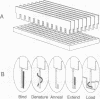
Abstract
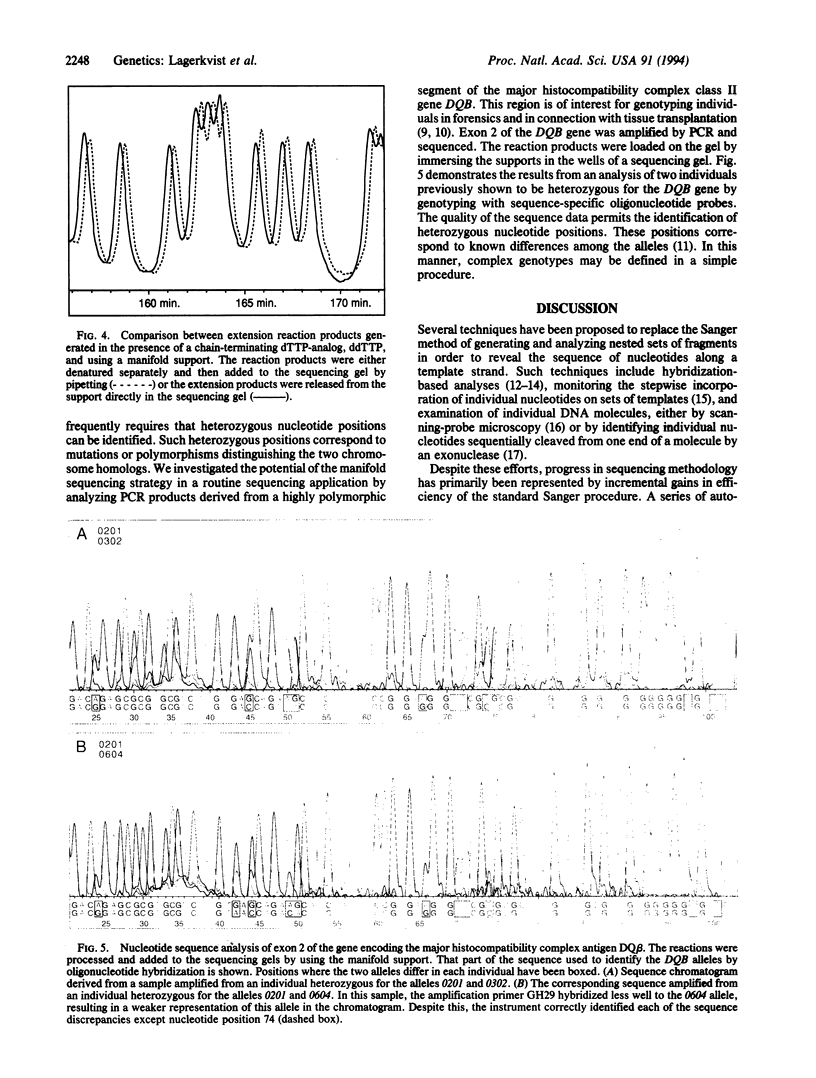
Automated instruments for DNA sequencing greatly simplify data collection in the Sanger sequencing procedure. By contrast, the so-called front-end problems of preparing sequencing templates, performing sequencing reactions, and loading these on the instruments remain major obstacles to extensive sequencing projects. We describe here the use of a manifold support to prepare and perform sequencing reactions on large sets of templates in parallel, as well as to load the reaction products on a sequencing instrument. In this manner, all reaction steps are performed without pipetting the samples. The strategy is applied to sequencing PCR-amplified clones of the human mitochondrial D-loop and for detection of heterozygous positions in the human major histocompatibility complex class II gene HLA-DQB, amplified from genomic DNA samples. This technique will promote sequencing in a clinical context and could form the basis of more efficient genomic sequencing strategies.
Full text
PDF
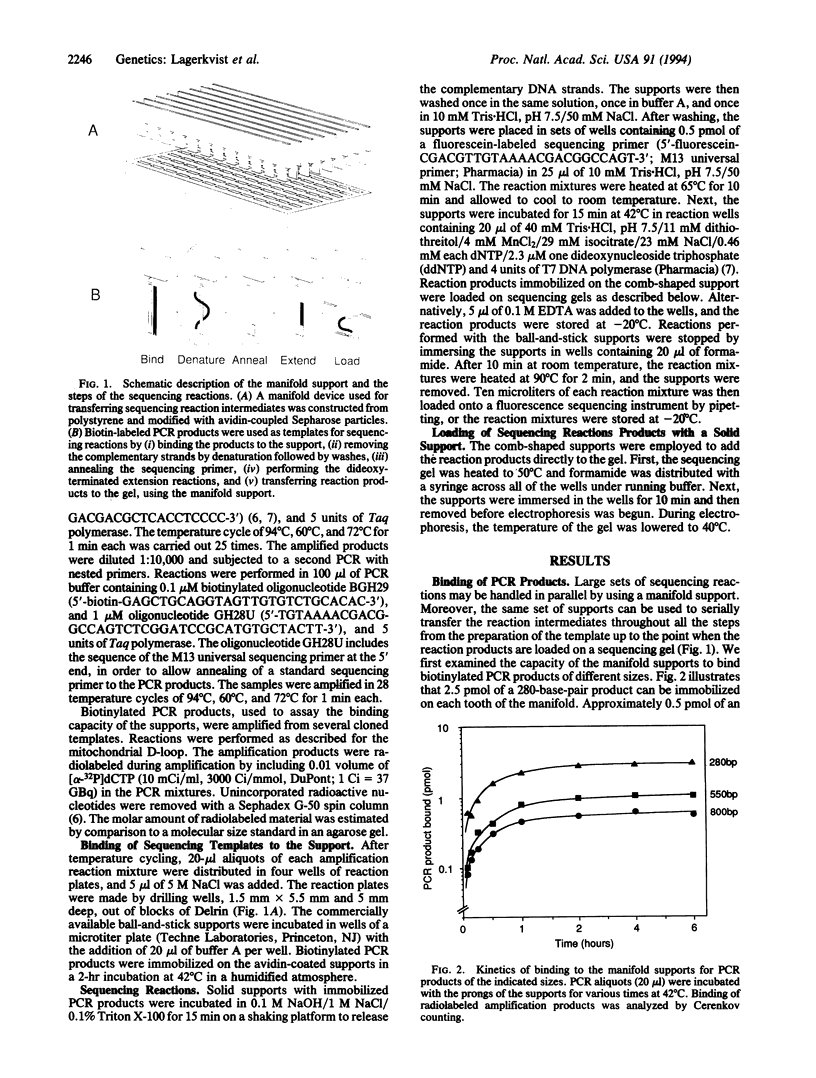
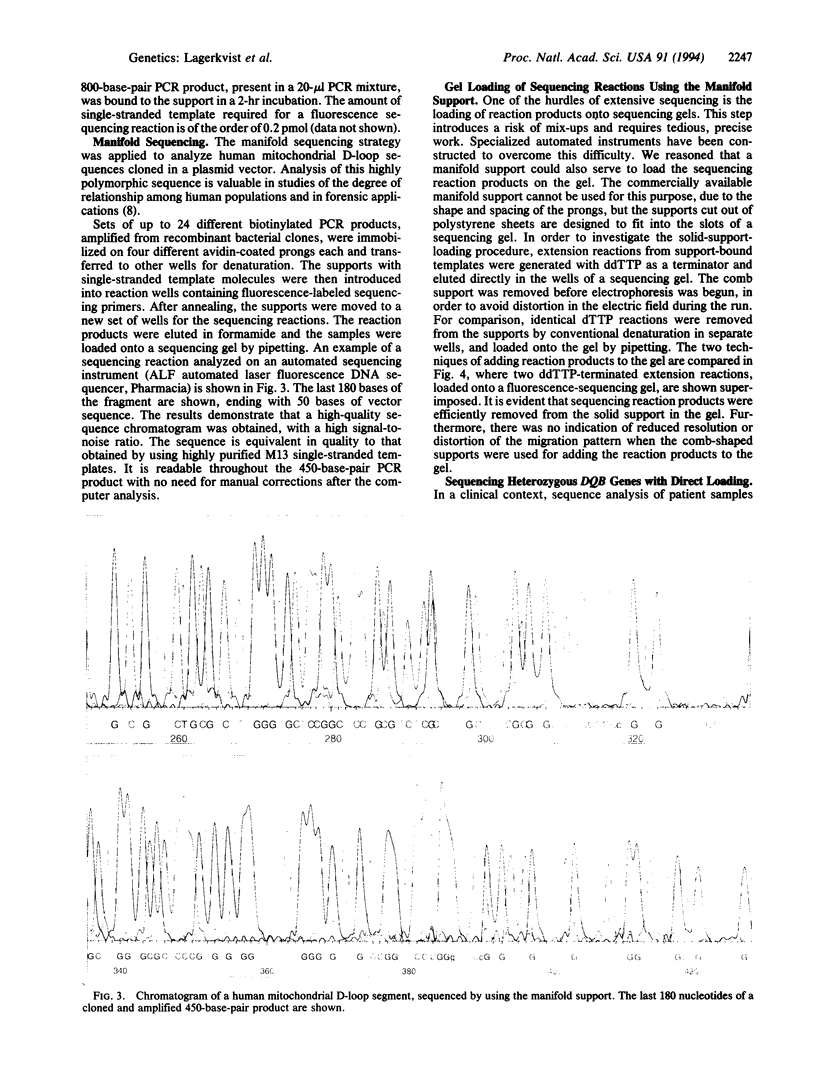

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M., Saldeen T., Pettersson U., Gyllensten U. Genetic typing of HLA class II genes in Swedish populations: application to forensic analysis. J Forensic Sci. 1993 May;38(3):554–570. [PubMed] [Google Scholar]
- Ansorge W., Sproat B. S., Stegemann J., Schwager C. A non-radioactive automated method for DNA sequence determination. J Biochem Biophys Methods. 1986 Dec;13(6):315–323. doi: 10.1016/0165-022x(86)90038-2. [DOI] [PubMed] [Google Scholar]
- Bugawan T. L., Erlich H. A. Rapid typing of HLA-DQB1 DNA polymorphism using nonradioactive oligonucleotide probes and amplified DNA. Immunogenetics. 1991;33(3):163–170. doi: 10.1007/BF01719235. [DOI] [PubMed] [Google Scholar]
- Davis L. M., Fairfield F. R., Harger C. A., Jett J. H., Keller R. A., Hahn J. H., Krakowski L. A., Marrone B. L., Martin J. C., Nutter H. L. Rapid DNA sequencing based upon single molecule detection. Genet Anal Tech Appl. 1991 Feb;8(1):1–7. doi: 10.1016/1050-3862(91)90002-9. [DOI] [PubMed] [Google Scholar]
- Drmanac R., Labat I., Brukner I., Crkvenjakov R. Sequencing of megabase plus DNA by hybridization: theory of the method. Genomics. 1989 Feb;4(2):114–128. doi: 10.1016/0888-7543(89)90290-5. [DOI] [PubMed] [Google Scholar]
- Hopgood R., Sullivan K. M., Gill P. Strategies for automated sequencing of human mitochondrial DNA directly from PCR products. Biotechniques. 1992 Jul;13(1):82–92. [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller T., Kaiser R. J., Koop B. F., Hood L. Large-scale and automated DNA sequence determination. Science. 1991 Oct 4;254(5028):59–67. doi: 10.1126/science.1925562. [DOI] [PubMed] [Google Scholar]
- Khrapko K. R., Lysov YuP, Khorlyn A. A., Shick V. V., Florentiev V. L., Mirzabekov A. D. An oligonucleotide hybridization approach to DNA sequencing. FEBS Lett. 1989 Oct 9;256(1-2):118–122. doi: 10.1016/0014-5793(89)81730-2. [DOI] [PubMed] [Google Scholar]
- Lindsay S. M., Philipp M. Can the scanning tunneling microscope sequence DNA? Genet Anal Tech Appl. 1991 Feb;8(1):8–13. doi: 10.1016/1050-3862(91)90003-a. [DOI] [PubMed] [Google Scholar]
- Marsh S. G., Bodmer J. G. HLA class II nucleotide sequences, 1992. Tissue Antigens. 1992 Nov;40(5):229–243. doi: 10.1111/j.1399-0039.1992.tb02050.x. [DOI] [PubMed] [Google Scholar]
- Middendorf L. R., Bruce J. C., Bruce R. C., Eckles R. D., Grone D. L., Roemer S. C., Sloniker G. D., Steffens D. L., Sutter S. L., Brumbaugh J. A. Continuous, on-line DNA sequencing using a versatile infrared laser scanner/electrophoresis apparatus. Electrophoresis. 1992 Aug;13(8):487–494. doi: 10.1002/elps.11501301103. [DOI] [PubMed] [Google Scholar]
- Ortlepp S. A., McKay I. A. Performing nucleic acid reactions using predispensed lyophilized reaction mixtures. Biotechniques. 1989 Nov-Dec;7(10):1110–1115. [PubMed] [Google Scholar]
- Parik J., Kwiatkowski M., Lagerkvist A., Lagerström Fermér M., Samiotaki M., Stewart J., Glad G., Mendel-Hartvig M., Landegren U. A manifold support for molecular genetic reactions. Anal Biochem. 1993 May 15;211(1):144–150. doi: 10.1006/abio.1993.1245. [DOI] [PubMed] [Google Scholar]
- Prober J. M., Trainor G. L., Dam R. J., Hobbs F. W., Robertson C. W., Zagursky R. J., Cocuzza A. J., Jensen M. A., Baumeister K. A system for rapid DNA sequencing with fluorescent chain-terminating dideoxynucleotides. Science. 1987 Oct 16;238(4825):336–341. doi: 10.1126/science.2443975. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Jung R., Hunger H. D. Solid-phase methods for sequencing of oligodeoxyribonucleotides and DNA. Methods Enzymol. 1987;155:301–331. doi: 10.1016/0076-6879(87)55022-4. [DOI] [PubMed] [Google Scholar]
- Smith L. M., Sanders J. Z., Kaiser R. J., Hughes P., Dodd C., Connell C. R., Heiner C., Kent S. B., Hood L. E. Fluorescence detection in automated DNA sequence analysis. Nature. 1986 Jun 12;321(6071):674–679. doi: 10.1038/321674a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M., Maskos U., Elder J. K. Analyzing and comparing nucleic acid sequences by hybridization to arrays of oligonucleotides: evaluation using experimental models. Genomics. 1992 Aug;13(4):1008–1017. doi: 10.1016/0888-7543(92)90014-j. [DOI] [PubMed] [Google Scholar]
- Trainor G. L. DNA sequencing, automation, and the human genome. Anal Chem. 1990 Mar 1;62(5):418–426. doi: 10.1021/ac00204a001. [DOI] [PubMed] [Google Scholar]
- Vigilant L., Pennington R., Harpending H., Kocher T. D., Wilson A. C. Mitochondrial DNA sequences in single hairs from a southern African population. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9350–9354. doi: 10.1073/pnas.86.23.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]