Abstract
Alzheimer's disease (AD) is a fatal progressive disease and the most common form of dementia without effective treatments. Previous studies support that the disruption of endoplasmic reticulum (ER) Ca2+ via overactivation of Ryanodine receptors (RYRs) plays an important role in the pathogenesis of AD. Normalization of intracellular Ca2+ homeostasis could be an effective strategy for AD therapies. Dantrolene, an antagonist of RYRs and an FDA approved drug for clinical treatment of malignant hyperthermia and muscle spasms, exhibits neuroprotective effects in multiple models of neurodegenerative disorders. Recent preclinical studies consistently support the therapeutic effects of dantrolene in various types of AD animal models and were summarized in the current review.
Keywords: Alzheimer's disease; calcium; endoplasmic reticulum; Ryanodine receptors; Inositol-1,4,5-trisphosphate receptors; dantrolene
Alzheimer's disease (AD), a fatal progressive disease and the most common form of dementia, threatens around 24 to 35 million people worldwide.1-5 It is estimated that this disease affects around 6 % of population aged over 65 years, with its incidence increasing with age. Patients suffer memory loss and cognitive function decline, and on average die nine years after diagnosis.6,7
Amyloid cascade hypothesis of AD is a prominent idea in the research field of AD pathogenesis.8-12 It arises from the observation that patients affected by AD are characterized by the accumulation of senile plaques containing a product of amyloid precursor protein (APP) metabolism called the Amyloid beta (Aβ) peptide.13-15 Histopathological and genetic evidence form the basis of the amyloid cascade hypothesis, which states that deposition of Aβ is the initiating event that triggers neuronal dysfunction and death.16-18 Aβ peptides constitute a major part of the neuritic plaques causing neurotoxicity, which are cleaved from APP by β- and γ-secretases.19 Hitherto, much of the research focus in the AD field has been on Aβ peptide generation and its mechanisms of action.20-22 Despite the efforts to characterize the molecular mechanisms underlying Aβ's toxicity, it remains unclear what triggers the accumulation of the peptide and whether such action is the primary cause of AD pathogenesis and cognitive dysfunction.18,23-26 However, the amyloid cascade hypothesis still dominates the search for AD disease treatments up to now. For example, researchers have tried to reduce Aβ production by developing molecules that inhibit γ-secrease activity, block Aβ aggregation and promote Aβ clearance.27-32 Unfortunately, none of the amyloid-targeting molecules that reached clinical trials have succeeded, despite of decades of basic and clinical research.
Calcium is one of the most important second messengers33 in the nervous system because it plays an essential role in wide range of cellular function including learning and memory, synaptic activity and neurotransmitter action, excitotoxicity, and cell death.34-37 Neurons maintain intracellular Ca2+ content mainly through Ca2+ signal transduction pathways. Neuronal Ca2+ influx is regulated by different Ca2+ channels, including voltage-dependent Ca2+ channels (VDCC), α-mino-3-hydroxy-5-methyl-4-isoxazolepropanoic acid (AMPA) receptors, nicotinic receptors, N-methyl-D-aspartate (NMDA) receptors and store-operated Ca2+ channels (SOC).38-40 Ca2+ can also be released from the primary intracellular stores of endoplasmic reticulum (ER).41-45 Neurons are highly sensitive to any changes in intracellular Ca2+ concentrations: insufficient intracellular Ca2+ content leads to abnormal functioning of neurons, whereas excessive Ca2+ levels cause cell death.40,46,47 Therefore, even small fluctuations of Ca2+ content can significantly change the physiological functions of cells.39,48-51
In 1987, Dr. Zaven Khachaturian suggested the Ca2+ hypothesis of AD that disruption in intracellular Ca2+ homeostasis leads to the final common pathway for AD and age-associated brain changes.48,52-55 This hypothesis is supported by the presence of AD-like symptoms in mouse models harboring presenilin's mutations and synaptic dysfunction due to ER Ca2+ concentration changes in the absence of Aβ pathology.56-58 The basis of the Ca2+ hypothesis in AD is that the disruption of intracellular Ca2+ signaling homoeostasis contributed to both the progressive decline in memory and the increase in neuronal cell apoptosis.59,60 Any neuropathology of AD, particularly sporadic AD, has to account for the slow progression of the disease and for the fact that the changes in synaptic physiology and onset of memory loss often precedes any evidence for the massive cell loss that characterizes the later stages of AD. Also, neuronal cell death were observed when Ca2+ levels exceeded the normal physiologic range, and the Ca2+-mediated signaling system is altered in the aging nervous system resulting in altered neuronal functioning and/or cell death.61,62 In addition, Ca2+ dysregulation is further implicated in AD since each of the genes currently known to influence AD susceptibility so far (APP, PSEN1, PSEN2, and APOE) affects intracellular Ca2+ levels and/or calcium signaling.63-65 Early changes in intraneuronal Ca2+ regulation may be also common observations in AD patients. Since intracellular Ca2+ homeostasis plays such an important role in both neuronal and synapse function, its disruption produce symptoms of brain aging, neuronal degeneration and death, synapse and cognitive dysfunction.66-69 Up to date, the proposed mechanisms responsible for Ca2+ dysregulation in AD primarily include overactivation of ryanodine receptors and InsP3R, which may contribute to early AD neuropathology and susceptibility.35,70-74
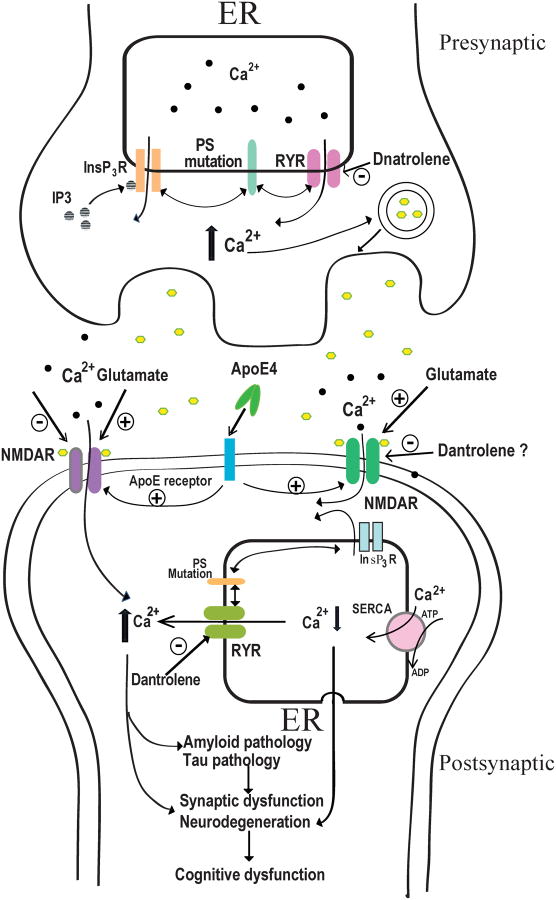
Two well studied Ca2+ channels release Ca2+ from the ER into cytosolic space: Inositol-1,4,5-trisphosphate receptors (InsP3Rs) and Ryanodine receptors (RYRs).75-78 As shown in figure 1, mutations in presenilin-1 (PS1) and presenilin-2 (PS2) associated with familial AD (FAD), significantly enhance the expression and activation of RYRs, as well as the activation of InsP3Rs, resulting in excessive Ca2+ release from the ER and abnormal cytosolic Ca2+ concentration [Ca2+]c elevation.50,79-81 On the other hand, the ApoE4 gene, a widely accepted genetic risk factor for sporadic AD, has been shown to disrupt intracellular Ca2+ homeostasis by abnormally increasing NMDA receptor activation, which may result in excessive Ca2+ influx, elevation of [Ca2+]c, and Ca2+-induced Ca2+ release (CICR) from the ER via RYRs and/or IP3Rs.
Figure 1. Calcium dysregulation in AD and the proposed effects of dantrolene.
Mutated Presenilins (PS) in familial AD (FAD) increase numbers and activation of RYRs and activation of InsP3Rs, resulting in excessive Ca2+ release from the ER. Overactivation of RYRs and subsequent disruption of intracellular Ca2+ homeostasis in AD can then cause the following pathological changes: 1). Increase presynaptic glutamate release and postsynaptic NMDA receptor activation and glutamate excitotoxicity; 2). Increase postsynaptic cytosolic [Ca2+]c and neurodegeneration; 3). Form a vicious cycle between amyloid pathology and Ca2+ dysregulation; 4). Promote Tau pathology and damage of microtubules; 5). Synapse dysfunction. These above pathological changes may result in synaptic dysfunction, neurodegeneration and cognitive dysfunction. Dantrolene is expected to restore intracellular Ca2+ homeostasis, resulting in inhibition of neuropathology and cognitive dysfunction in AD.
RYRs are expressed in soma, proximal dendrites as well as in distal processes and spines. The three isoforms of RYRs are RYR1, RYR2 and RYR3, all of which are expressed in the central nervous system.82-85 RYR1 is expressed in cerebellar Purkinje cells.84,86,87 RYR2 is found in the olfactory nerve layer, dentate gyrus, cerebral cortex, cerebellar granule cells, the facial nucleus and the motor trigeminal nucleus.39,88 Lastly, RYR3 is highly expressed in the hippocampal CA1 pyramidal cell layer, dorsal thalamus and caudate putamen.49,85 Overactivation of RYRs in the brain and excessive Ca2+ release from the ER may result in increased excitatory glutamate release from presynaptic spaces, neuronal death, neurodegeneration, and Aβ pathologies, which is considered as early pathogenic factors in AD (Figure 1).34,89-91 Thus, reducing Ca2+ over-release through the inhibition of RYRs is a reasonable way to mitigate the AD pathology and ameliorate the memory and cognitive problems.74,92,93
Dantrolene is an FDA approved drug for clinical treatment of malignant hyperthermia, and muscle spasms, which is an antagonist of RYRs.94-99 The most common side effects of dantrolene are dizziness, drowsiness, light headedness, headaches, anorexia, diarrhea, nausea, and vomiting. Chronic oral use can be associated with liver dysfunction. Rarely observed side effects are fatigue, weakness and rash.39,77, Although the common side effects of dantrolene originate in the central nervous system, several studies addressed the beneficial effects of dantrolene in AD pathology via acting on RYRs in recent years.39,77,100-103 In April of 2012, Peng et al.104 published a pioneer paper investigating the therapeutic effect of dantrolene on ethology and pathology in the triple transgenic Alzheimer mouse model (3xTg-AD). Wild type or 3xTg-AD mice from 2 to 13 months of age were treated with dantrolene continuously. Compared to control, 3xTg-AD mice treated with dantrolene exhibited significant improvement in memory retention and working memory. In fact, dantrolene treated 3xTg-AD mice performed the same memory and learning ability as the wild type control mice. In addition, there was no significant difference in motor function among all groups. Immunohistochemical analysis of phosphorylated GSK-3β and phosphorylated Tau in the cortex, and synaptic marker expression did not show any statistical significance among all treated groups. Notably, amyloid plaques in the hippocampus in dantrolene treated 3xTg-AD mice significantly reduced compared to its corresponding control group. Taken together, the results showed that early and chronic dantrolene treatment starting before the initiation of amyloid pathology significantly decreased amyloid plaque load in the hippocampus and memory deficits in 3xTg-AD mice.
In August of 2012, Oules et al.105 showed that APP contributes to ER Ca2+ homeostasis and in turn ER Ca2+ could influence Aβ production. The authors also found that over expression of wild-type human APP (APP695) in human SH-SY5Y neuroblastoma cells or the Swedish double mutation APP (APPswe) in APPswe-expressing mice (Tg2576) enhances RYR expression and increases ER Ca2+ release via RYRs. The authors' use of dantrolene to block RYRs decreased the release of RYR-mediated Ca2+ and contributed to the reduction of both intracellular and extracellular Aβ load in mouse Tg2576 primary cultured neurons and in human SH-SY5Y neuroblastoma cells. Also, Aβ in the hippocampus and cortex decreased in dantrolene-treated AD mice. The authors speculated that dantrolene reduces APP phosphorylation on Thr-668 residue through the regulation of RYR-mediated Ca2+ release by means of the modulation of GSk3β and Cdk5 kinase activities. Their study also notes that dantrolene decreases β- and γ-secretases activities. As a result, dantrolene prevents learning and memory decline by reducing C99 and Aβ production.
In December of 2012, Chakroborty et al.106 reported sub-chronically short-term (4 weeks) treatment of AD models with dantrolene. Using patch clamp recordings and 2-photon Ca2+ imaging of hippocampal slices, the authors found that ER Ca2+ signaling is fully normalized in dendritic compartments in dantrolene treated early and later-stage AD mice. In addition, the increased RYR2 levels and enhanced IP3R-mediated Ca2+ release in AD mice were restored to normal levels with dantrolene treatment. Thus, sub-chronic dantrolene treatment stabilizes RYRs' function and expression and inhibits abnormal CICR initiated through IP3R-mediated Ca2+ release. These aforementioned studies suggest that inhibition of RYRs with dantrolene exerts beneficial effects on AD pathology.
However, not all laboratory findings on dantrolene treatment in AD are consistent. When Zhang et al.107 investigated the role of presenilins in neuronal ER Ca2+ release in 2010, they found that the long-term oral dantrolene treatment of 2-8 month old AD (APP-PS1 mutant) mice increased amyloid load and neuronal atrophy in hippocampal and cortical regions along with loss of synaptic markers, raising some doubts about the therapeutic potential of dantrolene. It is not clear what the reasons are for the discrepancies among all these studies, but differences in mouse models, treatment duration, and route of administration may be contributing factors. Further studies in different AD animal models are urgently needed to investigate and confirm if dantrolene or other raynodine inhibitors will ameliorate or aggravate the neuropathology in AD.
In summary, excessive Ca2+ release from the ER modulates the amyloid genic processing pathway and other AD pathology, thereby promoting memory loss.43,57,74,108,109 Dantrolene, as an antagonist of RYRs on ER, mitigates AD pathology and may serve as a probe compound for future studies to treat Alzheimer's disease.
Acknowledgments
Supported by NIH (GM-073224, GM084979, GM084979-02 S1 to H.W.), and a March of Dimes Birth Defects Foundation Research Grant (#12-FY08-167 to H.W.). We appreciate the editing from Alex King, University of Pennsylvania.
Abbreviations
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- Aβ
Amyloid beta
- Ca2+
Calcium
- VDCC
voltage-dependent Ca2+ channels
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropanoic acid
- NMDA
N-methyl-D-aspartate
- SOC
store-operated Ca2+ channels
- ER
endoplasmic reticulum
- InsP3Rs
Inositol-1,4,5-trisphosphate receptors
- RYRs
Ryanodine receptors
- CICR
cytosolic Ca2+ concentration [Ca2+]c, Ca2+-induced Ca2+ release
References
- 1.Ridge PG, Ebbert MT, Kauwe JS. Genetics of Alzheimer's disease. BioMed research international. 2013;2013:254954. doi: 10.1155/2013/254954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang YJ. Alzheimer disease: Lessons from immunotherapy for Alzheimer disease. Nature reviews Neurology. 2014;10(4):188–189. doi: 10.1038/nrneurol.2014.44. [DOI] [PubMed] [Google Scholar]
- 3.Reitz C, Mayeux R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochemical pharmacology. 2014;88(4):640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantarci K. Molecular Imaging of Alzheimer Disease Pathology. AJNR American journal of neuroradiology. 2014 doi: 10.3174/ajnr.A3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bible E. Alzheimer disease: High serum levels of the pesticide metabolite DDE--a potential environmental risk factor for Alzheimer disease. Nature reviews Neurology. 2014;10(3):125. doi: 10.1038/nrneurol.2014.25. [DOI] [PubMed] [Google Scholar]
- 6.Yang B, Liang G, Khojasteh S, et al. Comparison of neurodegeneration and cognitive impairment in neonatal mice exposed to propofol or isoflurane. PloS one. 2014;9(6):e99171. doi: 10.1371/journal.pone.0099171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph JD, Peng Y, Mak DOD, et al. General Anesthetic Isoflurane Modulates Inositol 1, 4, 5-Trisphosphate Receptor Calcium Channel Opening. Anesthesiology. 2014 doi: 10.1097/ALN.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lelos MJ, Good MA. beta-Amyloid pathology alters neural network activation during retrieval of contextual fear memories in a mouse model of Alzheimer's disease. The European journal of neuroscience. 2014 doi: 10.1111/ejn.12527. [DOI] [PubMed] [Google Scholar]
- 9.Small DH, Hu Y, Bolos M, Dawkins E, Foa L, Young KM. beta-Amyloid precursor protein: function in stem cell development and Alzheimer's disease brain. Neuro-degenerative diseases. 2014;13(2-3):96–98. doi: 10.1159/000353686. [DOI] [PubMed] [Google Scholar]
- 10.Sultzer DL, Leskin LP, Melrose RJ, et al. Neurobiology of Delusions, Memory, and Insight in Alzheimer Disease. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2013 doi: 10.1016/j.jagp.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skaper SD. A Toll road to Alzheimer disease? CNS & neurological disorders drug targets. 2013;12(4):445–446. doi: 10.2174/18715273113129990067. [DOI] [PubMed] [Google Scholar]
- 12.Shah R. The role of nutrition and diet in Alzheimer disease: a systematic review. Journal of the American Medical Directors Association. 2013;14(6):398–402. doi: 10.1016/j.jamda.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Lane RM, Potkin SG, Enz A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2006;9(1):101–124. doi: 10.1017/S1461145705005833. [DOI] [PubMed] [Google Scholar]
- 14.Catalano SM, Dodson EC, Henze DA, Joyce JG, Krafft GA, Kinney GG. The role of amyloid-beta derived diffusible ligands (ADDLs) in Alzheimer's disease. Current topics in medicinal chemistry. 2006;6(6):597–608. doi: 10.2174/156802606776743066. [DOI] [PubMed] [Google Scholar]
- 15.Pimplikar SW. Reassessing the amyloid cascade hypothesis of Alzheimer's disease. The international journal of biochemistry & cell biology. 2009;41(6):1261–1268. doi: 10.1016/j.biocel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taher N, McKenzie C, Garrett R, Baker M, Fox N, Isaacs GD. Amyloid-beta alters the DNA methylation status of cell-fate genes in an Alzheimer's disease model. Journal of Alzheimer's disease : JAD. 2014;38(4):831–844. doi: 10.3233/JAD-131061. [DOI] [PubMed] [Google Scholar]
- 17.Portelius E, Zetterberg H, Dean RA, et al. Amyloid-beta(1-15/16) as a marker for gamma-secretase inhibition in Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2012;31(2):335–341. doi: 10.3233/JAD-2012-120508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayes J, Tinker-Mill C, Kolosov O, Zhang H, Tabner BJ, Allsop D. beta-Amyloid Fibrils in Alzheimer's Disease are not Inert When Bound to Copper Ions but can Degrade Hydrogen Peroxide and Generate Reactive Oxygen Species. The Journal of biological chemistry. 2014 doi: 10.1074/jbc.M113.525212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 20.Morawe T, Hiebel C, Kern A, Behl C. Protein homeostasis, aging and Alzheimer's disease. Molecular neurobiology. 2012;46(1):41–54. doi: 10.1007/s12035-012-8246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folk DS, Franz KJ. A prochelator activated by beta-secretase inhibits Abeta aggregation and suppresses copper-induced reactive oxygen species formation. Journal of the American Chemical Society. 2010;132(14):4994–4995. doi: 10.1021/ja100943r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boche D, Denham N, Holmes C, Nicoll JA. Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer's disease pathogenesis. Acta neuropathologica. 2010;120(3):369–384. doi: 10.1007/s00401-010-0719-5. [DOI] [PubMed] [Google Scholar]
- 23.Giacobini E, Gold G. Alzheimer disease therapy--moving from amyloid-beta to tau. Nature reviews Neurology. 2013;9(12):677–686. doi: 10.1038/nrneurol.2013.223. [DOI] [PubMed] [Google Scholar]
- 24.Drachman DA. The amyloid hypothesis, time to move on: Amyloid is the downstream result, not cause, of Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2014 doi: 10.1016/j.jalz.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Iqbal K, Liu F, Gong CX. Alzheimer disease therapeutics: Focus on the disease and not just plaques and tangles. Biochemical pharmacology. 2014;88(4):631–639. doi: 10.1016/j.bcp.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panza F, Solfrizzi V, Imbimbo BP, Tortelli R, Santamato A, Logroscino G. Amyloid-based immunotherapy for Alzheimer's disease in the time of prevention trials: the way forward. Expert review of clinical immunology. 2014;10(3):405–419. doi: 10.1586/1744666X.2014.883921. [DOI] [PubMed] [Google Scholar]
- 27.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nature reviews Drug discovery. 2011;10(9):698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 28.Rosenblum WI. Why Alzheimer trials fail: removing soluble oligomeric beta amyloid is essential, inconsistent, and difficult. Neurobiology of aging. 2014;35(5):969–974. doi: 10.1016/j.neurobiolaging.2013.10.085. [DOI] [PubMed] [Google Scholar]
- 29.Panza F, Logroscino G, Imbimbo BP, Solfrizzi V. Is there still any hope for amyloid-based immunotherapy for Alzheimer's disease? Current opinion in psychiatry. 2014;27(2):128–137. doi: 10.1097/YCO.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 30.Tiiman A, Palumaa P, Tougu V. The missing link in the amyloid cascade of Alzheimer's disease - metal ions. Neurochemistry international. 2013;62(4):367–378. doi: 10.1016/j.neuint.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Swerdlow RH, Burns JM, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis: Progress and perspectives. Biochimica et biophysica acta. 2013 doi: 10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta neuropathologica. 2013;126(4):479–497. doi: 10.1007/s00401-013-1177-7. [DOI] [PubMed] [Google Scholar]
- 33.Schousboe A, Belhage B, Frandsen A. Role of Ca+2 and other second messengers in excitatory amino acid receptor mediated neurodegeneration: clinical perspectives. Clinical neuroscience. 1997;4(4):194–198. [PubMed] [Google Scholar]
- 34.Berridge MJ. Calcium hypothesis of Alzheimer's disease. Pflugers Archiv : European journal of physiology. 2010;459(3):441–449. doi: 10.1007/s00424-009-0736-1. [DOI] [PubMed] [Google Scholar]
- 35.Wallace J. Calcium dysregulation, and lithium treatment to forestall Alzheimer's disease - a merging of hypotheses. Cell calcium. 2014 doi: 10.1016/j.ceca.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Saravanaraman P, Chinnadurai RK, Boopathy R. Why calcium channel blockers could be an elite choice in the treatment of Alzheimer's disease: a comprehensive review of evidences. Reviews in the neurosciences. 2014;25(2):231–246. doi: 10.1515/revneuro-2013-0056. [DOI] [PubMed] [Google Scholar]
- 37.Berridge MJ. Calcium regulation of neural rhythms, memory and Alzheimer's disease. The Journal of physiology. 2014;592(Pt 2):281–293. doi: 10.1113/jphysiol.2013.257527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeiger W, Vetrivel KS, Buggia-Prevot V, et al. Ca2+ influx through store-operated Ca2+ channels reduces Alzheimer disease beta-amyloid peptide secretion. The Journal of biological chemistry. 2013;288(37):26955–26966. doi: 10.1074/jbc.M113.473355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popugaeva E, Bezprozvanny I. Role of endoplasmic reticulum Ca2+ signaling in the pathogenesis of Alzheimer disease. Frontiers in molecular neuroscience. 2013;6:29. doi: 10.3389/fnmol.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riazantseva MA, Mozhaeva GN, Kaznacheeva EV. Calcium hypothesis of Alzheimer disease. Uspekhi fiziologicheskikh nauk. 2012;43(4):59–72. [PubMed] [Google Scholar]
- 41.Schon EA, Area-Gomez E. Mitochondria-associated ER membranes in Alzheimer disease. Molecular and cellular neurosciences. 2013;55:26–36. doi: 10.1016/j.mcn.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiology of aging. 2011;32(3):398–406. doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anekonda TS, Quinn JF. Calcium channel blocking as a therapeutic strategy for Alzheimer's disease: the case for isradipine. Biochimica et biophysica acta. 2011;1812(12):1584–1590. doi: 10.1016/j.bbadis.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diaz JC, Simakova O, Jacobson KA, Arispe N, Pollard HB. Small molecule blockers of the Alzheimer Abeta calcium channel potently protect neurons from Abeta cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3348–3353. doi: 10.1073/pnas.0813355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasar S, Corrada M, Brookmeyer R, Kawas C. Calcium channel blockers and risk of AD: the Baltimore Longitudinal Study of Aging. Neurobiology of aging. 2005;26(2):157–163. doi: 10.1016/j.neurobiolaging.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Supnet C, Bezprozvanny I. The dysregulation of intracellular calcium in Alzheimer disease. Cell calcium. 2010;47(2):183–189. doi: 10.1016/j.ceca.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conley YP, Mukherjee A, Kammerer C, et al. Evidence supporting a role for the calcium-sensing receptor in Alzheimer disease. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2009;150B(5):703–709. doi: 10.1002/ajmg.b.30896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Popugaeva E, Bezprozvanny I. Can the calcium hypothesis explain synaptic loss in Alzheimer's disease? Neuro-degenerative diseases. 2014;13(2-3):139–141. doi: 10.1159/000354778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Supnet C, Sun S, et al. The role of ryanodine receptor type 3 in a mouse model of Alzheimer disease. Channels. 2014;8(3) doi: 10.4161/chan.27471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Supnet C, Bezprozvanny I. Presenilins function in ER calcium leak and Alzheimer's disease pathogenesis. Cell calcium. 2011;50(3):303–309. doi: 10.1016/j.ceca.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X, Wu J, Lvovskaya S, Herndon E, Supnet C, Bezprozvanny I. Dantrolene is neuroprotective in Huntington's disease transgenic mouse model. Molecular neurodegeneration. 2011;6:81. doi: 10.1186/1750-1326-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khachaturian ZS. Hypothesis on the regulation of cytosol calcium concentration and the aging brain. Neurobiology of aging. 1987;8(4):345–346. doi: 10.1016/0197-4580(87)90073-x. [DOI] [PubMed] [Google Scholar]
- 53.Supnet C, Bezprozvanny I. Presenilins as endoplasmic reticulum calcium leak channels and Alzheimer's disease pathogenesis. Science China Life sciences. 2011;54(8):744–751. doi: 10.1007/s11427-011-4201-y. [DOI] [PubMed] [Google Scholar]
- 54.Bezprozvanny IB. Calcium signaling and neurodegeneration. Acta naturae. 2010;2(1):72–82. [PMC free article] [PubMed] [Google Scholar]
- 55.Nimmrich V, Ebert U. Is Alzheimer's disease a result of presynaptic failure? Synaptic dysfunctions induced by oligomeric beta-amyloid. Reviews in the neurosciences. 2009;20(1):1–12. doi: 10.1515/revneuro.2009.20.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Rosales-Corral SA, Acuna-Castroviejo D, Coto-Montes A, et al. Alzheimer's disease: pathological mechanisms and the beneficial role of melatonin. Journal of pineal research. 2012;52(2):167–202. doi: 10.1111/j.1600-079X.2011.00937.x. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez-Morales JC, Arranz-Tagarro JA, Calvo-Gallardo E, Maroto M, Padin JF, Garcia AG. Stabilizers of neuronal and mitochondrial calcium cycling as a strategy for developing a medicine for Alzheimer's disease. ACS chemical neuroscience. 2012;3(11):873–883. doi: 10.1021/cn3001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berridge MJ. Calcium signalling and Alzheimer's disease. Neurochemical research. 2011;36(7):1149–1156. doi: 10.1007/s11064-010-0371-4. [DOI] [PubMed] [Google Scholar]
- 59.Verkhratsky A, Toescu EC. Calcium and neuronal ageing. Trends in neurosciences. 1998;21(1):2–7. doi: 10.1016/s0166-2236(97)01156-9. [DOI] [PubMed] [Google Scholar]
- 60.Mattson MP, Guo Q, Furukawa K, Pedersen WA. Presenilins, the endoplasmic reticulum, and neuronal apoptosis in Alzheimer's disease. Journal of neurochemistry. 1998;70(1):1–14. doi: 10.1046/j.1471-4159.1998.70010001.x. [DOI] [PubMed] [Google Scholar]
- 61.Khachaturian ZS. Calcium and the aging brain: upsetting a delicate balance? Geriatrics. 1991;46(11):78–79. 83. [PubMed] [Google Scholar]
- 62.Mattson MP. Apoptosis in neurodegenerative disorders. Nature reviews Molecular cell biology. 2000;1(2):120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 63.Misra UK, Adlakha CL, Gawdi G, McMillian MK, Pizzo SV, Laskowitz DT. Apolipoprotein E and mimetic peptide initiate a calcium-dependent signaling response in macrophages. Journal of leukocyte biology. 2001;70(4):677–683. [PubMed] [Google Scholar]
- 64.LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nature reviews Neuroscience. 2002;3(11):862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 65.Mattson MP. Oxidative stress, perturbed calcium homeostasis, and immune dysfunction in Alzheimer's disease. Journal of neurovirology. 2002;8(6):539–550. doi: 10.1080/13550280290100978. [DOI] [PubMed] [Google Scholar]
- 66.Bordji K, Becerril-Ortega J, Buisson A. Synapses, NMDA receptor activity and neuronal Abeta production in Alzheimer's disease. Reviews in the neurosciences. 2011;22(3):285–294. doi: 10.1515/RNS.2011.029. [DOI] [PubMed] [Google Scholar]
- 67.Bardo S, Cavazzini MG, Emptage N. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends in pharmacological sciences. 2006;27(2):78–84. doi: 10.1016/j.tips.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 68.Goussakov I, Miller MB, Stutzmann GE. NMDA-mediated Ca(2+) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer's disease mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(36):12128–12137. doi: 10.1523/JNEUROSCI.2474-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Demuro A, Parker I, Stutzmann GE. Calcium signaling and amyloid toxicity in Alzheimer disease. The Journal of biological chemistry. 2010;285(17):12463–12468. doi: 10.1074/jbc.R109.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jensen LE, Bultynck G, Luyten T, Amijee H, Bootman MD, Roderick HL. Alzheimer's disease-associated peptide Abeta42 mobilizes ER Ca(2+) via InsP3R-dependent and -independent mechanisms. Frontiers in molecular neuroscience. 2013;6:36. doi: 10.3389/fnmol.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer's disease: a novel neuronal trigger for inflammation and Alzheimer's pathology. Journal of neuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seo J, Jo SA, Hwang S, et al. Trichostatin A epigenetically increases calpastatin expression and inhibits calpain activity and calcium-induced SH-SY5Y neuronal cell toxicity. The FEBS journal. 2013;280(24):6691–6701. doi: 10.1111/febs.12572. [DOI] [PubMed] [Google Scholar]
- 73.Daschil N, Obermair GJ, Flucher BE, et al. CaV1.2 calcium channel expression in reactive astrocytes is associated with the formation of amyloid-beta plaques in an Alzheimer's disease mouse model. Journal of Alzheimer's disease : JAD. 2013;37(2):439–451. doi: 10.3233/JAD-130560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chakroborty S, Stutzmann GE. Calcium channelopathies and Alzheimer's disease: Insight into therapeutic success and failures. European journal of pharmacology. 2013 doi: 10.1016/j.ejphar.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 75.Chakroborty S, Kim J, Schneider C, Jacobson C, Molgo J, Stutzmann GE. Early presynaptic and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer's disease mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(24):8341–8353. doi: 10.1523/JNEUROSCI.0936-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(19):5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stutzmann GE. Calcium dysregulation, IP3 signaling, and Alzheimer's disease. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2005;11(2):110–115. doi: 10.1177/1073858404270899. [DOI] [PubMed] [Google Scholar]
- 78.Ahn GJ, Lee BC, Hwang WS. Effect of IP3 and ryanodine treatments on the development of bovine parthenogenetic and reconstructed embryos. Journal of veterinary science. 2001;2(2):131–137. [PubMed] [Google Scholar]
- 79.Rodriguez R, Lopera F, Alvarez A, et al. Spectral Analysis of EEG in Familial Alzheimer's Disease with E280A Presenilin-1 Mutation Gene. International journal of Alzheimer's disease. 2014;2014:180741. doi: 10.1155/2014/180741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Canevelli M, Piscopo P, Talarico G, et al. Familial Alzheimer's disease sustained by presenilin 2 mutations: Systematic review of literature and genotype-phenotype correlation. Neuroscience and biobehavioral reviews. 2014;42C:170–179. doi: 10.1016/j.neubiorev.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 81.Larner AJ. Presenilin-1 mutations in Alzheimer's disease: an update on genotype-phenotype relationships. Journal of Alzheimer's disease : JAD. 2013;37(4):653–659. doi: 10.3233/JAD-130746. [DOI] [PubMed] [Google Scholar]
- 82.Lanner JT. Ryanodine receptor physiology and its role in disease. Advances in experimental medicine and biology. 2012;740:217–234. doi: 10.1007/978-94-007-2888-2_9. [DOI] [PubMed] [Google Scholar]
- 83.Mattson MP. ER calcium and Alzheimer's disease: in a state of flux. Science signaling. 2010;3(114):pe10. doi: 10.1126/scisignal.3114pe10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bruno AM, Huang JY, Bennett DA, Marr RA, Hastings ML, Stutzmann GE. Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer's disease. Neurobiology of aging. 2012;33(5):1001 e1001–1006. doi: 10.1016/j.neurobiolaging.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stutzmann GE, Smith I, Caccamo A, Oddo S, Parker I, Laferla F. Enhanced ryanodine-mediated calcium release in mutant PS1-expressing Alzheimer's mouse models. Annals of the New York Academy of Sciences. 2007;1097:265–277. doi: 10.1196/annals.1379.025. [DOI] [PubMed] [Google Scholar]
- 86.Chakroborty S, Goussakov I, Miller MB, Stutzmann GE. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(30):9458–9470. doi: 10.1523/JNEUROSCI.2047-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hayrapetyan V, Rybalchenko V, Rybalchenko N, Koulen P. The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein-protein interaction. Cell calcium. 2008;44(5):507–518. doi: 10.1016/j.ceca.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 88.Hwang HS, Nitu FR, Yang Y, et al. Divergent regulation of ryanodine receptor 2 calcium release channels by arrhythmogenic human calmodulin missense mutants. Circulation research. 2014;114(7):1114–1124. doi: 10.1161/CIRCRESAHA.114.303391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koran ME, Hohman TJ, Thornton-Wells TA. Genetic interactions found between calcium channel genes modulate amyloid load measured by positron emission tomography. Human genetics. 2014;133(1):85–93. doi: 10.1007/s00439-013-1354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Honarnejad K, Herms J. Presenilins: role in calcium homeostasis. The international journal of biochemistry & cell biology. 2012;44(11):1983–1986. doi: 10.1016/j.biocel.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 91.Stutzmann GE, Mattson MP. Endoplasmic reticulum Ca(2+) handling in excitable cells in health and disease. Pharmacological reviews. 2011;63(3):700–727. doi: 10.1124/pr.110.003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fan G, Zhou F, Feng C, et al. Lead-induced ER calcium release and inhibitory effects of methionine choline in cultured rat hippocampal neurons. Toxicology in vitro : an international journal published in association with BIBRA. 2013;27(1):387–395. doi: 10.1016/j.tiv.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 93.Goussakov I, Chakroborty S, Stutzmann GE. Generation of dendritic Ca2+ oscillations as a consequence of altered ryanodine receptor function in AD neurons. Channels. 2011;5(1):9–13. doi: 10.4161/chan.5.1.14124. [DOI] [PubMed] [Google Scholar]
- 94.van Karnebeek C, Horvath G, Murphy T, et al. Deep Brain Stimulation and Dantrolene for Secondary Dystonia in X-Linked Adrenoleukodystrophy. JIMD reports. 2014 doi: 10.1007/8904_2014_305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chou CC, Wen MS, Lee HL, et al. Dantrolene suppresses ventricular ectopy and arrhythmogenicity with acute myocardial infarction in a langendorff-perfused pacing-induced heart failure rabbit model. Journal of cardiovascular electrophysiology. 2014;25(4):431–439. doi: 10.1111/jce.12320. [DOI] [PubMed] [Google Scholar]
- 96.Bannister RA. Dantrolene-induced inhibition of skeletal L-type Ca2+ current requires RyR1 expression. BioMed research international. 2013;2013:390493. doi: 10.1155/2013/390493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Staats KA, Van Rillaer M, Scheveneels W, et al. Dantrolene is neuroprotective in vitro, but does not affect survival in SOD1(G(9)(3)A) mice. Neuroscience. 2012;220:26–31. doi: 10.1016/j.neuroscience.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 98.Ginz HF, Levano S, Girard T, Urwyler A, Hamel C. Dantrolene for severe rhabdomyolysis in Staphylococcus aureus toxic shock syndrome. European journal of anaesthesiology. 2012;29(3):161–162. doi: 10.1097/EJA.0b013e32834c7c9d. [DOI] [PubMed] [Google Scholar]
- 99.Makarewicz D, Zieminska E, Lazarewicz JW. Dantrolene inhibits NMDA-induced 45Ca uptake in cultured cerebellar granule neurons. Neurochemistry international. 2003;43(4-5):273–278. doi: 10.1016/s0197-0186(03)00012-3. [DOI] [PubMed] [Google Scholar]
- 100.Costa RO, Ferreiro E, Martins I, et al. Amyloid beta-induced ER stress is enhanced under mitochondrial dysfunction conditions. Neurobiology of aging. 2012;33(4):824 e825–816. doi: 10.1016/j.neurobiolaging.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 101.Nakajima M, Miura M, Aosaki T, Shirasawa T. Deficiency of presenilin-1 increases calcium-dependent vulnerability of neurons to oxidative stress in vitro. Journal of neurochemistry. 2001;78(4):807–814. doi: 10.1046/j.1471-4159.2001.00478.x. [DOI] [PubMed] [Google Scholar]
- 102.Mattson MP, Zhu H, Yu J, Kindy MS. Presenilin-1 mutation increases neuronal vulnerability to focal ischemia in vivo and to hypoxia and glucose deprivation in cell culture: involvement of perturbed calcium homeostasis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(4):1358–1364. doi: 10.1523/JNEUROSCI.20-04-01358.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu J, Tang TS, Tu H, et al. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(29):9148–9162. doi: 10.1523/JNEUROSCI.0660-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peng J, Liang G, Inan S, et al. Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neuroscience letters. 2012;516(2):274–279. doi: 10.1016/j.neulet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oules B, Del Prete D, Greco B, et al. Ryanodine receptor blockade reduces amyloid-beta load and memory impairments in Tg2576 mouse model of Alzheimer disease. J Neurosci. 32(34):11820–11834. doi: 10.1523/JNEUROSCI.0875-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chakroborty S, Briggs C, Miller MB, et al. Stabilizing ER Ca2+ channel function as an early preventative strategy for Alzheimer's disease. PloS one. 2012;7(12):e52056. doi: 10.1371/journal.pone.0052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang H, Sun S, Herreman A, De Strooper B, Bezprozvanny I. Role of presenilins in neuronal calcium homeostasis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(25):8566–8580. doi: 10.1523/JNEUROSCI.1554-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Armato U, Chiarini A, Chakravarthy B, et al. Calcium-sensing receptor antagonist (calcilytic) NPS 2143 specifically blocks the increased secretion of endogenous Abeta42 prompted by exogenous fibrillary or soluble Abeta25-35 in human cortical astrocytes and neurons-therapeutic relevance to Alzheimer's disease. Biochimica et biophysica acta. 2013;1832(10):1634–1652. doi: 10.1016/j.bbadis.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 109.Yamaguchi M. Role of regucalcin in brain calcium signaling: involvement in aging. Integrative biology : quantitative biosciences from nano to macro. 2012;4(8):825–837. doi: 10.1039/c2ib20042b. [DOI] [PubMed] [Google Scholar]