistic cooperation in non-human primates groups. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:3243–3254. doi: 10.1098/rstb.2009.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB. Ties that bond: the role of kinship in primate societies. In: Stone L, editor. New directions in anthropological kinship. Boulder, CO: Rowman and Littlefield; 2001. pp. 71–92. [Google Scholar]
- Southwick CH, Siddiqi MF. Population dynamics of rhesus monkeys in Northern India. In: HSH Prince Rainier III of Monaco; Bourne GE, editor. Primate Conservation. New York: Academic Press; 1977. pp. 339–362. [Google Scholar]
- Steenbeek R, van Schaik CP. Competition and group size in Thomas’s langurs [Presbytis thomasi]: the folivore paradox revisited. Behavioral Ecology and Sociobiology. 2001;49:100–110. [Google Scholar]
- van Noordwijk, van Schaik CP. The effects of dominance rnak and group size on female lifteime reproductive success in wild long-tailed macaques, Macaca fascicularis. Primates. 1999;40:105–130. doi: 10.1007/BF02557705. [DOI] [PubMed] [Google Scholar]
- van Schaik CP. Why are diurnal primates living in groups? Behaviour. 1983;87:120–144. [Google Scholar]
- Wilson ME, Gordon TP, Bernstein IS. Timing of births and reproductive success in rhesus monkey social groups. Journal of Medical Primatology. 1977;4:202–212. doi: 10.1159/000459880. [DOI] [PubMed] [Google Scholar]
- Wrangham RW, Wilson ML, Muller MN. Comparative rates of violence in chimpanzees and humans. Primates. 2006;1:14–26. doi: 10.1007/s10329-005-0140-1. [DOI] [PubMed] [Google Scholar]
- Yunes J. Mortality from Violent Causes in the Americas. Bulletin of PAHO. 1993;27:2. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Aerial view of the 2.0-hectare field station at the Laboratory of Comparative Ethology [LCE].
Correlation between number of severe fight wounds and infant mortality for each matriline. **p<0.01. Note: numbers next to symbols indacte the year of study, 1=2007; 2=2008; 3=2009; 4=2010; 5=2011; 6=2012.