Abstract
Background
The mechanisms behind natural control of HIV replication are still unclear, and several studies pointed that elite controllers are a heterogeneous group.
Methods
We performed analyses of virologic, genetic and immunologic parameters of HIV-1 controllers groups: 1) Elite Controllers (EC; VL <80 copies/mL); 2) Ebbing Elite Controllers (EEC; transient viremia/blips); and Viremic Controllers (VC; detectable viremia <5,000 copies/mL). Untreated non-controllers (NC), patients under suppressive HAART and HIV-1 negative individuals were analyzed as controls.
Results
Total and integrated HIV-1 DNA for EC were significantly lower than for NC and HAART groups. 2-LTR circles were detected in EEC (3/5) and VC (6/7) but not in EC. While EC and EEC maintain normal T cell counts over time, some VC displayed negative CD4+ T cells slopes. VC and EEC showed a higher percentage of activated CD8+ T cells and microbial translocation than HIV-1 negative controls. EC displayed a weaker Gag/Nef IFN-γ T cell response and a significantly lower proportion of anti-HIV IgG antibodies than EEC, VC and NC groups.
Conclusion
Transient/persistent low level viremia in HIV controllers may have an impact on immunologic and virologic profiles. Classify HIV controllers patients taking into account their virologic profile may decrease the heterogeneity of HIV controllers cohorts, which may help to clarify the mechanisms associated to the elite control of HIV.
Keywords: HIV-1, elite controllers, viral load blips, ongoing replication, immune activation and microbial translocation, immune response
INTRODUCTION
A small percentage (<1%) of HIV-1 infected individuals spontaneously control viral replication to levels below the limit of detection of standard clinical assays (50-400 RNA copies/mL). They are usually called HIV controllers (HIC) or elite controllers (EC) 1,2. An initial study described the EC as a homogenous group of HIV-infected patients 3, but subsequent analyses revealed a more heterogeneous scenario 4-6. While some EC maintain stable CD4+ T cell counts within the normal range and no evidence of chronic immune activation over time when compared to HIV negative subjects, 7 others display higher CD4+ and CD8+ T cell activation levels and a significant loss of CD4+ T cell 8-10. Markers of microbial translocations are higher in some EC than in HIV-negative individuals and correlate with T cell activation levels 8.
The role of the HIV-specific cellular immune response as a mechanism of natural control of HIV-1 replication in EC is also controversial. The overall level and functionality of HIV-specific CD8+ T cells in EC is highly variable among individuals 3,4,6,11-15. Indeed, based on the capacity of CD8+ T cells to suppress HIV infection ex vivo, the EC were classified as strong and weak responders 6. In addition, while various EC cohorts are enriched by some HLA class I alleles, including B*27 and B*57 that have been associated with HIV control 3,16-18, other EC individuals do not encode these alleles. Moreover, several HIV infected patients harboring B*27 and B*57 HLA alleles are not able to control viral replication at such low levels 4,19.
These data suggest that the EC phenotype is probably a multifactorial phenomenon that results from the combination of several host and/or viral factors. Despite this, few studies are able to perform comprehensive analyses associating virologic, genetic and immunologic data to characterize EC. Furthermore, the criteria used to classify EC patients in different cohorts varies based on the viral load (VL) cut-offs (<50 to <400 copies/mL) and time of HIV control (1 to >10 years), which also influence the underlying characteristics of the selected patients. Here, we performed a comprehensive analysis of virologic, genetic and immunologic profiles of a cohort of HIV-1-infected patients who naturally control viral replication at distinct levels.
PATIENTS AND METHODS
Patients
A cohort of 19 HIV controllers has been followed-up at the Instituto Nacional de Infectologia Evandro Chagas (INI), Rio de Janeiro, Brazil. For the present study, these HIV controllers were classified in three categories: (1) Elite controllers (EC), including patients presenting 100% of VL measures <80 copies/mL (n=7); (2) Ebbing elite controllers (EEC), including patients with occasional episodes (<30% of frequency) of transient viremia between 81-400 copies/mL (n=5); and (3) Viremic controllers (VC), patients with consistently detectable plasma viral load in the low range (<5,000 copies/mL) (n=7). Untreated non-controller (NC) patients (n=30) with high viral load levels (>10,000 copies/mL), patients under suppressive HAART (n=13) and HIV-1 negative controls (n=10) were also included in the analysis for comparisons. The cut-off value of 80 copies/mL used to classify the EC patients was determined based on the limit of detection of the Nucleic Acid Sequence Based Amplification System (NASBA) which was adopted by the Brazilian Ministry of Health from 1999 to 2008 to quantify the viral load of HIV infected patients. This period includes most viral load measures used to classify these patients. The present work was approved by the IPEC Institutional Review Board (Addendum 049/2010) and the Brazilian National Committee for Research Ethics (CONEP 840/2008). All subjects gave written informed consent.
CD4 T cell counts and RNA viral load quantification
Absolute CD4+ T cells counts were obtained using the MultiTest TruCount-kit and the MultiSet software on a FACSCalibur flow cytometer (BD Biosciences, USA). Plasma HIV-1 viral loads were measured using the nucleic acid sequence based amplification (NASBA) system (limit of detection: 80 copies/mL - Nuclisens, Organon Teknika) and the Versant HIV-1 3.0 RNA assay (limit of detection: 50 copies/mL - bDNA, Siemens Healthcare Diagnostics, USA).
HIV subtype classification
Subtype was determined based on the gag, pol and/or nef HIV-1 genomic regions. DNA extraction, PCR amplification, and sequencing were performed as previously described20,21. Subtype determination was inferred based on phylogenetic analysis (Neighbor-Joining with Kimura-2 parameters correction, as available in the Mega 5.1 package).
HLA and CCR5 genotyping
HLA-B typing was performed as previously described 22. The presence of the Δ32 variant in CCR5 was assessed by PCR amplification/agarose gel electrophoresis. For the wild-type allele, a 239bp fragment was detected and a 207bp fragment corresponded to the mutant allele. Primers are described in Supplementary table 1.
HIV-1 total, integrated and 2-LTR DNA quantification
Total and episomal DNA were extracted as previously described 23. Chromosomal DNA was purified from the SDS-precipitate recovered during the purification of episomal DNA, following the same protocol described for total DNA extraction. HIV total and integrated DNA and CCR5 were measured by a single step real time PCR protocol. PCR conditions were as previously described 23, primers and probes are detailed in Supplementary Table 1. Standard curves were generated using a plasmid containing two copies of HIV LTR and two copies of CCR5 gene. The values were normalized based on cell numbers estimated by CCR5 quantification and are expressed as the number of DNA copies/106 PBMC.
T-cell activation analysis
Cryopreserved PBMCs were thawed and immediately stained for anti-CD8-FITC/CD38-PE (BD Simultest, BD Biosciences, USA), anti-CD3-APC, and anti-HLA-DRPerCP (BD Biosciences, USA). Samples were acquired using a BD FACSCalibur flow cytometer, and analyses were performed with BD CellQuest software (BD Biosciences, USA).
Quantification of soluble CD14 (sCD14) plasma levels
The microbial translocation was estimated based on the level of sCD14 in plasma. Plasma levels of sCD14 were assayed in duplicate using ELISA assay-sCD14 Quantikine (R&D Systems, USA), according to the manufacturer’s protocol. The results were expressed as pg/mL.
Serological testing (BED-CEIA)
The proportion of anti-HIV-1 IgG in comparison to total IgG was measured in plasma samples by a quantitative competitive capture enzyme immunoassay - Calypte HIV-1 BED Incidence EIA (Calypte Biomedical Corporation, USA) 24.
IFN-γ ELISpot
The IFN-γ ELISPOT assay was performed as previously described 25. Peptides were derived from HIV-1 Gag and Nef consensus subtype B, C and F1, previously described (ref), and for each patient, was used a subtype homologue consensus peptides. Phytohemagglutinin-5μg/mL (Sigma, USA) was used as a positive control, and cells suspended only in culture medium served as a negative control. The spots were counted using an automated ELISPOT reader (CTL Analyzers LLC, Cellular Technology, USA). The results were expressed as spot-forming cells (SFC)/million PBMC. The response was considered positive if ≥ 50 SFC/106 PBMC were detected.
Statistical analysis
Statistical analyses were performed using GraphPad 5.0 (Prism Software, USA) and Epi Info Version 6 26. DNA quantification analysis, CD4 slope, CD8 T cell activation, sCD14 and ELISPOT IFN-γ data were analyzed using Mann-Whitney test to compare variables between two subjects groups. Chi-square tests (or Fisher’s exact tests, when appropriate) were used for HLA-B allelic frequencies and 2-LTR positive PCR percentages comparisons among groups. Correlations were performed using the Spearman test. All tests were considered significant if the P value was ≤ 0.05.
RESULTS
Clinical, epidemiological, and genetic characteristics of HIV controllers
The clinical, epidemiological and genetic data of the 19 HIV controllers included in the present study are displayed in Table 1. The median time of follow-up (estimated time of HIV control) was nine years (IQR: 7-12 years). The median age for the HIV controllers cohort was 44 years old (IQR: 42.5-44.0 years old), and females (58%) were more frequent than males. The detection of subtype B, F1, C and BF recombinants among HIV controllers, is in accordance with the overall HIV-1 molecular epidemiologic scenario in Rio de Janeiro state 27.
Table 1.
Characteristics of the cohort of HIV controllers followed-up at IPEC, Rio de Janeiro, Brazil
| Patient | Sex | Age | Year of HIV diagnosis |
HIV Risk Factor |
Years of Known HIV Suppression |
CD4 counts Median (IQR) |
VL Frequency | HLA-B alleles |
CCR-5 genotype |
HIV subtype | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <80cps/ml | 81-400cps/ml | 401-5000cps/ml | ||||||||||
| EC02 | F | 49 | 1997 | HET | 10 | 1229 (1077-1480) |
100% | B*48, B*52 | WT//WT | B | ||
| EC07 | F | 28 | 2003 | HET | 8 | 2308 (1931-2444) |
100% | B*48, B*81 | WT//WT | BF | ||
| EC08 | F | 41 | 1996 | HET | 15 | 1207 (993.8-1355) |
100% | B*45, B*57 | WT//WT | B | ||
| EC17 | F | 62 | 2001 | NI | 8 | 1674 (1494-1954) |
100% | B*14, B*15 | WT//WT | B | ||
| EC19 | M | 44 | 2006 | HET | 5 | 972 (826.5-1093) |
100% | B*44, B*58 | WT//WT | B | ||
| EC26 | M | 40 | 2008 | MSM | 4 | 1531 (1409-2124) |
100% | B*07, B*57 | WT/WT | BF | ||
| EC28 | M | 46 | 1996 | MSM | 13 | 1163 (891.5-1358) |
100% | B*51, B*58 | WT/Δ32 | B | ||
| EEC09 | M | 43 | 2001 | MSM | 10 | 888 (710-1054) |
79% | 21% | B*49, B*52 | WT/WT | B | |
| EEC11 | F | 45 | 1995 | HET | 10 | 1078 (959.5-1206) |
90% | 10% | B*49, B*81 | WT/δ32 | B | |
| EEC13 | F | 58 | 1993 | HET | 19 | 954 (718.3-1138) |
73% | 27% | B*15, B*51 | WT/WT | B | |
| EEC14 | F | 42 | 1999 | HET | 7 | 702 (652-795) |
75% | 25% | B*42, B*44 | WT/WT | F | |
| EEC18 | F | NI | 2001 | HET | 10 | 825 (673-940.5) |
86% | 14% | B*44, B*58 | WT/WT | B | |
| VC04 | F | 47 | 2007 | HET | 4 | 770 (718-895.3) |
28% | 72% | B*27, B*40 | WT/WT | C | |
| VC06 | M | 34 | 2000 | MSM | 7 | 1132 (913-1225) |
80% | 20% | B*15, B*48 | WT/δ32 | B | |
| VC10 | M | 48 | 1991 | NI | 18 | 1254 (1078-1460) |
22% | 52% | 26% | B*15, B*52 | WT/WT | B |
| VC12 | M | 40 | 2000 | MSM | 9 | 1022 (872.5-1131) |
23% | 15% | 62% | B*27, B*44 | WT/WT | B |
| VC15 | F | NI | 2001 | HET | 11 | 735 (677-793.8) |
100% | B*56, B*57 | WT/WT | B | ||
| VC16 | M | 45 | 1997 | MSM | 9 | 552 (530.5-623.3) |
29% | 47% | 24% | B*14, B*57 | WT/WT | B |
| VC27 | F | 36 | 1998 | HET | 10 | 895 (757-965.5) |
35% | 18% | 47% | B*08, B*27 | WT/WT | B |
|
Group
summary |
Frequency | Median | Median | Frequency | Median | Median | Mean | Mean | Mean | Frequency | Frequency | Frequency |
| EC | M=43% F=57% |
44 | 2001 | HET=57% MSM=29% |
8 | 1229 | 100% | 0% | 0% | B*27/B*57= 29% Non- B*27, B*57= 71% |
WT/Δ32=14% WT/WT=86% |
B=71% BF=29% |
| EEC | M=20% F=80% |
44 | 1999 | HET=80% MSM=20% |
10 | 888 | 80% | 20% | 0% | B*27/B*57= 0% Non- B*27, B*57= 100% |
WT/Δ32=20% WT/WT=80% |
B=80% F=20% |
| VC | M=57% F=43% |
42.5 | 2000 | HET=43% MSM=43% |
9 | 895 | 27% | 40% | 49% | B*27/B*57= 43% Non- B*27, B*57= 57% |
WT/Δ32=14% WT/WT=86% |
B=86% C=14% |
NI: Not informed; HET: Heterosexual; MSM: Man who have sex with man; WT: wild type.
The HLA-B alleles B*27 and B*57, classically associated with HIV-1 control, were found among our cohort of HIV controllers. Three individuals - all VC - presented HLAB*27 (allelic frequency= 7.9%); and four individuals – two EC and two VC – presented HLA-B*57 (allelic frequency= 10.5%). Three out of 19 patients (one EC, one EEC and one VC) were heterozygous for the CCR5Δ32, but we did not identify any patient homozygous for this mutation. No statistical difference was observed in the comparison of the host genetic markers among the three HIV controllers groups.
HIV controllers have a limited HIV-1 reservoir
The level of total and integrated HIV-1 DNA was determined for the three groups of HIV controllers as well as for NC and HAART groups (Figure 1A and 1B). Total and integrated HIV-1 DNA were significantly lower for the EC group in comparison to both NC (P= 0.0003 and P= 0.0005) and HAART (P= 0.0005 and P= 0.0004) groups. The levels of total and integrated HIV-1 DNA for the EEC were higher than the observed for EC, but also significantly lower in comparison to NC (P= 0.0028 and P= 0.0052). VC displayed HIV DNA levels in a mean range between EC/EEC and HAART/NC, significantly lower than NC (P= 0.0078 and P= 0.0492, respectively). Plasma HIV-1 RNA viral load correlated positively with both total (r = 0.78; P<0.0001) and integrated (r = 0.69; P<0.0001) HIV DNA for the patients analyzed here (Figure 1D and 1E). This correlation was still significant when undetectable viral loads were removed (r = 0.65; P=0.0003 and 0.52; P<0.0059, respectively).
Figure 1.
Quantification of total (A) and integrated (B) viral DNA and 2-LTR circles (C) for HIV controllers, HAART and NC groups. Relationship between plasma viral load and total HIV DNA (D), integrated HIV DNA (E), and 2-LTR circles (F). Negative 2-LTR PCR results are represented by open symbols. The number of positive 2-LTR PCR /number patients tested were as follows: EC=0/7, EEC=3/5, VC=6/7, HAART=9/13 and NC=18/19 (the percentages are indicated in the graph). The horizontal lines denote median values. P values for comparison between two groups were calculated using a 2-tailed Mann-Whitney test. Correlations were calculated using a nonparametric Spearman test. Data from EC, EEC, VC and NC were used to calculate correlations.
2-LTR circles are detected in EEC and VC but not in EC
In order to assess whether residual HIV replication occurs in these HIV controllers, we quantified the 2-LTR circles (Figure 1C). The 2-LTR circles were not detected in EC patients, but were detected in three out of five EEC (P = 0.0455) and six out of seven VC (P = 0.0047). The levels of 2-LTR circles were significantly lower for EEC and VC in comparison to NC (P = 0.0104 and P = 0.0192, respectively). Of note, nine out of 13 patients under HAART had detectable 2-LTR circles and in higher levels than the HIV controller groups. The levels of 2-LTR HIV DNA correlated positively with HIV-1 RNA viral load (Figure 1F), and with total and integrated HIV DNA (data not shown).
HIV replication at a low level may induce a loss of CD4+ T cells
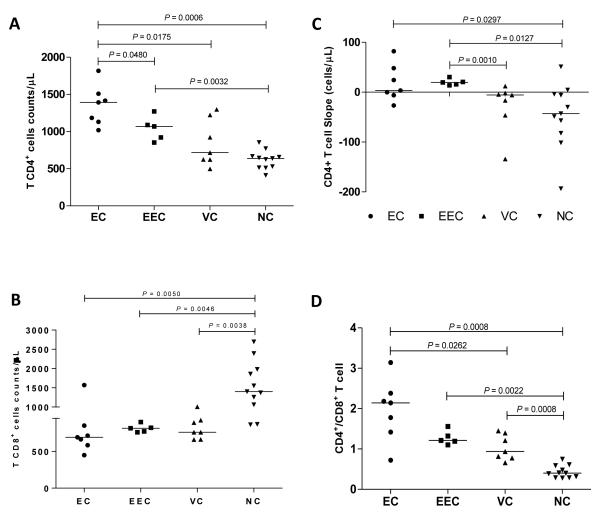
The median of CD4+ T cells counts in the peripheral compartment of EC was significantly higher than that of EEC, VC, and NC patients (P = 0.0480, 0.0175 and 0.0005, respectively) (Figure 2A). EEC patients also displayed significantly higher CD4+ T cells counts that NC patients (P = 0.0032) (Figure 2A). All groups of HIV controllers displayed CD8+ T cells counts significantly lower than the NC group (P < 0.01) (Figure 2B). All EC and EEC displayed nearly flat or positive CD4+ T cells slopes over time; whereas two VC and most NC displayed negative CD4+ T cells slopes (Figure 2C). The CD4+/CD8+ T cell ratio in all EC (except one) and EEC was ≥1; while three VC and all NC displayed a CD4+/CD8+ T cell ratio <1 (Figure 2D).
Figure 2.
Evaluation of peripheral CD4+ (A) and CD8+ (B) T cell populations, CD4+ T cell slope (C) and CD4/CD8 ratio among Elite Controllers (EC), Ebbing Elite Controllers (EEC), Viremic Controllers (VC) and Non-controllers (NC). The horizontal bars denote median values. P values for comparison between two groups were calculated using a 2-tailed Mann-Whitney test.
Transient and persistent low viral replication induce activation and microbial translocation
The level of activated CD8+ T cells in EC was comparable to the level observed in HIV-uninfected participants (Figure 3A). However, a higher activation was observed in EEC than in HIV-uninfected individuals (P = 0.0027), but lower than in NC (P = 0.0414) (Figure 3A). Despite of the high dispersion, the median of CD8+ T cell activation in VC was higher than in HIV-uninfected participants (P = 0.0020) and similar to NC (Figure 3A). The median of sCD14 concentration in plasma of HIV-infected individuals was higher than in HIV uninfected, although differences were only significant for EEC and NC groups (Figure 3B).
Figure 3.
Percentages of activated (CD38+HLA-DR+) CD8+ T cells (A) and level of sCD14 (pg/mL) in the plasma (B) among HIV-uninfected participants, Elite Controllers (EC), Ebbing Elite Controllers (EEC), Viremic Controllers (VC) and Non-Controllers (NC). The horizontal bars denote median values. P values for comparison between two groups were calculated using a 2-tailed Mann-Whitney test.
HIV specific immune responses were influenced by transient viral load
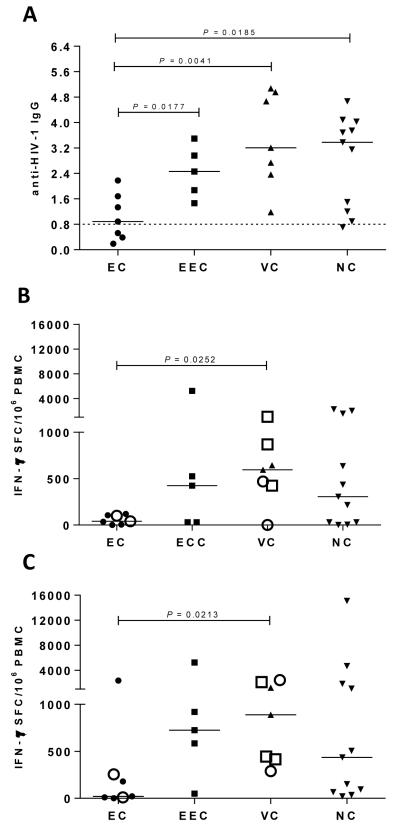
The proportion of anti-HIV-1 IgG antibodies and anti-HIV-1 IFN-γ T cells were analyzed in the different HIV-infected groups. No significant differences were observed in the proportion of anti-HIV-1 IgG among EEC, VC and NC groups, conversely, the median proportion of anti-HIV-1 IgG of EC was significantly lower than that found in the other groups (P = 0.0177; 0.0041; 0.0185; respectively) (Figure 4A). Three EC displayed a remarkably low proportion of anti-HIV-1 IgG [normalized optical density (ODn) < 0.8 of calibrator OD], comparable to those seen in recently infected individuals. The magnitude of IFN-γ T cell responses against both Gag and Nef subtype-specific epitopes in EC also showed a trend towards lower values when compared to the other groups, although significant differences were observed only between EC and VC groups (Figure 4B and 4C).
Figure 4.
Evaluation of HIV-1 specific responses. Proportion of anti-HIV-1 IgG determined by BED-CEIA found in Elite Controllers (EC), Ebbing Elite Controllers (EEC), Viremic Controllers (VC) and Non-Controllers (NC) (A). Gag- (B) and Nef- (C) specific PBMC responses among Elite Controllers (EC), Ebbing Elite Controllers (EEC), Viremic Controllers (VC) and Non-Controllers (NC) by IFN-γ ELISpot (SFC/106 PBMC). Open circles represent patients with B*57 and open squares represent patients with B*27 alelles. Horizontal dashed line represents the conventional cut-off value (0.8) below which a sample is classified as recent infection (153-day window period) based on the low proportion of HIV-specific IgG in the serum/plasma. The horizontal bars denote median values. P values for comparison between two groups were calculated using a 2-tailed Mann-Whitney test.
Plasma RNA viral load is associated with CD8+ T cell activation and anti-HIV-1 IgG antibodies
The percentage of activated CD8+ T cells was positively correlated with both plasma viral load (see Figure S1A, Supplemental Digital Content) and 2-LTR DNA viral load (see Figure S1B, Supplemental Digital Content) and negatively correlated with the CD4+ T cell slope (see Figure S1C, Supplemental Digital Content). No significant correlations were detected between the level of plasma sCD14 and percentage of activated CD8+ T cells, plasma viral load and 2-LTR HIV-1 DNA (see Figure S1D, S1E and S1F, Supplemental Digital Content). A positive correlation was also observed between plasma viral load and antibody response (see Figure S2A, Supplemental Digital Content), but not between plasma viral load and Gag- or Nef-specific T cell responses (see Figure S2B and S2C, Supplemental Digital Content). When undetectable viral load values are removed, all correlations remain significant, except for CD8+ T cell activation (data not shown).
DISCUSSION
Studies with EC offer a unique opportunity to understand the mechanisms underlying the natural control of HIV infection, giving valuable clues to the development of a therapeutic vaccine and strategies for inducing a durable viral remission in non-controllers patients. However, the EC status has been shown to be a multifactorial phenomenon, associated with virus, host genetics and immunity. Here, we show that the presence of transient viremic episodes and low level persistent HIV replication have relevance in the immune activation and specific immune responses.
Protective HLA class I alleles, mainly HLA-B*57, are enriched in EC cohorts 4,16,19. In the present study, we also noted a significant increase in HLA-B*27 and B*57 frequencies among HIV controllers. These frequencies were also significantly increased (P = 0.01 and P = 0.04, respectively) when compared with a Brazilian cohort of 218 individuals with distinct AIDS progression profiles 28. Concerning the CCR5 polymorphisms, the frequencies of the CCR5Δ32 mutation did not vary from that observed among the population of HIV seronegative individuals 4 in the present study, contrary to a previous description of a higher prevalence of CCR5Δ32 mutation in EC compared to that observed for the general population 17. In our study, neither the HLA-B alleles nor the CCR5Δ32 frequencies differed significantly among different HIV controllers groups (EC, EEC and VC), although the number of patients within each group was too small to detect potential differences.
HIV reservoirs are established very early after HIV infection 29. In the present study, we assessed viral reservoir by quantifying total and integrated HIV-1 DNA in PBMCs. We confirmed previous findings that HIV controllers have a smaller reservoir compared to HAART-treated and patients with a typical progression profile 30,31. Although there were no significant differences in the HIV reservoir size among different HIV controller groups, the mean of total and integrated HIV-1 DNA load in EC was lower than in EEC and VC. This is consistent with a more efficient long-term suppression of HIV replication in EC patients compared with the other groups and further suggests that the occurrence of transient and persistent replication in EEC and VC, even at low levels, contributes to replenishment of the viral reservoir over time. A small HIV reservoir is consistent with a long-term suppression of HIV replication in EC patients.
Despite the limited HIV reservoir, it has been consistently described that most EC maintain a persistent low-level viremia and that HIV continues to replicate and evolve overtime 32-35. We evaluated whether ongoing viral replication was taking place in our cohort of HIV controllers. Consistently, the levels of 2-LTR circles were lower for all groups of HIV controllers than to NC. 2-LTR circles were not detected in EC patients but were detected in three out of five EEC patients and six out of seven VC. Of note, the VC patient with undetectable 2-LTR circles also had undetectable viremia at this time point. To our knowledge, this study is the first to demonstrate that 2-LTR circles can be detected in EEC, which might be used as a predictive marker of transient viremic episodes. Further studies with a larger number of patients must be conducted to validate this hypothesis.
A previous study by Graf and colleagues 30 described increased levels of 2-LTR circles in PBMC from EC compared to viremic non-treated and HAART treated patients. The authors raised the possibility that a pre-integration restriction mechanism could be taking place in the EC, but this hypothesis was not confirmed by ex vivo analyses in that cohort. Our findings of undetectable 2-LTR levels in EC contradict the findings of Graf and colleagues, however our results strongly correlate with our HIV controllers clinical data. Since the EC patients have well documented long-term suppression of viremia, markers of ongoing viral replication are not expected to be detected at high levels ex vivo.
Since persistent viremia can be detected by using ultrasensitive methods in the majority of HIV controllers, the continuous exposure to viral products might induce a chronic immune activation and inflammation in these patients 32. Previous studies demonstrated that some HIV controllers have a higher level of T cell activation than HIV uninfected subjects 8,36, while others show similar levels 7. Here, we investigated the impact of different levels of viremia on T cell activation and, despite the limited number of patients included in this study, we found a significantly higher degree of CD8+ T cell activation in VC and EEC than in HIV uninfected individuals. This result indicates that even transient episodes of detectable viremia might induce chronic immune activation in HIV-infected individuals. Moreover, EEC also presented a higher level of sCD14 than EC. Chronic immune activation has been pointed as a major driving force of CD4+ T cell depletion in HIV-infected patients 37. In our study, CD8+ T cell activation levels were positively correlated with the RNA viral load and the 2-LTR DNA viral load, and negatively correlated with the CD4+ T cell slope, suggesting that ongoing viral replication accounts, at least in part, for systemic immune activation and possibly CD4+ T cell depletion in our cohort patients. The EEC presented lower CD4+ T cell counts than EC, although no significant decrease in CD4+ T cells was observed in this group. This result is in contrast with Boufassa and colleagues 38 that showed an association between the presence of blips and a decrease in CD4+ T cell count, although they observed a frequency of blips (≤ 50%) higher than in our study (≤ 30%).
Previous studies described the heterogeneity of CD8+ T cell responses in elite controllers 4,6 and based on this heterogeneity EC patients were classified as “strong responders” and “weak responders” 6. Sáez-Cirión et al.6 demonstrated a strong correlation between the frequency of IFN-γ-producing CD8+ T cells upon peptide stimulation, using IFN-γ ELISPOT and the HIV-suppressive capacity of unstimulated CD8+ T cells, among HIV controllers. Here, we did not evaluate the suppressive capacity of ex vivo CD8+ T cells, but based on IFN-γ ELISPOT results, we found that most “weak responders” were EC, whereas EEC and VC mostly correspond to “strong responders”. This is in agreement with previous studies that also observed an association between the presence of blips and a stronger CD8+ T cell response in EC 6 and HAART treated individuals 39, pointing out the need for a transient or continuous stimulus for maintaining a detectable long-term ex vivo HIV-1 specific CD8+ T cell response. The analyses of others parameters of T cell responses that have been considered important for the control of viral replication, such as polyfunctional cells 12, synthesis of cytotoxic granule components, such as granzyme and perforin 14,40 will certainly improve the knowledge of the impact of transient or persistent HIV replication on the quality of CD8+ T cell response. No correlation between stronger T cell response and presence of HLA-B*27 or B*57 was observed, corroborating previous findings 41. Conversely, a recent study evaluated the impact of HLA-B*57 on the HIV-specific CD8+ response in EC, confirming that this allele plays an important role in the high quality HIV-specific CD8+ T cell response displayed by these subjects 42. The association between HIV replication and immune response was also verified when HIV-specific antibodies were evaluated. The proportion of anti-HIV-1 IgG antibodies in EC was significantly lower than that observed in EEC, VC and NC. Furthermore, three EC subjects presented levels of anti-HIV-1 IgG lower than the cut-off that defines recent infection (<6 months from HIV infection), despite being measured more than 5 years after an HIV diagnosis. These results are fully consistent with those previously reported by our group 7 and others 43,44, and support the notion that transient or persistent HIV replication is also necessary to develop and maintain a high proportion of anti-HIV-1 IgG antibodies.
Although transient or persistent viremia in the detectable range (>50 copies/mL plasma) seems to be necessary for maintaining strong HIV-specific cellular and humoral immune responses over time, we detected one EC patient that presented a strong ex vivo HIV-1 specific CD8+ T cell INF-γ response and a high proportion of HIV-specific IgG antibodies. Notably, this subject also presented the highest level of CD8+ T cell counts and lowest CD4/CD8 ratio among EC, while normal levels of CD8+ T cell activation were observed. Taken together, our data reinforce the heterogeneity of the elite controller population and highlight the importance of viral load “blips” in the virologic and immunologic profile of these patients. In the present study, we adopted the nomenclature “ebbing elite controllers” to define the patients with “blips” due to their ability to suppress viral loads after loss of HIV control. Based on the data presented here, investigation of patients with the same characteristics in larger cohorts of HIV controllers is warranted in order to elucidate the role of transient viremic episodes in the evaluation of virus, host genetics and immunity.
Supplementary Material
Supplementary Figure 1. Relationship between CD8+ T cell activation level and plasma viral load (A), 2-LTR circles (B), CD4+ T cell slope (C) and sCD14 (D). Relationship between sCD14 and plasma viral load (E), and 2-LTR circles (F). Correlations were calculated using a nonparametric Spearman test. Data from Elite Controllers (EC), Ebbing Elite Controllers (EEC) and Viremic Controllers (VC) were used to calculate correlations between immunologic parameters and 2-LTR circles. For all others correlations, data from Elite Controllers (EC), Ebbing Elite Controllers (EEC), Viremic Controllers (VC) and Non-Controllers (NC) were used. Not significant (ns).
Supplementary Figure 2. Relationship between plasma viral load and anti-gp41-binding antibodies (A), Gag- (B), and Nef-specific (C) T cell responses. Correlations were calculated using a nonparametric Spearman test. Data from Elite Controllers (EC), Ebbing Elite Controllers (EEC), Viremic Controllers (VC) and Non-Controllers (NC) were used to calculate correlations. Not significant (ns).
Acknowledgments
We wish to thank the University of Miami – Department of International Students and Scholar Services for the support. We thank the Programa de Desenvolvimento Tecnológico em Insumos para Saúde -PDTIS/FIOCRUZ for use of its facilities. Most of all, we thank the patients. Without their participation and commitment this study would not have been possible.
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq [grant number 480875/2010-33]; by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ [grant numbers E-26/103.095/2011 and E-26/100.299/2011 to C.P.B.P] and by National Institutes of Allergy and Infectious Diseases of the National Institutes of Health [grant number U19 AI096109 to M.S.].
Footnotes
Conflicts of Interest and Source of Funding The authors declare no conflict of interest.
Results presented at: 19th Conference on Retroviruses and Opportunistic Infections, Seattle, WA, March, 2012 (abstract: 291). 30 years of HIV science: Imagine the future, Paris, France, May, 2013 (abstract: 109).
References
- 1.Madec Y, Boufassa F, Porter K, Meyer L. Spontaneous control of viral load and CD4 cell count progression among HIV-1 seroconverters. AIDS. 2005;19(17):2001–2007. doi: 10.1097/01.aids.0000194134.28135.cd. [DOI] [PubMed] [Google Scholar]
- 2.Okulicz JF, Marconi VC, Landrum ML, et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis. 2009;200(11):1714–1723. doi: 10.1086/646609. [DOI] [PubMed] [Google Scholar]
- 3.Lambotte O, Boufassa F, Madec Y, et al. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis. 2005;41(7):1053–1056. doi: 10.1086/433188. [DOI] [PubMed] [Google Scholar]
- 4.Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197(4):563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 5.Lambotte O, Ferrari G, Moog C, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. Aids. 2009;23(8):897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saez-Cirion A, Sinet M, Shin SY, et al. Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J Immunol. 2009;182(12):7828–7837. doi: 10.4049/jimmunol.0803928. [DOI] [PubMed] [Google Scholar]
- 7.Bello G, Velasco-de-Castro CA, Bongertz V, et al. Immune activation and antibody responses in non-progressing elite controller individuals infected with HIV-1. J Med Virol. 2009;81(10):1681–1690. doi: 10.1002/jmv.21565. [DOI] [PubMed] [Google Scholar]
- 8.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197(1):126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrade A, Bailey JR, Xu J, et al. CD4+ T cell depletion in an untreated HIV type 1-infected human leukocyte antigen-B*5801-positive patient with an undetectable viral load. Clin Infect Dis. 2008;46(8):e78–82. doi: 10.1086/529387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sedaghat AR, Rastegar DA, O’Connell KA, Dinoso JB, Wilke CO, Blankson JN. T cell dynamics and the response to HAART in a cohort of HIV-1-infected elite suppressors. Clin Infect Dis. 2009;49(11):1763–1766. doi: 10.1086/648081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams LD, Bansal A, Sabbaj S, et al. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J Virol. 2011;85(5):2316–2324. doi: 10.1128/JVI.01476-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Betts M, Nason M, West S, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owen RE, Heitman JW, Hirschkorn DF, et al. HIV+ elite controllers have low HIV-specific T-cell activation yet maintain strong, polyfunctional T-cell responses. AIDS. 2010;24(8):1095–1105. doi: 10.1097/QAD.0b013e3283377a1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hersperger AR, Pereyra F, Nason M, et al. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 2010;6(5):e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saez-Cirion A, Lacabaratz C, Lambotte O, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104(16):6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Migueles SA, Sabbaghian MS, Shupert WL, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A. 2000;97(6):2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereyra F, Jia X, McLaren PJ, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330(6010):1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fellay J, Shianna KV, Ge D, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317(5840):944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emu B, Sinclair E, Hatano H, et al. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J Virol. 2008;82(11):5398–5407. doi: 10.1128/JVI.02176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortes FH, Bello G, Vorsatz C, et al. Higher cross-subtype IFN-gamma ELISpot responses to Gag and Nef peptides in Brazilian HIV-1 subtype B- and F1- than in C-infected subjects. Vaccine. 2013;31(7):1106–1112. doi: 10.1016/j.vaccine.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 21.Passaes CP, Bello G, Lorete RS, et al. Genetic characterization of HIV-1 BC recombinants and evolutionary history of the CRF31_BC in Southern Brazil. Infect Genet Evol. 2009;9(4):474–482. doi: 10.1016/j.meegid.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Teixeira SL, Bastos FI, Hacker MA, Morgado MG. Distribution of CCR5 genotypes and HLA Class I B alleles in HIV-1 infected and uninfected injecting drug users from Rio de Janeiro, Brazil. Infect Genet Evol. 2009;9(4):638–642. doi: 10.1016/j.meegid.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Sharkey M, Babic DZ, Greenough T, Gulick R, Kuritzkes DR, Stevenson M. Episomal viral cDNAs identify a reservoir that fuels viral rebound after treatment interruption and that contributes to treatment failure. PLoS Pathog. 2011;7(2):e1001303. doi: 10.1371/journal.ppat.1001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parekh BS, Kennedy MS, Dobbs T, et al. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res Hum Retroviruses. 2002;18(4):295–307. doi: 10.1089/088922202753472874. [DOI] [PubMed] [Google Scholar]
- 25.Calarota SA, Foli A, Maserati R, et al. HIV-1-specific T cell precursors with high proliferative capacity correlate with low viremia and high CD4 counts in untreated individuals. J Immunol. 2008;180(9):5907–5915. doi: 10.4049/jimmunol.180.9.5907. [DOI] [PubMed] [Google Scholar]
- 26.Epi Info, Version 6: A word Processing, Database, and Statistics Program for Epidemiology on Microcomputers [computer program] Centre for Disease Control and Prevention; Atlanta, Georgia, USA: 1995. [Google Scholar]
- 27.Velasco-de-Castro CA, Grinsztejn B, Veloso VG, et al. HIV-1 diversity and drug resistance mutations among people seeking HIV diagnosis in voluntary counseling and testing sites in Rio de Janeiro, Brazil. PLoS One. 2014;9(1):e87622. doi: 10.1371/journal.pone.0087622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teixeira SL, de Sa NB, Campos DP, et al. Association of the HLA-B*52 allele with non-progression to AIDS in Brazilian HIV-1-infected individuals. Genes Immun. 2014 doi: 10.1038/gene.2014.14. [DOI] [PubMed] [Google Scholar]
- 29.Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387(6629):183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 30.Graf EH, Mexas AM, Yu JJ, et al. Elite suppressors harbor low levels of integrated HIV DNA and high levels of 2-LTR circular HIV DNA compared to HIV+ patients on and off HAART. PLoS Pathog. 2011;7(2):e1001300. doi: 10.1371/journal.ppat.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambotte O, Boufassa F, Madec Y, et al. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis. 2005;41(7):1053–1056. doi: 10.1086/433188. [DOI] [PubMed] [Google Scholar]
- 32.Pereyra F, Palmer S, Miura T, et al. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis. 2009;200(6):984–990. doi: 10.1086/605446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009;83(1):329–335. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mens H, Kearney M, Wiegand A, et al. HIV-1 continues to replicate and evolve in patients with natural control of HIV infection. J Virol. 2010;84(24):12971–12981. doi: 10.1128/JVI.00387-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connell KA, Brennan TP, Bailey JR, Ray SC, Siliciano RF, Blankson JN. Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus. J Virol. 2010;84(14):7018–7028. doi: 10.1128/JVI.00548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereyra F, Lo J, Triant VA, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. Aids. 2012;26(18):2409–2412. doi: 10.1097/QAD.0b013e32835a9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev. 2013;254(1):326–342. doi: 10.1111/imr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boufassa F, Saez-Cirion A, Lechenadec J, et al. CD4 dynamics over a 15 year-period among HIV controllers enrolled in the ANRS French observatory. PLoS One. 2011;6(4):e18726. doi: 10.1371/journal.pone.0018726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karlsson AC, Younger SR, Martin JN, et al. Immunologic and virologic evolution during periods of intermittent and persistent low-level viremia. Aids. 2004;18(7):981–989. doi: 10.1097/00002030-200404300-00005. [DOI] [PubMed] [Google Scholar]
- 40.Migueles S, Osborne C, Royce C, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29(6):1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomescu C, Duh FM, Hoh R, et al. Impact of protective killer inhibitory receptor/human leukocyte antigen genotypes on natural killer cell and T-cell function in HIV-1-infected controllers. Aids. 2012;26(15):1869–1878. doi: 10.1097/QAD.0b013e32835861b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lecuroux C, Saez-Cirion A, Girault I, et al. Both HLA-B*57 and plasma HIV RNA levels contribute to the HIV-specific CD8+ T cell response in HIV controllers. J Virol. 2014;88(1):176–187. doi: 10.1128/JVI.02098-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashida T, Gatanaga H, Tanuma J, Oka S. Effects of low HIV type 1 load and antiretroviral treatment on IgG-capture BED-enzyme immunoassay. AIDS Res Hum Retroviruses. 2008;24(3):495–498. doi: 10.1089/aid.2007.0150. [DOI] [PubMed] [Google Scholar]
- 44.Wendel SK, Mullis CE, Eshleman SH, et al. Effect of natural and ARV-induced viral suppression and viral breakthrough on anti-HIV antibody proportion and avidity in patients with HIV-1 subtype B infection. PLoS One. 2013;8(2):e55525. doi: 10.1371/journal.pone.0055525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Relationship between CD8+ T cell activation level and plasma viral load (A), 2-LTR circles (B), CD4+ T cell slope (C) and sCD14 (D). Relationship between sCD14 and plasma viral load (E), and 2-LTR circles (F). Correlations were calculated using a nonparametric Spearman test. Data from Elite Controllers (EC), Ebbing Elite Controllers (EEC) and Viremic Controllers (VC) were used to calculate correlations between immunologic parameters and 2-LTR circles. For all others correlations, data from Elite Controllers (EC), Ebbing Elite Controllers (EEC), Viremic Controllers (VC) and Non-Controllers (NC) were used. Not significant (ns).
Supplementary Figure 2. Relationship between plasma viral load and anti-gp41-binding antibodies (A), Gag- (B), and Nef-specific (C) T cell responses. Correlations were calculated using a nonparametric Spearman test. Data from Elite Controllers (EC), Ebbing Elite Controllers (EEC), Viremic Controllers (VC) and Non-Controllers (NC) were used to calculate correlations. Not significant (ns).