Abstract
Objective. Aberrant rho kinase (ROCK) activity is implicated in the pathogenesis of several vascular diseases and is associated with Th17 differentiation. Th17 immune response is recognized in the pathogenesis of GCA. The aim of this study was to assess ROCK activity in GCA.
Methods. All patients who underwent temporal artery biopsy (TAB) at a tertiary care centre over 5 years were identified and charts reviewed. Subjects were categorized into three groups: TAB-positive GCA, TAB-negative GCA and age- and sex-matched controls. TABs were stained for phosphorylated ezrin/radixin/moesin (pERM), a surrogate of ROCK activity, and reviewed by a pathologist blinded to clinical status. Three areas were scored for staining intensity on a scale of 0–2, with a maximum possible score of 6.
Results. Nineteen subjects with TAB-positive GCA, 17 with TAB-negative GCA and 18 controls were analysed. Compared with controls, GCA subjects with either positive or negative TABs had significantly higher pERM intensity scores (P = 0.0109). Adjusting for diabetes, hypertension, prednisone and statin use, GCA subjects still had higher pERM scores [odds ratio 7.3 (95% CI 1.9, 25.9), P = 0.0046]. The high pERM score had a sensitivity of 90% and a negative predictive value of 91% for the diagnosis of GCA in those with a negative TAB, compared with 51% sensitivity for histopathology alone.
Conclusion. Subjects with GCA had more intense pERM staining in TAB specimens compared with age- and sex-matched controls, regardless of whether TAB was positive or negative by routine histopathology, suggesting increased ROCK activity in GCA. The ROCK pathway warrants further investigation in GCA, as it may have diagnostic significance in enhancing the sensitivity of TAB.
Keywords: giant cell arteritis, vasculitis, rho-associated kinases
Introduction
GCA is an inflammatory disorder of medium and large blood vessels affecting adults >50 years of age [1]. Vasculitic involvement of the aorta and its branches, especially cranial branches, is typical in GCA. Histopathological evidence of vascular inflammatory infiltrate is the gold standard for the diagnosis of GCA and temporal artery biopsy (TAB) is recommended to confirm the diagnosis in patients with suspected GCA [2]. While the aetiology of GCA is complex, activation and recruitment of two populations of CD4+ T cells—the Th1 and Th17 subsets—are implicated in the pathogenesis. T cells with a Th17 signature are found in increased number in the peripheral blood and vascular inflammatory infiltrate in patients with active GCA [3]. Expression of Th17-related cytokines, IL-17 and IL-21, is similarly increased in TABs from patients with active GCA and levels of IL-17 have been demonstrated to correlate with treatment responsiveness [4, 5].
RhoA is an intracellular GTPase that, along with its downstream target rho kinase (ROCK), regulates several cellular processes including regulation of gene expression and cytoskeletal organization. Phosphorylation by ROCK has been shown to activate IFN regulatory factor 4, a transcription factor required for expression of IL-17 and IL-21 [6]. The influence of ROCK over the Th17 immune response offers emerging evidence that abnormal ROCK activation plays a role in autoimmune diseases [7, 8].
Activated ROCK also mediates multiple processes through effects on the actin cytoskeleton. ROCK phosphorylates several cytoskeletal proteins, such as ezrin/radixin/moesin (ERM), resulting in enhanced cytoskeletal contraction and thereby regulating cell migration and permeability [9]. In the vascular system, the ROCK pathway mediates smooth muscle tone and vascular remodelling. Increased ROCK activity is implicated in a variety of vascular diseases, including coronary artery disease, pulmonary and systemic hypertension (HTN) and cerebrovascular disease [10]. ROCK inhibition, with orally available small molecule inhibitors, is being studied as a potential therapy in human vascular disease [11]. One of the proposed pleotropic, non-lipid-lowering beneficial effects of statins is via down-regulation of the ROCK pathway [12].
ROCK activation is associated with Th17 differentiation, inflammatory cell recruitment and vascular remodelling, all of which are implicated in GCA pathogenesis; however, the relevance of ROCK in GCA is unknown. We aimed to assess ROCK activity via staining for phosphorylated ERM (pERM), a downstream target and surrogate of ROCK, in TAB specimens of patients with GCA compared with controls.
Patients and methods
Subjects
All TABs performed at our tertiary care institution between 2007 and 2012 were identified via a pathology database and the medical records were reviewed. A standardized case report form was used to record the following data: age, sex, number of ACR criteria for GCA [13], prednisone dose at the time of biopsy, statin use and the presence of HTN and diabetes mellitus. Subjects for whom clinical information was not available were excluded. Subjects were categorized into three groups: biopsy-positive GCA, biopsy-negative GCA and subjects without GCA. TAB was considered positive if there was demonstration of a granulomatous inflammatory mononuclear cell infiltrate with or without the presence of multinucleated giant cells. The subjects with GCA and negative TAB met three or four ACR criteria and were treated as GCA for at least 6 months with no alternative diagnosis. Our control group was derived from those with negative TAB and in whom review of the medical records did not support the clinical diagnosis of GCA; they were matched on age and sex to biopsy-positive GCA subjects. This study was approved by the institutional review board of the Hospital for Special Surgery, New York and New York-Presbyterian Hospital-Weill Cornell Medical Center, New York, NY, USA.
Methods
Paraffin-embedded TAB specimens were sectioned and treated with an immunohistochemical stain for pERM (Cell Signaling Technology, Danvers, MA, USA). Antibody standardization and staining were performed at the Weill Cornell Translational Research Laboratory. pERM stained TABs were then assessed by a single pathologist who was blinded to clinical status for the negative biopsies. The intensity of pERM staining in each specimen was measured on a scale from 0 (no staining) to 2 (high-intensity staining) in three separate areas of the vessel (intimal endothelium, adventitia and vasa vasorum) for a maximum possible composite score of 6.
Outcomes and statistical analysis
The primary outcome of interest was the pERM intensity score in subjects with GCA compared with age- and sex-matched controls without GCA. The pERM intensity score was dichotomized into a high pERM score (>4) or a low pERM score (≤4) based on the distribution of the data. Secondary outcomes included analysis of the pERM score as a continuous variable (range 0–6) in GCA subjects vs controls. A chi-square test was employed to detect the association between pERM scores in GCA subjects compared with controls and to compare categorical demographic data. Student’s t test was used to compare continuous data. A simple logistic regression model was used to analyse the difference in pERM intensity score in GCA subjects compared with controls. A second logistic regression model was built to adjust for known confounders of ROCK activity, including age, sex, HTN or diabetes mellitus, prednisone dose and statin use. Spearman’s rho was used to assess the correlation between pERM intensity score and ESR. Statistical analyses were performed using SAS statistical software, version 9.2 (SAS Institute, Cary, NC, USA).
Results
Subjects
Two hundred and eight TABs were performed at our institution over the study period. Nineteen subjects, three of whom had a positive TAB, were excluded due to missing clinical information. Of the remaining subjects, review of clinical information resulted in categorization of 43 subjects with GCA and 146 without GCA. Of the subjects with GCA, TAB was positive in 19 and negative in 17. Eighteen age- and sex-matched controls were selected from the cohort of subjects without GCA.
The mean age of the subjects was 77.9 years (s.d. 9.1) and 81% were female. All subjects were receiving steroids at the time of TAB with a mean prednisone dose of 51.1 mg (s.d. 21.1; range 10–100). There were no differences between the three groups in terms of demographic characteristics, prednisone dose, statin use or the prevalence of HTN or diabetes mellitus (Table 1). The mean ESR was substantially elevated (>50) in all three groups: GCA subjects with a positive TAB, 89.1 (s.d. 30.6); GCA subjects with a negative TAB, 95.7 (s.d. 31.8); controls, 66.1 (s.d. 31.7). Median ESR in GCA subjects with positive biopsy was 87.5 [interquartile range (IQR) 66–112], in GCA subjects with negative biopsy was 97 (IQR 73–120) and in controls was 71 (IQR 50–91).
Table 1.
Baseline demographics and pERM intensity scores in GCA patients compared with controls
| GCA (n = 36) |
Controls (n = 18) | P-value | Adjusted P-valuea | ||
|---|---|---|---|---|---|
| GCA biopsy positive (n = 19) | GCA biopsy negative (n = 17) | ||||
| Age, mean (s.d.), years | 78.4 (9.2) | 76.6 (7.9) | 78.4 (10.5) | 0.81 | |
| Female, n (%) | 14 (73.7) | 16 (94.1) | 14 (77.8) | 0.2 | |
| Prednisone, mean (s.d.), mg | 55.6 (17.9) | 50.6 (17.1) | 47.2 (27.2) | 0.5 | |
| Hypertension, n (%) | 8 (42.1) | 10 (58.8) | 12 (66.7) | 0.39 | |
| Diabetes mellitus, n (%) | 1 (5.5) | 6 (35.3) | 4 (22.2) | 0.11 | |
| Statin, n (%) | 11 (61.1) | 8 (47.1) | 5 (26.3) | 0.13 | |
| pERM intensity score | 0.0109 | 0.0046 | |||
| Dichotomousb | |||||
| Low, n (%) | 4 (21.1) | 1 (5.9) | 10 (55.6) | ||
| High, n (%) | 15 (78.9) | 16 (94.1) | 8 (44.4) | ||
| pERM intensity score | |||||
| Continuousc | |||||
| Mean (s.d.) | 5.4 (1.06) | 5.6 (0.79) | 4.1 (1.41) | 0.0009 | |
aAdjusted for age, sex, hypertension, diabetes mellitus, and prednisone and statin use. bDichotomous pERM intensity score with low score ≤4 and high score >4. cContinuous pERM intensity score range 0–6. pERM: phosphorylated ezrin/radixin/moesin.
pERM intensity score
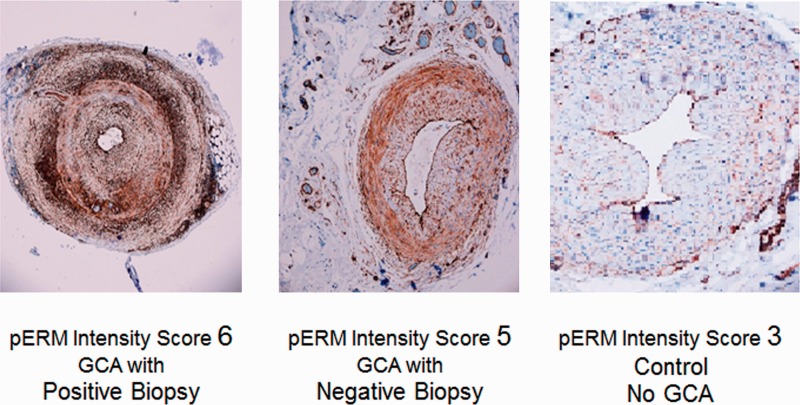
High pERM intensity scores were recorded in 31 (86.1%) subjects with GCA compared with only 8 (44.4%) controls (P = 0.0109) (Table 1). Compared with controls, GCA subjects with either positive (78.9%) or negative (94.1%) biopsy were statistically significantly more likely to have a high pERM intensity score (P = 0.035 and P = 0.0083, respectively). All but one of the GCA subjects with negative TAB had a high pERM intensity score. Analysing pERM intensity score as a continuous variable, mean scores in subjects with GCA were higher than the mean score in the control population (P = 0.0009). After adjusting for age, sex, HTN, diabetes mellitus, prednisone dose and statin use, GCA subjects were still significantly more likely to have a high pERM intensity score compared with controls [odds ratio (OR) 7.3 (95% CI 1.9, 25.9), P = 0.0046]. pERM intensity score was only weakly correlated with ESR value [ρ = 0.35 (95% CI 0.09, 0.57)]. pERM staining was noted predominantly in endothelial cells comprising the intima and vasa vasorum and adventitial fibroblasts (Fig. 1).
Fig. 1.
pERM staining
pERM: phosphorylated ezrin/radixin/moesin.
pERM diagnostic test characteristics
Evaluating high pERM intensity score as a diagnostic test for those with negative TAB, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated. In subjects with negative TAB, high pERM intensity score had 90% sensitivity and a NPV of 91% for diagnosis of GCA. This was compared with sensitivity of 51% for routine histopathology. While the specificity of high pERM intensity score was low (56%), these test characteristics suggest that those with negative TAB and low pERM intensity score have a very low likelihood of having GCA.
Discussion
This exploratory study suggests that ROCK activity is increased in the vasculature of subjects with GCA compared with matched controls. Interestingly, increased ROCK activity was demonstrated in all subjects with GCA regardless of whether TAB was positive by routine histopathology. This represents the first attempt to evaluate the ROCK pathway, which has been demonstrated as aberrant in many forms of vascular disease, in vasculitis. While small numbers in our study limit the ability to generalize these findings without evaluation in larger cohorts, our results may offer a new pathogenic pathway that could have diagnostic and therapeutic utility in a disease for which better diagnostic tools and therapeutic options are needed.
TAB has limited sensitivity in the diagnosis of GCA. Due to the discontinuous nature of inflammation in GCA, skip lesions may render a false negative biopsy, especially if the TAB is not of sufficient length and multiple sections are not examined. Large series of TABs have shown that only 20–35% of all biopsies obtained are positive [14, 15]. Even in GCA patients, the sensitivity of TAB is estimated in the 70% range [16]. Attempts to improve the sensitivity of TAB with the addition of imaging modalities such as US have been of limited clinical utility in many regions, in part related to accessibility and operator dependence [17]. Because of the risk of ischaemic complications, including irreversible visual loss, in early, untreated GCA, the recommendation for suspected GCA is for initiation of high-dose corticosteroid therapy, which itself may cause substantial morbidity [18]. A test that could increase the sensitivity of standard histopathology would be helpful to distinguish patients with biopsy-negative GCA from those without GCA to prevent prolonged, potentially toxic treatment in those with an alternative diagnosis. By including the clinically relevant group of patients with biopsy-negative GCA, we were able to demonstrate that pERM intensity score may in fact be a test that could improve the sensitivity of TAB and help distinguish patients with biopsy-negative GCA from those without the disease. In this cohort, adding pERM intensity score to standard histopathology greatly increased the diagnostic sensitivity of TAB. These results were bolstered by the fact that our pathologist was blinded to clinical status, which was crucial in assessing those with negative biopsies.
In this study, ROCK activity was not directly assessed and pERM was instead used as a surrogate, which is a limitation. Phosphorylation of ERM proteins allows for a conformational change that enables interaction of ERM proteins with the actin cytoskeleton and results in cytoskeletal contraction, which influences cellular migration and adhesion. Several studies have previously used pERM as a surrogate of ROCK activity, as phosphorylation of ERM proteins by ROCK has been demonstrated in several cell types, with a temporal relationship between ROCK activation and increased pERM detection and a blunting of ERM phosphorylation and amelioration of pathological phenotype with administration of ROCK inhibitors [8].
In designing our scoring system, we included areas of the artery that we thought were central in disease pathogenesis and where high ROCK activity would likely represent aberrancy. Because of the important physiological role ROCKs are assumed to play in vascular smooth muscle in both normal and disease states, we decided to omit the vascular media, which consists predominantly of vascular smooth muscle cells, from our score. The adventitia in GCA has been touted by Weyand et al. [19] as the initial site of immunological disturbance. The vasa vasorum was also included as infiltrating T cells and macrophages, which cause the histopathological panarteritis in GCA, enter the vessel via the vasa vasorum, making this area key in early disease pathogenesis. In addition, subtypes of GCA with isolated vasa vasorum vasculitis have been described [20]. Finally, we included the intima in our pERM intensity score, as pathological intimal hyperplasia is responsible for ischaemic complications in GCA, and ROCK helps to regulate vascular remodelling.
In this cohort, all but one of the biopsy-negative GCA subjects had a high pERM intensity score. There are several potential and not mutually exclusive explanations for this observation. In addition to regulating Th17 differentiation, aberrant ROCK activity is well known to be associated with a number of vascular disorders. It is thus possible that the biopsy-negative GCA subjects had intrinsic vascular ROCK dysfunction, which may have contributed to disease development. Alternatively, with antigenic stimulation of adventitial dendritic cells early in disease pathogenesis, we can speculate that the inflammatory milieu driven by local activation of innate immune cells may cause ROCK activation in the adventitia, one of the areas scored in our pERM intensity score, prior to detectable infiltration of lymphocytes.
There is overlap between the downstream products of ROCK activation and the resultant vessel changes and the cytokine milieu and vascular phenotype in GCA, lending mechanistic plausibility to our findings. ROCK plays a key role in the vasculature and immune response via regulation of gene expression promoting Th17 differentiation, alteration of cell migration allowing for local recruitment of inflammatory cells and control of cytoskeletal organization, which can promote vascular remodelling; all of these features are present in GCA. By comparing GCA subjects to an age- and sex-matched control population without GCA, we were able to show that the high-intensity pERM staining demonstrated in subjects with GCA was likely due to the disease state itself. These findings were strengthened by controlling for several major factors known to influence ROCK activity.
The ROCK pathway warrants further study in GCA and other forms of large-vessel vasculitis such as aortitis, as it may prove to be of both diagnostic and therapeutic significance.
Rheumatology key messages.
Addition of pERM intensity score to routine histopathology may greatly enhance the sensitivity of temporal artery biopsy for GCA diagnosis.
The rho-kinase pathway may be of relevance in GCA.
Acknowledgements
L.L. received support through a National Institutes of Health training grant (T32-AR007517-30).
Funding: This study was funded by the Hospital for Special Surgery Scleroderma, Vasculitis and Myositis Center.
Disclosure statement: A.P. has received research support from Kadmon Pharmaceuticals. All other authors have declared no conflicts of interest.
References
- 1.Salvarani C, Cantini F, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. Lancet. 2008;372:234–45. doi: 10.1016/S0140-6736(08)61077-6. [DOI] [PubMed] [Google Scholar]
- 2.Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2009;68:318–23. doi: 10.1136/ard.2008.088351. [DOI] [PubMed] [Google Scholar]
- 3.Weyand CM, Younge BR, Goronzy JJ. IFN-γ and IL-17: the two faces of T cell pathology in giant cell arteritis. Curr Opin Rheumatol. 2011;23:43–9. doi: 10.1097/BOR.0b013e32833ee946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espígol-Frigolé G, Corbera-Bellalta M, Planas-Rigol E, et al. Increased IL-17A expression in temporal artery lesions is a predictor of sustained response to glucocorticoid treatment in patients with giant-cell arteritis. Ann Rheum Dis. 2013;72:1481–7. doi: 10.1136/annrheumdis-2012-201836. [DOI] [PubMed] [Google Scholar]
- 5.Terrier B, Geri G, Chaara W, et al. IL-21 modulates Th1 and Th17 responses in giant cell arteritis. Arthritis Rheum. 2012;64:2001–11. doi: 10.1002/art.34327. [DOI] [PubMed] [Google Scholar]
- 6.Biswas PS, Gupta S, Chang E, et al. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest. 2010;120:3280–95. doi: 10.1172/JCI42856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas PS, Bhagat G, Pernis AB. IRF4 and its regulators: evolving insights into the pathogenesis of inflammatory arthritis? Immunol Rev. 2010;233:79–96. doi: 10.1111/j.0105-2896.2009.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Harada T, Juang YT, et al. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. J Immunol. 2007;178:1938–47. doi: 10.4049/jimmunol.178.3.1938. [DOI] [PubMed] [Google Scholar]
- 9.Matsui T, Maeda M, Doi Y. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–57. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedrose Nunes K, Rigsby CS, Webb RC. RhoA/Rho-kinase and vascular diseases: what is the link? Cell Mol Life Sci. 2010;67:3823–36. doi: 10.1007/s00018-010-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satoh K, Fukumoto Y, Shimokawa H. Rho-kinase: important new therapeutic target in cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2011;301:287–96. doi: 10.1152/ajpheart.00327.2011. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Q, Liao JK. Pleiotropic effects of statins: basic research and clinical perspectives. Circ J. 2010;74:818–26. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunder GG, Bloch DA, Michel BA, et al. American College of Rheumatology 1990 criteria for the classification of giant cell (temporal) arteritis. Arthritis Rheum. 1990;33:1122–8. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 14.Younge BE, Cook BE, Jr, Barley GB, et al. Initiation of glucocorticoid therapy: before or after temporal artery biopsy? Mayo Clin Proc. 2004;79:483–91. doi: 10.4065/79.4.483. [DOI] [PubMed] [Google Scholar]
- 15.Varma D, O’Neill D. Quantification of the role of temporal artery biopsy in diagnosing clinically suspected giant cell arteritis. Eye. 2004;18:384–8. doi: 10.1038/sj.eye.6700677. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Gay MA, Garcia-Porrua C, Llorca J, et al. Biopsy-negative giant cell arteritis: clinical spectrum and predictive factors for positive temporal artery biopsy. Semin Arthritis Rheum. 2001;30:249–56. doi: 10.1053/sarh.2001.16650. [DOI] [PubMed] [Google Scholar]
- 17.Karassa FB, Matsagas MI, Schmidt WA, et al. Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med. 2005;142:359–69. doi: 10.7326/0003-4819-142-5-200503010-00011. [DOI] [PubMed] [Google Scholar]
- 18.Proven A, Gabriel SE, Orces C, et al. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum. 2003;49:703–8. doi: 10.1002/art.11388. [DOI] [PubMed] [Google Scholar]
- 19.Weyand CM, Ma-Krupa W, Goronzy JJ. Immunopathways in giant cell arteritis and polymyalgia rheumatica. Autoimmun Rev. 2004;3:46–53. doi: 10.1016/S1568-9972(03)00064-8. [DOI] [PubMed] [Google Scholar]
- 20.Restuccia G, Cavazza A, Boiardi L, et al. Small-vessel vasculitis surrounding an uninflamed temporal artery and isolated vasa vasorum vasculitis of the temporal artery: two subsets of giant cell arteritis. Arthritis Rheum. 2012;64:549–56. doi: 10.1002/art.33362. [DOI] [PubMed] [Google Scholar]