Abstract
Purpose
Dysregulated signaling of nuclear transcription factors vitamin D receptor (VDR) and Forkhead box M1 (FOXM1) play important roles in transformation and tumorigenesis. In this study, we sought to determine whether VDR signaling causally impacted FOXM1 signaling in and pathogenesis of pancreatic ductal adenocarcinoma (PDAC).
Experimental Design
Genetic and pharmacologic approaches were used to manipulate VDR signaling. The impacts of altered VDR signaling on FOXM1 expression and function in PDAC cells was determined using molecular and biochemical methods, whereas that on PDAC cell biology and tumorigenicity was determined using in vitro and in vivo experimental systems. The clinical relevance of our findings was validated by analyzing human PDAC specimens.
Results
There was a striking inverse correlation between reduced expression of VDR and increased expression of FOXM1 in human PDAC cells and tissues. Treatment of PDAC cells with 1,25-dihydroxyvitamin D3 (1,25D), its synthetic analog EB1089, and VDR transgenics drastically inhibited FOXM1 signaling and markedly suppressed tumor stemness, growth and metastasis. Mechanistically, 1,25D and EB1089 repressed FOXM1 transcription and reduced the expression level of nuclear FOXM1 protein.
Conclusion
Inactivation of Vitamin D/VDR signaling is a critical contributor to PDAC development and progression via elevated expression and function of FOXM1 and enhanced PDAC cell stemness, invasion, and metastasis.
Keywords: VDR, FOXM1, Pancreatic cancer, Progression, Stem cell
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer deaths in industrialized countries, with a mortality rate near 75% within 1 year after diagnosis and a 5-year survival rate of less than 6%.1 Furthermore, the incidence of this disease appears to be increasing.2 The dismal prognosis for pancreatic cancer is attributable to its tendency toward late presentation, early metastasis, and resistance to therapy.3,4 Better understanding the mechanisms underlying the aggressiveness of and dismal prognosis for PDAC would help develop novel, effective prevention and therapeutic modalities to save the lives of patients with PDAC.5–7
Forkhead box M1 (FOXM1) is a transcription factor in the FOX protein superfamily.8–9 FOXM1 essentially regulates multiple aspects of tumor cell biology.10–12 Overexpression of FOXM1 occurs frequently in a wide variety of human tumors and contributes to human cancer pathogenesis,12–14 including that of PDAC.15,16 However, the molecular mechanisms underlying FOXM1 dysregulation and its impact on PDAC pathogenesis remain unclear.12 Interestingly, both FOXM1 and Vitamin D receptor (VDR) interact with β-catenin and regulate cellular functions.17–20 However, whether the expression and function of VDR and FOXM1 are causally related and whether dysregulation of their crosstalk if exists impacts cellular transformation and tumorigenesis remain unknown.
VDR, which belongs to the family of trans-acting transcriptional regulatory factors and exhibits sequence similarity with the steroid and thyroid hormone receptors, binds to the active form of Vitamin D, 1,25-dihydroxyvitamin D3 (1,25D).21 Also, Vitamin D binds to nuclear VDR, which activates the receptor to form a heterodimer with the retinoid X receptor and interacts with the Vitamin D response element (VDRE). Transcription repressors occupying the VDRE are then replaced by transcription activators to initiate transcription of targeted genes.21 Microarray analyses have identified many genes with VDREs in their promoter regions, all of which are potential targets of the Vitamin D/VDR complex.22–24 Vitamin D directly alters patterns of gene expression via the VDR as well as VDR-independent mechanisms,25,26 and regulates transcriptome,27–30 and exerts antitumor effects.31–35 These genomic effects can result from the classical mechanism of VDR recruitment of co-activators to VDREs and nonclassical interactions of VDR with activated β-catenin on other promoters.24 VDR can also influence the level of nuclear β-catenin in colon cancer cells and can therefore attenuate the impact of oncogenic mutations that activate the Wnt/β-catenin pathway.18 Thus, Vitamin D deficiency may play an important role in cancer development and progression and that Vitamin D and its synthetic analogs may have therapeutic potential.36–40 However, in a pancreatic cancer clinical trial, EB1089 (EB or Seocalcitol, a synthetic analog of 1,25D), is well tolerated but has no objective anti-tumour activity in advanced disease,41 while the underlying mechanisms for this refractory nature is unknown.
In the present study, we sought to determine whether VDR signaling causally regulates the expression and function of FOXM1 in and pathogenesis of PDAC. We demonstrated that inactivation of Vitamin D/VDR signaling critically impacted to PDAC cell stemness and invasive and metastatic phenotypes via elevated expression and function of FOXM1.
Materials and Methods
Cell Lines and Culture Conditions
The human PDAC cell lines AsPC-1, BxPC-3, CaPan-1, CaPan-2, FG, Hs766T, MiaPaCa-2, mPanc96, PANC-1, MDA-28, MDA-48, and PA-TU-8902 and human embryonic kidney 293 (HEK293) cells were purchased from the American Type Culture Collection (Manassas, VA) or obtained as described previously.16,42 All of the cancer cell lines were maintained in plastic flasks as adherent monolayers in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), sodium pyruvate, nonessential amino acids, L-glutamine, and a vitamin solution. The immortalized normal human pancreatic ductal epithelial cell line HPDE (provided by Dr. Ming-Sound Tsao, Ontario Cancer Institute) was maintained in keratinocyte serum-free medium supplemented with epidermal growth factor and bovine pituitary extract (Invitrogen, Carlsbad, CA). The cell lines were obtained directly from ATCC that performs cell line characterizations or authentication by the short tandem repeat profiling and passaged in our laboratory for fewer than 6 months after receipt.
Lentiviral VDR Expression Vector Construction and Transfection
For generation of a lentiviral VDR expression vector, the PCR primers 5′-ctagtgaattcggtaccgaggagatctgccgc-3′ and 5′-tcgcgggatcccgtttaaaccttatcgtcgtc-3′ were used in a PCR with pCMV6-VDR used as a template. The PCR product was subcloned into the EcoRI and BamH1 sites of a pLVX-Puro vector, and the resultant vector was used to package lentiviral particles using the Lenti-X HT packaging system (Clontech Laboratories, Mountain View, CA). All vector constructs were confirmed using DNA sequence analysis. For generation of stable cell lines, 5×105 PANC-1 or mPanc96 cells were incubated for 5 hours with 1×106 particles of either L-EGFP or L-VDR in 2 mL of complete DMEM in the presence of polybrene (4 μg/mL).16,42 Cells infected with the lentivirus were subjected to selection in 5 μg/mL puromycin for 10 days before use.
Measurement of Cell Proliferation, Migration, Invasion, and Spheroid Colony Formation
Pancreatic cancer cells were treated with different doses of 1,25D (Sigma-Aldrich, St. Louis, MO), EB (Tocris Bioscience, Bristol, UK), or a vehicle control (EtOH) in DMEM containing 4% FBS for 2–6 days. Cell proliferation, migration, invasion, and spheroid colony formation were measured using procedures described previously.16,42
Immunocytochemical Analysis
PANC-1 and mPanc96 cells were seeded in Falcon chamber slides (Becton Dickinson, Franklin Lakes, NJ) at 1×105 cells per well in DMEM supplemented with 10% FBS for overnight culture. The cells were then treated with Vitamin D or EB (100 nM) or a vehicle control (EtOH) in complete DMEM containing 4% FBS for 48 hours. Cells were then fixed in a 4% paraformaldehyde solution for 8 minutes. After being washed twice with phosphate-buffered saline (PBS), the cells were incubated with a specific anti-FOXM1, anti-β-catenin, or anti-E-cadherin antibody and then incubated with a Texas red-labeled secondary antibody. Nuclear staining of the cells was accomplished via incubation in a solution containing 10 μg/mL 4′,6-diamidino-2-phenylindole (Sigma-Aldrich). Fluorescent imaging of cell cultures was performed using an Axiophoto 2 microscope and the Photoshop CS4 software program.
Western Blot Analysis
Standard Western blotting was performed using whole-cell protein lysates or cytoplasmic and nuclear protein lysates; primary antibodies against FOXM1, β-catenin, c-Myc, cyclin D1, Skp2, histone H1, α-tubulin, GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA), p27 (Cell Signaling Technology, Danvers, MA), E-cadherin (BD Biosciences), and VDR (OriGene Technologies, Inc.); and a secondary antibody (anti-rabbit IgG or anti-mouse IgG; Santa Cruz Biotechnology). Equal protein-sample loading was monitored using an anti-GAPDH antibody (Santa Cruz Biotechnology).
SiRNA
RNA interference was performed using synthetic siRNA oligos to VDR (Santa Cruz Biotechnology). Briefly, PANC-1 or mPanc96 cells were seeded to 80% confluence in six-well plates in triplicate and transiently transfected with VDR siRNA or a control siRNA (100 pmol/well) using Lipofectamine 2000CD (Invitrogen). Cell and protein samples were harvested 48 hours after transfection and processed for Western blot analysis.
Flow Cytometry Analysis
Single-cell suspensions of PDAC cells were prepared in Dulbecco’s PBS (DPBS)/3% FBS at a concentration of 1–5×106 cells/mL. Anti-CD44-PE antibody (BD Biosciences Pharmingen, San Diego, CA) was added to the suspensions, which were then incubated on ice for 30 minutes. After being washed twice with DPBS/3% FBS, the cells were resuspended in DPBS/3% FBS and analyzed using a FACSCalibur flow cytometer equipped with the CellQuest software program (Becton Dickinson). In some experiments, single-cell suspensions were prepared after treatment with EB or a vehicle control and then analyzed using flow cytometry.
Real-Time Reverse Transcriptase-PCR
Total RNA was isolated from cell cultures using TRIzol reagent (Invitrogen) and reverse-transcribed into cDNA using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). The cDNA products were used in quantitative PCR analysis of indicated gene expression using the FOXM1 and β-catenin PCR primers and probe sets (Applied Biosystems, Foster City, CA). Each real-time reverse transcriptase-PCR experiment was performed in triplicate. Relative quantitation of the gene expression was carried out using the comparative CT method, and the relative level of expression of an individual target gene was normalized according to the expression of both the HPRT1 gene (Applied Biosystems) and a calibrator sample was run on the same plate. Relative RNA expression calculations were performed using a commercially available software program (SDS, version 1.2; Applied Biosystems).
Human Tissue Samples and Immunohistochemical Analysis
Expression of VDR and FOXM1 was analyzed using human pancreatic tumor and normal tissue microarrays (PA2081; US Biomax, Inc., Rockville, MD). The pancreatic tumor microarray contained 42 ductal adenocarcinoma, 3 adenosquamous carcinoma, 1 islet cell carcinoma, 6 metastatic carcinoma, 10 islet cell tumor, 2 hyperplasia, 10 inflamed tissue, 20 adjacent normal tissue, and 10 normal tissue specimens obtained at autopsy. Use of the tissue samples was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. Standard immunohistochemical procedures were carried out using an anti-VDR (OriGene Technologies, Inc.) or anti-FOXM1 (Sigma-Aldrich) antibody. The staining results were scored by two investigators blinded to the clinical data as described previously.16,42 As negative controls, the primary antibodies were omitted and replaced with a related strain of IgG used as a negative control.
Mouse Model of Pancreatic Tumor Growth and Metastasis
Pathogen-free female athymic BALB/c nude mice were purchased from the National Cancer Institute (Bethesda, MD). The animals were maintained in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with the current regulations and standards of the U.S. Department of Agriculture and U.S. Department of Health and Human Services. Pancreatic tumor cells in exponential growth phase were prepared, and 1×106 or as indicated of them were injected into the pancreas or subcutis in 7- to 8-week-old mice. The animals were killed 4–5 weeks after inoculation, and their primary tumors were harvested, weighed, and processed for further analysis of related molecular marker expression.
VDR Expression Vector and Gene Transfection
The plasmid pCMV6-Myc-DDK-VDR (OriGene Technologies, Inc., Rockville, MD) and the control vector pcDNA3.1 were transfected into PDAC cells using Lipofectamine 2000CD (Invitrogen, Carlsbad, CA). Functional assays for cell proliferation and migration were carried out 48 hours or as indicated after transfection.
FOXM1 Promoter Reporter Activity Assay
A 2.496-kb fragment of FOXM1 containing 5′ FOXM1 sequences from −2430 to +66 bp relative to the transcription initiation site was subcloned into the Asp718 and XhoI sites of a pGL3-basic vector (Promega, Madison, WI). The final resulting full-length reporter plasmid, designated as pFOXM1-2496. All constructs were verified by sequencing the inserts in and flanking regions of the plasmid. Pancreatic cancer cells were transfected with promoter reporters and then treated with 1,25D and/or EB1089 (EB, Santa Cruz Biotechnology, Dallas, TX), a synthetic analog of 1,25D, or co-transfected with the indicated pCMV6-Myc-DDK-VDR–specific gene expression plasmids. The FOXM1 promoter activity in these cells was normalized via co-transfection of a β-actin/Renilla luciferase reporter containing a full-length Renilla luciferase gene.42 The luciferase activity in the cells was quantified using a dual luciferase assay system (Promega) 48 hours after transfection.
Statistical Analysis
All the in vitro and in vivo experiments were repeated at least once, while one exprement of two or three with similar results was represented. The significance of the data on patient specimens was determined using the two-tailed χ2 test and Fisher exact test. The significance of the in vitro and in vivo data was determined using the Student t-test (two-tailed), the Mann-Whitney test (two-tailed), or one-way analysis of variance. P values less than 0.05 were considered significant. The SPSS software program (version 12.0; IBM Corporation, Armonk, NY) was used for all statistical analyses.
Results
Inverse Correlation of VDR Expression with FOXM1 Expression in Pancreatic Tissue Specimens and Association with Clinicopathological Features of PDAC
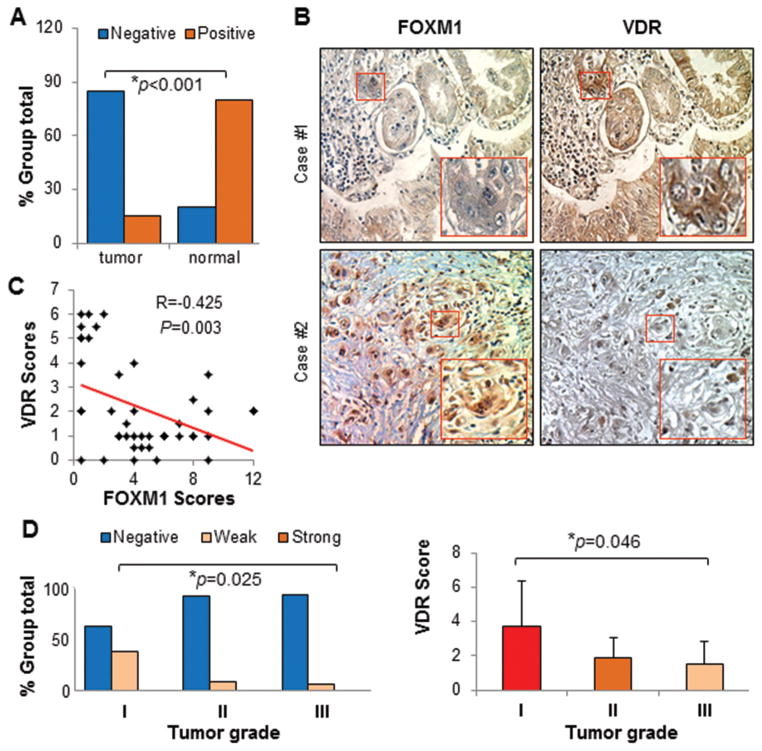
Previous studies have shown an elevated expression of FOXM1 in human PDAC.16,17 To determine the potential regulation of FOXM1 expression by Vitamin D/VDR and its clinical relevance of VDR-FOXM1 signaling to PDAC pathogenesis, we first sought to measure the expression of VDR in 46 primary pancreatic tumor, 6 metastatic pancreatic tumor, and 10 normal pancreatic tissue specimens in a tissue microarray. We observed VDR-positive or weak VDR-positive staining in the nuclei of normal pancreatic cells, whereas we observed VDR-negative staining in pancreatic tumor cells. However, expression of FOXM1 occurred predominantly in tumor cells (Figure 1A & 1B). We detected a pronounced inverse correlation between the levels of VDR and FOXM1 expression in PDAC specimens (Figures 1B & 1C). Moreover, the levels of VDR expression correlated with tumor differentiation, as there was a significant difference between well (grade I) and poorly (grade III) differentiated tumors (Figure 1D). These clinical findings support that VDR interacts with FOXM1 and critically impacts PDAC pathogenesis and that VDR and FOXM1 are potentially valuable biomarkers.
Figure 1.
Inverse correlation between the expression of VDR and FOXM1 in human PDAC specimens. The expression of VDR and FOXM1 protein was determined in tissue microarrays of paired normal pancreatic and PDAC specimens. (A) Expression of VDR protein was higher in normal pancreatic tissue (10 cases) than in pancreatic tumors (46 cases). The χ2 test demonstrated significant differences in VDR and FOXM1 protein expression in normal tissue and tumor specimens (P<0.05). (B) Representative photos of VDR and FOXM1 protein expression. (C) A significant inverse correlation between the levels of VDR and FOXM1 expression was observed in PDAC specimens (P<0.01). (D) Increased VDR expression correlated with increased tumor differentiation and a significant difference between well (grade I) and poorly (grade III) differentiated tumors (P<0.05).
Downregulation of the Expression of FOXM1 and its Downstream Target Genes by Activation of VDR Signaling
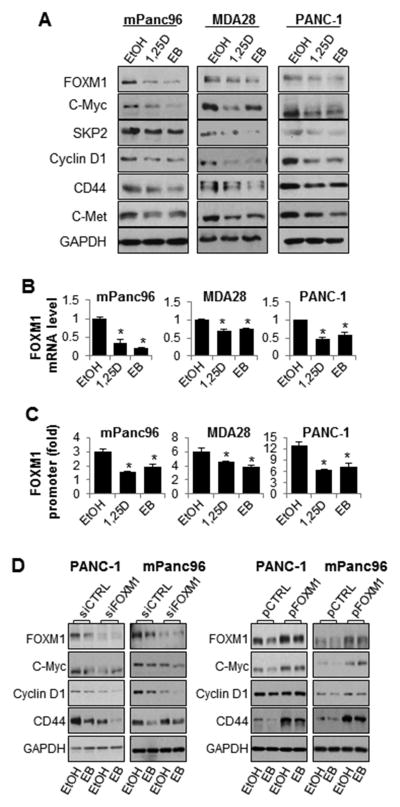
To determine whether Vitamin D/VDR causally regulates the expression of FOXM1, we pharmacolocally activated VDR signaling or genetically overexpressed VDR in PDAC cells by using active form of Vitamin D, i.e., 1,25D and its synthetic analog, EB1089, i.e., EB. Treatment of mPanc96, MDA-28, and PANC-1 cells with 1,25D and EB suppressed the expression of FOXM1 and its downstream target genes, including Cyclin D1, Skp2, c-Myc, CD44, and c-Met (Figure 2A). This downregulation of FOXM1 protein expression was consistent with suppression of FOXM1 promoter activity and mRNA expression (Figures 2B and 2C).
Figure 2.
Treatment with 1,25D and EB inhibits expression of FOXM1 and its downstream genes. mPanc96, MDA-28, and PANC-1 cells were treated with 1,25D and EB (100 nM for 48 hours). (A) Total cell lysate proteins were extracted for Western blot analysis using specific antibodies, and (B) mRNA was extracted for quantitative PCR analysis. (C) The FOXM1 promoter reporter was transfected into pancreatic cancer cells, which were then treated with 1,25D or EB (100 nM for 48 hours). The promoter activity was assessed 48 hours after treatment using a dual luciferase assay kit. Both mPanc96 and PANC-1 cells were transfected with (D) FOXM1 siRNA or control siRNA; or FOXM1 expression vector (pFOXM1) or control vector (pCTRL). Total cell lysate proteins were extracted for Western blot analysis of gene expression using specific antibodies. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; CTRL, control.
Next, we either knocked down the expression or induced overexpression of FOXM1 in mPanc96 and PANC-1 cells and then exposed the cells to EB. The treatment resulted in further reduction of expression of FOXM1 and its downstream target genes caused by FOXM1 knockdown (Figure 2D), whereas overexpression of FOXM1 attenuated downregulation of expression of FOXM1 target genes caused by treatment with EB (Figure 2D). This suggests an essential role for FOXM1 in EB-mediated downregulation of Cyclin D1, c-Myc, and CD44 expression.
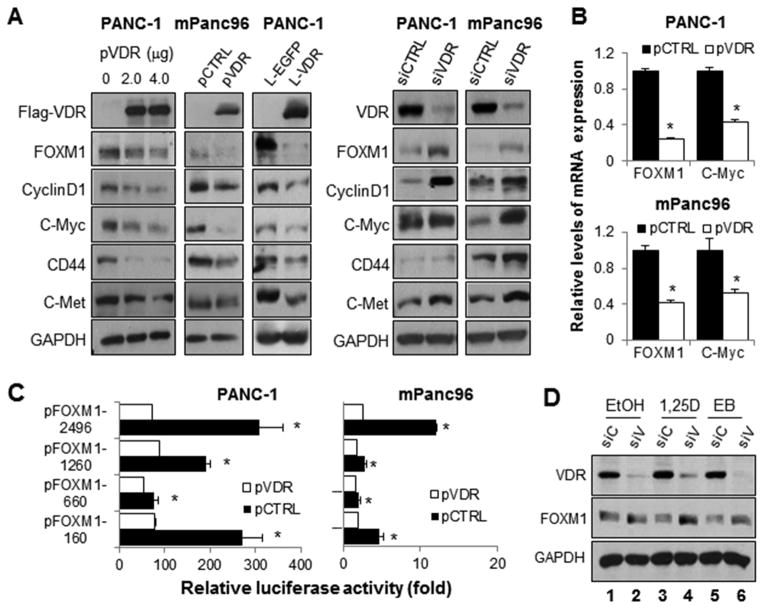
Furthermore, overexpression of VDR induced by either gene transfection or lentiviral gene transfer caused downregulation of FOXM1 and its downstream targets, while VDR knockdown increased the expression of FOXM1 and its downstream targets (Figure 3A). Downregulation of FOXM1 protein expression was consistent with decreased FOXM1 mRNA expression (Figure 3B). VDR transfection led to the suppression of FOXM1 promoter activity (Figure 3C).
Figure 3.
Impact of altered expression of VDR on FOXM1 expression. PANC-1 and mPanc96 cells were transfected with pVDR or pCTRL or infected with a lentivirus with control enhanced green fluorescent protein (EGFP [L-EGFP]) or a lentivirus with VDR (L-VDR). PANC-1 and mPanc96 cells were transfected with VDR siRNA (siVDR) or control siRNA (siCTRL). (A) Total cell lysate proteins were extracted for Western blot analysis of gene expression using specific antibodies, and (B) mRNA was extracted for quantitative PCR analysis. (C) Luciferase assays using mPanc96 and PANC-1 cells demonstrated that altered VDR recruitment was consistent with changes in FOXM1 promoter activity. (D) mPanc96 cells were transfected with VDR siRNA (siV) or control siRNA (siC) and then treated with 1,25D, EB (100 nM), or a dissolvent for 48 hours. Total cell lysate proteins were extracted for Western blot analysis of gene expression using specific antibodies.
Although the constitutive expression levels of VDR did not significantly correlate with those of FOXM1 (Supplementary Figure S1A), knockdown of VDR increased FOXM1 promoter activities (Supplementary Figure S1B); and clearly attenuated the 1,25D- and EB-mediated repression of both FOXM1 protein expression (Figure 3C) and promoter activity (Supplementary Figure S1C), indicating that expression of VDR is necessary for 1,25D- and EB-mediated repression of the expression of FOXM1 and its downstream targets. In contrast, overexpression of VDR sensitized the suppression of FOXM1 expression to treatment with EB (Supplementary Figure S1D).
Suppression of Expression of Nuclear FOXM1 by Activation of VDR Signaling
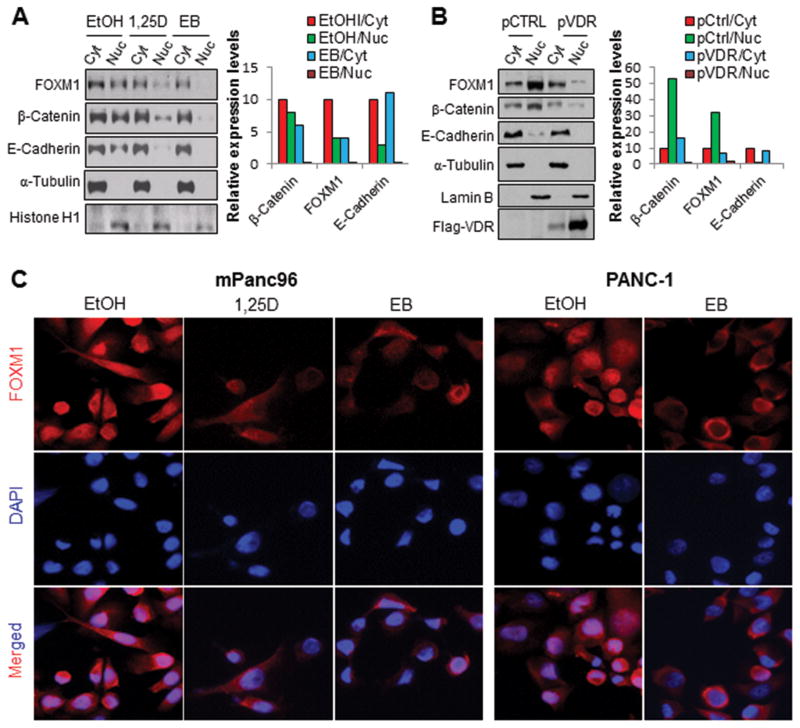
FOXM1 contains a functional nuclear localization signal domain and shuttles between the cytoplasm and the nucleus. FOXM1 also is a downstream component of the Wnt signaling pathway and is critical for β-catenin’s transcriptional function in tumor cells. Specifically, FOXM1 binds directly to β-catenin and enhances its nuclear localization and transcriptional activity.42 We therefore examined whether the VDR signaling plays a role in nuclear FOXM1 expression. Treatment with 1,25D and EB (Figure 4A) and VDR transfection (Figure 4B) were sufficient to decrease nuclear FOXM1 and β-catenin expression in PDAC cells. Further experiments using double cell immunofluorescence staining demonstrated that treatment with 1,25D and EB decreased the expression of FOXM1 and β-catenin and especially the nuclear FOXM1 and β-catenin in PDAC cells (Figure 4C; Supplementary Figure S2).
Figure 4.
Impact of altered VDR signaling on subcellular localization of FOXM1. mPanc96 cells were treated with 1,25D, EB, or a dissolvent (100 nM for 48 hours) (A) or transfected with control vector (pCTRL) or VDR vector (pVDR) (B). Cytosolic (Cyt) and nuclear (Nuc) proteins were extracted for Western blot analysis of gene expression using specific antibodies (respective left panel) and relative expression levels of proteins were quantitated (respective right panels). Protein localization in the PDAC cells was determined using immunofluorescent staining. DAPI, 4′,6-diamidino-2-phenylindole (C).
Increased FOXM1 expression induced by transfection prevented downregulation of β-catenin expression caused by VDR overexpression in PDAC cells (Supplementary Figure S3A), whereas knockdown of FOXM1 expression potentiated the downregulation of β-catenin expression caused by VDR overexpression (Supplementary Figure S3B).
Moreover, treatment with 1,25D significantly decreased the expression of β-catenin but slightly decreased that of E-cadherin in PDAC cells (Supplementary Figure S2). In untreated cells, E-cadherin was predominantly expressed in non-nuclear compartments, e.g., cytosolic and/or membrane-bound, whereas β-catenin was expressed in both nuclear and non-nuclear compartments (Supplementary Figure S2). In contrast, treatment with 1,25D decreased the expression of β-catenin in both nuclear and non-nuclear compartments, predominantly the nuclear β-catenin (less so for non-nuclear β-catenin). Consistently, cell fractionation experiments showed that EB treatment and VDR transfection decreased the expression levels of nucler FOXM1, β-catenin and E-Cadherin, while the levels of non-nuclear E-Cadhering were relatively stable (Figure 4A and 4B). These data suggested that interactions among β-catenin, FOXM1 and E-cadherin play important roles in their expression in nuclear and non-nuclear compartments.
Suppression of the Growth, Migration, Invasion, and Stemness of Pancreatic Cancer Cells by Activation of VDR Signaling
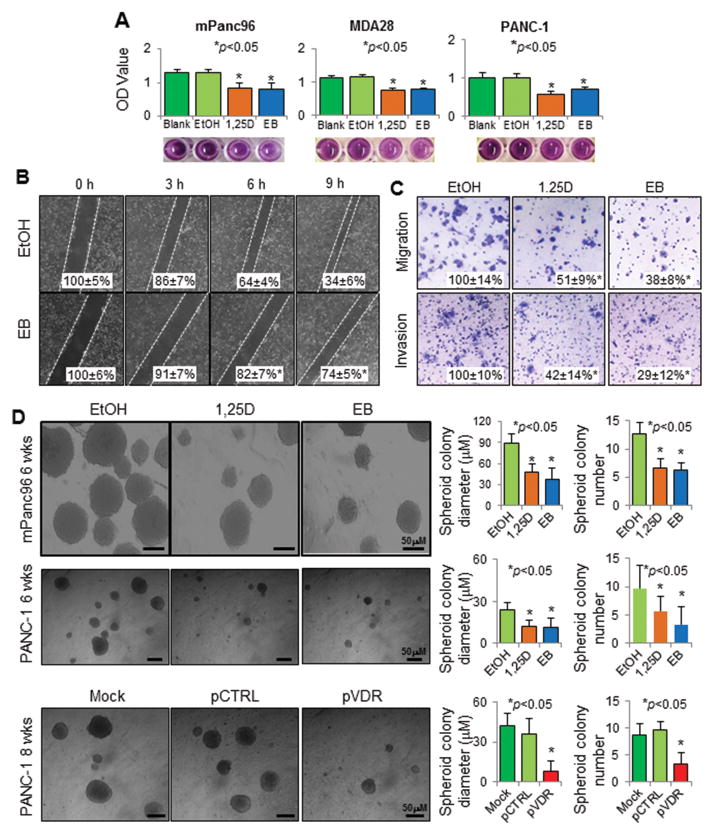
Researchers have demonstrated the anti-proliferative activity of Vitamin D and VDR in several types of tumors.27–35 Indeed, both 1,25D and EB inhibited the growth of mPanc96, PANC-1, MDA-28, PA-TU-8902, BxPC-3, and FG cells in vitro in a time- and dose-dependent manner (Figure 5A; Supplementary Figures S4). Interestingly, 1,25D and EB also suppressed the migration, invasion of, and, most importantly, spheroid formation by PDAC cells (Figure 5B, 5C, 5D; Supplementary Figures S5). Attenuation of tumor growth was consistent with suppression of FOXM1 expression in the tumors (Supplementary Figure 6A and 6B). Thus, we clearly established for the first time that 1,25D and EB inhibit PDAC cell stemness, invasion, and metastasis.
Figure 5.
Impact of altered VDR signaling on pancreatic cancer cell biology. Treatment with 1,25D and/or EB at 100 nM for 48 hours or as indicated inhibited the (A) growth of mPanc96, PANC-1, and MDA-28 cells in vitro (MTT assay); (B) horizontal migration of mPanc96 cells (gap-closing assay); (C) vertical migration and invasion of mPanc96 cells (Boyden chamber assay); and (D) stemness of PANC-1 and mPanc96 cells (spheroid colony formation assay). Moreover, transfection of PANC-1 cells with a VDR expression vector (pVDR) suppressed cell stemness more so than did transfection with a control vector (pCTRL). *P<0.01 as with the proper control.
Impact of Genetic and Pharmacological Manipulation of VDR Expression and Function on PDAC Cell stemness and Metastasis
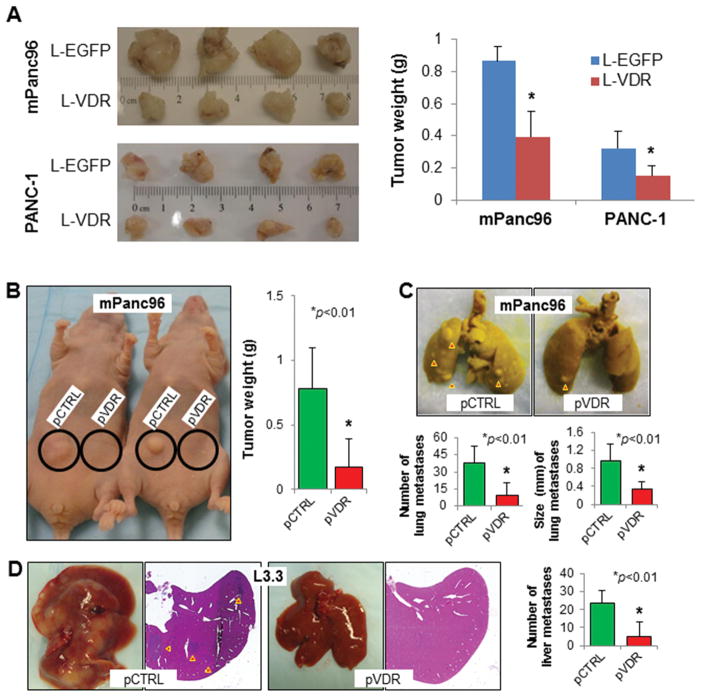
Finally, increased expression of VDR by using lentivirus-mediated gene transfer suppressed the growth of PDAC cells in orthotopic (Figure 6A) and ectopic (Figure 6B) nude mouse models of PDAC. Moreover, overexpression of VDR suppressed both experimental lung (Figure 6C) and liver (Figure 6D) metastasis in these models. Importantly, we isolated the tumor stem cells from mPanc96, and their tumorigenic potential was determined (Supplementary Figure S7A). Their tumor-spheroid formation (Supplementary Figure S7B) and in vivo tumorigenicity were suppressed by the treatment of 1,25D or EB (Supplementary Figure S7C). Thus, activation of VDR signaling produced significant anti-stemness and anti-tumor activity in PDAC.
Figure 6.
Impact of altered VDR signaling on pancreatic tumor growth and metastasis. (A) mPanc96 and PANC-1 cells were transfected with L-VDR or L-EGFP. The cells were then injected into the pancreases of nude mice. The mice were killed 35 days after tumor-cell injection, and their tumors were harvested: Photos of gross tumors (left panels) and Tumor weights (right panels). The cells were injected into the subcutis (B), tail vein for experimental lung metastasis(C), or ileocolic vein for experimental liver metastasis (D). Affected mice (B, left panel), mouse tumor weights (B, right panel), experimental lung metastases (C, upper panel), numbers and sizes of experimental lung metastases (C, lower panels), photos and sections of liver metastases (D, left panels), and numbers of liver metastases (D, right panel) were shown.
Discussion
In the present study, we discovered a novel Vitamin D/VDR/FOXM1 signaling pathway in regulation of PDAC pathogenesis. First, VDR expression was drastically reduced in PDAC cell lines and tissues, and was inversely correlated with that of FOXM1. Reduced or lost VDR expression correlated with PDAC progression. Second, activation of the Vitamin D/VDR pathway suppressed the proliferation, migration, stemness, tumorigenicity and metastasis of PDAC cells. Third, treatment with 1,25D or EB inhibited the expression of FOXM1 and its downstream targets by repressing FOXM1 transcription and by blockade of nuclear FOXM1 expression. Fourth, treatment with 1,25D or EB and VDR transgenics reduced the stemness of PDAC cells. These novel clinical and mechanistic findings strongly indicate that inactivation of the Vitamin D/VDR pathway and consequential elevation of FOXM1 expression and function promotes PDAC progression.
FOXM1 expression is elevated in human PDAC, 12,16,17 and is a key regulator of PDAC biology.12,15–17 Our current study has shown that the levels of FOXM1 expression correlate with tumor grade and differentiation, further substantiating the clinical significance of FOXM1 expression in PDAC pathogenesis. However, we observed that VDR expression was pronouncedly reduced in primary PDAC cells and tissues, and correlated with advanced stage and poor pathological grade. Those results suggest that both FOXM1 and VDR are potentially novel biomarkers for predicting prognosis of PDAC patients. For example, the frequently reduced VDR in advanced PDAC could be responsible for no objective anti-tumour activity of Seocalcitol clinical trials.41 Seocalcitol and other approaches to activate VDR signaling could have significant impact on this malignancy in early or minimal disease states.
Besides the inverse correlation of their expressions in PDAC, FOXM1 and VDR exhibit opposite cellular functions in a variety of contexts.43–45 In additional to its anti-proliferation activity,33–35 we have demonstrated that VDR expression and activation suppressed the migration, and invasion of PDAC cells and, most importantly, attenuate the stemness of PDAC cells. In contrast, overexpression of FOXM1 promotes many aspects of PDAC cell biology, including stemness.12 As regulatory molecules, VDR and FOXM1 opposingly regulates the expression and/or function of various genes related to cell cycle, stemness and mesenchymal cell markers,27–30 i.e., many downstream molecules of VDR are in fact those of FOXM1. Significantly, VDR suppressed the expression of FOXM1, suggesting that the impact of VDR activation on its downstream targets and consequential anti-tumor activities may likely be executed through suppression of FOXM1 expression. Evidently, VDR negatively regulates FOXM1 expression and function by two potentially distinct mechanisms, i.e., repressing FOXM1 gene transcription and attenuating nuclear FOXM1 expression.
Moreover, VDR activation caused concomitant changes of expression levels and subcellular distribution of both FOXM1 and β-catenin, i.e., predominantly reduced expression levels of nuclear FOXM1 and β-catenin. This indicates the existence of a novel VDR/FOXM1/β-catenin axis. This notion is supported by our early study, which has shown that FOXM1 interacts directly with β-catenin and facilitate the nuclear translocation of β-catenin,42 and other early reports, which have shown that VDR interacts with β-catenin and influences its nuclear content in colorectal cancer cells.18,19 Presumbly, VDR can influence the nuclear level of β-catenin via increased binding of β-catenin to membrane E-cadherin.44 This notion is supported by our study, showing that the expression of non-nuclear E-Cadherin correlated directly with the relatively stable levels of non-nuclear β-catenin and FOXM1. However, we clearly observed that VDR reduced the expression of both FOXM1 and β-catenin in both nuclear and non-nuclear compartments. We believe that VDR-mediated downregulation of FOXM1 critically contributed to the reduction of nuclear β-catenin, given that FOXM1 critically regulates the nuclear translocalization of β-catenin and that FOXM1 is a positive transactivator of β-catenin gene expression.42 Thus, VDR-indeced downregulation of FOXM1 could result in an decrease in overall expression and particularly nuclear accumulation of β-catenin protein.
Finally, the great potential of Vitamin D as a cancer chemopreventive and therapeutic agent has spurred investigations into the molecular mechanisms that govern its effects on cancer and the development of Vitamin D/VDR-based strategies for prevention and treatment of cancer.17,24,45–47 However, the mechanisms underlying VDR underexpression in cancer cells remain unclear. VDR promoter methylation and overexpression of negative regulators potentially contribute to the silencing of VDR signaling pathway.47–49 Future study is clearly warranted to develop strategies to restore VDR expression and function and translate those relevant findings to PDAC interventions.
In summary, a reduced or lost expression of VDR and its attenuated signaling led to the overexpression of FOXM1 and its downstream targets, thus promoting PDAC cell proliferation, stemness, invasion, and metastasis. The clinicopathological relevance and significance of this aberrant Vitamin D/VDR/FOXM1 signaling in PDAC pathogenesis has been demonstrated by using molecular biology, animal models and human PDAC specimens. Therefore, activation and/or restoration of Vitamin D/VDR signaling likely produces an antitumor effect by repressing FOXM1 signaling. Further investigations into molecular mechanisms underlying dysregulation of this novel pathway would help identify promising targets for designing novel preventive and therapeutic modalities to control PDAC.
Supplementary Material
Translational Relevance.
We used a pancreatic tumor tissue microarray, molecular biology, and animal models to evaluate the inactivation and function of the Vitamin D receptor (VDR)/Forkhead box M1 (FOXM1) pathway in pancreatic cancer cells. Our clinical and mechanistic findings indicated that FOXM1 is a direct transcriptional target of VDR and that dysregulation of VDR expression, which occurs frequently, leads to aberrant FOXM1 expression. Moreover, VDR negatively regulated stemness, growth and metastasis of pancreatic cancer cells, suggesting a novel molecular basis for the critical role of VDR inactivation in pancreatic cancer progression. It also suggests that dysregulated VDR/FOXM1 signaling is a promising new molecular target for designing novel preventive and/or therapeutic strategies to control this malignancy. Therefore, our findings may have a major effect on clinical management of pancreatic cancer.
Acknowledgments
The authors thank Don Norwood for editorial comments.
Funding
Supported in part by grants R01-CA129956, R01-CA148954, R01-CA152309 and R01-CA172233 (to K.X.) and grants R01-CA116528 and R01-CA157933 (to S.H.); and MD Anderson Cancer Center Support Grant CA016672 from the National Institutes of Health; and grant LC2013C28 (to Z.L.) from the Heilongjiang Province Foundation for Returnees of China; and grant 81172265/H1617 (to Y.Z.) from the National Natural Science Foundation of China.
Abbreviations used in this paper
- DMEM
Dulbecco’s modified Eagle’s medium
- DPBS
Dulbecco’s phosphate-buffered saline
- EB
EB1089
- EGFP
enhanced green fluorescent protein
- EMT
epithelial-to-mesenchymal transition
- EtOH
ethanol
- FBS
fetal bovine serum
- FOXM1
Forkhead box M1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- 1, 25D
1,25-dihydroxyvitamin D3
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PDAC
pancreatic ductal adenocarcinoma
- VDR
Vitamin D receptor
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63(5):318–48. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144(6):1316–26. doi: 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 4.Hartwig W, Werner J, Jäger D, Debus J, Büchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14(11):e476–85. doi: 10.1016/S1470-2045(13)70172-4. [DOI] [PubMed] [Google Scholar]
- 5.Abel EV, Simeone DM. Biology and clinical applications of pancreatic cancer stem cells. Gastroenterology. 2013;144(6):1241–8. doi: 10.1053/j.gastro.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 6.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144(6):1230–40. doi: 10.1053/j.gastro.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanger BZ, Hebrok M. Control of cell identity in pancreas development and regeneration. Gastroenterology. 2013;144(6):1170–9. doi: 10.1053/j.gastro.2013.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wierstra I. The transcription factor FOXM1 (Forkhead box M1): proliferation-specific expression, transcription factor function, target genes, mouse models, and normal biological roles. Adv Cancer Res. 2013;118:97–398. doi: 10.1016/B978-0-12-407173-5.00004-2. [DOI] [PubMed] [Google Scholar]
- 9.Kalin TV, Ustiyan V, Kalinichenko VV. Multiple faces of FoxM1 transcription factor: lessons from transgenic mouse models. Cell Cycle. 2011;10(3):396–405. doi: 10.4161/cc.10.3.14709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raychaudhuri P, Park HJ. FoxM1: a master regulator of tumor metastasis. Cancer Res. 2011;71:4329–4333. doi: 10.1158/0008-5472.CAN-11-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halasi M, Gartel AL. FOX(M1) news--it is cancer. Mol Cancer Ther. 2013;12(3):245–54. doi: 10.1158/1535-7163.MCT-12-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C, Du J, Xie K. FOXM1 and its oncogenic signaling in pancreatic cancer pathogenesis. Biochim Biophys Acta. 2014;1845(2):104–16. doi: 10.1016/j.bbcan.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Ahmad A, Li Y, Banerjee S, Kong D, Sarkar FH. Forkhead box M1 transcription factor: a novel target for cancer therapy. Cancer Treat Rev. 2010;36(2):151–6. doi: 10.1016/j.ctrv.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koo CY, Muir KW, Lam EW. FOXM1: From cancer initiation to progression and treatment. Biochim Biophys Acta. 2012;1819:28–37. doi: 10.1016/j.bbagrm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Banerjee S, Kong D, Li Y, Sarkar FH. Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007;67:8293–8300. doi: 10.1158/0008-5472.CAN-07-1265. [DOI] [PubMed] [Google Scholar]
- 16.Huang C, Qiu Z, Wang L, Peng Z, Jia Z, Logsdon CD, Le X, Wei D, Huang S, Xie K. A novel FoxM1-Caveolin signaling pathway promotes pancreatic cancer invasion and metastasis. Cancer Res. 2012;72:655–65. doi: 10.1158/0008-5472.CAN-11-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah S, Islam MN, Dakshanamurthy S, et al. The molecular basis of vitamin D receptor and β-catenin crossregulation. Mol Cell. 2006;21:799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 18.Larriba MJ, González-Sancho JM, Barbáchano A, Niell N, Ferrer-Mayorga G, Muñoz A. Vitamin D Is a Multilevel Repressor of Wnt/β-Catenin Signaling in Cancer Cells. Cancers (Basel) 2013;5(4):1242–60. doi: 10.3390/cancers5041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beildeck ME, Islam M, Shah S, Welsh J, Byers SW. Control of TCF-4 expression by VDR and vitamin D in the mouse mammary gland and colorectal cancer cell lines. PLoS One. 2009;4:e7872. doi: 10.1371/journal.pone.0007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans PM, Chen X, Zhang W, Liu C. KLF4 interacts with β-catenin/TCF4 and blocks p300/CBP recruitment by beta-catenin. Mol Cell Biol. 2010;30:372–81. doi: 10.1128/MCB.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy DA, Cooley K, Skidmore B, Fritz H, Campbell T, Seely D. Vitamin d: pharmacokinetics and safety when used in conjunction with the pharmaceutical drugs used in cancer patients: a systematic review. Cancers (Basel) 2013;5(1):255–80. doi: 10.3390/cancers5010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin R, Nagai Y, Sladek R, Bastien Y, Ho J, Petrecca K, et al. Expression profiling in squamous carcinoma cells reveals pleiotropic effects of vitamin D3 analog EB1089 signaling on cell proliferation, differentiation, and immune system regulation. Mol Endocrinol. 2002;16:1243–1256. doi: 10.1210/mend.16.6.0874. [DOI] [PubMed] [Google Scholar]
- 23.Wood RJ, Tchack L, Angelo G, Pratt RE, Sonna LA. DNA microarray analysis of vitamin D-induced gene expression in a human colon carcinoma cell line. Physiol Genomics. 2004;17:122–129. doi: 10.1152/physiolgenomics.00002.2003. [DOI] [PubMed] [Google Scholar]
- 24.Byers SW, Rowlands T, Beildeck M, Bong YS. Mechanism of action of vitamin D and the vitamin D receptor in colorectal cancer prevention and treatment. Rev Endocr Metab Disord. 2012;13(1):31–8. doi: 10.1007/s11154-011-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakai Y, Demay MB. Evaluation of keratinocyte proliferation and differentiation in vitamin D receptor knockout mice. Endocrinology. 2000;141:2043–9. doi: 10.1210/endo.141.6.7515. [DOI] [PubMed] [Google Scholar]
- 26.Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134(2):123–39. doi: 10.1111/j.1365-2567.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorne J, Campbell MJ. The vitamin D receptor in cancer. Proc Nutr Soc. 2008;67:115–27. doi: 10.1017/S0029665108006964. [DOI] [PubMed] [Google Scholar]
- 28.Pálmer HG, Sánchez-Carbayo M, Ordóñez-Morán P, Larriba MJ, Cordón-Cardó C, Muñoz A. Genetic signatures of differentiation induced by 1alpha,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res. 2003;63:7799–806. [PubMed] [Google Scholar]
- 29.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19:2685–95. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 30.Mullin GE, Dobs A. Vitamin d and its role in cancer and immunity: a prescription for sunlight. Nutr Clin Pract. 2007;22:305–22. doi: 10.1177/0115426507022003305. [DOI] [PubMed] [Google Scholar]
- 31.Spina CS, Ton L, Yao M, et al. Selective vitamin D receptor modulators and their effects on colorectal tumor growth. J Steroid Biochem Mol Biol. 2007;103:757–62. doi: 10.1016/j.jsbmb.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 32.de Lyra EC, da Silva IA, Katayama ML, et al. 25(OH)D3 and 1,25(OH)2D3 serum concentration and breast tissue expression of 1alpha-hydroxylase, 24-hydroxylase and Vitamin D receptor in women with and without breast cancer. J Steroid Biochem Mol Biol. 2006;100:184–92. doi: 10.1016/j.jsbmb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Zugmaier G, Jäger R, Grage B, Gottardis MM, Havemann K, Knabbe C. Growth-inhibitory effects of vitamin D analogues and retinoids on human pancreatic cancer cells. Br J Cancer. 1996;73(11):1341–6. doi: 10.1038/bjc.1996.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanemaru M, Maehara N, Chijiiwa K. Antiproliferative effect of 1α,25-dihydroxyvitamin D3 involves upregulation of cyclin-dependent kinase inhibitor p21 in human pancreatic cancer cells. Hepatogastroenterology. 2013;60(125):1199–205. doi: 10.5754/hge11073. [DOI] [PubMed] [Google Scholar]
- 35.Kawa S, Nikaido T, Aoki Y, Zhai Y, Kumagai T, Furihata K, Fujii S, Kiyosawa K. Vitamin D analogues up-regulate p21 and p27 during growth inhibition of pancreatic cancer cell lines. Br J Cancer. 1997;76(7):884–9. doi: 10.1038/bjc.1997.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Persons KS, Eddy VJ, Chadid S, Deoliveira R, Saha AK, Ray R. Anti-growth effect of 1,25-dihydroxyvitamin D3–3-bromoacetate alone or in combination with 5-amino-imidazole-4-carboxamide-1-beta-4-ribofuranoside in pancreatic cancer cells. Anticancer Res. 2010;30(6):1875–80. [PubMed] [Google Scholar]
- 37.Ghous Z, Akhter J, Pourgholami MH, Morris DL. Inhibition of Hepatocellular Cancer by EB1089: In Vitro and In Vivo Study. Anticancer Res. 2008;28:3757–3762. [PubMed] [Google Scholar]
- 38.Mouratidis PX, Dalgleish AG, Colston KW. Investigation of the mechanisms by which EB1089 abrogates apoptosis induced by 9-cis retinoic acid in pancreatic cancer cells. Pancreas. 2006;32:93–100. doi: 10.1097/01.mpa.0000191648.47667.4f. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Jiang F, Li P, Li C, Ma Q, Nicosia SV, Bai W. Growth suppression of ovarian cancer xenografts in nude mice by vitamin D analogue EB1089. Clin Cancer Res. 2005;11:323–328. [PubMed] [Google Scholar]
- 40.Milliken EL, Zhang X, Flask C, Duerk JL, MacDonald PN, Keri RA. EB1089, a vitamin D receptor agonist, reduces proliferation and decreases tumor growth rate in a mouse model of hormone-induced mammary cancer. Cancer Lett. 2005;229:205–215. doi: 10.1016/j.canlet.2005.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans TR, Colston KW, Lofts FJ, Cunningham D, Anthoney DA, Gogas H, de Bono JS, Hamberg KJ, Skov T, Mansi JL. A phase II trial of the vitamin D analogue Seocalcitol (EB1089) in patients with inoperable pancreatic cancer. Br J Cancer. 2002;86(5):680–5. doi: 10.1038/sj.bjc.6600162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, Huang H, Xue J, Liu M, Wang Y, Sawaya R, Xie K, Yung WK, Medema RH, He X, Huang S. FoxM1 promotes β-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20(4):427–42. doi: 10.1016/j.ccr.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hendrickson WK, Flavin R, Kasperzyk JL, Fiorentino M, Fang F, Lis R, Fiore C, Penney KL, Ma J, Kantoff PW, Stampfer MJ, Loda M, Mucci LA, Giovannucci E. Vitamin D receptor protein expression in tumor tissue and prostate cancer progression. J Clin Oncol. 2011;29(17):2378–85. doi: 10.1200/JCO.2010.30.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pálmer HG, González-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Muñoz A. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154(2):369–87. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dalhoff K, Dancey J, Astrup L, et al. A phase II study of the vitamin D analogue Seocalcitol in patients with inoperable hepatocellular carcinoma. Br J Cancer. 2003;89:252–7. doi: 10.1038/sj.bjc.6601104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho M, Peddi PF, Ding K, Chen L, Thomas D, Wang J, Lockhart AC, Tan B, Wang-Gillam A. Vitamin D deficiency and prognostics among patients with pancreatic adenocarcinoma. J Transl Med. 2013;11:206. doi: 10.1186/1479-5876-11-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marik R, Fackler M, Gabrielson E, et al. DNA methylation-related vitamin D receptor insensitivity in breast cancer. Cancer Biol Ther. 2010;10:44–53. doi: 10.4161/cbt.10.1.11994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larriba MJ, Martín-Villar E, García JM, et al. Snail2 cooperates with Snail1 in the repression of vitamin D receptor in colon cancer. Carcinogenesis. 2009;30:1459–68. doi: 10.1093/carcin/bgp140. [DOI] [PubMed] [Google Scholar]
- 49.Yang H, Zhang Y, Zhou Z, Jiang X, Shen A. Snail-1 regulates VDR signaling and inhibits 1,25(OH)-D(3) action in osteosarcoma. Eur J Pharmacol. 2011;670:341–6. doi: 10.1016/j.ejphar.2011.09.160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.