Abstract
A comparative analysis of cellular and soluble markers of immune activation in HIV-infected women on combination antiretroviral therapy (cART) showed that the upper (UGT) compared to the lower female genital (LGT) tract was characterized by higher frequencies of potential HIV target cells and increased inflammatory molecules. Despite the activated UGT milieu, HIV RNA could not be detected in paired samples of plasma, cervicovaginal (CVL) or endometrial lavage (EML). As ARV concentrations were ≥3 fold higher in the endometrium than the in the lower genital tract, high ARV penetration and/or metabolism may limit viral replication in the UGT.
Keywords: HIV, female, uterus, tenofovir, pharmacokinetics, inflammation
INTRODUCTION
The relationship between elevated blood plasma HIV viral load and increased risk of sexual and vertical transmission is well documented.1,2 However, viral shedding persists in the female genital tract despite the suppression of plasma viral load even in women taking combination antiretroviral therapy (cART).3–7 Therefore, blood plasma viral load is not a reliable surrogate marker for viral activity or HIV infectivity in the female genital tract.
Most mechanistic research on heterosexual transmission of HIV has focused on the vagina and cervix [lower genital tract (LGT)]. 8–18 However, the uterine endometrium [upper genital tract (UGT)] contains activated CCR5+ T-cells, which are a target for HIV transmission.19 It is plausible that the UGT could serve as site of HIV acquisition, replication, and viral shedding.20–27 This feasibility study was conducted to better understand the biology of HIV in the UGT.
METHODS
Study Participants
This study was approved by the University of North Carolina Institutional Review Board. We enrolled 16 HIV-1-positive menstruating women, aged 25–48 years, who were fully suppressed by cART with plasma HIV RNA ≤ 40 copies/mL for at least 6 months. Ten HIV-negative women were enrolled to pilot the study procedures, and 4 were enrolled as immunologic study controls. Exclusion criteria included (i) pregnancy or planning pregnancy, (ii) unwillingness to use 2 forms of contraception (condoms + hormonal) or previous bilateral tubal ligation procedure, (iii) irregular menses (not between 21–42 days), (iv) untreated cervical infection (N. gonorrhea, C. trachomatis), (v) intrauterine device in place, or (vi) use of immunosuppressive medications. Study samples were taken in the secretory phase of the menstrual cycle based on reported menstrual history.
Specimen Collection
Cervicovaginal fluid (CVF) was collected as described.28,29 Cervicovaginal lavage was collected using 10 mL phosphate-buffered saline and spun to remove cells.4 Endometrial lavage (EML) specimens were collected using a Goldstein sonohysterography cathether passed through the internal cervical os, inflated with 1.5 mL of air, and 8 mL of normal saline advanced and withdrawn. Cells recovered from EML were stained for flow cytometric analysis. Endometrial biopsy (EMB) specimens were collected via a 3mm plastic pipelle following EML collection. The EMB was snap frozen in liquid nitrogen and stored at −80ºC; cells were isolated as described for mucosal tissues.30 Whole blood was collected in EDTA tubes with plasma isolated and stored at −80°C.
Measurement of immune activation
Cellular activation was assessed by staining mononuclear cell suspensions with antibodies to CD3, CD4, CD8, CCR5, CD69, and Ki67 in all participants. Naïve, effector, and memory T cells were characterized by CD45RA and CCR7 in HIV-infected women. Samples were stained as described,30 acquired on a LSRII instrument and analyzed using FlowJo software (FlowJo, Ashland, OR).
Soluble mediators in CVL and EML were analyzed in all participants using the Human Cytokine and Chemokine Panel I Kit (Millipore, Billerica, MA) using a MagPix instrument, or by ELISA (sCD163: Trillenium Diagnostics, LLC, Brewer, ME; sCD14: and R&D Systems, Minneapolis, MN). CVL specimens contained predominantly sloughed dead epithelial cells, and therefore, no further cellular evaluation was performed.
HIV RNA testing
HIV RNA was measured in plasma, EML and CVL using the Abbott RealTime HIV-1 Assay, and in EMB by real-time PCR31 after extraction with the RNeasy Fibrous Tissue Mini Kit (Qiagen). Lower limit of quantification was 40 copies/mL.
Quantification of antiretroviral concentrations
Plasma, CVF, and endometrial tissue concentrations of tenofovir (TFV) and emtricitabine (FTC) were measured using previously published liquid chromatography-tandem mass spectrometry (LC-MS/MS) and LC-UV methods.32–34 Samples below the limit of detection (BLD) were reported as 1/2 the lower limit of quantification (LLOQ). Analyte concentrations for both parent and intracellular metabolite are reported as nanomolar (nM). Solid matrices were converted to nM units assuming a tissue density of 1 g/ml.
RESULTS
Study population
Data were obtained on 12 of 16 HIV-1 positive women who participated in the study. Two participants came for more than one visit. Five samples were not evaluable due to cervical stenosis (n=2) or vaginal bleeding (n=3). Median age of participants was 39 years (range 25–44); 73% of women self-identified as Black or African-American and 27% were Caucasian. All women were either sexually abstinent (56%) or had undergone tubal ligation procedures (44%). The 4 HIV-negative women sampled for immunologic studies were all Caucasian with a median age of 26 years (range 20–42). These women used either oral contraceptive pills plus condoms (75%) or had a prior tubal ligation (25%) for contraception. Participants were taking TFV containing regimens described in Table 1 as well as etravirine/raltegravir/darunavir/ritonavir and atazanavir/ritonavir/abacavir/lamivudine regimens.
Table 1.
Nucleoside/tide Reverse Transcriptase Inhibitor (N(t)RTI) a and Phosphorylated Metabolite Concentration. Concentrations are reported as median (min, max).
| TFV (nM) | TFV-dp (nM) | FTC (nM) | FTC-tp (nM) | |
|---|---|---|---|---|
| Plasma (N=14) | 316 (0.44, 1122) b | N/A | 977 (155, 9628) | N/A |
| CVF (N=13) | 394 (4, 7869) c | N/A | 8900 (765, 34021) | N/A |
| Endometrial Tissue (N=3) | 91 (74, 181) | 31 (14, 34) | 1204 (939, 1617) | 161 (115, 163) |
The following regimens are represented: TFV/FTC/raltegravir, TFV/FTC/darunavir/ritonavir, TFV/FTC/atazanavir/ritonavir, TFV/FTC/rilpivirine, TFV/FTC/fosamprenavir/ritonavir, and TFV/FTC/elvitegravir/cobicistat.
One sample was below the limit of detection (BLD) and reported as 1/2 the lower limit of detection (LLOQ), 0.25ng/ml.
Two samples were below the limit of detection (BLD) and reported as 1/2 the lower limit of detection (LLOQ), 2ng/ml.
Immune activation of the UGT
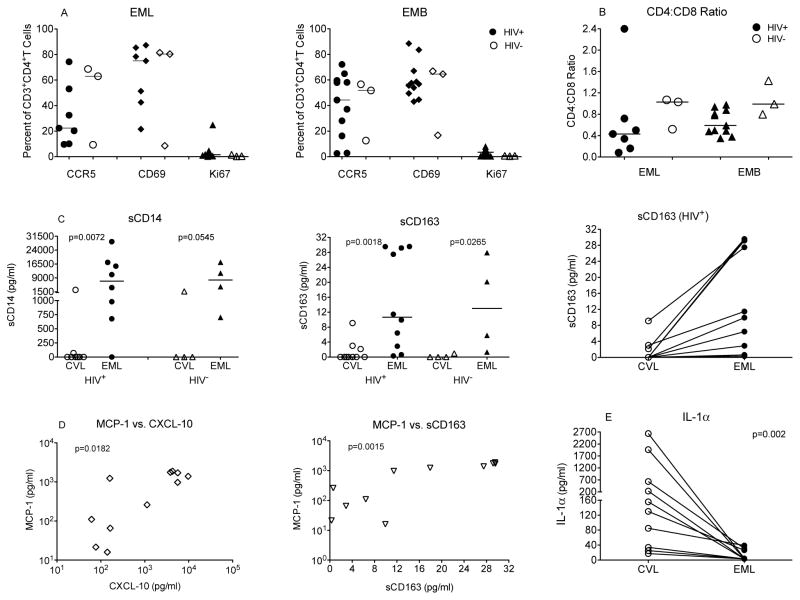
Cellular immune activation was measured in EML and EMB cells (n=10). Both EML and EMB specimens contained high frequencies of activated CD4+ and CD8+ T cells. Despite great variation from one woman to another, within the same patient, activation in EML and EMB samples were comparable. Frequencies of potential HIV target cells, CCR5-positive CD4+T cells, ranged from 9.7% to 74.3% and from 2.4 to 72.3% in EML and EMB samples, respectively (Figure 1A). Similarly, a large percentage of CD4+ T cells expressed the activation marker CD69 (Figure 1A). As >80% of T cells expressed markers typical for memory T cells (data not shown), CD69+ cells likely represented tissue resident memory T cells. This conclusion was supported by the apparent lack of proliferation (Ki67) among CD4+ and CD8+T (Figure 1A).
Figure 1. Immune Activation in the upper and lower female genital tract.
Panel A: T cell activation in EML and EMB samples of HIV-positive (closed symbols) and HIV-negative (open symbols) individuals as assessed by CCR5, CD69 and Ki67 expression on CD4+T cells. Panel B: Consistent with HIV-1 infection, the CD4:CD8 T cell ratio is reduced in HIV-positive compared to HIV-negative women in EML samples. Panel C: Levels of sCD14 and sCD163 in CVL and EML samples from HIV-positive and –negative women. The right graph illustrates that despite patient-to-patient variation, in each of the HIV-positive women, inflammatory markers (example of sCD163 shown) were expressed at lower levels in CVL compared to EML samples. Panel D: Several of the inflammatory cytokines in EML were positively correlated to each other suggesting that they all serve as surrogate for immune activation. Examples shown include the correlation between MCP-1 and CXCL-10 and MCP-1 and sCD163. Panel E: IL-1α was the only cytokine that was expressed at significantly higher levels in CVL than in EML samples.
To determine whether increased immune activation in the upper FGT was the result of HIV infection, or represented a normal physiologic condition, we analyzed samples from 4 HIV-uninfected women. Of note, there was no EMB sample from one participant and 1 EML sample was only used to assess for cytokine/chemokine studies. Consistent with their HIV-1 infection status, the group of HIV-infected women had a slightly lower CD4:CD8 T ratio than the HIV-uninfected women (Figure 1B). T cell activation, however, was similar in HIV-positive and negative women (Figure 1A).
The EML concentrations of several inflammatory molecules, including MCP-1, CXCL-10 and IL-7, were significantly higher in EML than in CVL fluids (Figure 1C–D). In contrast, IL-1α levels were higher in CVL than in EML samples (Figure 1E). These differences were observed regardless of whether we compared samples from the LGT to the UGT of all women or from a single woman (Figure 1E). Markers of monocyte activation, such sCD14 and sCD163, were also elevated in EML compared to CVL samples of HIV-infected and uninfected women (Figure 1C). Several of the increased inflammatory mediators of the UGT were positively correlated with each other. (Figure 1D).
Detection of virus and antiretroviral concentrations
HIV RNA was not detected in matched samples of plasma, CVL and EML (n=8). Proviral DNA was not detected in EMB tissue samples (n=2). This lack of HIV RNA in genital fluids was consistent with antiretroviral drug concentrations in the CVF similar to, or greater than, blood plasma. The active drug concentrations of TFV and FTC (TFVdp and FTCtp) in EMB specimens were greater than concentrations previously documented in the lower genital tract.34,35 (Table 1).
DISCUSSION
This is the first study to simultaneously assess immunologic, virologic and pharmacologic characteristics of both the UGT and LGT in HIV-1 positive women. These findings supporting the potential for the UGT to exist as a separate compartment for HIV activity are relevant for the prevention and cure of HIV infection.
Since HIV preferentially replicates in activated cells, we studied the cell distribution and state of immune activation of the UGT. Research related to the immunologic effect of HIV on the UGT is limited though the data available shows the endometrium in a chronic inflammatory state in HIV-infected women.36–38 Enhanced immune activation in endometrial samples of HIV-negative women has previously been reported;19 this is consistent with our findings that immune activation is high in the endometrium of both HIV-infected and HIV-negative women. These high levels of immune activation imply that there are target cells for HIV transmission, replication and shedding in the endometrium. Increased immune activation has also been described in plasma cells after TFV exposure. 39,40 Our study showed that the immune activation of the endometrium was similar in both HIV-infected women on TDF/FTC and healthy HIV-negative women not taking ARVs in the secretory phase of the menstrual cycle, implying that immune activation of the female UGT represents a normal physiological state.
Genital HIV RNA/DNA shedding has been associated with elevated plasma viral loads and genital tract infection or inflammation.41–43 Successful suppression of HIV in blood using ART does not eliminate shedding measured in the LGT.7,44–46 HIV shedding has been assumed to be derived from the mucosal surfaces of the vagina and cervix. However, the endometrium is in direct communication with these LGT sites and it is biologically plausible for virus to pass from UGT to LGT. A study of untreated HIV-infected women after intrauterine device (IUD) placement found significant increases in genital HIV-1 RNA shedding in women with evidence of chronic endometritis; however, HIV-1 RNA was only measured in the cervix.38 Ours is the first investigation to measure virus from the LGT and UGT simultaneously and separately in order to localize the exact source of the shedding. Though we did not demonstrate the presence of HIV RNA in the endometrium, our findings were limited by the lower limit of the viral RNA assay of 40 copies/mL and small sample size. It is also possible that because the endometrial lining is routinely shed, there is not enough time within a menstrual cycle to establish a viral reservoir within this compartment.
The observation that suppression of HIV RNA in blood plasma does not always correlate with suppression of virus in the genital tract suggests that the virus may have inadequate ARV exposure in the genital tract. Variable penetration of ARV in different anatomic compartments has been demonstrated in both HIV-infected and healthy women. 29,34,35,47,48 Concentrations of TFV and FTC in CVF in our study were consistent with previously reported concentrations in HIV-infected and healthy females. 34,48 However, our EMB TFV and FTC concentrations were approximately 4-fold higher than vaginal tissue and TFVdp and FTCtp concentrations were 18 and 3-fold higher, respectively.34 Previous reports of TFVdp in vaginal tissue after oral dosing identify approximately 2% of TFV is converted to TFVdp. 49 In our study, we found a median conversion rate of 18 (17–37)% for TFVdp and 12 (10–13)% for FTCtp. These data suggest that there are different drug distribution influences within the UGT with different intracellular activation kinetics. Though no drug-drug interaction has been demonstrated between TFV and estrogen,50 an open label study of healthy women reported lower concentrations of serum and intracellular TFV and TFV-dp in those using oral and injectable hormonal contraception.51. It is possible that the higher concentrations we noted were due to none of our participants using hormonal contraception; however both the LGT and UGT are equally influenced by sex hormones, and we did not see this difference manifest in the LGT exposure.
To date, the optimal technique to measure HIV shedding in the female genital tract has not been identified. One goal of this study was to develop a sampling and distribution algorithm that would allow the collection of multiple LGT and UGT samples for multidisciplinary analyses. Through unique EML sampling, we were able to obtain cellular and immunologic profiles analogous to those of the endometrium while minimizing trauma and blood contamination associated with EMB samples. Although both sampling methods are effective at studying the female endometrium, the EML may be less invasive and painful due to use of the narrow diameter catheter and lack of suction curettage. Since immunologic studies correlated EML and EMB samples, in the future, we will be able to collect EML samples for immunology while sharing EMB samples between virology and pharmacology labs as needed.
In summary, we found that the endometrium contains activated immune cells. The penetration of TFV and FTC into the endometrium may be at concentrations high enough to prevent viral replication. Though findings from this study do not support the endometrium as a source of genital tract shedding, further research is needed in order to make definitive conclusions on the role of the female UGT in HIV pathogenesis.
Acknowledgments
Sources of support: National Institutes of Health, National Institute of General Medical Sciences
This research was funded by a developmental grant from the University of North Carolina Center for AIDS Research (CFAR), an NIH funded program (P30 AI50410), NIH Award R37 DK49381, the National Institute of General Medical Sciences grant number 5T32GM086330 and the Eunice Kennedy Shriver NICHD/NIH grant R01HD067721, SLY. The content is solely the responsibility of the authors and does not necessarily represent the official views of the supporting agencies listed above.
Footnotes
No financial conflicts of interest.
References
- 1.Montano M, Russell M, Gilbert P, et al. Comparative prediction of perinatal human immunodeficiency virus type 1 transmission, using multiple virus load markers. J Infect Dis. 2003;188:406–413. doi: 10.1086/376838. [DOI] [PubMed] [Google Scholar]
- 2.Lehman DA, Farquhar C. Biological mechanisms of vertical human immunodeficiency virus (HIV-1) transmission. Rev Med Virol. 2007;17:381–403. doi: 10.1002/rmv.543. [DOI] [PubMed] [Google Scholar]
- 3.Si-Mohammed A, Kazatchkine MD, Heard I, et al. Selection of drug-resitant variant in the female genital tract of human immunodeficiency virus type-1 infected women recieving antiretroviral therapy. J Infect Dis. 2000;182:112–122. doi: 10.1086/315679. [DOI] [PubMed] [Google Scholar]
- 4.Fiore JR, Suligoi B, Saracino A, et al. Correlates of HIV-1 shedding in cervicovaginal secretions and effects of antiretroviral therapies. AIDS. 2003;17:169–176. doi: 10.1097/00002030-200310170-00004. [DOI] [PubMed] [Google Scholar]
- 5.Vettore MV, Schechter M, Melo MF, Boechat LJ, Barroso PF. Genital HIV-1 viral load is correlated with blood plasma HIV-1 viral load in Brazillian women and is reduced by antiretroviral therapy. J Infect. 2006;52:290–293. doi: 10.1016/j.jinf.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Cu-Uvin S, Caliendo AM, Reinert S, et al. Effect of highly active antiretroviral therapy on cervicovaginal HIV-1 RNA. AIDS. 2000;14:415–421. doi: 10.1097/00002030-200003100-00015. [DOI] [PubMed] [Google Scholar]
- 7.Neely MN, Benning L, Xu J, et al. Cervical shedding of HIV-1 RNA among women with low levels of viremia while receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44:38–42. doi: 10.1097/01.qai.0000248352.18007.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spira AI, Marx PA, Patterson BK, et al. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of Simian immunodeficiency virus into Rhesus Macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Schuler T, Zupancic M, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 10.Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infectes intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veazey RS, Marx PA, Lackner AA. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in Simian immunodeficiency virus infection. J Infect Dis. 2003;187:769–776. doi: 10.1086/368386. [DOI] [PubMed] [Google Scholar]
- 12.Miller CJ, Li Q, Abel K, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nature Med. 2000;6:475–479. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- 14.Kawamura T, Cohen SS, Borris DL, et al. Candidate microbicides block HIV-1 infection of human immature Langerhans cells within epithelial tissue explants. J Exp Med. 2000;192:1491–1500. doi: 10.1084/jem.192.10.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta P, Collins KB, Ratner D, et al. Memory CD4+ cells are the earliest detectable Human Immunodeficiency Virus Type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol. 2002;76:9868–9876. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Q, Frank I, Williams V, et al. Blockade of attachment and fusion receptors inhibits HIV-1 infection in human cervical tissue. J Exp Med. 2004;199:1065–1075. doi: 10.1084/jem.20022212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hladik F, Sakchalathorn P, Ballweber L, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saba E, Origoni M, Taccagni G, et al. Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol. 2013;6:1081–1090. doi: 10.1038/mi.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shanmugasundaram U, Critchfield JW, Pannell J, et al. Phenotype and functionality of CD4+ and CD8+ cells in the upper reporductive tract of healthy premenopausal women. Am J Reprod Immunol. 2014;71:95–108. doi: 10.1111/aji.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asin SN, Wildt-Perinc D, Mason SI, Howell AL, Wira CR, Fanger MW. Human immunodeficiency type 1 infection of human uterine epithelial cells: viral shedding and cell contact-mediated infectivity. J Infect Dis. 2003;187:1522–1533. doi: 10.1086/374782. [DOI] [PubMed] [Google Scholar]
- 21.Saidi H, Magri G, Nasreddine N, Requena M, Belec L. R5- and X4-HIV-1 use differentially the endometrial epithelial cells HEC-1A to ensure their own spread: Implications for mechanisms of sexual transmission. Virol. 2007;358:55–68. doi: 10.1016/j.virol.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 22.Saidi H, Magri G, Carbonneil C, Bouhlal J, Hocini H, LB Apical interaction of HIV type 1 with polarized HEC-1 cell monolayer modulate R5-HIV type 1 spread by submucosal macrophages. AIDS Res Hum Retroviruses. 2009;25:497–509. doi: 10.1089/aid.2008.0156. [DOI] [PubMed] [Google Scholar]
- 23.Yeaman GR, Howell AL, Weldon S, et al. Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology. 2003;109:137–146. doi: 10.1046/j.1365-2567.2003.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulayim N, Palter SF, Kayisli UA, Senturk L, Arici A. Chemokine receptor expression in human endometrium. Biol Reprod. 2003;68:1491–1495. doi: 10.1095/biolreprod.102.009639. [DOI] [PubMed] [Google Scholar]
- 25.Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN. Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab. 1997;82:1621–1628. doi: 10.1210/jcem.82.5.3919. [DOI] [PubMed] [Google Scholar]
- 26.King AE, Critchley HO, Kelly RW. Presence of secretory leukocyte protease inhibitor in human endometrium and first trimester decidua suggests an antibacterial protective role. Mol Hum Reprod. 2000;6:191–196. doi: 10.1093/molehr/6.2.191. [DOI] [PubMed] [Google Scholar]
- 27.Fahey JV, Wira CR. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis. 2002;185:1606–1613. doi: 10.1086/340512. [DOI] [PubMed] [Google Scholar]
- 28.Adams JL, Patterson KB, Prince HM, et al. Single and multiple dose pharmacokinetics of dolutegravir in the genital tract of HIV-negative women. Antivir Ther. 2013;18:1005–1013. doi: 10.3851/IMP2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumond JB, Patterson KB, Pecha AL, et al. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:546–553. doi: 10.1097/QAI.0b013e3181ae69c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marthas ML, Van Rompay KK, Abbott Z, et al. Partial efficacy of a VSV-SIV/MVA-SIV vaccine against oral SIV challenge in infact macaques. 2011;29:3124–3137. doi: 10.1016/j.vaccine.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Israel-Ballard K, Ziermann R, Leutenegger C, et al. TaqMan RT-PCR and VERSANT HIV-1 RNA 3.0 (bDNA) assay Quantification of HIV-1 RNA viral load in breast milk. J Clin Virol. 2005;34:253–256. doi: 10.1016/j.jcv.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Rezyk NL, Crutchley RD, Kashuba AD. Simultaneous quantification of emtricitabine and tenofovir in human plasma using high-performance liquid chromatography after solid phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;822:201–208. doi: 10.1016/j.jchromb.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Jung BH, Rezyk NL, Bridges AS, Corbett AH, Kashuba AD. Simultaneous determination of 17 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography-tandem mass spectometry. Biomed Chromatogr. 2007;21:1095–1104. doi: 10.1002/bmc.865. [DOI] [PubMed] [Google Scholar]
- 34.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;2011:112re114. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louissaint NA, Cao Y, Skipper PL, et al. Single dose phamacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses. 2013;29:1443–50. doi: 10.1089/aid.2013.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peuchmaur M, Emilie D, Vazeux R, et al. HIV-associated endometritis. AIDS`. 1989;3:239–241. doi: 10.1097/00002030-198904000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Johnstone FD, Williams ARW, Bird GA, Bjornsson S. Immunohistochemical characterization of endometrial lymphoid cell populations in women infected with Human Immunodeficiency Virus. Obstet Gynecol. 1994;83:586–593. doi: 10.1097/00006250-199404000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Coleman J, Hitti J, Bukusi EA, et al. Infectious correlates of HIV-1 shedding in the female upper and lower genital tracts. AIDS. 2007;21:755–759. doi: 10.1097/QAD.0b013e328012b838. [DOI] [PubMed] [Google Scholar]
- 39.Naranbhai V, Abdool Karim SS, Altfeld M, et al. Innate immune activation enhances HIV acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J Infect Dis. 2012;206:993–1001. doi: 10.1093/infdis/jis465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biswas N, Rodiguez-Garcia M, Crist SG, et al. Effect of tenofovir on nucleotidases and cytokins in HIV-1 target cells. PLOS One. 2013;8:e78814. doi: 10.1371/journal.pone.0078814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell C, Balkus JE, McKernan-Mullin J, et al. Associations between genital tract infections, genital tract inflammation, and cervical cytobrush HIV-1 DNA in US versus Kenyan women. J Acquir Immune Defic Syndr. 2013;62:143–148. doi: 10.1097/QAI.0b013e318274577d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blish CA, McClelland RS, Richardson BA, et al. Genital inflammation predicts HIV-1 shedding independent of plasma viral load and systemic inflammation. J Acquir Immune Defic Syndr. 2012;61:436–440. doi: 10.1097/QAI.0b013e31826c2edd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fornabaio C, Carvalho AC, Lillo F, et al. Cervical human papillomavirus infection and shedding of human immunodeficiency virus in cervicovaginal fluids: a cross-setional study. J Acquir Immune Defic Syndr. 2012;61:78–82. doi: 10.1097/QAI.0b013e31826327a0. [DOI] [PubMed] [Google Scholar]
- 44.Kovacs A, SSW, Burns D, et al. Determinants of HIV-1 shedding in the genital tract of women. Lancet. 2001;358:1593–1601. doi: 10.1016/S0140-6736(01)06653-3. [DOI] [PubMed] [Google Scholar]
- 45.Graham SM, Holte SE, Peshu NM, et al. Initiation of antiretroviral therapy leads to a rapid decline in cervical and vaginal HIV-1 shedding. AIDS. 2007;21:501–507. doi: 10.1097/QAD.0b013e32801424bd. [DOI] [PubMed] [Google Scholar]
- 46.Prazuck T, Chaillon A, Avettand-Fenoel V, et al. HIV-DNA in the genital tract of women on long-term effective therapy is associated with residual viremia and previous AIDS-defining illnesses. PLOS One. 2013;8:e69686. doi: 10.1371/journal.pone.0069686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumond JB, Yeh RF, Patterson KB, et al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS. 2007;21:1899–1907. doi: 10.1097/QAD.0b013e328270385a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dumond JB, Nicol MR, Kendrick RN, et al. Pharmacokinetic modelling of efavirenz, atazanavir, lamivudine and tenofovir in the female genital tract of HIV-infected pre-menopausal women. Clin Pharmacokinet. 2012;51:809–822. doi: 10.1007/s40262-012-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hendrix CW, Chen BA, Guddera V, et al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLOS One. 2013;8:e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kearney BP, Mathias A. Lack of effect of tenofovir disoproxil fumarate on pharmacokinetics or hormonal contraceptives. Pharmacotherapy. 2009;29:924–929. doi: 10.1592/phco.29.8.924. [DOI] [PubMed] [Google Scholar]
- 51.Coleman JS, Chaturvedula A, Hendrix CW the MTN-001 Protocol Team. Method of hormonal contraception is associated with lower tenofovir concentration in healthy women (MTN-001): implications for pre-exposure prophylaxis. XIX International AIDS Conference; Washington, DC. 2012. [Google Scholar]