Abstract
Purpose
Few studies have evaluated the effects of anti-retroviral therapy on skeletal metabolism in Asian populations infected with human immunodeficiency virus (HIV).
Methods
We performed a secondary analysis of bone turnover markers (BTM) at baseline and two years in stored plasma samples collected from 2/2009 – 1/2013 as part of a multi-center trial. Two groups were compared: 1) treatment-naïve patients initiated on zidovudine (AZT)/lamivudine (3TC) plus nevirapine (NVP), and 2) patients who failed first-line therapy and were switched to tenofovir (TDF)/3TC plus lopinavir/ritonavir (LPVr). Tests included the bone resorption marker, C-terminal cross-linking telopeptide of type-1 collagen (CTX) and the bone formation marker procollagen type 1 N-terminal propeptide (P1NP).
Results
In the TDF/3TC+LPVr group, samples were available from 59 patients at baseline and 56 patients at two years. Of these, 36 patients had samples available from both time points. In the AZT/3TC+NVP group, plasma samples were analyzed from 82 participants at baseline and of those, 61 had samples at two years. Median change over two years was greater in the TDF/3TC+LPVr group for both CTX (+0.24 ng/mL, IQR 0.10–0.43 vs. +0.09ng/mL, IQR −0.03 to 0.18, p=0.001) and P1NP (+25.5ng/mL, IQR 2.4–51.3 vs. +7.11 ng/mL, IQR −4.3 to 21.6, p=0.012). Differences remained after adjusting for age, sex, and body mass index.
Conclusions
Switching to TDF/3TC+LPVr after treatment failure resulted in greater increases in BTMs than initiation with AZT/3TC+NVP in Chinese patients with HIV. Following this change, bone resorption marker levels increased by nearly 60%, which is greater than the 25–35% increase from baseline described previously in non-Chinese populations. Further studies are warranted to elucidate these findings.
Keywords: Bone Turnover, Resorption, HIV, Chinese, Tenofovir
INTRODUCTION
Over the past decade, a growing number of studies have demonstrated the deleterious effects of human immunodeficiency virus (HIV) and antiretroviral therapies (ART) on skeletal metabolism [1,2,3]. In particular, initiation of ART has been shown to lead to a rapid rise in markers of bone resorption in the first 3–6 months of therapy followed by delayed rise in markers of bone formation creating a “catabolic window” during which bone resorption predominates. These two closely coupled processes plateau by approximately one year, however levels of resorption have not been shown to return to baseline over two to three years of follow up [4–6]. Corresponding studies evaluating outcomes such as bone mineral density (BMD) and fractures have also shown that over time, patients with HIV on ART lose bone mass [7–9] and have increased rates of fracture [10–13] compared with uninfected individuals.
Certain ARTs appear to exert an especially profound effect on bone. Tenofovir disoproxil fumarate (TDF) has been consistently associated with a 1–2% greater decline in BMD compared to other nucleoside reverse transcriptase inhibitors when used in combination with other ARTs [6,14–16]. The mechanism of TDF’s effect on the skeleton remains uncertain, and may be related to direct effects on bone cells, or indirect effects due to secondary hyperparathyroidism and disruption of calcium-phosphate homeostasis resulting in inadequate bone mineralization [17–20]. Protease inhibitor (PI)-containing regimens have been shown to be associated with greater bone loss than non-PI containing regimens in some [16,21,22] but not all studies [8]. Depending on the specific agent, PIs appear to impact bone by differentially activating osteoclasts or inhibiting osteoblasts [16,23,24].
It is estimated that 780,000 persons live with HIV/AIDS in China and as China continues to broaden HIV/AIDS surveillance and treatment networks, the number of individuals diagnosed and started on treatment each year continues to rise [25,26]. Under the China National Free Antiretroviral Treatment Programme (NFATP), individuals with HIV that have a CD4 count <350 per μL are referred for free ART. As of 2011, the total number of people ever receiving and currently receiving treatment were 155,530 and 126,448 respectively [27]. In 2012, TDF, which was previously obtainable only as second line therapy in combination with ritonavir-boosted lopinavir (LPVr), was made available as first line therapy in response to increasing data demonstrating the cost-effectiveness of first line TDF therapy in low-resource settings [28,29].
Little data exists regarding the impact of ART on bone metabolism in Asian populations and other middle-income countries, and few studies have specifically evaluated the impact of TDF administration. As HIV screening, diagnosis and treatment programs expand in China and neighboring middle-income countries, understanding the skeletal consequences of prolonged survival on ART in Asian populations is essential. In an effort to better assess the impact of widespread availability of TDF in China in the coming years, we retrospectively compared changes in bone turnover markers (BTM) over two years in a cohort of Chinese individuals with HIV treated with first-line zidovudine (AZT)/lamivudine (3TC) plus nevirapine (NVP) therapy, to a group of patients who were switched to TDF/3TC+LPVr after failing first line AZT- or stavudine-based therapy, and hypothesized that bone turnover marker (BTM) levels at two years would be greater in the latter group.
METHODS
Study Design and Study Population
We examined BTM levels at baseline and after two years using stored plasma samples collected from 2/2009 – 1/2013 as part of a large multi-center clinical trial in eight cities across China: Beijing, Shanghai, Zhengzhou, Fuzhou, Guangzhou, Shenzhen, Xi’an, and Yunnan (ClinicalTrials.gov identifier: NCT00872417). Details of the recruitment and follow up of the original parent study have been described previously [30,31]. All participants enrolled in the parent trial had clinical and laboratory evaluations performed at week 0, 4, 12, and every 12 weeks thereafter. Plasma samples were collected fasting in the morning and stored at −80°C. The parent study was approved by the institutional review board of Peking Union Medical College Hospital (PUMCH) and all patients provided written informed consent before participating in any trial procedures.
Samples were drawn from two cohorts. The first cohort consisted of 87 patients who failed first-line ART and were switched to TDF/3TC+LPVr (called the TDF+LPVr group). Of these 87 patients, 59 had samples available at baseline and 56 had samples available at two years. Thirty-six participants in this group had samples available from both time points. Inclusion criteria included: (1) men and women 18–65 years of age, (2) first-line ART failure defined as receiving first-line ART (e.g., AZT/3TC + NVP or efavirenz (EFV); stavudine (D4T)/3TC + NVP or EFV; AZT/didanosine (DDI) + NVP or EFV; and D4T/DDI + NVP or EFV) for at least one year with plasma HIV-1 viral load (VL) more than 400 copies per milliliter, appearance of new opportunistic infections or AIDS-related cancers, or inability to tolerate first line regimen. The main exclusion criteria were (1) patients who had received initial ART regimes containing TDF and/or protease inhibitors (PIs) (2) serum creatinine >1.5 times the upper limit of normal, (3) eGFR< 50ml/min/1.73m2, and (4) anticipated poor adherence.
We then randomly selected 87 participants from a second cohort of 184 treatment-naïve patients initiated on AZT/3TC+NVP (called the AZT+NVP group) to compare with the TDF+LPVr group. Of these, 82 participants had plasma samples available from baseline and 61 participants had samples available at both time points. The inclusion criteria for this study group included: men and women aged 18–65 years with documented HIV-1 infection and a CD4+ count <350 cell/mm3 for over 1 month. Exclusion criteria included: acute HIV infection, currently active AIDS-defining illness, pregnancy, breastfeeding, women of child-bearing age not on contraception, current injection drug use or alcohol abuse, acute or chronic pancreatitis, peripheral neuropathy, severe psychiatric or neurologic diseases, severe peptic ulcers, WBC < 2.0 × 109/L, Hemoglobin <90 g/L, platelet count < 75 × 109/L, transaminase, alkaline phosphatase, bilirubin, amylase or creatinine kinase levels 3 times upper limit of normal (ULN), or serum creatinine 1.5 times ULN.
Baseline demographic and clinical data, including sex, age, BMI, ethnicity, education level, alcohol use and smoking history, route of transmission, CD4+ cell counts, HIV-1 viral load, and Hepatitis C status were collected for all participants as part of the parent study. This study was reviewed and exempt by the institutional review board of PUMCH and the human investigations committee of Yale School of Medicine.
Laboratory Testing
All laboratory testing was performed in the Clinical Laboratory Department of PUMCH. BTMs tested included the bone resorption marker, C-terminal cross-linking telopeptide of type-1 collagen (CTX), and bone formation marker, procollagen type 1 N-terminal propeptide (P1NP). 25-hydroxy vitamin D (25OHD), intact PTH (iPTH) and phosphorus were assayed as additional indicators of bone homeostasis. CTX, P1NP, 25OHD, and iPTH were assayed by an electrochemiluminescence immunoassay (MODULAR ANALYTICS E170, cobase 601, Roche Diagnostics, Mannheim, Germany). Phosphorus was assessed using a molybdate-based method (Beckman Coulter AU5800 Chemistry Analyzer, Beckman Coulter Inc., Brea CA, USA).
Plasma HIV-1 RNA viral load was measured by the COBAS Ampliprep/TaqMan 48 according to the manufacturer’s instructions (Roche Molecular Systems, Pleasanton, CA, USA; reference range 40–1,000,000 copies/mL). CD4+ T cell count was determined by 3-color flow cytometry (Epics XL flow cytometer, Beckman Coulter, USA). Freshly collected EDTA-anticoagulated whole blood was incubated with a panel of fluorescence-labeled monoclonal antibodies and isotype controls (FITC-CD4/PE-CD8/PE-Cy5-CD3, FITC-IgG1/PE-IgG1/PE-Cy5-IgG1). Cell number was acquired by flow cytometer after red blood cell lysis.
Statistical Analysis
All statistical analyses were performed using Stata Intercooled 13 (StataCorp, College Station, TX). Descriptive statistics were used to report means, standard deviations, medians, interquartile ranges (IQR), and frequencies. Student’s t-test and Wilcoxon Rank Sum tests were used to compare measures of central tendency across treatment groups for normally distributed and non-parametric continuous variables, respectively. Comparisons of categorical variables were performed using Pearson’s χ2 and Fisher’s exact tests. Spearman’s rank correlation was used to measure correlation between CTX and P1NP within each treatment group at baseline and two years. For the participants that had samples available from both time points, the median absolute difference in BTM was calculated as well as median percent change from baseline. Differences between treatment groups were then compared using the Wilcoxon Rank Sum test. Separate univariate linear regression models were used to assess the relationships between baseline characteristics (age, sex, BMI, treatment regimen, alcohol use and smoking history, baseline levels of CTX and P1NP, 25OHD and iPTH levels, CD4+ cell count, HIV viral load, and Hepatitis C status) and levels of CTX and P1NP at two years. We subsequently estimated multivariable linear regression models after assessing all independent variables for possible multicollinearity, and removing smoking history from the model due to a high correlation coefficient (r > 0.40). We fit multivariable models using backward elimination beginning with all variables that were hypothesized to be related to bone turnover and/or that were significant (p-value < 0.10) in the univariate analyses. To obtain a parsimonious model, we removed non-significant variables one at a time beginning with the least significant; in each step, remaining parameter estimates remained largely unchanged (<20%). We further performed a subgroup analysis to identify predictors of increased BTM levels for patients treated with TDF+LPVr. Again, univariate linear regression was performed with CTX and P1NP as dependent variables, followed by multivariable regression analysis using backward elimination to fit a parsimonious model. We retained age, sex and BMI in all final multivariable models due to the strong historical influence of these factors on skeletal metabolism.
RESULTS
Baseline characteristics
Table 1 outlines the baseline characteristics of the participants included in this study, grouped by treatment regimen. Sex distribution, BMI, ethnicity (Han Chinese vs. all other Chinese ethnicities), current alcohol use (any vs. none), and smoking history (ever vs. never) were similar in both groups. Compared with patients treated with AZT+NVP, participants in the treatment-failure (TDF+LPVr) group primarily came from one study site, Zhengzhou (88.6 vs. 19.5%, p<0.001), where blood-borne transmission is the primary source of infection (83.1 vs. 12.4%, p<0.001), rates of HCV co-infection are high (72.7 vs. 14.8%, p<0.001), and level of education is low. The mean age at enrollment was older in the TDF+LPVr group (42 ± 8.0 vs. 38 ± 9.8 years, p=0.003) and the median number of months since HIV diagnosis to enrollment was significantly longer (61 vs. 1 month, p<0.001). Baseline median HIV-1 viral load and CD4+ cell counts were similar in both groups.
Table 1.
Baseline characteristics of study participants, by treatment group
| Characteristic | AZT+NVP group | TDF+LPVr group | p |
|---|---|---|---|
| Male, n/N(%) | 56/81 (68.3) | 55/77 (71.4) | 0.667 |
| Age at enrollment, mean years ± SD | 38 ± 9.8 | 42 ± 8.0 | 0.003 |
| BMI, mean kg/m2 ± SD | 21.4 ± 2.8 | 21.8 ± 2.6 | 0.422 |
| Han Chinese, n/N(%) | 75/81 (92.6) | 76/77 (98.7) | 0.062 |
| Education level, n/N(%) | |||
| Illiterate/poorly literate | 3/81 (3.7) | 10/77 (13.0) | <0.001 |
| Primary/junior high school | 43/81 (53.1) | 57/77 (74.0) | |
| High school/college/beyond | 35/81 (43.2) | 10/77 (13.0) | |
| Study Site = Zhengzhou, n/N(%) | 16/82 (19.5) | 70/79 (88.6) | <0.001 |
| Any alcohol use, n/N(%) | 20/81 (24.7) | 19/77 (24.7) | 0.998 |
| Smoker ever, n/N(%) | 20/81 (24.7) | 26/77 (33.8) | 0.209 |
| Route of Transmission, n/N(%) | |||
| Sexual transmission | 65/81 (80.3) | 10/77 (13.0) | <0.001 |
| Blood-borne | 10/81 (12.4) | 64/77 (83.1) | |
| Months since HIV diagnosis, median (IQR) | 1 (0–11) | 61 (56–72) | <0.001 |
| CD4+ T cell count, median cells/mm3(IQR) | 154 (64–258.5) | 156.5 (62.5–272.5) | 0.559 |
| HIV viral load, median copies/mL (IQR) | 37,443 (8029–128697) | 24,509 (5595–62079) | 0.102 |
| HCV Antibody positive, n/N(%) | 12/77 (15.6) | 56/70 (80.0) | <0.001 |
SD: standard deviation; IQR: interquartile range; BMI: body mass index; AZT: zidovudine; NVP: nevirapine; TDF: tenofovir; LPVr: lopinavir/ritonavir; HIV: human immunodeficiency virus; HCV: hepatitis C virus.
Changes in Bone Turnover Markers
Within-group comparisons of change in BTM found a slight increase from baseline to two years in median levels of CTX that did not reach statistical significance in the AZT+NVP group (0.34 ng/mL, IQR 0.22–0.46 vs. 0.39 ng/mL, IQR 0.32–0.51, p= 0.058) accompanied by a small but significant increase in P1NP (41.53 ng/mL, IQR 27.58–57.09 vs. 48.73 ng/mL, 41.44–56.55, p=0.008; Figures 1A and 1B). By comparison, in the TDF+LPVr group, there was a pronounced increase observed in both markers at two years (CTX: 0.33 ng/mL, IQR 0.19–0.49 vs. 0.72 ng/mL, IQR 0.56–0.93, p<0.001; P1NP: 39.33 ng/mL, IQR 27.05–62.39 vs. 85.76 ng/mL, IQR 59.16–104.80, p<0.001).
Figure 1. Change in bone turnover markers, vitamin D, and parathyroid hormone over two years, by treatment group.
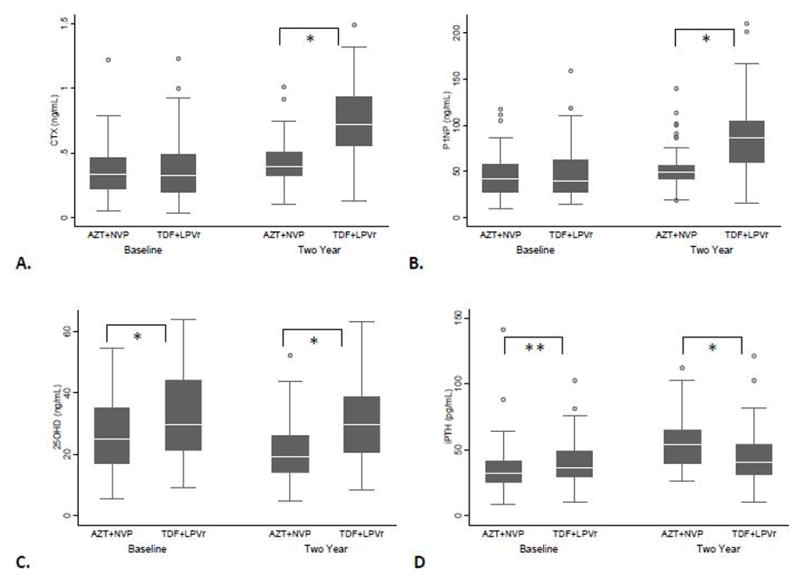
A. Serum levels of C-terminal cross-linking telopeptide of type-1 collagen (CTX) B. Serum levels of procollagen type 1 N-terminal propeptide (P1NP) C. Serum levels of 25-hydroxy vitamin D (25OHD)D. Serum levels of intact parathyroid hormone (iPTH). AZT+NVP = zidovudine+nevirapine; TDF+LPVr = tenofovir+lopinavir/ritonavir; * p≤0.01; ** p=0.05.
Between-group comparisons showed that while there was no significant difference at baseline between levels of BTM in the two treatment groups (CTX: p=0.652, P1NP: p=0.876), at two years BTM levels were significantly increased in the TDF+LPVr group compared with the AZT+NVP group (CTX: p<0.001, P1NP: p<0.001; Figures 1A and 1B).
In a subgroup analysis of participants with samples available from both time points (Table 2), serum CTX increased by 59.6% over two years in the TDF+LPVr group while only increasing by 31.3% in the AZT+NVP group (median absolute differences: +0.24 ng/mL, IQR 0.10–0.43 vs. +0.09ng/mL, IQR −0.03 to 0.18, p=0.001). P1NP increased by 42.4% in the TDF+LPVr group and by 15.3% in the participants treated with AZT+NVP (median absolute differences: +25.47ng/mL, IQR 2.35–51.31 vs. +7.11 ng/mL, IQR −4.26 to 21.55, p=0.012).
Table 2.
Absolute difference and percent change in BTM, by treatment group
| Bone Turnover Marker | AZT+NVP (N=61 pairs) | TDF+LPVr (N=36 pairs) | p |
|---|---|---|---|
| CTX ng/mL | |||
| Absolute Difference, median (IQR) | 0.09 (−0.03 – 0.18) | 0.24 (0.10 – 0.43) | 0.001 |
| Percent Change, median (IQR) | 31.27 (−12.03 – 80.54) | 59.59 (16.31 – 166.53) | 0.018 |
| P1NP ng/mL | |||
| Absolute Difference, median (IQR) | 7.11 (−4.26 – 21.55) | 25.47 (2.35 – 51.31) | 0.012 |
| Percent Change, median (IQR) | 15.30 (−8.11 – 77.30) | 42.43 (6.11 – 129.74) | 0.067 |
CTX: C-terminal cross-linking telopeptide of type-1 collagen; P1NP: procollagen type 1 N-terminal propeptide; AZT: zidovudine; NVP: nevirapine; TDF: tenofovir; LPVr: lopinavir/ritonavir; IQR: interquartile range.
Levels of CTX and P1NP were strongly correlated both at baseline and two years in both treatment groups indicating a coupled increase in bone turnover (Figure 2).
Figure 2. Correlation between BTM levels, by treatment group and time point.
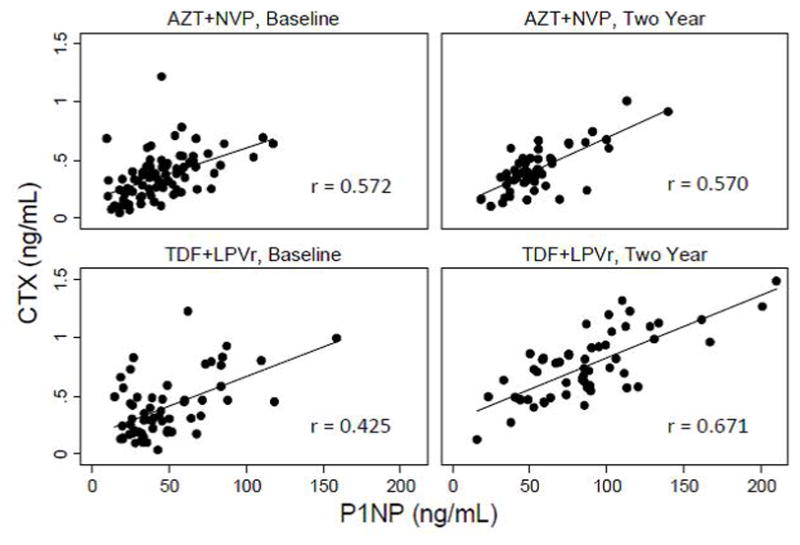
CTX: C-terminal cross-linking telopeptide of type-1 collagen; P1NP: procollagen type 1 N-terminal propeptide; AZT: zidovudine; NVP: nevirapine; TDF: tenofovir; LPVr: lopinavir/ritonavir.
Changes in 25OHD, iPTH, and Phosphorus
Within-group comparisons showed that 25OHD remained stable from baseline to two years in patients receiving TDF+LPVr (29.80 ng/mL, IQR 21.46–44.18 vs. 29.73 ng/mL, IQR 20.82–38.64; p=0.568), but decreased in the AZT+NVP group (24.88 ng/mL, IQR 17.02–34.89 vs. 19.31 ng/mL, IQR 14.26–26.18, p=0.007). Coincident with the decline in 25OHD, iPTH increased from baseline to two years in the AZT+NVP group (32.27 pg/mL, IQR 25.41–41.76 vs. 53.92 pg/mL, IQR 39.18–65.08, p<0.001) but did not change the TDF+LPVr group (Figure 1C and 1D). Mean phosphorus levels did not change significantly from baseline to two years within either treatment group (AZT+NVP: 1.10 ± 0.22 mmol/L vs. 1.09 ± 0.25 mmol/L, p=0.717; TDF+LPVr: 1.16 ± 0.20 mmol/L vs. 1.2 ± 0.29, p=0.407).
Between-group comparisons showed that at baseline participants in the TDF+LPVr group had higher median levels of 25OHD (p=0.010) and iPTH (p=0.050). However over time 25OHD decreased and iPTH increased in the AZT+NVP such that at two years 25OHD was lower (p<0.001) and iPTH was higher (p=0.003) in the AZT+NVP group (Figure 1C and 1D). Phosphorus levels did not differ at baseline between the two treatment groups (p=0.076), however serum phosphorus was slightly higher at two years (p=0.022) in the TDF+LPV group.
Linear Regression Analysis
Univariate linear regression found that TDF+LPVr treatment was the strongest predictor for increased CTX and P1NP levels at two years (Table 3). Other significant variables in the univariate analysis included BMI (for CTX only), baseline BTM level, Hepatitis C antibody (HCV Ab) status, and 25OHD level (for CTX only). In the multivariable model, variables that remained significant included TDF+LPVr therapy, BMI, baseline BTM level, and baseline CD4+ cell count (for CTX only).
Table 3.
Univariate and multivariable linear regression analysis
| CTX Level at Two Years | P1NP Level at Two Years | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Entire Cohort | Univariate | Multivariable (R2=0.46) | Univariate | Multivariable (R2=0.27) | ||||
|
|
|
|||||||
| β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | |
| Age | 0.003 | −0.003 to 0.009 | −0.002 | −0.006 to 0.002 | 0.06 | −0.64 to 0.76 | −0.09 | −0.66 to 0.49 |
| Sex | −0.02 | −0.13 to 0.10 | 0.02 | −0.07 to 0.11 | 3.62 | −10.10 to 17.34 | 3.62 | −7.31 to 14.54 |
| BMI | −0.02 | −0.04 to −0.002b | −0.02 | −0.03 to −0.001b | −2.20 | −4.67 to 0.28a | −2.06 | −4.11 to −0.01b |
| TDF+LPVr regimen | 0.34 | 0.26 to 0.43d | 0.28 | 0.20 to 0.38d | 33.09 | 21.66 to 44.52d | 29.03 | 15.14 to 42.91c |
| Smoker ever | 0.03 | −0.09 to 0.14 | -- | -- | −2.16 | −16.41 to 12.10 | -- | -- |
| Any alcohol use | −0.12 | −0.22 to 0.01a | -- | -- | −14.75 | −30.31 to 0.81a | -- | -- |
| Baseline CTX level | 0.47 | 0.28 to 0.67d | 0.23 | 0.03 to 0.42b | -- | -- | -- | -- |
| Baseline P1NP level | -- | -- | -- | -- | 0.37 | 0.16 to 0.59d | 0.32 | 0.060 to 0.58b |
| Baseline 25OHD level | 0.01 | 0.0004 to 0.01b | -- | -- | −.02 | −0.32 to 0.29a | -- | -- |
| Baseline iPTH level | −0.0001 | −0.003 to 0.003 | -- | -- | 0.17 | −0.21 to 0.54 | -- | -- |
| Baseline CD4+ count | −0.0004 | −0.001 to 0.0001a | −0.0004 | −0.001 to −0.00002b | −0.03 | −0.08 to 0.02 | -- | -- |
| Baseline HIV Viral load | −4.6e-08 | −1.3e-07 to 3.5e-08 | -- | -- | −4.3e-06 | −0.00001 to 5.6e-06 | -- | -- |
| HCV antibody status | 0.28 | 0.19 to 0.38d | -- | -- | 24.29 | 11.22 to 36.94d | −10.99 | −25.11 to 3.14 |
| TDF+LPVr Subgroup | Univariate | Multivariable(R2=0.46) | Univariate | Multivariable (R2=0.28) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | |
| Age | −0.001 | −0.01 to 0.008 | −0.001 | −0.01 to 0.01 | −0.70 | −1.96 to 0.55 | −0.19 | −1.28 to 0.89 |
| Sex | −0.05 | −0.21 to 0.12 | 0.004 | −0.16 to 0.17 | 1.86 | −20.65 to 24.38 | 2.74 | −17.21 to 22.68 |
| BMI | −0.04 | −0.07 to −0.01c | −0.04 | −0.07 to −0.01d | −4.24 | −8.40 to −0.87d | −4.49 | −8.28 to −0.71d |
| Smoker ever | −0.06 | −0.22 to 0.10 | -- | -- | −11.37 | −32.88 to 10.13 | -- | -- |
| Any alcohol use | −0.18 | −0.37 to 0.005a | -- | -- | −20.07 | −45.97 to 5.83 | -- | -- |
| Baseline CTX level | 0.45 | 0.15 to 0.75c | 0.08 | −0.27 to 0.42 | -- | -- | -- | -- |
| Baseline P1NP level | -- | -- | -- | -- | 0.38 | 0.04 to 0.73d | 0.31 | −0.03 to 0.65 |
| Baseline 25OHD level | 0.0002 | −0.01 to 0.01 | -- | -- | −0.16 | −1.01 to 0.71 | -- | -- |
| Baseline iPTH level | −0.0004 | −0.01 to 0.01 | -- | -- | 0.40 | −0.28 to 1.07 | -- | -- |
| Baseline CD4+ count | −0.001 | −0.001 to −0.0003c | −0.001 | −0.001 to −0.0001d | −0.06 | −0.13 to 0.01 | -- | -- |
| Baseline HIV Viral load | −9.0e-08 | −3.2e-07 to 1.4e-07 | -- | -- | −0.00002 | −0.0001 to 9.8e-06 | -- | -- |
| HCV antibody status | 0.17 | −0.01 to 0.36a | -- | -- | 3.90 | −24.62 to 32.43 | -- | -- |
< 0.1;
< 0.05;
< 0.01;
< 0.001
BMI: body mass index; TDF: tenofovir; LPVr: lopinavir/ritonavir; CTX: C-terminal cross-linking telopeptide of type-1 collagen; P1NP: procollagen type 1 N-terminal propeptide; 25OHD: 25-OH vitamin D; iPTH: intact parathyroid hormone; HIV: human immunodeficiency virus; HCV: hepatitis C virus.
A separate regression was performed for those treated with TDF+LPVr to examine which variables were predictors for increased levels of BTM markers at two years in this group (Table 3). The univariate analysis found BMI, baseline BTM level, and baseline CD4+ cell count (for CTX only) were significantly associated with increased CTX and P1NP at two years. In the multivariable model only BMI and baseline CD4+ cell count (for CTX only) remained significantly associated with increase in BTM levels at two years. Of note, we found that BMI was inversely correlated with baseline BTM levels in the TDF+LPVr group as well (data not shown).
DISCUSSION
To our knowledge, this study is the first to investigate the impact of TDF+LPVr therapy on bone metabolism in a cohort of Chinese individuals with HIV. Our findings demonstrate a marked increase in bone resorption, as assessed by CTX, at two years among treatment-failure patients switched to TDF+LPVr that is greater than the 25–35% change previously observed in Western populations switched to TDF-based therapy from other NRTI-based regimens [32–34]. P1NP also increased in the TDF+LPVr group compared with the AZT+NVP group at two years, although the magnitude of increase was more comparable to that seen in prior studies. Taken together these findings suggest a significant increase in the skeletal remodeling rate at two years, with a particularly robust resorptive component.
It is unclear whether the striking increase in CTX levels observed is primarily associated with TDF therapy, or whether the concurrent administration of LPVr in our study augmented the effect on bone turnover. The limited studies that have measured bone turnover markers in patients treated with protease inhibitors versus other classes of ARTs have not found a significant increase in BTM in the PI groups [1,2,35]. However, it is important to note that all of these studies combined data from patients treated with different PIs so there are no isolated data regarding the BTM effects in patients on LPVr. Jain and Lenhard investigated the effects of individual PIs ex vivo on osteoblasts, osteoclasts and adipocytes and found that while RTV lead to slightly increased osteoclast activity in a rodent calvaria model, LPV did not [23]. Instead, these investigators showed that LPV inhibits human mesenchymal stem cell (hMSC) differentiation to osteoblasts. Furthermore, Hernandez-Vallejo, et al. showed that LPV with or without RTV induces premature senescence in hMSC [24]. Combined, these findings suggest that the primary impact of LPVr is to inhibit bone formation, while RTV may have a small role in increasing osteoclast activity. Therefore in our study, we suspect that TDF is the primary driver for the observed increase in bone resorption. It is possible that the relatively smaller rise in P1NP is due to the suppressive effect of LPVr on osteoblast activity. Additional studies are needed in Chinese patients with HIV treated with TDF without concurrent PI therapy to determine if the changes in bone formation and resorption observed in the present study persists in the absence of LPVr.
It is also possible TDF may have an enhanced effect on bone in Chinese individuals. Hu, et al. enrolled fourteen healthy Chinese volunteers who were given either a single dose or multiple dose regimen of TDF [36]. Despite the conclusion that TDF exhibited a similar pharmacokinetic profile to historical comparisons with healthy Western participants, certain pharmacokinetic parameters such as Cmax and Tmax differed by as much as 8–21% between the study participants and historical Western controls. While this study is limited by the use of historical controls and is not directly relevant to HIV-infected patients, it suggests that there may be differences in TDF pharmacokinetics in Chinese as compared to other populations. Larger-scale studies are necessary to make a meaningful comparison between Chinese and Western populations.
Several additional factors have the potential to influence to our findings. Unlike prior TDF switch studies that enrolled virologically suppressed patients, individuals in our cohort of patients were switched to TDF due to treatment-failure and therefore at baseline had lower CD4+ cell counts, higher median viral loads, and lower BMIs compared with the prior cohorts. More severe HIV disease parameters and low BMI are independent risk factors for bone disease, therefore it is possible that our cohort was at greater risk for an exaggerated bone turnover response to ART [1]. Furthermore, the majority of patients in the TDF+LPVr cohort were farmers from rural regions of Henan province, where HIV-1 transmission occurred primarily as a result of contaminated plasma donation practices and as a result the co-infection rate with Hepatitis C virus, which has also been independently associated with increased bone turnover, is high [37]. Based upon these differences, we may have expected to see a higher rate of bone turnover in the TDF+LPVr group at baseline, however baseline BTM levels measured in our TDF+LPVr cohort fell within the range of baseline CTX and P1NP levels seen in the previously published switch studies, and were not significantly different from the baseline values of BTM in our treatment-naïve cohort. Finally, in this region HIV-1 Subtype B predominates [38], however to our knowledge there have been no studies suggesting that populations infected with different subtypes of HIV-1 demonstrate different bone turnover profiles in response to ART.
In the univariate analysis, increased BTM at two years was independently associated with TDF+LPVr treatment, lower BMI (for CTX only), higher baseline BTM level, positive HCV Antibody status, and higher baseline 25OHD level (for CTX only). In the multivariable model, only treatment with TDF+LPVr, lower BMI, higher baseline BTM level, and lower baseline CD4+ cell count (for CTX only) remained significantly associated with higher BTM levels at two years. These findings suggest that treatment withTDF+LPVr is an independent predictor of increased bone turnover at two years among Chinese individuals with HIV, and also supports prior studies demonstrating BMI, baseline BTM levels, and HIV disease severity to impact bone turnover and bone density among patients with HIV. When we focused on predictors for increased BTM at two years within the TDF+LPVr group, we found that in the univariate analysis BMI, baseline BTM and baseline CD4+ cell count (for CTX only) were significant. However in the multivariable model, only lower BMI and higher baseline CD4+ cell count (for CTX only) remained significantly associated with increased BTM levels at two years.
Our study also measured levels of 25OHD, iPTH, and phosphorus to examine the impact of ART on other key regulators of bone homeostasis. Van den Bout-Van den Buekel, et al. showed that patients on NNRTIs such as NVP demonstrated higher rates of 25OHD deficiency and elevated PTH compared with PIs and with untreated patients [39]. This is consistent with our findings in the AZT+NVP group where median 25OHD levels fell from the insufficient to deficient range over two years, with a corresponding increase in levels of PTH during this period as well, likely as a secondary response to declines in 25OHD levels. Although increases in PTH have generally been inversely correlated with 25OHD levels, recent investigations with TDF suggest that with this drug the increase in PTH may be more directly linked to 1,25-OH vitamin D levels. Havens, et al. showed that adolescent patients with HIV treated with Tenofovir had elevations in PTH levels regardless of 25OHD status, and that Vitamin D3 supplementation also decreased levels of PTH regardless of baseline 25OHD status [40]. They hypothesized that perhaps treatment with Vitamin D3 corrected a functional deficiency in vitamin D that was responsible for the elevated PTH levels and demonstrated that high plasma tenofovir concentrations are associated with elevations in vitamin D binding protein and lower free 1,25-OHD levels [41]. Interestingly, their study did not find a decrease in BTM levels despite decrease in PTH with D3 supplementation. Tenofovir has also been shown to induce mild renal phosphate wasting, likely resulting from a combination of renal tubular dysfunction and the propensity of this drug to cause secondary hyperparathyroidism [42].
In our study patients in the TDF+LPVr cohort had higher baseline 25OHD levels compared with the AZT+NVP cohort, potentially attributable to high levels of sun exposure in an agriculturally-based lifestyle. Over two years 25OHD, iPTH, and phosphorus levels all remained stable in this group. Consistent with the observation in Havens’ study, BTM levels in our cohort still increased significantly despite apparently stable hormonal mediators of skeletal metabolism.
Our study had some important limitations. Ideally, we would have been able to measure the impact of TDF+LPVr in a treatment-naïve cohort as the demographic and biologic characteristics of a treatment failure cohort differ in many respects from a treatment-naïve population. However, since TDF was not available in China prior to 2012 as first-line treatment, it was not possible to enroll such a cohort. Furthermore, the participants in the TDF+LPVr cohort were mainly from one site in the Henan province, and differences in HIV-1 transmission patterns and subtype exist between the two groups. As discussed previously, while these characteristics had the potential to influence baseline measurements, they do not seem to have done so disproportionately. Moreover, because these characteristics generally remained stable within the TDF+LPVr group over time, we do not have reason to believe that they would have differentially impacted the magnitude of change in laboratory markers at two years. Finally, bone density data were not available in this cohort; however at present access to dual-energy x-ray absorptiometry (DXA) technology is very limited in China. Ultimately studies powered for changes in bone density and fracture rates are needed. Our study supports the need for such additional investigations.
In conclusion, our study provides new insights regarding BTM responses in an Asian population of individuals with HIV after two years of treatment with TDF+LPVr compared with AZT+NPV. We found that while patients on AZT+NPV sustained only slight increases in BTM at two years, a significant elevation in the bone resorption marker levels out of proportion to the bone formation marker levels remained in the TDF+LPVr group at two years. Furthermore, the differences found in our study appear to be greater in magnitude compared with previous TDF switch studies performed in Western virologically suppressed populations. The number of individuals with HIV exposed to treatment with TDF in China and neighboring middle-income countries is expanding rapidly; however, infrastructure for diagnosis and management of osteoporosis and fractures remains highly variable across regions. Therefore, future prospective studies addressing the knowledge gaps and limitations presented here are essential for understanding the long-term clinical impact of widespread TDF use on bone health in this vulnerable population.
Acknowledgments
This study was funded by the China National Key Technologies R&D Program for the 12th Five-year Plan (2012ZX10001-003). E.H. is funded by the Rheumatology Research Foundation Scientist Development Award. L.F. is supported by NIAMS K24 AR060231-01. X.J. is supported by a grant from the Chinese Ministry of Human Resources and Social Security (2011). Our deepest gratitude to all the study participants and to the participating centers of the original parent study: The Infectious Disease Hospital of Henan Province; Shanghai Public Health Clinical Center, Fudan University; Fuzhou Infectious Diseases Hospital, Fujian Medical University; Guangzhou No.8 People’s Hospital; Shenzhen Third People’s Hospital; Beijing You’an Hospital, Capital Medical University; Beijing Ditan Hospital, Capital Medical University; Yunnan AIDS Care Center; The First People’s Hospital of Honghe State; Tangdu Hospital, Xi’an Fourth Military Medical University; Kunming Third People’s Hospital; Shanghai University of Chinese Medicine. Special thanks to Christine Simpson for her contribution to the biomarker testing procedures, Lixia Zhang, Qu Cui, Ling Luo and Yijia Li.
Footnotes
CONFLICT OF INTEREST: Evelyn Hsieh, Liana Fraenkel, Weibo Xia, Ying Ying Hu, Yang Han, Karl Insogna, Michael T. Yin, Jing Xie, Ting Zhu1 and Taisheng Li state that they have no conflicts of interest.
ETHICAL STANDARDS: This study was reviewed and exempt by the institutional review board of PUMCH and the human investigations committee of Yale School of Medicine prior to initiation, and therefore was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All samples and data used were previously collected and deidentified prior to analysis.
References
- 1.Mondy K, Yarasheski K, Powderly WG, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36(4):482–901. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 2.Piso RJ, Rothen M, Rothen JP, Stahl M. Markers of bone turnover are elevated in patients with antiretroviral treatment independent of the substance used. J Acquir Immune Defic Syndr. 2011;56(4):320–4. doi: 10.1097/QAI.0b013e31820cf010. [DOI] [PubMed] [Google Scholar]
- 3.Yin MT, McMahon DJ, Ferris DC, et al. Low bone mass and high bone turnover in postmenopausal human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2010;95:620–629. doi: 10.1210/jc.2009-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Vonderen MG, Mallon P, Murray B, et al. Changes in bone biomarkers in antiretroviral naïve HIV-infected men randomized to nevirapine/lopinavir/ritonavir or zidovudine/lamivudine/lopinavir/ritonavir help explain limited loss of bone mineral density over first 12 months after antiretroviral therapy initiation. 18th Conference on Retroviruses and Opportunistic Infections; Feb 27–Mar2, 2011; Boston, MA.. [Google Scholar]
- 5.Squires KE, Gulick R, Tebas P, et al. A comparison of stavudine plus lamivudine versus zidovudine plus lamivudine in combination with indinavir in antiretroviral naive individuals with HIV infection: selection of thymidine analog regimen therapy (START I) AIDS. 2000;14(11):1591–600. doi: 10.1097/00002030-200007280-00015. [DOI] [PubMed] [Google Scholar]
- 6.Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51(8):963–72. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 7.Grund B, Peng G, Gibert CL, et al. Continuous antiretroviral therapy decreases bone mineral density. AIDS. 2009;23:1519–1529. doi: 10.1097/QAD.0b013e32832c1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51:554–561. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 9.Hansen AB, Obel N, Nielson H, Pedersen C, Gerstoft J. Bone mineral density changes in protease inhibitor-sparing vs. nucleoside reverse transcriptase inhibitor-sparing highly active antiretroviral therapy: data from a randomized trial. HIV Medicine. 2011;12:157–165. doi: 10.1111/j.1468-1293.2010.00864.x. [DOI] [PubMed] [Google Scholar]
- 10.Guerri-Fernandez R, Vestergaard P, Carbonell C, et al. HIV infection is strongly associated with hip fracture risk, independently of age, gender and comorbidities: a population-based cohort study. J Bone Miner Res. 2013;28:1259–1263. doi: 10.1002/jbmr.1874. [DOI] [PubMed] [Google Scholar]
- 11.Hansen AB, Gerstoft J, Kronborg F, et al. Incidence of low and high-energy fractures in persons with and without HIV infection: a Danish population-based cohort study. AIDS. 2012;26:285–93. doi: 10.1097/QAD.0b013e32834ed8a7. [DOI] [PubMed] [Google Scholar]
- 12.Womack JA, Goulet JL, Gibert C, et al. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One. 2011;6:e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203(12):1791–801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26:825–31. doi: 10.1097/QAD.0b013e32835192ae. [DOI] [PubMed] [Google Scholar]
- 16.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. Journal Am Med Assoc. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 17.Fux CA, Rauch A, Simcock M, et al. Tenofovir use is associated with an increase in serum alkaline phosphatase in the Swiss HIV Cohort Study. Antivir Ther. 2008;13:1077–1082. [PubMed] [Google Scholar]
- 18.Childs KE, Fishman SL, Constable C, et al. Short communication: Inadequate vitamin D exacerbates parathyroid hormone elevations in tenofovir users. AIDS Res Hum Retroviruses. 2010;26(8):855–9. doi: 10.1089/aid.2009.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masiá M, Padilla S, Robledano C, López N, Ramos JM, Gutiérrez F. Short communication: Early changes in parathyroid hormone concentrations in HIV-infected patients initiating antiretroviral therapy with tenofovir. AIDS Res Hum Retroviruses. 2012;28(3):242–246. doi: 10.1089/AID.2011.0052. [DOI] [PubMed] [Google Scholar]
- 20.Focà E, Motta D, Borderi M, et al. Prospective evaluation of bone markers, parathormone and 1,25-(OH)2 vitamin D in HIV positive patients after the initiation of tenofovir/emtricitabine with atazanavir/ritonavir or efavirenz. BMC Infectious Diseases. 2012;12:38. doi: 10.1186/1471-2334-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duvivier C, Kolta S, Assoumou L, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS. 2009;23(7):817–24. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- 22.Rivas P, Górgolas M, García-Delgado R, Díaz-Curiel M, Goyenechea A, Fernández-Guerrero ML. Evolution of bone minderal density in AIDS patients on treatment with zidovudine/lamivudine plus abacavir or lopinavir/ritonavir. HIV Medicine. 2008;9:89–95. doi: 10.1111/j.1468-1293.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 23.Jain RG, Lenhard JM. Select HIV protease inhibitors alter bone and fat metabolism ex vivo. J Biol Chem. 2002;277(22):19247–19250. doi: 10.1074/jbc.C200069200. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Vallejo SJ, Beaupere C, Larghero J, Capeau J, Lagathu C. HIV protease inhibitors induce senescence and alter osteoblastic potential of human bone marror mesenchymal stem cells: beneficial effect of pravastatin. Aging Cell. 2013 doi: 10.1111/acel.12119. [DOI] [PubMed] [Google Scholar]
- 25.Zhang F, Dou Z, Ma Y, et al. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis. 2011;11(7):516–24. doi: 10.1016/S1473-3099(11)70097-4. [DOI] [PubMed] [Google Scholar]
- 26.Dou Z, Chen RY, Xu J, et al. Changing baseline characteristics among patients in the China National Free Antiretroviral Treatment Program, 2002–09. Int J Epidemiol. 2010;39:ii56–64. doi: 10.1093/ije/dyq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ministry of Health of the People’s Republic of China. China AIDS Response Progress Report. 2012 Mar 31; https://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/
- 28.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach – 2010 rev. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. [PubMed]
- 29.Von Wyl V, Cambiano V, Jordan MR, et al. Cost-effectiveness of tenofovir instead of zidovudine for use in first-line antiretroviral therapy in settings without virological monitoring. PLoS ONE. 7(8):e42834. doi: 10.1371/journal.pone.0042834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kou H, Du X, Li Y, Xie J, Qiu Z, et al. Comparison of Nevirapine Plasma Concentrations between Lead-In and Steady-State Periods in Chinese HIV-Infected Patients. PLoS ONE. 2013;8(1):e52950. doi: 10.1371/journal.pone.0052950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao Y, Han Y, Xie J, et al. Impact of a tenofovir disoproxil fumarate plus ritonavir-boosted protease inhibitor-based regimen on renal function in HIV-infected individuals: a prospective, multicenter study. BMC Infectious Diseases. 2013;13:301. doi: 10.1186/1471-2334-13-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cotter A, Vrouenraets SME, Brady JJ, et al. Impact of switching from Zidovudine to Tenofovir Disoproxil Fumarate on Bone Mineral Density and markers of bone metabolism in virologically suppressed HIV-1 infected patients; a substudy of the PREPARE study. J Clin Endocrinol Met. 2013;98(4):1659–1666. doi: 10.1210/jc.2012-3686. [DOI] [PubMed] [Google Scholar]
- 33.Haskelberg H, Hoy JF, Amin J, et al. Changes in bone turnover and bone loss in HIV-infected patients changing treatment to tenofovir-emtricitabine or abacavir-lamivudine. PLoS ONE. 2012;7(6):e38377. doi: 10.1371/journal.pone.0038377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen TA, Jensen D, Tolstrup M, et al. Comparison of bone and renal effects in HIV-infected adults switching to abacavir or tenofovir based therapy in a randomized trial. PLoS ONE. 2012;7(3):e32445. doi: 10.1371/journal.pone.0032445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Menezes Barbosa EG, de Paula FJ, Machado AA, de Assis Pereira F, Barbosa F, Júnior, Navarro AM. Impact of anitretroviral therapy on bone metabolism markers in HIV-seropositive patients. Bone. 2013;57(1):62–7. doi: 10.1016/j.bone.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Hu CY, Liu YM, Liu Y, et al. Pharmacokinetics and tolerability of tenofovir disoproxil fumarate 300mg once daily: an open-label, single- and multiple-dose study in healthy Chinese subjects. Clin Ther. 2013 doi: 10.1016/j.clinthera.2013.09.020. S0149-2918(13)00971-5. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Uenishi R, Hase S, et al. Explosive HIV-1 subtype B′ epidemics in Asia driven by geographic and risk group founder events. Virology. 2010;401:223–227. doi: 10.1016/j.virol.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Sun GQ, Liang SJ, et al. Different Distribution of HIV-1 Subtype and Drug Resistance Were Found among Treatment Naïve Individuals in Henan, Guangxi, and Yunnan Province of China. PLoS ONE. 2013;8(10):e75777. doi: 10.1371/journal.pone.0075777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Den Bout-Van Den Beukel CJ, Fievez L, Michels M, et al. Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: Effects of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24(11):1375–1382. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- 40.Havens PL, Stephensen CB, Hazra R, et al. Vitamin D3 decreases parathyroid hormone in HIV-infected youth being treated with tenofovir: a randomized, placebo-controlled trial. Clin Infect Dis. 2012 Apr;54(7):1013–25. doi: 10.1093/cid/cir968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Havens PL, Kiser JJ, Stephensen CB, et al. Association of higher plasma vitamin D binding protein and lower free calcitriol levels with tenofovir disoproxil fumarate use and plasma and intracellular tenofovir pharmacokinetics: cause of a functional vitamin D deficiency? Antimicrob Agents and Chemother. 2013;57(11):5619–5628. doi: 10.1128/AAC.01096-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bech A, Van Bentum P, Nabbe K, Gisolf J, Richter C, De Boer H. Fibroblast growth factor 23 in hypophosphataemic HIV-positive adults on tenofovir. HIV Med. 2012;13(9):558–63. doi: 10.1111/j.1468-1293.2012.01015.x. [DOI] [PubMed] [Google Scholar]


