Abstract
Background
Digoxin remains commonly used for rate control in atrial fibrillation, but very limited data exist supporting this practice and some studies have shown an association with adverse outcomes. We examined the independent association between digoxin and risks of death and hospitalization in adults with incident atrial fibrillation and no heart failure.
Methods and Results
We performed a retrospective cohort study of 14,787 age, gender and high-dimensional propensity score-matched adults with incident atrial fibrillation and no prior heart failure or digoxin use in the AnTicoagulation and Risk factors In Atrial fibrillation-Cardiovascular Research Network (ATRIA-CVRN) Study within Kaiser Permanente Northern and Southern California. We examined the independent association between newly initiated digoxin and the risks of death and hospitalization using extended Cox regression. During a median 1.17 (interquartile range 0.49–1.97) years of follow-up among matched patients with atrial fibrillation, incident digoxin use was associated with higher rates of death (8.3 vs. 4.9 per 100 person-years, P<0.001) and hospitalization (60.1 vs. 37.2 per 100 person-years, P<0.001). Incident digoxin use was independently associated with a 71% higher risk of death (hazard ratio [HR] 1.71, 95%CI:1.52–1.93) and a 63% higher risk of hospitalization (HR 1.63, 95%CI:1.56–1.71). Results were consistent in subgroups of age and gender and when using “intent-to-treat” or “on-treatment” analytic approaches.
Conclusions
In adults with atrial fibrillation, digoxin use was independently associated with higher risks of death and hospitalization. Given other available rate control options, digoxin should be used with caution in the management of atrial fibrillation.
Keywords: arrhythmia, atrial fibrillation, digoxin, morbidity, mortality
Digitalis has been used for more than a century for heart rate control in patients with atrial fibrillation,1 and it remains very commonly used for this indication worldwide.2 Current clinical practice guidelines for the management of atrial fibrillation recommend the use of digoxin alone for resting heart rate control in sedentary individuals, in combination with beta-blockers for resting and exercise heart rate control, and in the setting of concurrent systolic heart failure.3, 4 However, these guidelines are based on small, older clinical studies with very limited follow-up of days to weeks that did not assess the long-term effects of digoxin on mortality or hospitalization.5–14 While a randomized trial assessing the association between digoxin use and adverse outcomes such as death would be optimal, such a study is unlikely to be performed. Observational studies may be of particular utility for evaluating this association because the indication for digoxin use in AF is heart rate control, which has been shown to have no impact on adverse outcomes such as death in large randomized trials,15 thus minimizing the risk of confounding by indication. Recent observational studies and post-hoc analyses from clinical trials have suggested that digoxin may be linked to excess mortality in atrial fibrillation,16–19 but these studies were limited by size and their ability to adjust for confounders and other studies have shown no effect of digoxin on the risk of death.20, 21 Importantly, all previous studies evaluating the association between digoxin and mortality in patients with AF included high numbers of patients with concurrent heart failure, and the pharmacologic risks and benefits may be very different in these patients than in those with AF alone.
Given the availability of other alternatives to digoxin for achieving heart rate control, and the limited and conflicting evidence about digoxin and risk of death in atrial fibrillation, we evaluated the independent association between newly initiated digoxin and the risks of death and hospitalization in a large, diverse, community-based cohort of adults with newly diagnosed atrial fibrillation and no documented heart failure.
Methods
Identification and characterization of patients with incident atrial fibrillation
We identified all adults aged ≥21 years who were diagnosed with atrial fibrillation (and/or atrial flutter) in Kaiser Permanente Northern California and Southern California, two large, integrated health care delivery systems that care for >6.7 million persons who are highly representative of the local and statewide population, except for slightly lower representation at the extremes of age and income (Figure 1).22, 23 Using information from comprehensive health plan clinical and administrative databases,24 atrial fibrillation was defined as meeting the following criteria between January 1, 2006 and June 30, 2009: one or more inpatient admission with a primary discharge diagnosis; or two or more outpatient, non-emergency department encounters for atrial fibrillation based on International Classification of Diseases, Ninth Edition [ICD-9] codes 427.31, or 427.32 with electrocardiographic evidence of atrial fibrillation or atrial flutter. The index date was assigned based on the first qualifying atrial fibrillation diagnosis, and we focused on the subset of patients with presumed incident atrial fibrillation by excluding patients with any previous inpatient or outpatient diagnosis of atrial fibrillation between 2001 and cohort entry date. We also excluded patients with unknown gender, <12 months of continuous membership or drug benefit before index date, no membership after index date, documented heart failure, or prior cardiac or renal transplant using previously described methods.25
Figure 1.
Age, gender, and high-dimensional propensity score-matched cohort assembly of patients with incident atrial fibrillation and no history of heart failure or digoxin use between January 1, 2006 and June 31, 2009.
Institutional review boards of the Kaiser Foundation Research Institute and Kaiser Permanente Southern California approved the study. A waiver of informed consent was obtained due to the nature of the study.
Longitudinal exposure to digoxin
We implemented a “new user” design 26, 27 by excluding all patients with evidence of digoxin use up to four years before study entry in order to focus on outcomes associated with incident digoxin use and remove biases associated with including prevalent drug users.
We characterized use of digoxin in two ways (“intent-to-treat” and time-varying “on-treatment” exposure) based on estimated day supply information per dispensed prescription and observed refill patterns found in health plan pharmacy databases using previously validated methods.25, 28 Briefly, for any two consecutive prescriptions, we examined the time between the projected end date of the first prescription and the date of the next filled prescription. Given that dose adjustment is not uncommon, we allowed a “grace period” of 30 days between dispensed prescriptions. Thus, if the time between the projected end date of the first prescription and the fill date of the next prescription was ≤30 days, we considered that individual to be continuously receiving digoxin therapy. If the refill interval was >30 days, then the individual was considered off digoxin therapy starting the day after the projected end date of the first prescription until the date of next filled prescription, if any. Because hospitalized patients receive their medications from the inpatient pharmacy and do not use their outpatient medication supply, we subtracted the number of hospitals days from the subsequent refill interval if there was an interim hospitalization.
Follow-up and outcomes
Patients were followed through June 30, 2009 for the outcomes of all-cause death and hospitalization from any cause which was the latest date complete data were available at the time of analysis. Patients were censored at the time of health plan disenrollment or the end of follow-up. Death from any cause was identified from health plan databases (inpatient deaths, proxy report of outpatient deaths), annual California state death certificate files and Social Security Administration Death Master File quarterly updated data files.29, 30 All-cause and heart failure-related hospitalizations were identified using comprehensive hospital discharge and billing claims databases; heart failure-related hospitalizations were based on primary discharge diagnoses of heart failure based on validated ICD-9 codes as previously described.31, 32
Age, gender, and high-dimensional propensity score matching
A high-dimensional propensity score for the initiation of digoxin was calculated for each person using logistic regression methods that included demographic characteristics and multidimensional patient characteristics. As opposed to standard propensity scoring which includes a limited group of pre-selected variables, the high dimensional propensity score is generated automatically by an algorithm that scans through all available data in the Kaiser Permanente electronic medical record from the three dimensions of medication prescriptions, diagnoses and procedures. The algorithm selects the most frequent 200 items from each of these three dimensions within a five-year look-back period and after that selects the 300 best matched parameters out of the initial 600 items for use in the high-dimensional propensity score.33 This methodology has been shown to approximate point estimates of risk from randomized clinical trials substantially better than standard propensity scoring or regression methodologies.33, 34 Each digoxin user was matched to a maximum of three non-digoxin users (without replacement) based on age (+/− one year), gender and high-dimensional propensity score for the initiation of digoxin (caliper width of +/− 0.01) on the calendar date of the first digoxin prescription (model c statistic =0.68).
Data on age, gender, self-reported race/ethnicity, and socioeconomic status were obtained from health plan databases. We ascertained relevant medical history documented up to five years before cohort entry using previously validated approaches based on ICD-9 diagnosis and procedure codes, Current Procedure Terminology (CPT) procedure codes, laboratory records and pharmacy records.25, 31, 35–39 This included cardiovascular diseases (acute myocardial infarction, unstable angina, ischemic stroke, transient ischemic attack, intracranial hemorrhage, peripheral arterial disease, valvular heart disease), prior ventricular arrhythmias (ventricular tachycardia or fibrillation), prior cardiac procedures (percutaneous coronary intervention or coronary artery bypass surgery), other cardiovascular risk factors (hypertension, diabetes mellitus, and dyslipidemia), and other coexisting medical illnesses (dementia, depression, thyroid disease, prior gastrointestinal bleed, other bleeding, cancer, lung disease, liver disease) (codes available on request). We ascertained body mass index and blood pressure up to 365 days before cohort entry from ambulatory visit information in health plan electronic medical records. We also characterized baseline kidney function using outpatient serum creatinine concentration values based on an IDMS-traceable assay and estimated glomerular filtration rate (eGFR, ml/min/1.73 m2) using the CKD-EPI equation.40
We characterized baseline exposure to other relevant cardiovascular medications using similar methods as described above for digoxin based on information from ambulatory pharmacy records for the following medications: warfarin, α-adrenergic receptor antagonists, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, diuretics, beta-blockers, aldosterone receptor antagonists, calcium channel blockers, nitrates, hydralazine, statins, other lipid lowering agents, anti-platelet agents, and diabetic medications.31, 32
Statistical Analysis
All analyses were performed using SAS statistical software, version 9.2 (Cary, N.C.). We compared the baseline characteristics for the overall cohort and the matched patients prescribed or not prescribed digoxin during follow-up using t-test or Wilcoxon rank sum test for continuous variables and the χ2 test for categorical variables. Subsequent analyses were performed in the age, gender and high-dimensional propensity score-matched cohort to minimize confounding.
We next calculated rates (per 100 person-years) with associated 95% confidence limits for death and hospitalization for those who received digoxin during follow-up compared with those who did not receive digoxin. We generated Kaplan-Meier survival curves for the outcomes of mortality and hospitalization, censoring patients at the time of death or loss to follow-up. We conducted extended Cox regression models to examine the independent association between digoxin use in the propensity score-matched cohort and the risk of adverse outcomes. We conducted additional analyses stratified by age and gender.
To assess whether changes in covariates in the follow-up period may confound the relationship between digoxin use and the outcomes of interest, we performed a secondary analysis with additional adjustment for time-updated covariates. In this secondary analysis, we performed extended Cox regression in the propensity score-matched cohort to examine the association of incident digoxin use and the risk of adverse outcomes with additional adjustment for time-updated comorbidities including development of heart failure, targeted laboratory results, and longitudinal medication use (Supplemental Table 1).
Finally, because previous observational studies on this topic have shown discordant findings depending on whether they performed their analyses using an “intent to treat” or “on-treatment” analytic method,16, 21 we performed a secondary analysis in which we examined digoxin use as a time-varying exposure within the propensity score-matched cohort. In this “on-treatment” analysis, the outcomes of death and hospitalization were only assigned to the digoxin use group if patients were classified as receiving the medication at the time of the event.
Results
Baseline Characteristics
Between January 2006 and June 2009, we identified 27,288 adults who had incident atrial fibrillation and no prior digoxin use or a history of heart failure (Figure 1), of whom 4858 (17.8%) initiated digoxin during follow-up. The age, gender, and high-dimensional propensity score-matched cohort included 14,787 adults, of whom 4231 (28.6%) initiated digoxin during follow-up. Digoxin users were successfully matched to three non-users in 65.4% of cases.
After age, gender and high-dimensional propensity score matching, digoxin users and non-users were very similar in terms of characteristics at study entry (Table 1). However, digoxin users were slightly older, had lower household income, and had lower body mass index and blood pressure; digoxin users were also more likely to have a history of non-gastrointestinal bleeding, cancer and chronic lung disease. Non-digoxin users were more likely to have a history of acute myocardial infarction and coronary artery bypass surgery and they were more likely to be treated with beta blockers and statins.
Table 1.
Baseline characteristics of adults with atrial fibrillation between January 2006 – June 2009 with no known heart failure or prior digoxin use, overall and stratified by new digoxin use during follow-up, in the full cohort and the age, gender and high-dimensional propensity score-matched cohort.
| Full Digoxin Cohort | Age, Gender, and High-dimensional Propensity Score Matched Cohort (1:3) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Characteristic | Overall (N=27,288) |
Digoxin Users (N=4858) |
Non-users (N=22,430) |
P value | Overall (N=14,787) |
Digoxin Users (N=4231) |
Matched Non-users (N=10,556) |
P value |
| Age, year, mean (SD) | 70.7 (12.9) | 71.9 (11.9) | 70.5 (13.1) | <0.001 | 71.7 (11.3) | 72.1 (11.4) | 71.6 (11.2) | 0.01 |
| Gender, N (%) | ||||||||
| Men | 15,191 (55.7) | 2441 (50.2) | 12,750 (56.8) | <0.001 | 7714 (52.2) | 2164 (51.1) | 5550 (52.6) | 0.12 |
| Women | 12,097 (44.3) | 2417 (49.8) | 9680 (43.2) | <0.001 | 7073 (47.8) | 2067 (48.9) | 5006 (47.4) | 0.12 |
| Race, N (%) | <0.001 | 0.43 | ||||||
| White | 20,969 (76.8) | 3932 (80.9) | 17,037 (76.0) | 11,790 (79.7) | 3399 (80.3) | 8391 (79.5) | ||
| Black | 1582 (5.8) | 297 (6.1) | 1285 (5.7) | 858 (5.8) | 249 (5.9) | 609 (5.8) | ||
| Native American | 58 (0.2) | 12 (0.2) | 46 (0.2) | 36 (0.2) | 11 (0.3) | 25 (0.2) | ||
| Asian | 2043 (7.5) | 257 (5.3) | 1786 (8.0) | 891 (6.0) | 233 (5.5) | 658 (6.2) | ||
| Other/unknown | 2636 (9.7) | 360 (7.4) | 2276 (10.1) | 1212 (8.2) | 339 (8.0) | 873 (8.3) | ||
| Hispanic ethnicity, N (%) | 3197 (11.7) | 507 (10.4) | 2690 (12.0) | 0.002 | 1550 (10.5) | 445 (10.5) | 1105 (10.5) | 0.93 |
| Socioeconomic status, N (%) | ||||||||
| Low annual household income | 3514 (12.9) | 722 (14.9) | 2792 (12.4) | <0.001 | 1925 (13.0) | 610 (14.4) | 1315 (12.5) | 0.001 |
| Low educational attainment | 5778 (21.2) | 1110 (22.8) | 4668 (20.8) | 0.002 | 3111 (21.0) | 937 (22.1) | 2174 (20.6) | 0.04 |
| Cardiovascular disease, N (%) | ||||||||
| Acute myocardial infarction | 999 (3.7) | 125 (2.6) | 874 (3.9) | <0.001 | 454 (3.1) | 110 (2.6) | 344 (3.3) | 0.04 |
| Unstable angina | 613 (2.2) | 85 (1.7) | 528 (2.4) | 0.010 | 279 (1.9) | 74 (1.7) | 205 (1.9) | 0.44 |
| Ischemic stroke | 1037 (3.8) | 155 (3.2) | 882 (3.9) | 0.01 | 508 (3.4) | 142 (3.4) | 366 (3.5) | 0.74 |
| Transient ischemic attack | 728 (2.7) | 116 (2.4) | 612 (2.7) | 0.18 | 369 (2.5) | 100 (2.4) | 269 (2.5) | 0.51 |
| Intracranial hemorrhage | 141 (0.5) | 20 (0.4) | 121 (0.5) | 0.26 | 66 (0.4) | 17 (0.4) | 49 (0.5) | 0.61 |
| Peripheral arterial disease | 949 (3.5) | 160 (3.3) | 789 (3.5) | 0.44 | 433 (2.9) | 141 (3.3) | 292 (2.8) | 0.06 |
| Valvular heart disease | 328 (1.2) | 43 (0.9) | 285 (1.3) | 0.03 | 134 (0.9) | 35 (0.8) | 99 (0.9) | 0.52 |
| Ventricular arrhythmias, N (%) | ||||||||
| Ventricular tachycardia | 87 (0.3) | 11 (0.2) | 76 (0.3) | 0.21 | 43 (0.3) | 11 (0.3) | 32 (0.3) | 0.66 |
| Ventricular fibrillation | 6 (0.0) | 0 (0.0) | 6 (0.0) | 0.25 | 4 (0.0) | 0 (0.0) | 4 (0.0) | 0.21 |
| Cardiovascular procedure history, N (%) | ||||||||
| Percutaneous coronary intervention | 1175 (4.3) | 150 (3.1) | 1025 (4.6) | <0.001 | 527 (3.6) | 138 (3.3) | 389 (3.7) | 0.21 |
| Coronary artery bypass surgery | 825 (3.0) | 81 (1.7) | 744 (3.3) | <0.001 | 316 (2.1) | 75 (1.8) | 241 (2.3) | 0.05 |
| Other cardiovascular risk factors, N (%) | ||||||||
| Hypertension | 21,163 (77.6) | 3707 (76.3) | 17,456 (77.8) | 0.02 | 11,573 (78.3) | 3260 (77.1) | 8313 (78.8) | 0.02 |
| Diabetes mellitus | 6498 (23.8) | 1116 (23.0) | 5382 (24.0) | 0.13 | 3416 (23.1) | 974 (23.0) | 2442 (23.1) | 0.88 |
| Dyslipidemia | 15,112 (55.4) | 2517 (51.8) | 12,595 (56.2) | <0.001 | 8006 (54.1) | 2249 (53.2) | 5757 (54.5) | 0.13 |
| Other coexisting medical illnesses, N (%) | ||||||||
| Dementia | 1470 (5.4) | 248 (5.1) | 1222 (5.4) | 0.34 | 721 (4.9) | 209 (4.9) | 512 (4.9) | 0.82 |
| Depression | 4757 (17.4) | 860 (17.7) | 3897 (17.4) | 0.58 | 2626 (17.8) | 744 (17.6) | 1882 (17.8) | 0.73 |
| Thyroid disease | 4196 (15.4) | 765 (15.7) | 3431 (15.3) | 0.43 | 2368 (16.0) | 671 (15.9) | 1697 (16.1) | 0.75 |
| Gastrointestinal bleeding | 596 (2.2) | 96 (2.0) | 500 (2.2) | 0.27 | 284 (1.9) | 82 (1.9) | 202 (1.9) | 0.92 |
| Other bleeding | 95 (0.3) | 25 (0.5) | 70 (0.3) | 0.03 | 55 (0.4) | 24 (0.6) | 31 (0.3) | 0.01 |
| Cancer | 1761 (6.5) | 371 (7.6) | 1390 (6.2) | <0.001 | 947 (6.4) | 312 (7.4) | 635 (6.0) | 0.002 |
| Lung disease | 6162 (22.6) | 1253 (25.8) | 4909 (21.9) | <0.001 | 3322 (22.5) | 1036 (24.5) | 2286 (21.7) | <0.001 |
| Liver disease | 618 (2.3) | 95 (2.0) | 523 (2.3) | 0.11 | 304 (2.1) | 83 (2.0) | 221 (2.1) | 0.61 |
| Medications, N (%) | ||||||||
| Warfarin | 9363 (34.3) | 1517 (31.2) | 7846 (35.0) | <0.001 | 4989 (33.7) | 1420 (33.6) | 3569 (33.8) | 0.77 |
| Alpha-adrenergic receptor antagonist | 3821 (14.0) | 615 (12.7) | 3206 (14.3) | 0.003 | 1941 (13.1) | 555 (13.1) | 1386 (13.1) | 0.98 |
| Angiotensin converting enzyme inhibitor | 9873 (36.2) | 1628 (33.5) | 8245 (36.8) | <0.001 | 5288 (35.8) | 1442 (34.1) | 3846 (36.4) | 0.007 |
| Angiotensin receptor blocker | 2465 (9.0) | 402 (8.3) | 2063 (9.2) | 0.04 | 1313 (8.9) | 364 (8.6) | 949 (9.0) | 0.45 |
| Diuretic | 10,399 (38.1) | 1793 (36.9) | 8606 (38.4) | 0.06 | 5683 (38.4) | 1576 (37.2) | 4107 (38.9) | 0.06 |
| Beta-blocker | 15,926 (58.4) | 2557 (52.6) | 13,369 (59.6) | <0.001 | 8497 (57.5) | 2351 (55.6) | 6146 (58.2) | 0.003 |
| Aldosterone receptor antagonist | 345 (1.3) | 58 (1.2) | 287 (1.3) | 0.63 | 186 (1.3) | 49 (1.2) | 137 (1.3) | 0.49 |
| Calcium channel blocker | 7920 (29.0) | 1369 (28.2) | 6551 (29.2) | 0.15 | 4194 (28.4) | 1224 (28.9) | 2970 (28.1) | 0.33 |
| Nitrates | 2326 (8.5) | 346 (7.1) | 1980 (8.8) | <0.001 | 1136 (7.7) | 304 (7.2) | 832 (7.9) | 0.15 |
| Hydralazine | 504 (1.8) | 76 (1.6) | 428 (1.9) | 0.11 | 237 (1.6) | 67 (1.6) | 170 (1.6) | 0.91 |
| Statin | 12,173 (44.6) | 1960 (40.3) | 10,213 (45.5) | <0.001 | 6385 (43.2) | 1767 (41.8) | 4618 (43.7) | 0.03 |
| Other lipid lowering agent | 1185 (4.3) | 176 (3.6) | 1009 (4.5) | 0.007 | 619 (4.2) | 164 (3.9) | 455 (4.3) | 0.23 |
| Antiplatelet agent | 2018 (7.4) | 283 (5.8) | 1735 (7.7) | <0.001 | 960 (6.5) | 260 (6.1) | 700 (6.6) | 0.28 |
| Diabetes medications | 4183 (15.3) | 692 (14.2) | 3491 (15.6) | 0.02 | 2127 (14.4) | 606 (14.3) | 1521 (14.4) | 0.89 |
| Baseline CHADS2 score, mean (SD) | 1.56 (1.04) | 1.55 (1.00) | 1.56 (1.05) | 0.59 | 1.56 (1.01) | 1.56 (1.00) | 1.56 (1.02) | 0.89 |
| Body mass index, kg/m2, N (%) | <0.001 | <0.001 | ||||||
| <25 | 5959 (21.8) | 1012 (20.8) | 4947 (22.1) | 2865 (19.4) | 908 (21.5) | 1957 (18.5) | ||
| 25–29 | 7077 (25.9) | 1179 (24.3) | 5898 (26.3) | 3421 (23.1) | 1065 (25.2) | 2356 (22.3) | ||
| 30–39 | 5783 (21.2) | 1008 (20.7) | 4775 (21.3) | 2846 (19.2) | 900 (21.3) | 1946 (18.4) | ||
| >=40 | 1197 (4.4) | 245 (5.0) | 952 (4.2) | 577 (3.9) | 215 (5.1) | 362 (3.4) | ||
| Missing | 7272 (26.6) | 1414 (29.1) | 5858 (26.1) | 5078 (34.3) | 1143 (27.0) | 3935 (37.3) | ||
| Systolic blood pressure, mmHg, N (%) | <0.001 | <0.001 | ||||||
| < 120 | 6926 (25.4) | 1434 (29.5) | 5492 (24.5) | 3345 (22.6) | 1285 (30.4) | 2060 (19.5) | ||
| 120–129 | 4886 (17.9) | 764 (15.7) | 4122 (18.4) | 2281 (15.4) | 704 (16.6) | 1577 (14.9) | ||
| 130–139 | 4704 (17.2) | 724 (14.9) | 3980 (17.7) | 2350 (15.9) | 635 (15.0) | 1715 (16.2) | ||
| >=140 | 4266 (15.6) | 648 (13.3) | 3618 (16.1) | 2208 (14.9) | 569 (13.4) | 1639 (15.5) | ||
| Missing | 6506 (23.8) | 1288 (26.5) | 5218 (23.3) | 4603 (31.1) | 1038 (24.5) | 3565 (33.8) | ||
| Diastolic blood pressure, mmHg, N (%) | <0.001 | <0.001 | ||||||
| < 80 | 14,687 (53.8) | 2404 (49.5) | 12,283 (54.8) | 7016 (47.4) | 2167 (51.2) | 4849 (45.9) | ||
| 80–84 | 2912 (10.7) | 510 (10.5) | 2402 (10.7) | 1502 (10.2) | 453 (10.7) | 1049 (9.9) | ||
| 85–89 | 1480 (5.4) | 274 (5.6) | 1206 (5.4) | 749 (5.1) | 248 (5.9) | 501 (4.7) | ||
| >=90 | 1703 (6.2) | 383 (7.9) | 1320 (5.9) | 918 (6.2) | 326 (7.7) | 592 (5.6) | ||
| Missing | 6506 (23.8) | 1287 (26.5) | 5219 (23.3) | 4602 (31.1) | 1037 (24.5) | 3565 (33.8) | ||
| Baseline laboratory results | ||||||||
| Estimated glomerular filtration rate category, ml/min/1.73 m2, N (%) | <0.001 | 0.001 | ||||||
| 90–150 | 3305 (12.1) | 521 (10.7) | 2784 (12.4) | 1296 (8.8) | 431 (10.2) | 865 (8.2) | ||
| 60–89 | 11,819 (43.3) | 2156 (44.4) | 9663 (43.1) | 6568 (44.4) | 1881 (44.5) | 4687 (44.4) | ||
| 45–59 | 6031 (22.1) | 1129 (23.2) | 4902 (21.9) | 3552 (24.0) | 1000 (23.6) | 2552 (24.2) | ||
| 30–44 | 2686 (9.8) | 511 (10.5) | 2175 (9.7) | 1486 (10.0) | 428 (10.1) | 1058 (10.0) | ||
| <30 | 817 (3.0) | 125 (2.6) | 692 (3.1) | 412 (2.8) | 111 (2.6) | 301 (2.9) | ||
| Missing | 2630 (9.6) | 416 (8.6) | 2214 (9.9) | 1473 (10.0) | 380 (9.0) | 1093 (10.4) | ||
Outcomes According to Digoxin Exposure
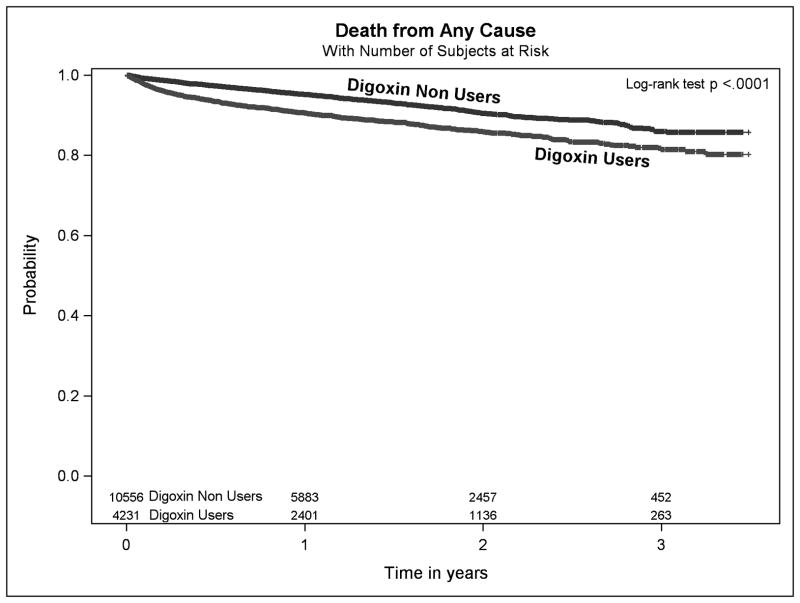
Median follow up time in the propensity-matched cohort was 1.17 (interquartile range 0.49–1.97) years. There were 1140 deaths (473 in digoxin users, 667 in non-users of digoxin), during follow-up, with a significantly higher rate of death in digoxin users compared with non-users (8.3 vs. 4.9 per 100 person years, respectively, P<0.001, Figure 2). Digoxin use was associated with a 71% higher risk of death (hazard ratio [HR] 1.71, 95%CI: 1.52–1.93, Figure 3).
Figure 2.
Kaplan-Meier survival curves for the outcome of death from any cause in age, gender and high-dimensional propensity score matched adults with incident atrial fibrillation and no heart failure between 2006–2009 for digoxin users vs non-users.
Figure 3.

Risk of death from any cause in age, gender and high-dimensional propensity score matched adults with incident atrial fibrillation and no heart failure between 2006–2009 for digoxin users vs non-users.
During follow-up, there were 8456 hospitalizations for any cause in the propensity-matched cohort (3411 in digoxin users vs. 5045 in non-users of digoxin). In the matched cohort 2176 patients (632 digoxin users, 1544 non-users of digoxin) were hospitalized and subsequently died during follow-up, and 11 patients (4 digoxin users, 7 non-users of digoxin) died during hospitalization. There were 904 hospitalizations for heart failure in the propensity matched cohort (512 in digoxin users vs 391 in non-users of digoxin. The rate of hospitalization was higher for patients who received digoxin compared with those who did not receive digoxin (60.1 vs. 37.2 per 100 person years, respectively, P<0.001, Figure 4). Digoxin use was associated with a 63% higher risk of hospitalization (HR 1.63, 95%CI: 1.56–1.71, Figure 5).
Figure 4.
Kaplan-Meier curves for the outcome of hospitalization from any cause in age, gender and high-dimensional propensity score matched adults with incident atrial fibrillation and no heart failure between 2006–2009 for digoxin users vs non-users.
Figure 5.

Risk of hospitalization from any cause in age, gender and high-dimensional propensity score matched adults with incident atrial fibrillation and no heart failure between 2006–2009 for digoxin users vs non-users.
Outcomes According to Digoxin Use in Subgroups of Age and Gender
The association between incident digoxin use and the outcomes of death and hospitalization were consistent in subgroups of patient age, with 45–115% higher risks of death and hospitalization across all age strata (Figures 3 and 5). Similarly, in analyses stratified by gender, we observed 60–82% higher risks of death and hospitalization for both men and women (Figures 3 and 5).
Additional Adjustment for Time-updated Covariates and “On Treatment” Analysis
We performed two secondary analyses using different analytic approaches to examine the consistency of our primary results.
Results were consistent in analyses in which we additionally adjusted for potentially relevant time-updated comorbidities including heart failure, targeted laboratory results, and medications. Digoxin use was associated with a 62% higher adjusted risk of death (adjusted HR 1.62, 95%CI: 1.43–1.84) and a 45% higher adjusted risk of hospitalization for any cause (adjusted HR 1.45, 95%CI: 1.39–1.52).
Similarly, results were consistent in analyses in which we treated digoxin use as a time-varying variable (“on treatment” analysis). Digoxin use was associated with a 40% higher risk of death (HR 1.40, 95%CI: 1.23–1.6) and a 53% higher risk of hospitalization (HR 1.53, 95%CI: 1.46–1.60).
Digoxin Prescription Dosages and Serum Digoxin Concentrations
Among digoxin users in the matched cohort, mean daily dose of digoxin was 0.164 mg. Mean daily dose of digoxin was not statistically different among those who died compared with those who did not die (0.162 mg daily vs. 0.164 mg daily, P=0.30).
Among digoxin users in the matched cohort, serum digoxin concentration was never measured in 31%, measured once in 27%, measured twice in 17%, and three or more times in 25% during follow-up. Mean serum digoxin concentration was 0.964 ng/ml. Mean serum digoxin concentration was higher among those who died compared with those who did not die (1.151 vs. 0.935, P<0.001).
Discussion
In a large, age, gender and high-dimensional propensity score-matched cohort of adults with newly diagnosed atrial fibrillation and no known prior digoxin use or history of heart failure treated in the community, we found that incident digoxin use was independently associated with a 71% higher risk of death and a 63% higher risk of hospitalization. These results were consistent across strata of age and gender. Results were also consistent in secondary analyses in which we included additional adjustment for time-updated comorbidities and medications, and in secondary analyses in which we treated digoxin as a time-varying variable (“on treatment” analysis).
Digoxin remains commonly used for heart rate control in patients with AF. In a recent survey of AF patients from 9 countries in Europe, it was prescribed in 19.4% of study subjects,2 and it was prescribed in 17.8% of incident AF in our cohort. Current clinical practice guidelines3, 4 endorse digoxin use in patients with atrial fibrillation based primarily on small clinical studies designed to assess the short-term efficacy of heart rate control.5–14 However, these studies had very limited follow-up of days to weeks, and did not evaluate long-term mortality or hospitalization. A large randomized trial specifically assessing the long-term safety of digoxin for heart rate control in patients with atrial fibrillation would be optimal for studying this question, but one has not been conducted and is very unlikely to ever be performed. However, well conducted observational studies may be of particular utility in evaluating the association between digoxin use in AF and adverse events because heart rate control has been shown in large randomized studies not to be associated with adverse outcomes,15, 41 minimizing the risk of confounding by indication.
Our results support and substantially extend recent reports from smaller, more limited observational studies demonstrating incident digoxin use is independently associated with a higher risk of death,16–18 and contradict the findings of two studies showing no association between digoxin and mortality.20, 21 Notably two post-hoc studies of data from the AFFIRM trial42 showed conflicting results on this topic depending on the analytic strategy used, with one study showing an increased risk of mortality associated with digoxin using an “on treatment” analytic strategy18 and another study showing no association with mortality using a “intent-to-treat” analytic strategy.21 We showed a consistent and substantial increase in mortality and hospitalization risk using both analytic methods. Importantly, our study is also the first to exclude patients with heart failure. All previous studies on this topic included substantial numbers of patients with comorbid heart failure, and the mechanisms of risk and benefit may be very different than for these patients compared with those who have AF alone.
While we were not able to assess the specific causes of death in our cohort, digoxin toxicity is well known to be a cause of arrhythmic death.43–45 Approximately 30% of the digoxin users in our study never had a serum digoxin concentration measurement and an additional 27% only had it measured once, reflecting the fact that routine surveillance of digoxin levels is not commonly performed in community practice. Among digoxin users who did have serum digoxin concentration measured, levels were significantly higher among those who died compared with those who did not die, which may also be consistent with an arrhythmic etiology for the increased mortality risk.
As an observational study of outcomes associated with a therapy used outside of a randomized trial, we cannot completely rule out residual confounding as an explanation for our findings. However, we implemented multiple design and analytic approaches to mitigate treatment selection bias. First, we studied contemporary patients with newly diagnosed atrial fibrillation and no known heart failure, and therefore we captured the full natural history of patients and prevented confounding by indication due to concomitant heart failure. Second, we employed a “new user” design 26 to focus on outcomes associated with newly initiated digoxin therapy to avoid biases associated with examining prevalent therapy and outcomes and captured longitudinal exposure to digoxin throughout follow-up. Third, we performed matching using age, gender and the recently developed high-dimensional propensity score33 so that the digoxin users and non-users we compared were very similar with regards to potential confounders, including sociodemographic factors, comorbidity, laboratory results and therapies. Results were also consistent in secondary analyses in which we included additional adjustment for time-updated comorbidities including heart failure, laboratories and medications, and in secondary analyses in which we treated digoxin as a time-varying exposure (“on treatment” analysis).
Our study was conducted within two large health care delivery systems in California, so the results may not be fully generalizable to all populations and practice settings, although our study included the largest and most diverse sample of adults with incident atrial fibrillation treated in clinical practice reported to date, which argues for greater generalizability. In addition, our results cannot be applied to those with concurrent heart failure and atrial fibrillation as we specifically excluded patients with known heart failure to mitigate confounding by indication.
In conclusion, we found that incident digoxin use was independently associated with higher risks of death and hospitalization in patients with atrial fibrillation and no known heart failure. These results were consistent across strata of age and gender and in secondary analyses employing different analytic strategies. Given other available rate control options, digoxin should be used with caution in the management of atrial fibrillation.
Supplementary Material
Acknowledgments
Funding Sources: Supported by grants 1RC2 HL101589 and U19 HL91179 from the National Heart, Lung and Blood Institute, National Institutes of Health, United States and grant 0875162N for the American Heart Association Pharmaceutical Roundtable-Spina Cardiovascular Outcomes Research Center program.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Lewis T. Clinical disorders of the heartbeat: A handbook for practitioners and students. New York: Hoeber; 1910. [Google Scholar]
- 2.Lip GY, Laroche C, Dan GA, Santini M, Kalarus Z, Rasmussen LH, Oliveira MM, Mairesse G, Crijns HJ, Simantirakis E, Atar D, Kirchhof P, Vardas P, Tavazzi L, Maggioni AP. A prospective survey in european society of cardiology member countries of atrial fibrillation management: Baseline results of eurobservational research programme atrial fibrillation (eorp-af) pilot general registry. Europace. 2014;16:308–319. doi: 10.1093/europace/eut373. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Smith SC, Jr, Priori SG, Estes NA, 3rd, Ezekowitz MD, Jackman WM, January CT, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Hochman JS, Kushner FG, Ohman EM, Tarkington LG, Yancy CW. 2011 accf/aha/hrs focused updates incorporated into the acc/aha/esc 2006 guidelines for the management of patients with atrial fibrillation: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2011;123:e269–367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 4.European Heart Rhythm A, European Association for Cardio-Thoracic S. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: The task force for the management of atrial fibrillation of the european society of cardiology (esc) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 5.Roberts SA, Diaz C, Nolan PE, Salerno DM, Stapczynski JS, Zbrozek AS, Ritz EG, Bauman JL, Vlasses PH. Effectiveness and costs of digoxin treatment for atrial fibrillation and flutter. Am J Cardiol. 1993;72:567–573. doi: 10.1016/0002-9149(93)90353-e. [DOI] [PubMed] [Google Scholar]
- 6.Segal JB, McNamara RL, Miller MR, Kim N, Goodman SN, Powe NR, Robinson K, Yu D, Bass EB. The evidence regarding the drugs used for ventricular rate control. J Fam Pract. 2000;49:47–59. [PubMed] [Google Scholar]
- 7.Tamariz LJ, Bass EB. Pharmacological rate control of atrial fibrillation. Cardiol Clin. 2004;22:35–45. doi: 10.1016/s0733-8651(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 8.Farshi R, Kistner D, Sarma JS, Longmate JA, Singh BN. Ventricular rate control in chronic atrial fibrillation during daily activity and programmed exercise: A crossover open-label study of five drug regimens. J Am Coll Cardiol. 1999;33:304–310. doi: 10.1016/s0735-1097(98)00561-0. [DOI] [PubMed] [Google Scholar]
- 9.Hou ZY, Chang MS, Chen CY, Tu MS, Lin SL, Chiang HT, Woosley RL. Acute treatment of recent-onset atrial fibrillation and flutter with a tailored dosing regimen of intravenous amiodarone. A randomized, digoxin-controlled study. Eur Heart J. 1995;16:521–528. doi: 10.1093/oxfordjournals.eurheartj.a060945. [DOI] [PubMed] [Google Scholar]
- 10.Singh S, Saini RK, DiMarco J, Kluger J, Gold R, Chen YW. Efficacy and safety of sotalol in digitalized patients with chronic atrial fibrillation. The sotalol study group. Am J Cardiol. 1991;68:1227–1230. doi: 10.1016/0002-9149(91)90200-5. [DOI] [PubMed] [Google Scholar]
- 11.Falk RH, Knowlton AA, Bernard SA, Gotlieb NE, Battinelli NJ. Digoxin for converting recent-onset atrial fibrillation to sinus rhythm. A randomized, double-blinded trial. Ann Intern Med. 1987;106:503–506. doi: 10.7326/0003-4819-106-4-503. [DOI] [PubMed] [Google Scholar]
- 12.Jordaens L, Trouerbach J, Calle P, Tavernier R, Derycke E, Vertongen P, Bergez B, Vandekerckhove Y. Conversion of atrial fibrillation to sinus rhythm and rate control by digoxin in comparison to placebo. Eur Heart J. 1997;18:643–648. doi: 10.1093/oxfordjournals.eurheartj.a015310. [DOI] [PubMed] [Google Scholar]
- 13.Capucci A, Boriani G, Rubino I, Della Casa S, Sanguinetti M, Magnani B. A controlled study on oral propafenone versus digoxin plus quinidine in converting recent onset atrial fibrillation to sinus rhythm. Int J Cardiol. 1994;43:305–313. doi: 10.1016/0167-5273(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 14.Intravenous digoxin in acute atrial fibrillation. Results of a randomized, placebo-controlled multicentre trial in 239 patients. The digitalis in acute atrial fibrillation (daaf) trial group. Eur Heart J. 1997;18:649–654. doi: 10.1093/oxfordjournals.eurheartj.a015311. [DOI] [PubMed] [Google Scholar]
- 15.Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, Bergsma-Kadijk JA, Cornel JH, Kamp O, Tukkie R, Bosker HA, Van Veldhuisen DJ, Van den Berg MP. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 16.Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, Josephson RA, Kellen JC, Klein RC, Krahn AD, Mickel M, Mitchell LB, Nelson JD, Rosenberg Y, Schron E, Shemanski L, Waldo AL, Wyse DG. Relationships between sinus rhythm, treatment, and survival in the atrial fibrillation follow-up investigation of rhythm management (affirm) study. Circulation. 2004;109:1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 17.Hallberg P, Lindback J, Lindahl B, Stenestrand U, Melhus H. Digoxin and mortality in atrial fibrillation: A prospective cohort study. Eur J Clin Pharmacol. 2007;63:959–971. doi: 10.1007/s00228-007-0346-9. [DOI] [PubMed] [Google Scholar]
- 18.Whitbeck MG, Charnigo RJ, Khairy P, Ziada K, Bailey AL, Zegarra MM, Shah J, Morales G, Macaulay T, Sorrell VL, Campbell CL, Gurley J, Anaya P, Nasr H, Bai R, Di Biase L, Booth DC, Jondeau G, Natale A, Roy D, Smyth S, Moliterno DJ, Elayi CS. Increased mortality among patients taking digoxin-analysis from the affirm study. European Heart Journal. 2013;34:1481–1488. doi: 10.1093/eurheartj/ehs348. [DOI] [PubMed] [Google Scholar]
- 19.Turakhia MP, Santangeli P, Winkelmayer WC, Xu X, Ullal AJ, Than CT, Schmitt S, Holmes TH, Frayne SM, Phibbs CS, Yang F, Hoang DD, Ho PM, Heidenreich PA. Increased mortality associated with digoxin in contemporary patients with atrial fibrillation: Findings from the treat-af study. J Am Coll Cardiol. 2014;64:660–668. doi: 10.1016/j.jacc.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friberg L, Hammar N, Rosenqvist M. Digoxin in atrial fibrillation: Report from the stockholm cohort study of atrial fibrillation (scaf) Heart. 2010;96:275–280. doi: 10.1136/hrt.2009.175786. [DOI] [PubMed] [Google Scholar]
- 21.Gheorghiade M, Fonarow GC, van Veldhuisen DJ, Cleland JG, Butler J, Epstein AE, Patel K, Aban IB, Aronow WS, Anker SD, Ahmed A. Lack of evidence of increased mortality among patients with atrial fibrillation taking digoxin: Findings from post hoc propensity-matched analysis of the affirm trial. Eur Heart J. 2013;34:1489–1497. doi: 10.1093/eurheartj/eht120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, Chen W, Jacobsen SJ. Sociodemographic characteristics of members of a large, integrated health care system: Comparison with us census bureau data. Perm J. 2012;16:37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieger N. Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Go AS, Magid DJ, Wells B, Sung SH, Cassidy-Bushrow AE, Greenlee RT, Langer RD, Lieu TA, Margolis KL, Masoudi FA, McNeal CJ, Murata GH, Newton KM, Novotny R, Reynolds K, Roblin DW, Smith DH, Vupputuri S, White RE, Olson J, Rumsfeld JS, Gurwitz JH. The cardiovascular research network: A new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes. 2008;1:138–147. doi: 10.1161/CIRCOUTCOMES.108.801654. [DOI] [PubMed] [Google Scholar]
- 25.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: The anemia in chronic heart failure: Outcomes and resource utilization (anchor) study. Circulation. 2006;113:2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 26.Ray WA. Evaluating medication effects outside of clinical trials: New-user designs. Am J Epidemiol. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 27.Johnson ES, Bartman BA, Briesacher BA, Fleming NS, Gerhard T, Kornegay CJ, Nourjah P, Sauer B, Schumock GT, Sedrakyan A, Sturmer T, West SL, Schneeweiss S. The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf. 2013;22:1–6. doi: 10.1002/pds.3334. [DOI] [PubMed] [Google Scholar]
- 28.Go AS, Yang J, Gurwitz JH, Hsu J, Lane K, Platt R. Comparative effectiveness of different beta-adrenergic antagonists on mortality among adults with heart failure in clinical practice. Arch Intern Med. 2008;168:2415–2421. doi: 10.1001/archinternmed.2008.506. [DOI] [PubMed] [Google Scholar]
- 29.Newman TB, Brown AN. Use of commercial record linkage software and vital statistics to identify patient deaths. J Am Med Inform Assoc. 1997;4:233–237. doi: 10.1136/jamia.1997.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arellano MG, Petersen GR, Petitti DB, Smith RE. The california automated mortality linkage system (camlis) Am J Public Health. 1984;74:1324–1330. doi: 10.2105/ajph.74.12.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 32.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 33.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20:512–522. doi: 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garbe E, Kloss S, Suling M, Pigeot I, Schneeweiss S. High-dimensional versus conventional propensity scores in a comparative effectiveness study of coxibs and reduced upper gastrointestinal complications. Eur J Clin Pharmacol. 2013;69:549–557. doi: 10.1007/s00228-012-1334-2. [DOI] [PubMed] [Google Scholar]
- 35.Selby JV, Ray GT, Zhang D, Colby CJ. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care. 1997;20:1396–1402. doi: 10.2337/diacare.20.9.1396. [DOI] [PubMed] [Google Scholar]
- 36.Executive summary of the third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 37.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 38.K/doqi clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 39.Fireman BH, Fehrenbacher L, Gruskin EP, Ray GT. Cost of care for patients in cancer clinical trials. J Natl Cancer Inst. 2000;92:136–142. doi: 10.1093/jnci/92.2.136. [DOI] [PubMed] [Google Scholar]
- 40.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groenveld HF, Crijns HJ, Van den Berg MP, Van Sonderen E, Alings AM, Tijssen JG, Hillege HL, Tuininga YS, Van Veldhuisen DJ, Ranchor AV, Van Gelder IC. The effect of rate control on quality of life in patients with permanent atrial fibrillation: Data from the race ii (rate control efficacy in permanent atrial fibrillation ii) study. J Am Coll Cardiol. 2011;58:1795–1803. doi: 10.1016/j.jacc.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 42.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD Atrial Fibrillation Follow-up Investigation of Rhythm Management I. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 43.Hauptman PJ, Kelly RA. Digitalis. Circulation. 1999;99:1265–1270. doi: 10.1161/01.cir.99.9.1265. [DOI] [PubMed] [Google Scholar]
- 44.Lip GY, Metcalfe MJ, Dunn FG. Diagnosis and treatment of digoxin toxicity. Postgrad Med J. 1993;69:337–339. doi: 10.1136/pgmj.69.811.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Dart RC. 2010 annual report of the american association of poison control centers’ national poison data system (npds): 28th annual report. Clin Toxicol (Phila) 2011;49:910–941. doi: 10.3109/15563650.2011.635149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.