Abstract
Background
Postoperative atrial fibrillation (PoAF) is common after coronary artery bypass grafting (CABG). We previously showed that AF susceptibility single nucleotide polymorphisms (SNPs) at the chromosome 4q25 locus are associated with PoAF. Here, we tested the hypothesis that a combined clinical and genetic model incorporating AF risk SNPs would be superior to a clinical-only model.
Methods and Results
We developed and externally validated clinical and clinical/genetic risk models for PoAF. The discovery and validation cohorts included 556 and 1164 patients, respectively. Clinical variables previously associated with PoAF and 13 SNPs at loci associated with AF in genome wide association studies were considered. PoAF occurred in 30% and 29% of patients in the discovery and validation cohorts, respectively. In the discovery cohort, a logistic regression model with clinical factors had good discrimination, with an area under the receiver operator characteristic (ROC) curve of 0.76. The addition of 10 SNPs to the clinical model did not improve discrimination (area under ROC curve: 0.78, P=0.14 for difference between the two models). In the validation cohort, the clinical model had good discrimination (area under the ROC curve: 0.69) and addition of genetic variables resulted in a marginal improvement in discrimination (area under ROC curve: 0.72, P<0.0001).
Conclusions
We developed and validated a model for the prediction of PoAF containing common clinical variables. Addition of AF susceptibility SNPs did not improve model performance. Tools to accurately predict PoAF are needed to risk-stratify patients undergoing CABG and identify candidates for prophylactic therapies.
Keywords: atrial fibrillation, genetics, risk model, cardiac surgery, postoperative complication arrhythmia
Introduction
Atrial fibrillation (AF), the most common sustained cardiac arrhythmia in clinical practice, frequently occurs after cardiac surgery.1-3 Postoperative AF (PoAF) is associated with longer intensive care unit and hospital stays, increased morbidity and mortality, and higher utilization of healthcare resources,2,4-6 and represents a major potentially preventable adverse outcome. Clinical risk factors for PoAF are well described, and include age, hypertension (HTN), prior AF, heart failure (HF), obesity, prolonged PR interval, tobacco use, and history of myocardial infarction (MI).1,3,4,6-10 Accordingly, several statistical models to predict PoAF have been developed and validated.3,11
AF is increasingly recognized as a genetic disorder. Genome-wide association studies (GWAS) have identified novel common variants associated with AF.12-14 We previously demonstrated that a common AF risk allele at the chromosome 4q25 locus was also associated with the development of PoAF.15 Whether additional common genetic variants confer risk for PoAF is unknown. The addition of these genetic factors to PoAF risk prediction models might improve the ability to accurately predict which patients will develop this important and potentially preventable complication. Using data from two large prospective cohort studies of patients undergoing coronary artery bypass grafting (CABG), we developed and validated a risk model for the prediction of PoAF and tested the hypothesis that a combined clinical and genetic model incorporating multiple common AF risk alleles would be superior to a model containing only clinical factors.
Methods
Study subjects
The discovery cohort included patients in the prospective Vanderbilt Cardiac Surgery Registry (VCSR) who underwent CABG without concurrent valve surgery from November 1999 until November 2004. The validation cohort included patients in the prospective CABG Genomics Program at Brigham and Women's Hospital and the Texas Heart Institute. Patients who had CABG without valve surgery after August 2001 were included in the analysis. All analyses were restricted to self-reported white patients to minimize genetic heterogeneity. Only patients who were in sinus rhythm at the time of surgery were included in the analysis. The study complies with the Declaration of Helsinki, the Institutional Review Boards at each participating institution approved the registries, and all patients gave written informed consent.
Patient demographics, biometrics, clinical comorbidities, and other variables were prospectively entered into both registries using standard definitions. PoAF, the primary study endpoint, was defined as electrocardiographically documented AF, as assessed by inpatient telemetry and 12-lead ECGs during the index hospitalization after surgery. In order to be considered a case, the episode of AF needed to be of sufficient duration to require specific therapy. Otherwise, there was no specific minimum duration of AF necessary to be considered a case.
Genotyping
Genotyping was performed for thirteen single nucleotide polymorphisms (SNPs) associated with prevalent AF in large GWAS studies12-14: rs13376333 and rs6666258 at 1q21; rs3903239 at 1q24; rs2200733, rs10033464, and rs6817105 at 4q25; rs3807989 at 7q31; rs10821415 at 9q22; rs10824026 at 10q22; rs1152591 at 14q23; rs7164883 at 15q24; and rs2106261 and rs7193343 at 16q22. Genotyping was performed using the Sequenom platform (San Diego, CA).
Risk model for post-operative atrial fibrillation
Multivariable logistic regression using age, sex, previous AF, diabetes mellitus (DM), HTN, left ventricular ejection fraction (LVEF), PR interval, smoking, chronic obstructive pulmonary disease, and use of beta-blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, statins, aspirin, and cyclooxygenase (COX)-2 inhibitors was performed to predict PoAF in the discovery cohort. Age, previous AF, and PR interval were highly significant and carried forward in further analyses. DM, HTN, LVEF<40%, pre-operative use of beta-blockers, and post-operative use of beta-blockers were non-significant in the initial model but were also included in further analyses as they were previously established as PoAF risk factors.1,4,7
The combined clinical and genetic model included all of the clinical factors plus rs13376333 (T allele), rs6666258 (C), rs3903239 (A), rs2200733 (T), rs3807989 (A), rs10821415 (A), rs10824026 (G), rs1152591 (A), rs2106261 (A), and rs7193343 (C), using an additive model (0, 1, or 2 copies of the minor allele) for each SNP. Genotyping for the other 3 SNPs failed quality control measures and therefore these were not included in the analysis. Receiver operator characteristic (ROC) curves were generated for the clinical-only and combined clinical/genetic models in both cohorts. A calibration curve was generated to assess the performance of the combined clinical/genetic model in the validation cohort.16 For this, grouped predicted probabilities for the development of PoAF were plotted against observed probabilities within each group and a curve was fit for the grouped observations. For an ideal prediction model, the curve would be a straight line with slope equal to 1. The distribution of the grouped observations and the fitted curve provide information about how the model performs for different predicted probabilities.
We derived a simple weighted risk score for PoAF using clinical data from the discovery cohort. Based on the magnitude of odds ratios in our logistic regression model, we assigned 1 point each for male sex, HTN, DM, LVEF <40%, and PR interval >200ms, 2 points for age >60 years, and 3 points for a previous history of AF. This resulted in a possible risk score from 0 to 10 points. We then tested the performance of the risk score by applying it to the validation cohort. All statistical analyses were conducted using R version 3.0 with the rms package, STATA v12, or SPSS v22.
Results
Baseline patient characteristics and genotypes
Baseline patient characteristics for the discovery and validation cohorts are presented in Table 1. In the discovery cohort of 556 patients without missing variables, mean age was 62±11 years, 72% were male, and 10% had a previous history of AF. In the validation cohort of 1164 patients, mean age was 64±10, 82% were male, and 4.3% had a previous history of AF.
Table 1.
Baseline clinical characteristics for the discovery and validation cohorts
| Discovery | Validation | Between-cohort P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (N=556) | No PoAF (N=391) | PoAF (N=165) | P value | Total (N=1164) | No PoAF (N=828) | PoAF (N=336) | P value | ||
| Age (years) | 62±11 | 60±11 | 66±10 | <0.001 | 64±10 | 63±10 | 68±10 | <0.001 | <0.001 |
| Sex (male) | 402 (72%) | 272 (70%) | 130 (79%) | 0.03 | 952 (82%) | 676 (82%) | 276 (82%) | 0.84 | <0.001 |
| Previous AF | 58 (10%) | 15 (3.8%) | 43 (26%) | <0.001 | 50 (4.3%) | 21 (2.5%) | 29 (8.6%) | <0.001 | <0.001 |
| PR interval | 165 [164–168] | 162 [160–165] | 173 [170–176] | <0.001 | 170 [168–172] | 167 [166–168] | 176 [172–180] | <0.001 | 0.001 |
| Diabetes | 200 (36%) | 136 (35%) | 64 (39%) | 0.37 | 329 (28%) | 242 (29%) | 87 (26%) | 0.25 | 0.001 |
| Hypertension | 444 (80%) | 300 (77%) | 144 (87%) | 0.005 | 873 (75%) | 603 (73%) | 270 (80%) | 0.007 | 0.03 |
| LVEF<40% | 107 (19%) | 67 (17%) | 40 (24%) | 0.05 | 132 (11%) | 86 (10%) | 46 (14%) | 0.11 | <0.001 |
| Pre-op beta blockers | 351 (63%) | 254 (65%) | 97 (59%) | 0.17 | 910 (78%) | 648 (78%) | 262 (78%) | 0.92 | <0.001 |
| Post-op beta blockers | 487 (88%) | 347 (89%) | 140 (85%) | 0.20 | 1103 (95%) | 776 (94%) | 327 (97%) | 0.01 | <0.001 |
Age presented as mean ± standard deviation. PR interval presented as median [bootstrap 95% confidence interval] in milliseconds. AF: atrial fibrillation. PoAF: post-operative atrial fibrillation. LVEF: left ventricular ejection fraction. P values were calculated for differences between PoAF and no PoAF with chi-square for nominal variables and Mann-Whitney U test for continuous variables. Between-cohort P values reflect differences between the 2 entire cohorts, irrespective of PoAF status.
Minor allele frequencies for the discovery and validation cohorts are presented in Table 2. In the discovery cohort, rs10033464 (at 4q25) was not in Hardy-Weinberg equilibrium and was excluded from further analysis. In the validation cohort, rs6817105 at 4q25 and rs7164883 at 15q24 failed genotyping. Otherwise, all genotyping assays met pre-specified quality control measures with >95% call rate and results were in Hardy-Weinberg equilibrium, and genotypes for each of the 10 SNPs were available for every patient included in the main analysis.
Table 2.
Minor allele frequencies for atrial fibrillation susceptibility loci
| Discovery | Validation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (N=556) | No PoAF (N=391) | PoAF (N=165) | P value | Total (N=1164) | No PoAF (N=828) | PoAF (N=336) | P value | Between cohort P value | ||
| Chr. 1q21 | rs13376333 (C/T) | 30.3% | 28% | 35.8% | 0.04 | 29.6% | 28.7% | 31.8% | 0.24 | 0.74 |
| rs6666258 (G/C) | 30.8% | 28% | 37.3% | 0.01 | 25% | 24.2% | 27.2% | 0.06 | <0.001 | |
| Chr. 1q24 | rs3903239 (G/A) | 39.1% | 38.7% | 40% | 0.80 | 56.8% | 57.9% | 54.3% | 0.14 | <0.001 |
| Chr. 4q25 | rs2200733 (C/T) | 11.1% | 10.5% | 12.4% | 0.62 | 12.8% | 10.7% | 17.9% | <0.001 | 0.25 |
| Chr. 7q31 | rs3807989 (G/A) | 45.1% | 44.2% | 47.3% | 0.55 | 41% | 41% | 41.1% | 0.90 | 0.009 |
| Chr. 9q22 | rs10821415 (C/A) | 37.2% | 36.6% | 38.8% | 0.80 | 44.3% | 44.3% | 44.2% | 0.53 | 0.001 |
| Chr. 10q22 | rs10824026 (A/G) | 18.8% | 19.6% | 17% | 0.17 | 15.5% | 16.7% | 12.6% | 0.02 | 0.05 |
| Chr. 14q23 | rs1152591 (G/A) | 42.5% | 42.7% | 42.1% | 0.70 | 47.7% | 48.3% | 46.3% | 0.52 | 0.02 |
| Chr. 16q22 | rs2106261 (G/A) | 17.9% | 17.8% | 18.2% | 0.44 | 14.4% | 13.4% | 17% | 0.04 | 0.01 |
| rs7193343 (C/T) | 16.5% | 16.2% | 17% | 0.94 | 15.3% | 14.4% | 17.6% | 0.16 | 0.57 | |
PoAF: Postoperative atrial fibrillation
Risk prediction models for post-operative atrial fibrillation
PoAF during the index hospitalization following surgery, the primary study endpoint, occurred in 165 (30%) subjects in the discovery cohort. In the clinical-only model, variables significantly associated with PoAF included age (P<0.0001), longer PR interval (P=0.0006), and a previous history of AF (P<0.0001)(Table 3). The largest effect size was seen with previous AF, with an odds ratio (OR) of developing PoAF of 6.46 (95% confidence interval [CI] 3.36 to 12.4). In the combined clinical and genetic model, age, PR interval, and previous AF remained statistically significant. However, none of the SNPs were significantly associated with PoAF. ROC curves for both models showed very good discrimination, with areas under the ROC curves of 0.763 and 0.78 in the clinical-only and combined clinical/genetic models, respectively (Figure 1a, red and blue curves). However, the difference between the models was not statistically significant (P=0.14 for difference in discrimination between the two models by likelihood ratio test).
Table 3.
Multivariable logistic regression models for the development of post-operative atrial fibrillation in the discovery cohort
| Combined clinical/genetic model | Clinical-only model | |||||
|---|---|---|---|---|---|---|
| Variable | β (SE) | OR (95% CI) | P value | β (SE) | OR (95% CI) | P value |
| Age (per 5 years) | 0.2435 (0.0105) | 1.28 (1.25 to 1.3) | <0.0001 | 0.2355 (0.0103) | 1.27 (1.24 to 1.29) | <0.0001 |
| PR interval (per 20 ms) | 0.154 (0.0044) | 1.36 (1.35 to 1.37) | 0.0005 | 0.292 (0.0042) | 1.34 (1.33 to 1.35) | 0.0006 |
| Previous AF | 1.9475 (0.3449) | 7.01 (3.57 to 13.8) | <0.0001 | 1.8653 (0.3335) | 6.46 (3.36 to 12.4) | <0.0001 |
| Hypertension | 0.6006 (0.3013) | 1.82 (1.01 to 3.29) | 0.05 | 0.5527 (0.2983) | 1.74 (0.97 to 3.12) | 0.06 |
| Diabetes | 0.1283 (0.2243) | 1.14 (0.73 to 1.76) | 0.57 | 0.1552 (0.2162) | 1.17 (0.76 to 1.78) | 0.47 |
| LVEF<40% | 0.356 (0.2648) | 1.43 (0.85 to 2.4) | 0.18 | 0.3806 (0.2605) | 1.46 (0.88 to 2.44) | 0.14 |
| Pre-op beta blocker | −0.0148 (0.2258) | 0.99 (0.63 to 1.53) | 0.95 | −0.0749 (0.2196) | 0.93 (0.6 to 1.43) | 0.73 |
| Post-op beta blocker | −0.2621 (0.3163) | 0.77 (0.41 to 1.43) | 0.41 | −0.1911 (0.3084) | 0.83 (0.45 to 1.51) | 0.54 |
| rs13376333 (T) | −0.9951 (0.8129) | 0.37 (0.08 to 1.82) | 0.22 | |||
| rs6666258 (C) | 1.3839 (0.797) | 3.99 (0.84 to 19) | 0.08 | |||
| rs3903239 (A) | −0.158 (0.1631) | 0.85 (0.62 to 1.18) | 0.33 | |||
| rs2200733 (T) | 0.2145 (0.2398) | 1.24 (0.77 to 1.98) | 0.37 | |||
| rs3807989 (A) | 0.0531 (0.1589) | 1.05 (0.77 to 1.44) | 0.74 | |||
| rs10821415 (A) | 0.1051 (0.1482) | 1.11 (0.83 to 1.49) | 0.48 | |||
| rs10824026 (G) | −0.2249 (0.1959) | 0.8 (0.54 to 1.17) | 0.25 | |||
| rs1152591 (A) | 0.212 (0.1506) | 1.24 (0.92 to 1.66) | 0.16 | |||
| rs2106261 (A) | −0.2831 (0.333) | 0.75 (0.39 to 1.45) | 0.4 | |||
| rs7193343 (C) | −0.1107 (0.3475) | 0.9 (0.45 to 1.77) | 0.75 | |||
SE: standard error. OR: odds ratio. CI: confidence interval. ms: milliseconds. AF: atrial fibrillation. LVEF: left ventricular ejection fraction
Figure 1A.
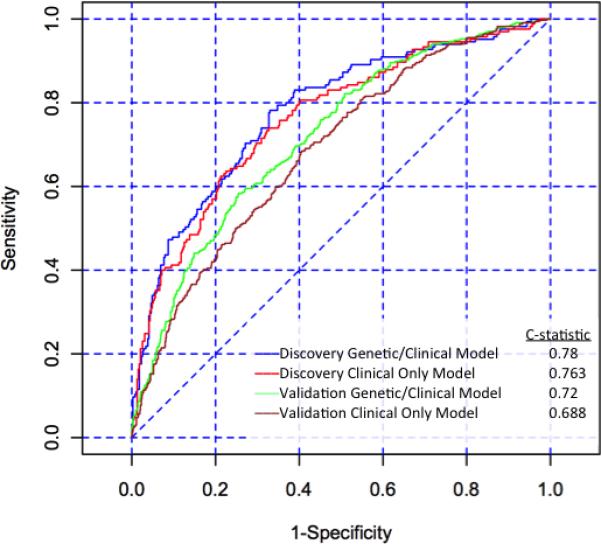
Receiver operator characteristic (ROC) curves for the discovery cohort combined clinical/genetic model (blue line, area under the ROC curve 0.78), discovery cohort clinical-only model (red line, area under the ROC curve 0.763), validation cohort combined clinical/ genetic model (green line, area under the ROC curve 0.72), and validation cohort clinical-only model (brown line, area under the ROC curve 0.688).
The VCSR clinical-only and combined clinical/genetic models were applied to the CABG Genomics validation cohort. The clinical-only model showed good discrimination, with an area under the ROC curve of 0.688 (Figure 1a). Application of the combined clinical/genetic model to the validation cohort resulted in a marginal improvement in discrimination (area under the ROC curve: 0.72, P=4.2 × 10−6 for difference between the models). A calibration curve showed overall good calibration, though there was over-prediction for the high probability groups, with lower-than-expected actual probabilities of developing PoAF in groups who had the highest predicted probability (Figure 1b).
Figure 1B.
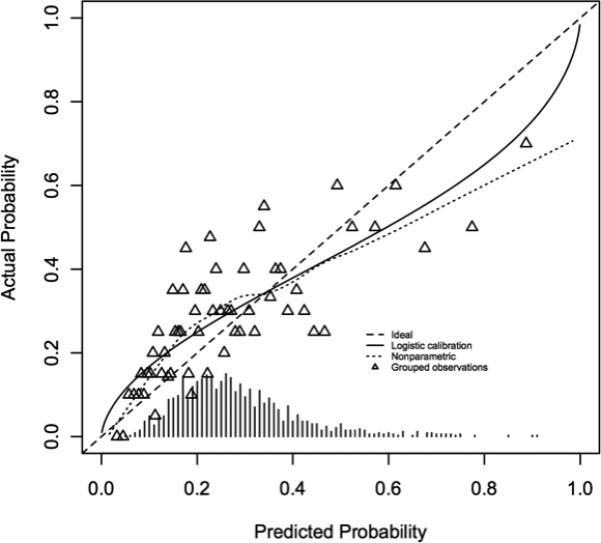
Calibration curve for the validation cohort combined clinical/genetic model. Patients were grouped based on predicted probability of developing post operative atrial fibrillation, predicted probabilities were plotted against actual probabilities, and a curve was fit to the data. For an ideal prediction model, this curve would represent a straight line with slope equal to 1.
We derived a simple risk score for PoAF that included age >60 years, male sex, DM, HTN, LVEF <40%, PR interval >200ms, and previous history of AF. The score performed well in both the discovery and validation cohorts, with incremental observed incidence of PoAF with increasing scores (Chi square P<0.001 in both cohorts, Table 4).
Table 4.
Simple risk score for the prediction of post-operative atrial fibrillation
| Discovery cohort (N=556) | Validation cohort (N=1164) | |||
|---|---|---|---|---|
| Score | Number of patients with score | Incidence of PoAF | Number of patients with score | Incidence of PoAF |
| 0-1 | 66 | 7.6% | 130 | 8.5% |
| 2 | 100 | 12% | 195 | 19.5% |
| 3 | 113 | 19.5% | 243 | 28.4% |
| 4 | 125 | 34.4% | 322 | 33.2% |
| 5 | 83 | 44.6% | 186 | 39.8% |
| ≥6 | 69 | 66.7% | 88 | 42% |
PoAF: postoperative atrial fibrillation. Calculation of score: 1 point each for male sex, hypertension, diabetes mellitus, left ventricular ejection fraction < 40%, and PR interval >200ms; 2 points for age >60 years; and 3 points for a previous history of atrial fibrillation.
Discussion
We developed and independently validated a clinical risk model for the prediction of PoAF. The model had good discrimination in the discovery and validation cohorts, with areas under the ROC curves of 0.76 and 0.69, respectively. We hypothesized that the addition of common AF risk SNPs to the model would improve discrimination. However, there was no difference between the clinical model and the combined clinical/genetic model in the discovery cohort, and only a marginal difference in the validation cohort. These findings call into question how much added value genetic information contributes to an already well-fitted model utilizing only clinical variables for the prediction of PoAF. While SNPs in AF susceptibility genes have been independently associated with the common ambulatory forms of AF in multiple studies, our results suggest a limited role of these SNPs in predicting PoAF. One reason for this finding might be that the triggers for PoAF are primarily driven by clinical risk factors and common AF risk alleles do not have a significant impact on the incidence of PoAF, especially if only a limited number of genetic variables are considered. Alternatively, one or more of the SNPs we studied might be strongly associated with clinical predictors (especially prior history of AF) and therefore the predictive information of these SNPs might already be accounted for by the clinical factors in our model.
PoAF is a common adverse event after cardiac surgery. In a large cohort of more than 18,000 patients undergoing cardiac surgery, the prevalence of PoAF was 19% and did not differ between on-pump and off-pump cases.11 In another large multicenter Veterans Administration (VA) study including more than 3800 patients, the overall incidence of PoAF was 30% and was highest among patients undergoing CABG with mitral valve replacement (60%) and lowest among patients having CABG without valve surgery (28%).4
PoAF is an important predictor of morbidity and mortality. In a case-control study of over 6400 patients who underwent CABG at a single center, PoAF was independently associated with stroke (OR 2.0), in-hospital mortality (OR 1.7), and long-term mortality (OR 1.5).17 The aforementioned VA study found that PoAF was independently associated with stroke (OR 2.2), HF (OR 3.3), intensive care unit (ICU) re-admission (OR 3.3), re-intubation (OR 4.3), in hospital mortality (OR 2.0), and 6 month mortality (OR 2.2).4 In a separate study of approximately 6700 patients who underwent CABG, PoAF was associated with increased mortality at 1, 5, and 10 years after surgery.18 Importantly, in the modern era of healthcare cost containment, the average length of stay for patients who developed PoAF in another study was increased by 4.9 days after full adjustment for covariates, corresponding to an increase in hospital cost of $10,055 per patient.1 PoAF also predicted ICU and hospital length of stay in a separate multicenter study (3.6 days vs. 2 days and 10 days vs. 7 days, respectively).4
Multiple strategies for preventing PoAF have been studied, including therapy with beta-blockers, anti-arrhythmic drugs (AADs), magnesium, colchicine, atrial pacing, and posterior pericardiectomy.19 Oral beta-blockers have been consistently shown to reduce the development of PoAF and are recommended for virtually all patients undergoing cardiac surgery.20 However, even with beta-blockade, the incidence of PoAF remains high. Several studies have shown that prophylactic use of amiodarone or sotalol in the perioperative period reduces the incidence of the arrhythmia. In a meta-analysis of randomized trials, the OR (95% CI) for development of PoAF for sotalol versus beta-blocker was 0.42 (0.26 to 0.65) and for amiodarone versus placebo was 0.48 (0.4 to 0.57).21 In fact, the use of amiodarone or sotalol for PoAF prophylaxis in selected patients has been recommended by the American College of Cardiology,22 the American College of Chest Physicians,23 and the Canadian Cardiovascular Society.24 However, these recommendations have not been widely incorporated into clinical practice.
It is unclear why clinicians do not routinely utilize prediction tools and prophylactic strategies (other than use of beta-blockers) to prevent PoAF after cardiac surgery. We and others have postulated that there is a reluctance to expose patients to the potential adverse effects of prophylactic AADs.8 An individualized approach, whereby only high-risk patients are selected for perioperative use of AADs might maximize benefits for these patients while minimizing the exposure of low-risk patients to the potential adverse drug effects. Thus, highly accurate methods of predicting which patients will develop PoAF are needed. However, in order to be widely accepted into clinical practice, prediction tools must also be simple to use. We therefore developed a simple risk score for the prediction of PoAF. The score performed well in our discovery cohort and also in our independent validation cohort. In both cohorts, a score ≥5 predicted a >40% risk of PoAF. Our score should assist clinicians in risk stratifying patients and identifying those who would benefit from additional preventative therapies such as prophylactic AADs.
Clinical risk factors for the development of PoAF including age, HTN, prior AF, HF, obesity, prolonged PR interval, tobacco use, and history of MI, have been thoroughly studied.1,3,4,6-10 As a result, several risk assessment tools to predict PoAF have been developed.3,21 We previously conducted a multi-center study of the association of 4q25 SNPs with PoAF.15 Seven 4q25 SNPs were independently associated with PoAF and addition of 4q25 genotypes to a clinical risk prediction model improved discrimination (area under the ROC curve 0.720 vs. 0.702, P<0.0001). These data suggested that the addition of genetic information could improve the ability to identify patients at high risk for PoAF. However, we were not able to reproduce this finding in our current study.
Our study has several important limitations that should be considered when interpreting the results. As with all retrospective studies, ours is prone to the effects of bias and unmeasured confounders. The final patient cohorts analyzed were selected from larger cardiac surgery registries based on the presence of complete data, and it is possible that our findings were affected by non-random missing variables. There were significant differences in clinical and genetic factors between the discovery and validation cohorts (Tables 1 and 2) that might account for the divergent results between the cohorts with respect to improved model performance with the addition of genetic factors. The definition of our primary endpoint, PoAF documented by 12 lead ECG or telemetry of sufficient duration to warrant specific therapy, was chosen in order to be consistent with previous studies.1,3,5,7,11 However, use of a more precise definition (e.g., PoAF of at least 5 minutes duration) might have led to different study results. In order to reduce variability, we chose a priori to limit our study to patients undergoing CABG without any concurrent procedures. However, excluding patients undergoing concurrent valve and structural procedures might limit the generalizability of our findings to broader patient populations.
In conclusion, we developed an accurate risk prediction model for PoAF in patients undergoing CABG based on readily available clinical variables. Importantly, we validated our model in a geographically independent cohort, a crucial step before any risk prediction model can be implemented in routine clinical practice. However, we were unable to prove our primary hypothesis that addition of AF susceptibility alleles would result in a clinically meaningful improvement in risk prediction model performance. Even in the current era of near ubiquitous use of post-operative beta-blockers, the risk for the development of AF after cardiac surgery remains high. Other preventative strategies including the use of AADs for selected high-risk patients have been recommended but are seldom utilized. The ability to accurately identify patients at high risk for PoAF through the use of a prediction model should allow for an individualized approach wherein AADs, with their inherent potential toxicities, are used in patients who stand to benefit the most and avoided in others, potentially maximizing clinical benefits while reducing adverse drug reactions.
Acknowledgments
Funding Sources: This work was supported by grants from the National Heart, Lung, and Blood Institute at the National Institutes of Health [grant numbers HL65962, HL068774, HL056693, and HL092217].
Footnotes
Conflicts of Interest Disclosures: None.
References
- 1.Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, Collins JJ, Cohn LH, Burstin HR. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94:390–397. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 2.Maisel W, Rawn J, Stevenson W. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001;135:1061–1073. doi: 10.7326/0003-4819-135-12-200112180-00010. [DOI] [PubMed] [Google Scholar]
- 3.Amar D, Shi W, Hogue CW, Zhang H, Passman RS, Thomas B, Bach PB, Damiano R, Thaler HT. Clinical prediction rule for atrial fibrillation after coronary artery bypass grafting. J Am Coll Cardiol. 2004;44:1248–1253. doi: 10.1016/j.jacc.2004.05.078. [DOI] [PubMed] [Google Scholar]
- 4.Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, Tarazi R, Shroyer AL, Sethi GK, Grover FL, Hammermeister KE. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–510. doi: 10.1097/00000658-199710000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filardo G, Hamilton C, Hebeler RF, Hamman B, Grayburn P. New-onset postoperative atrial fibrillation after isolated coronary artery bypass graft surgery and long-term survival. Circ Cardiovasc Qual Outcomes. 2009;2:164–169. doi: 10.1161/CIRCOUTCOMES.108.816843. [DOI] [PubMed] [Google Scholar]
- 6.Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010;37:1353–1359. doi: 10.1016/j.ejcts.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 7.Shen J, Lall S, Zheng V, Buckley P, Damiano RJ, Schuessler RB. The persistent problem of new-onset postoperative atrial fibrillation: A single-institution experience over two decades. J Thorac Cardiovasc Surg. 2011;141:559–570. doi: 10.1016/j.jtcvs.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banach M, Rysz J, Drozdz J, Okonski P, Misztal M, Barylski M, Irzmanski R, Zaslonka J. Risk factors of atrial fibrillation following coronary artery bypass grafting: a preliminary report. Circ J. 2006;70:438–441. doi: 10.1253/circj.70.438. [DOI] [PubMed] [Google Scholar]
- 9.Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah AS, Habib RH. Obesity and risk of new-onset atrial fibrillation after cardiac surgery. Circulation. 2005;112:3247–3255. doi: 10.1161/CIRCULATIONAHA.105.553743. [DOI] [PubMed] [Google Scholar]
- 10.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 11.El-Chami MF, Kilgo PD, Elfstrom KM, Halkos M, Thourani V, Lattouf OM, Delurgio DB, Guyton RA, Leon AR, Puskas JD. Prediction of new onset atrial fibrillation after cardiac revascularization surgery. Am J Cardiol. 2012;110:649–654. doi: 10.1016/j.amjcard.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 12.Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, Arking DE, Müller-Nurasyid M, Krijthe BP, Lubitz SA, Bis JC, Chung MK, Dörr M, Ozaki K, Roberts JD, Smith JG, Pfeufer A, Sinner MF, Lohman K, Ding J, Smith NL, Smith JD, Rienstra M, Rice KM, Van Wagoner DR, Magnani JW, Wakili R, Clauss S, Rotter JI, Steinbeck G, Launer LJ, Davies RW, Borkovich M, Harris TB, Lin H, Völker U, Völzke H, Milan DJ, Hofman A, Boerwinkle E, Chen LY, Soliman EZ, Voight BF, Li G, Chakravarti A, Kubo M, Tedrow UB, Rose LM, Ridker PM, Conen D, Tsunoda T, Furukawa T, Sotoodehnia N, Xu S, kamatani N, Levy D, Nakamura Y, Parvez B, Mahida S, Furie KL, Rosand J, Muhammad R, Psaty BM, Meitinger T, Perz S, Wichmann H-E, Witteman JCM, Kao WHL, Kathiresan S, Roden DM, Uitterlinden AG, Rivadeneira F, McKnight B, Sjögren M, Newman AB, Liu Y, Gollob MH, Melander O, Tanaka T, Stricker BHC, Felix SB, Alonso A, Darbar D, Barnard J, Chasman DI, Heckbert SR, Benjamin EJ, Gudnason V, Kääb S. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaab S, Darbar D, van Noord C, Dupuis J, Pfeufer A, Newton-Cheh C, Schnabel R, Makino S, Sinner MF, Kannankeril PJ, Beckmann BM, Choudry S, Donahue BS, Heeringa J, Perz S, Lunetta KL, Larson MG, Levy D, MacRae CA, Ruskin JN, Wacker A, Schomig A, Wichmann H-E, Steinbeck G, Meitinger T, Uitterlinden AG, Witteman JC, Roden DM, Benjamin EJ, Ellinor PT. Large scale replication and meta-analysis of variants on chromosome 4q25 associated with atrial fibrillation. Eur Heart J. 2008;30:813–819. doi: 10.1093/eurheartj/ehn578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MCY, Baum L, So WY, Wong KS, Chan JCN, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, Smith EE, Rosand J, Hillert J, Ma RCW, Ellinor PT, Thorgeirsson G, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 15.Body SC, Collard CD, Shernan SK, Fox AA, Liu K-Y, Ritchie MD, Perry TE, Muehlschlegel JD, Aranki S, Donahue BS, Pretorius M, Estrada J-C, Ellinor PT, Newton-Cheh C, Seidman CE, Seidman JG, Herman DS, Lichtner P, Meitinger T, Pfeufer A, Kaab S, Brown NJ, Roden DM, Darbar D. Variation in the 4q25 chromosomal locus predicts atrial fibrillation after coronary artery bypass graft surgery. Circ Cardiovasc Genet. 2009;2:499–506. doi: 10.1161/CIRCGENETICS.109.849075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Villareal RP, Hariharan R, Liu BC, Kar B, Lee V-V, Elayda M, Lopez J, Rasekh A, Wilson JM, Massumi A. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742–748. doi: 10.1016/j.jacc.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Mariscalco G, Engström KG. Postoperative atrial fibrillation is associated with late mortality after coronary surgery, but not after valvular surgery. Ann Thorac Surg. 2009;88:1871–1876. doi: 10.1016/j.athoracsur.2009.07.074. [DOI] [PubMed] [Google Scholar]
- 19.Arsenault KA, Yusuf AM, Crystal E, Healey JS, Morillo CA, Nair GM, Whitlock RP. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013;1:CD003611. doi: 10.1002/14651858.CD003611.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK. Management of Patients With Atrial Fibrillation (Compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS Recommendations): A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:1916–1926. doi: 10.1161/CIR.0b013e318290826d. [DOI] [PubMed] [Google Scholar]
- 21.Burgess DC, Kilborn MJ, Keech AC. Interventions for prevention of post-operative atrial fibrillation and its complications after cardiac surgery: a meta-analysis. Eur Heart J. 2006;27:2846–2857. doi: 10.1093/eurheartj/ehl272. [DOI] [PubMed] [Google Scholar]
- 22.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 23.Bradley D, Creswell LL, Hogue CW, Epstein AE, Prystowsky EN, Daoud EG. American College of Chest Physicians. Pharmacologic prophylaxis: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:39S–47S. doi: 10.1378/chest.128.2_suppl.39s. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell LB, CCS Atrial Fibrillation Guidelines Committee Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention and treatment of atrial fibrillation following cardiac surgery. Can J Cardiol. 2011;27:91–97. doi: 10.1016/j.cjca.2010.11.005. [DOI] [PubMed] [Google Scholar]


