Abstract
Objective
World Health Organization (WHO) prospective surveys of acquired HIV drug resistance (HIVDR) evaluate HIVDR emerging after the first year of antiretroviral therapy (ART) and associated factors.
Methods
Consecutive ART starters in 2009 were enrolled at three sentinel sites in Namibia. Genotyping was performed at start and after 12 months in patients with HIV viral load (VL) >1000 copies/mL. HIVDR outcomes were: HIVDR Prevention (VL ≤1000 copies/mL), Possible HIVDR (VL>1000 copies/mL without detectable HIVDR or loss to follow-up (LTFU) or ART stop), and HIVDR (VL>1000 copies/mL with detectable HIVDR). Adherence was assessed using medication possession ratio (MPR).
Results
Of 394 starters, at 12 months 80% were on first-line ART, 1% died, 4% transferred out, 1% stopped ART, <1% switched to second-line and 15% were LTFU. Among patients on first-line, 77% had VL testing. 94% achieved VL ≤1000 copies/mL. At baseline, 7% had HIVDR. After 12 months, among patients with VL testing, 5% had HIVDR. A majority of patients failing therapy had high level resistance to non-nucleoside reverse transcriptase inhibitors but none to protease inhibitors. All sites achieved WHO target of ≥70% HIVDR Prevention. Factors associated with not achieving HIVDR Prevention were: baseline resistance to non-nucleoside reverse transcriptase inhibitors (OR 3.0, p=0.023), WHO stage 3 or 4 at baseline (OR 2.0, p=0.012), and MPR<75% (OR 4.9, p=0.021).
Conclusions
Earlier ART initiation and removal of barriers to on-time drug pickups may help to prevent HIVDR. These data inform decisions at national and global levels on the effectiveness of first- and second-line regimens.
Keywords: HIV, AIDS, Africa, anti-retroviral agents, drug resistance, adherence
Introduction
In the context of global antiretroviral therapy (ART) scale-up, population-level emergence of HIV drug resistance (HIVDR) is inevitable. HIVDR and associated treatment failure pose major challenges to successful ART scale-up and sustainability in resource-limited countries and necessitate surveillance of acquired HIVDR in populations receiving ART. Equally important within the context of the public health model of ART delivery is the identification of ART program practices that can be optimized to improve the quality of care and minimize HIVDR emergence [1]. In this context, the World Health Organization (WHO) recommends standardized methods for the assessment and prevention of HIVDR [2].
Namibia is a resource-limited country in sub-Saharan Africa that has been severely affected by the HIV epidemic. Approximately 13.3% of Namibia’s 2.1 million people are known to be infected with HIV [3]. The epidemic is predominantly spread via heterosexual contact and among pregnant women 15–49 years of age, approximately 18.8% are infected with HIV [4]. ART has been available in Namibia’s private sector since 1998 and in the public sector since 2003. In the public sector, ART is provided free of charge following a public health model of care [5–6]. Namibia has one of the highest ART coverage rates (78%) in Sub-Saharan Africa with 162,900 patients on ART as of December 2013 [Namibia Ministry of Health and Social Services (MoHSS), unpublished data]. As of March 2014, ART is available at 46 main public sites and at an additional 100 satellite/outreach service points, as well as 84 Integrated Management of Adolescent and Adult Illness (IMAI) clinics [Namibia MoHSS, unpublished data].
Individual patient HIVDR testing is not routinely available due to its high cost and Namibia’s limited laboratory infrastructure. Therefore, surveillance of population-level HIVDR to support public health decision making regarding choice of nationally recommended first- and second-line ART regimens is essential. In 2006, the Namibia MoHSS created an HIVDR Technical Working Group (TWG) and adopted the WHO strategy for the surveillance of HIVDR [7]. In 2006 Namibia completed its first survey of transmitted HIVDR (TDR) and documented low levels of TDR in the capital, Windhoek [Namibia MoHSS, unpublished data]. In 2010, 2013 and 2014, national HIVDR Early Warning Indicator (EWI) results highlighted high rates of lost to follow-up 12 months after ART initiation, raising concern about emergence of preventable HIVDR due to treatment interruptions amongst those lost from care [8–10].
WHO prospective surveys of acquired HIVDR monitor prevention of acquired HIVDR emergence 12 months after ART initiation at sentinel sites. These surveys also identify ART program factors that can be adjusted at the level of the ART site or program to minimize emergence of preventable HIVDR [11]. The specific objectives of the present surveys were to: 1) estimate the proportion of ART initiators at each site with HIVDR prior to starting of first-line ART and characterize baseline drug resistance mutations, 2) estimate the proportion of the patients receiving ART at each site 12 months after ART initiation that achieves prevention of HIVDR (defined as viral load (VL) suppression (<1,000 copies/mL)) and characterize HIVDR mutations at 12 months, 3) identify factors potentially associated with the prevention (or non-prevention) of HIVDR.
Methods
In accordance with WHO guidance at the time of the survey [11], we conducted prospective HIVDR surveys at three sentinel ART sites: Katutura State Hospital, Oshakati Intermediate Hospital, and Rundu Hospital, large referral hospitals located in three important geographic regions. These sites had ART available for at least five years prior to the start of the survey, provided ART according to national guidelines, and utilized national record keeping systems.
A cohort of consecutive ART initiators at each site was enrolled. A blood draw for HIVDR genotyping was collected on the day of the first ART drug pick-up. Previous antiretroviral drug (ARV) exposure including prevention of mother to child transmission (PMTCT) was captured by a survey questionnaire. Patients were followed prospectively for 12 months. At endpoint, each individual’s status was classified into one of the following categories: on first-line ART at 12 months, switch to second line ART, lost to follow-up (LTFU), ART stop by physician, or death [10]. For participants who reached the endpoints of on first-line ART at 12 months and switch to second-line ART, a blood draw was collected for VL quantitation; HIVDR genotyping was performed on specimens with VL >1000 copies/ml.
Basic patient information was abstracted from patient records including socio-demographics, ART regimen, date of ART start, CD4 cell count, WHO clinical stage, dates of drug pickups, ART regimen picked up, and numbers of days of pills dispensed.
Ethics statement
Ethical approval was obtained from the institutional review board at Tufts University School of Medicine in Boston, USA and the Republic of Namibia Ministry of Health and Social Services Ethics and Research Committee in Windhoek, Namibia. Written informed consent was obtained from all participants.
Study population
Survey participants satisfied the following inclusion criteria: 1) adults ≥18 years, 2) confirmed diagnosis of HIV-1 infection, 3) eligible for ART initiation, and 4) initiating ART at the site during the survey period, regardless of previous ARV exposures. Patients were excluded if they were: 1) enrolled in a clinical trial or research study, 2) part of observational cohort for whom more follow-up efforts were made than for other patients, 3) restarting ART at the site (having previously started and stopped ART by the physician at the site), 4) transferring in from another site on ART.
Viral Load Testing and Genotyping
Specimens from baseline and endpoint blood draws were sent to the National Institute of Communicable Diseases (NICD) Johannesburg, South Africa for VL quantitation (using Ampliprep Taqman V1 assays) and genotyping (using an in-house assay certified by the Virology Quality Assessment Program) [12]. Predicted HIVDR for each drug was determined by Stanford database (HIVdb) scoring (baseline and endpoint) [13]. Resistance to protease inhibitors (PIs) without ritonavir boosting was not counted. At baseline the prevalence of mutations on the WHO surveillance drug resistance mutations list (SDRM) was also determined [14].
HIVDR at Baseline and Endpoint
Baseline HIVDR classifications were: HIVDR (HIVDR detected by genotyping), Possible HIVDR (HIVDR not detected but patient with a history of ARV exposure) and No HIVDR (HIVDR not detected and patient with no history of ARV exposure). Patients were included in these categories if they had a successfully amplified baseline genotype.
Endpoint HIVDR outcomes were: HIVDR Prevention (VL≤1000 copies/mL), HIVDR (VL>1000 copies/mL and HIVDR detected), and Possible HIVDR (VL>1000 copies/mL and no detected HIVDR, patients LTFU, or patients who stopped ART). Patients who died or transferred out were censored from this analysis. Patients who did not receive a 12-month VL test were censored from this analysis. All patients with a classifiable endpoint VL>1000 copies/mL and a successfully amplified genotype were included.
ART Adherence
Adherence to ART was assessed using two different adherence measures: medication possession ratio (MPR) and on-time pill pickup (PPU). MPR measures the time an individual is in possession of his/her pills as a proportion of the time between two drug pickups [15]. MPR was calculated over the entire duration of time from ART initiation until survey endpoint with the formula: (1 – (number of days late for pick-up/number of days between first and last ARV pickup)) X 100. Patients were classified into three groups based on MPR <80, 80–94, and ≥95. Patients were also classified above or below a 75% MPR threshold based on previous operational research [16].
PPU per WHO EWI guidance [17] was assessed by calculating the number of days late a patient picked up pills from the day he/she would have run out of pills if taken according to schedule (pill run-out-date). The pill run-out-date was determined by adding the number of days of pills dispensed and the number of days of pills with which the patient returned (pill count). A pill pickup was defined as “late” if it occurred more than 2 days after the pill run-out-date [17]. Patients were classified based on how many late pickups they experienced (e.g. no late pickups, late 1 time, or late ≥2 times). Patients were also classified based on percentage of total pickups that were “late”, using ≥20% as the threshold for non-adherent.
Sample size at each site
The goal sample size at each sentinel site was 96 individuals classifiable at endpoint after censoring deaths and transfers of care to other clinics (transfers out). This is the minimum sample size needed to estimate the proportion of all ART starters with HIVDR Prevention 12 months after start of ART with a 95% confidence interval width of +/−10%. We enrolled 130 ART starters at each site in order to accommodate for the numbers anticipated to transfer out or to have died during the survey period so that each site would achieve the goal of 96. These calculations were based on outcomes from the first 100 patients starting ART in the same quarter at the site in the previous year. Sample sizes were not intended to be sufficiently large to assess for statistical significance of factors potentially associated with HIVDR prevention.
Statistical Analysis
Bivariate associations were examined between factors and outcomes using chi square tests for categorical variables and Wilcoxon Rank Sum tests for ordinal and continuous measures. Three separate analyses were performed: 1) factors associated with baseline non-nucleoside reverse transcriptase inhibitor (NNRTI) drug resistance, 2) factors associated with VL failure 12 months after ART initiation, and 3) factors associated with not achieving HIVDR prevention (HIVDR or Possible HIVDR) 12 months after ART initiation.
For analysis 1, factors analyzed included baseline NNRTI drug resistance with history of PMTCT exposure (in women), prior ARV exposure, and a combination of PMTCT or previous ARV exposure. For analysis 2 and 3, bivariate statistics were obtained for each variable included in this analysis such as ART site, sex, baseline CD4 cell count, baseline WHO stage, baseline ART regimen, baseline ARV and PMTCT exposure, and pharmacy adherence measures. Frequencies and distributions were examined for unusual values. For all statistical analyses, an alpha of 0.05 was used to evaluate statistically significant differences or associations. All analyses were performed using STATA version 12 (College Station Texas).
Results
Study flow
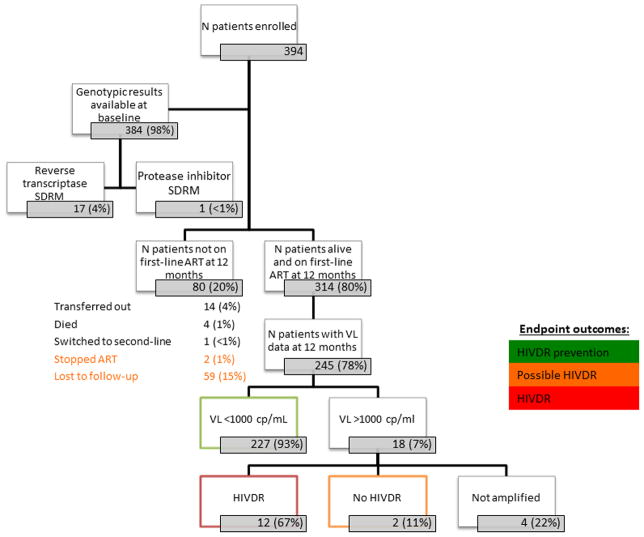
A total of 394 patients were enrolled, of which 384 had baseline HIVDR genotypes available for analysis. (10 specimens were not successfully amplified). (Figure 1) Of the 384 with a baseline genotype, 5% had any detected SDRM (17 had reverse transcriptase and 1 had PI mutations). At endpoint, 314 of 394 patients (80%) were alive and on first-line ART while 80 patients (20%) were not on first-line ART. Fifty-nine (15%) were LTFU, 4 (1%) died, 2 (1%) stopped ART, 14 transferred out to another ART site, and 1 (<1%) was switched to second-line ART. Of the 314 patients alive and on first-line ART at 12 months, 245 (78%) had VL testing. Two hundred and twenty-seven of the 245 (93%) had VL suppression. Of those not suppressed at 12 months and successfully genotyped, 12 of 14 (86%) had any predicted HIVDR per Stanford HIVdb.
FIGURE 1. Flow diagram of individuals enrolled in survey: from baseline to 12-month endpoints.
SDRM= WHO surveillance drug resistance mutations list
ART=antiretroviral therapy
VL=viral load
cp/mL=copies/mL
HIVDR=HIV drug resistance
Patient Characteristics
Table 1 displays the patient characteristics of 394 participants at ART initiation. Median age was 35 years and 71% were female. Fifty-seven percent (57%) had a CD4 cell count < 200 cells/mL. Thirty percent (30%) had WHO clinical stage 3 or 4. Eighteen percent (18%) reported previous ARV exposure or previous PMTCT. Twenty-one percent (21%) of women reported previous PMTCT at baseline. Ninety-nine percent (99%) were started on NNRTI-based regimens.
TABLE 1.
Patient Characteristics
| Characteristics | Katutura State Hospital | Oshakati Hospital | Rundu Hospital | Overall |
|---|---|---|---|---|
|
| ||||
| n=134 | n=131 | n=129 | N=394 | |
|
| ||||
| N (%), Median (Q1, Q3), or numerator/denominator (%) | N (%), Median (Q1, Q3), or numerator/denominator (%) | N (%), Median (Q1, Q3), or numerator/denominator (%) | N (%), Median (Q1, Q3), or numerator/denominator (%) | |
| Age at ART start (years) | 33.6 (29.9, 39.4) | 37.1 (31.7, 43.2) | 34.4 (29.3, 41.7) | 35.0 (30.2, 41.5) |
| Sex | ||||
| Male | 26 (19%) | 57 (44%) | 30 (23%) | 113 (29%) |
| Female | 108 (81%) | 74 (56%) | 99 (77%) | 281 (71%) |
| CD4 cell count at baseline | ||||
| <200 cells/mL | 46 (34%) | 95 (73%) | 85 (66%) | 226 (57%) |
| 200–350 cells/mL | 80 (60%) | 20 (15%) | 42 (33%) | 142 (36%) |
| ≥351 cells/mL | 3 (2%) | 14 (11%) | 2 (2%) | 19 (5%) |
| Missing | 5 (4%) | 2 (2%) | 0 (0%) | 7 (2%) |
| WHO Clinical Stage at baseline | ||||
| Stage 1 | 88 (66%) | 22 (17%) | 14 (11%) | 124 (31%) |
| Stage 2 | 28 (21%) | 53 (40%) | 70 (54%) | 151 (38%) |
| Stage 3 | 14 (10%) | 40 (31%) | 37 (29%) | 91 (23%) |
| Stage 4 | 3 (2%) | 16 (12%) | 8 (6%) | 27 (7%) |
| Missing | 1 (1%) | 0 (0%) | 0 (0%) | 1 (0%) |
| Previous ARV exposure or Previous PMTCT at baseline | 38 (28%) | 19 (15%) | 13 (10%) | 70 (18%) |
| Previous ARV exposure at baseline | 4 (3%) | 9 (7%) | 0 (0%) | 13 (3%) |
| Previous PMTCT at baseline (% of women) | 36 (33%) | 11 (15%) | 13 (13%) | 60 (21%) |
| ART regimen at baseline | ||||
| ZDV+3TC+NVP | 85 (63%) | 72 (55%) | 68 (52%) | 225 (57%) |
| ZDV+3TC+EFV | 23 (17%) | 20 (15%) | 8 (6%) | 51 (13%) |
| d4T+3TC+NVP | 2 (2%) | 18 (14%) | 16 (12%) | 36 (9%) |
| d4T+3TC+EFV | 4 (3%) | 5 (4%) | 5 (4%) | 14 (4%) |
| TDF+3TC+NVP | 11 (8%) | 10 (8%) | 22 (17%) | 43 (11%) |
| ZDV+TDF+3TC+EFV | 9 (7%) | 5 (4%) | 8 (6%) | 22 (6%) |
| +3TC+LPV/r | 0 (0%) | 1 (1%) | 0 (0%) | 1 (0%) |
| TDF+ZDV+3TC+LPV/r | 0 (0%) | 0 (0%) | 2 (2%) | 2 (1%) |
| Medication Possession Ratio | n=96 | n=96 | n=110 | N=302 |
| Missing | 3 | 5 | 5 | 13 |
| ≥95% | 65 (70%) | 62 (68%) | 83 (79%) | 210 (73%) |
| 80–94% | 24 (26%) | 23 (25%) | 18 (17%) | 65 (22%) |
| <80% | 4 (4%) | 6 (7%) | 4 (4%) | 14 (5%) |
| ≥75% | 90 (97%) | 88 (97%) | 102 (97%) | 280 (97%) |
| <75% | 3 (3%) | 3 (3%) | 3 (3%) | 9 (3%) |
| On-time Pill Pickup (>2 days late from pill run-out date) | n=96 | n=96 | n=110 | N=302 |
| Missing | 3 | 5 | 5 | 13 |
| Never late | 38 (41%) | 26 (29%) | 53 (50%) | 117 (40%) |
| Late 1 time | 43 (45%) | 45 (47%) | 36 (33%) | 124 (41%) |
| Late ≥ 2 times | 12 (12%) | 30 (21%) | 16 (15%) | 48 (15%) |
| ≥20% of pickups late (out of total pickups) | 22 (24%) | 20 (22%) | 24 (23%) | 66 (23%) |
| 12-month HIV RNA result available among patients alive and on ART | 73/103 (71%) | 76/104 (73%) | 96/107 (90%) | 245/314 (78%) |
| 12-month HIV RNA suppressed (VL≤1000 copies/mL) | 70/73 (96%) | 70/76 (92%) | 87/96 (91%) | 227/245 (93%) |
ART=antiretroviral therapy
ARV=antiretroviral
NVP=nevirapine; EFV=efavirenz, TDF=tenofovir; 3TC=lamivudine; ZDV=zidovudine; d4T=stavudine; LPV/r=lopinavir/ritonavir
VL=viral load
RNA=ribonucleic acid
Amongst all patients with pill pickup data available, 73% of patients had MPR ≥95% and 97% had MPR ≥75%. (Table 1) Forty-three percent (43%) of patients had no “late” pill pickups and 22% had ≥20% of all pickups “late”.
The proportion with 12-month on treatment VL suppression was 93% (96% Katutura State Hospital, 92% Oshakati Hospital and 91% Rundu Hospital). (Table 1)
Baseline HIVDR Classifications and Characterization
Seventy-eight percent (78%) of patients were classified as having No HIVDR at baseline (67% Katutura State Hospital, 83% Oshakati Hospital, 84% Rundu Hospital), 16% as Possible HIVDR (25% Katutura State Hospital, 14% Oshakati Hospital, 8% Rundu Hospital) and 7% as HIVDR (9% Katutura State Hospital, 3% Oshakati Hospital, 9% Rundu Hospital). (Figure 2a)
FIGURE 2. Baseline HIVDR classifications, drug resistance and mutation prevalence.
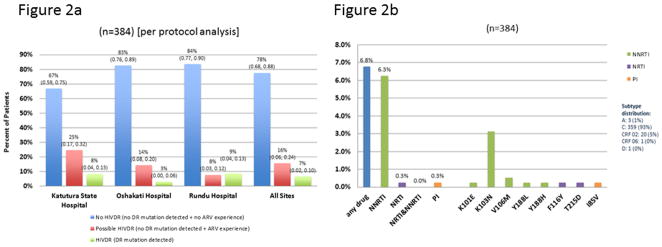
Figure 2a.
Denominator = classifiable baseline genotype
HIVDR=HIV drug resistance
DR=drug resistance
ARV=antiretrovirals
Figure 2b.
Excludes resistance to unboosted PIs
Predicted HIVDR per Stanford HIVdb
Mutations as defined by 2009 WHO surveillance drug resistance mutations (SDRM) list
NNRTI=non-nucleoside reverse transcriptase inhibitors
NRTI=nucleoside/nucleotide reverse transcriptase inhibitors
PI=protease inhibitors
Out of 384 patients at baseline, 6.8% had predicted resistance to any drug, 6.3% to NNRTIs, 0.3% to nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), and 0.3% to PIs. SDRMs detected were K103N (3.1%), K101E (0.3%), V106M (0.5%), Y188L (0.3%), Y188H (0.3%), T215D (0.3%), and I85V (0.3%). (Figure 2b) Ninety-three percent (93%) were subtype C.
Endpoint HIVDR Outcomes and Characterization
Seventy-five percent (75%) of patients were classified as having HIVDR Prevention (73% Katutura State Hospital, 73% Oshakati Hospital, 79% Rundu Hospital), 21% as Possible HIVDR (24% Katutura State Hospital, 22% Oshakati Hospital, 17% Rundu Hospital), and 4% as HIVDR (3% Katutura State Hospital, 5% Oshakati Hospital, 4% Rundu Hospital). (Figure 3a) All sites met the WHO target of ≥70% HIVDR Prevention. Most Possible HIVDR were due to patients classified as LTFU as few stopped ART or had virological failure without detected HIVDR.
FIGURE 3. Endpoint HIVDR classification and drug resistance.
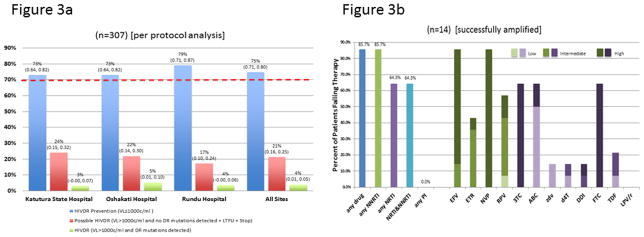
Figure 3a.
Denominator = classifiable endpoint genotype + LTFU + stopped ART – (died + transferred out)
HIVDR=HIV drug resistance
VL=viral load
DR=drug resistance
LTFU=lost to follow-up
c/mL=copies/mL
Figure 3b.
Predicted HIVDR per Stanford HIVdb
ZDV+TDF+3TC+LPV/r is the recommended second-line regimen in Namibia
NNRTI=non-nucleoside reverse transcriptase inhibitor
NRTI=nucleoside/nucleotide reverse transcriptase inhibitor
PI=protease inhibitor
NVP=nevirapine; EFV=efavirenz, ETR=etravirine, RPV=rilpivirine, TDF=tenofovir; 3TC=lamivudine; ABC=abacavir, ZDV=zidovudine; d4T=stavudine; DDI=didanosine, FTC=emtricitabine, LPV/r=lopinavir/ritonavir
Of the 14 patients with endpoint genotyping, 85.7% had predicted resistance to any drug class, 85.7% to NNRTIs, 64.3% to NRTIs, 64.3% to NRTIs and NNRTIs. (Figure 3b) There was no predicted PI resistance.
Factors Associated with Baseline NNRTI Resistance
History of PMTCT exposure (in women), prior ARV exposure, and a combination of PMTCT or prior ARV exposure were not found to be significantly associated with NNRTI resistance at baseline. (Supplemental Table 1)
Factors Associated with Viral Load Suppression
Baseline NNRTI drug resistance (OR 8.8, p<0.001) was significantly associated with virological failure at 12 months. (Supplemental Table 2) Pharmacy adherence measures were found to be associated with VL >1000 copies/mL at 12 months, specifically MPR (MPR<95% OR 4.8, p=0.002; MPR<80% OR 5.5, p=0.019) and on-time pill pickup (having experience one “late” pill pickup OR 6.3, p=0.004; ≥20% late pickups OR 14.3, p<0.001).
Factors Associated with HIVDR Outcomes
Baseline NNRTI drug resistance (OR 3.0, p=0.023), and WHO stage 3 or 4 at time of ART start (OR 2.0, p=0.012) were significantly associated with not achieving HIVDR prevention. (Table 2) The pharmacy adherence measure, MPR was found to be associated with not achieving HIVDR Prevention at 12 months (MPR<75 OR 4.9, p=0.021).
TABLE 2.
Factors associated with HIVDR or Possible HIVDR at 12 months
| Factors | n | OR (95 CI) | p-value |
|---|---|---|---|
| Site | 302 | ||
| Katutura State Hospital (reference) | Ref | ||
| Oshakati Hospital | 1 (0.5, 1.9) | 0.99 | |
| Rundu Hospital | 0.7 (0.4, 1.4) | 0.30 | |
| Female | 302 | 1.1 (0.6, 1.9) | 0.86 |
| Age | 302 | 0.98 (0.96, .01) | 0.17 |
| CD4 cell count Baseline | 297 | ||
| CD4 <200 cells/mL (reference) | Ref | ||
| CD4 200–350 cells/mL | 0.9 (0.5, 1.6) | 0.80 | |
| CD4 >351 cells/mL | 1.1 (0.3, 3.8) | 0.84 | |
| WHO stage at baseline | 301 | ||
| 1 (reference) | Ref | ||
| 2 | 1.1 (0.6, 2.2) | 0.75 | |
| 3 | 2.1 (1.0, 4.4) | 0.038 | |
| 4 | 2.2 (0.8, 6.3) | 0.14 | |
| WHO stage 3 or 4 at baseline | 301 | 2.0 (1.2, 3.5) | 0.012 |
| Baseline ART Regimens | |||
| EFV based vs NVP based (reference) | 301 | 1.3 (0.7, 2.4) | 0.40 |
| TDF based (reference) | 301 | Ref | |
| ZDV based | 1.6 (0.7, 3.5) | 0.25 | |
| d4T based | 1.5 (0.6, 4.1) | 0.40 | |
| Baseline NNRTI drug resistance | 295 | 3.0 (1.2, 7.7) | 0.023 |
| PMTCT exposure (women only) | 219 | 0.5 (0.2, 1.2) | 0.12 |
| Previous ARV exposure | 302 | 0.9 (0.2, 4.2) | 0.85 |
| Prior ARV or PMTCT exposure | 302 | 0.6 (0.3, 1.3) | 0.20 |
| MPR continuous | 289 | 0.95 (0.92, 0.98) | 0.003 |
| MPR groups | 289 | ||
| <0.80 | 3.3 (1.1, 10.0) | 0.036 | |
| 0.80–0.94 | 1.6 (0.8, 3.0) | 0.19 | |
| ≥0.95 (reference) | Ref. | -- | |
| MPR <75 | 289 | 4.9 (1.3, 18.8) | 0.021 |
| MPR <95 | 289 | 1.8 (1.0, 3.3) | 0.054 |
ART=antiretroviral therapy
ARV=antiretroviral
NVP=nevirapine; EFV=efavirenz, TDF=tenofovir; ZDV=zidovudine; d4T=stavudine
NNRTI=non-nucleoside reverse transcriptase inhibitors
MPR=medication possession ratio
PMTCT=prevention of mother to child transmission
HIVDR=HIV drug resistance
Discussion
These surveys provide the first data from Namibia on baseline (pre-treatment) and acquired HIVDR and contribute valuable information for evidence-based decision making. Specifically, results support the ongoing effectiveness of currently available first-line ART regimens in Namibia and identify country-specific practices associated with prevention of HIVDR amongst those receiving ART. Results also contribute to discussions on the selection of first- and second-line ART regimens, as well as regimens for prophylaxis including PMTCT.
Baseline HIVDR was predominantly to NNRTIs with very little resistance to NRTIs or PIs. The most commonly detected SDRM was K103N, which is not unexpected in a population treated with primarily NNRTI-based regimens. Baseline HIVDR of 6.8% was observed and is consistent with the 2012 WHO HIVDR Global Report which found 5.0% baseline HIVDR (4.3% African Region and 5.1% Southern African sub-region) [18]. Pre-treatment HIVDR in Namibia could be due to TDR or HIVDR acquired as a result of previous ARV exposure (for example PMTCT) and warrants further studies to determine its etiology. The Namibia TWG is currently analyzing national TDR using the antenatal care sentinel survey data.
All three sentinel sites achieved the WHO target for HIVDR Prevention after 12 months (≥70% HIVDR Prevention) [11]. The three sites combined achieved 75% HIVDR Prevention, which is comparable to data reported in the WHO African Region of 76.6% HIVDR Prevention, but lower than Southern Africa (80.3%) [18]. These favorable results are due primarily to the high levels of virological suppression (93%) in patients on first-line ART at 12 months, which suggests that current recommended first-line ART regimens are highly effective in this population. Among all ART starters, detected HIVDR after 12 months was 4%, which is consistent with the reported 4.7% in Africa and 4.7% in Southern Africa. HIVDR detected among patients genotyped was 85.7% compared to 69.5% in the African region and 73.3% in the Southern African region [18].
In patients failing ART at 12 months the majority had predicted HIVDR to NNRTIs, EFV and NVP with very little resistance to PIs. High levels of resistance were present to second-generation NNRTIs, etravirine (43%) and rilpivirine (57%), predominantly driven by Y181C. These results suggest caution in the use of these agents for second-line or salvage therapy options. Nonetheless it is reassuring that these survey results show that the vast majority with virological failure at 12 months would be expected to achieve virological suppression if switched to a WHO-recommended 2nd line regimen [19].
At 12 months, 21% were classified as Possible HIVDR compared to 18.8% in the African and 15.0% in the Southern African regions [18]. Possible HIVDR can be due to patients LTFU or patients with virological failure at 12 months but no detectable HIVDR. Not detecting HIVDR in patients failing ART may be accounted for by the fact that HIVDR may have been present but predominantly reverted to drug-sensitive wild-type virus. Moreover, standard population-based sequencing (standard commercial and laboratory assays) only detects HIVDR if it is present at about 10–20% of the virus population [20–21]. Notably, HIVDR present as minority variants may pass undetected, persisting for months or years after treatment and may re-emerge in the viral population after treatment is reinitiated, impacting treatment outcomes adversely [22–24]. Possible HIVDR in Namibia was primarily due to LTFU (not virological failure without detected HIVDR). Patients LTFU are more likely to have experienced treatment interruptions. Treatment interruptions of NNRTI-based regimens of 48 hours or longer are associated with the selection of NNRTI drug resistance and increased risk of virological failure [25–26]. Results from this survey and data from Namibia’s EWIs underscore the urgent need to improve retention of patients on ART. To start addressing this need, the ART program has initiated operational research to characterize LTFU and test interventions to improve retention on ART including an effort to intensify defaulter tracing mechanisms.
These data suggest that patients with NNRTI drug resistance at start of therapy are at high risk of failing first-line NNRTI-based therapy, which is not unexpected. However, further cost-effectiveness analyses needs to be conducted to determine whether individual genotype testing is warranted in all patients initiating ART in Namibia and/or other resource-limited settings.
These data indicate that patients with more advanced disease (WHO stage) at start of ART initiation are at higher risk of not achieving HIVDR Prevention at 12 months, a finding consistent with data in other settings that point towards earlier start of ART [27]. Therefore, the ART program should focus efforts on early diagnosis and treatment in an effort to minimize emergence of HIVDR. Additionally, patients who are LTFU before ART initiation are at higher risk of death or starting ART at a more advanced HIV disease state [28]. Therefore, to start addressing concerns about attrition from time of diagnosis to start of ART, the country is currently conducting a study to quantify retention in care prior to treatment initiation.
Pharmacy adherence measures were found to be associated with not achieving HIVDR Prevention and virologic failure at 12 months. Therefore, MPR and PPU may be useful tools to identify patients at risk of failing therapy and developing HIVDR at 12 months. Additionally, in a recent publication, we found that MPR was associated with short-term virological response (VL at 6 months), suggesting its utility for early identification of patients at high risk for virologic failure and emergence of HIVDR [16]. Therefore, the ART program is considering the use of a combination of MPR and on-time pill pickup to identify patients at risk of early ART failure for targeted adherence intervention to remove barriers to on-time pill pickup.
This study has some limitations. The three sentinel sites were selected to represent the large ART sites in Namibia’s different geographic areas and are not representative of the national ART program. Additionally, there was a proportion of patients who were on first-line ART at 12 months and did not receive VL testing due to ART clinic error. We assessed patient characteristics between those that received VL testing and those that did not and did not find any significant differences. However, there may have still been bias introduced which affected VL suppression rates. Finally, the recent changes to more tenofovir use instead of zidovudine may limit the current generalizability of these findings.
In conclusion, results from these surveys of HIVDR demonstrate the sentinel sites are functioning well to optimize levels of virologic suppression and minimize emergence of HIVDR. Additionally, these surveys demonstrate an important level of baseline HIVDR which necessitates the need for nationally representative estimates of pre-treatment and acquired HIVDR. Namibia plans to implement WHO’s updated 2014 guidance to estimate national prevalence estimates of HIVDR [29–30], which will facilitate trend analyses and national program decision making. These surveys will facilitate country-specific cost-effectiveness analyses of interventions such as increased frequency of VL testing and individual patient HIVDR testing prior to start of ART. Additionally, results from these surveys will better support discussions and evidence-based decisions on the effectiveness of internationally recommended first- and second-line regimens, discussions on vaccine design, development of microbicides for HIV prevention and the selection of ARV regimens for prophylaxis, including PMTCT.
Supplementary Material
Acknowledgments
The authors would like to thank the study participants, the Republic of Namibia Ministry of Health and Social Services (Andrew Ndishishi, Norbert P. Forster, Ella Shihepo, Anna-Maria Nitschke), Namibia Institute of Pathology, Management Sciences for Health/Strengthening Pharmaceutical Systems funded by USAID, WHO-Namibia (Magda Robalo, Tiruneh Desta), WHO-Geneva (Silvia Bertagnolio), University of Namibia School of Medicine (Peter Nyarango, Philip Odonkor). We would also like to make a special acknowledgement of James Mukamba (WHO-Namibia) who made considerable contributions to this work but passed away in 2013.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest.
Financial disclosure: This work was supported by the Spanish Government Grant AF/NAM/BBA/701/XU/08, the Bill and Melinda Gates Foundation #38180, the National Institutes of Health (NIH), NIH 1K23AI097010-01A1 (SYH), NIH L30 AI080268-02 (SYH), NIH T32 AI007438-16 (SYH), NIH K23 AIO74423-05 (MRJ), Lifespan/Tufts/Brown Center for AIDS Research P30 AI042853 (AMT), and Harold Williams, MD Medical Student Research Fellowship, Tufts University School of Medicine (KL), Christine E. Driscoll O’Neill and James M. Driscoll, Driscoll-O’Neill Charitable Foundation (AT).The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–10. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. [Accessed 4 August 2014];WHO global strategy for the surveillance and monitoring of HIV drug resistance. 2012 http://www.who.int/hiv/pub/drugresistance/drug_resistance_strategy/en/index.html.
- 3.Central Intelligence Agency. Namibia. [Accessed 4 August 2014];The World Factbook. 2014 https://www.cia.gov/library/publications/the-world-factbook/geos/wa.html.
- 4.Republic of Namibia Ministry of Health and Social Services. Report on the 2012 National HIV Sentinel Survey [Google Scholar]
- 5.Republic of Namibia Ministry of Health and Social Services Directorate of Special Programs. [Accessed 18 October 2011];National Guidelines for Antiretroviral Therapy. (3). 2010 http://www.who.int/hiv/pub/guidelines/namibia_art.pdf.
- 6.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [Accessed 18 October 2011];Recommendations for a public health approach. 2013 Jun; http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [PubMed]
- 7.Bennett DE, Bertagnolio S, Sutherland D, et al. The World Health Organization’s global strategy for prevention and assessment of HIV drug resistance. Antivir Ther. 2008;13(Suppl 2):1–13. [PubMed] [Google Scholar]
- 8.Hong SY, Jonas A, Dumeni E, et al. Population-based Monitoring of HIV Drug Resistance in Namibia with Early Warning Indicators. J Acquir Immune Defic Syndr. 2010 Dec;55(4):27–31. doi: 10.1097/QAI.0b013e3181f5376d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonas A, Gwesha J, Siboleka M, et al. HIV Drug Resistance Early Warning Indicators in Namibia for Public Health Action. PLoS ONE. 2013;8(6):e65653. doi: 10.1371/journal.pone.0065653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonas A, Sumbi V, Mwinga S, et al. HIV Drug Resistance Early Warning Indicators in Namibia with Updated World Health Organization Guidance. PLoS One. 2014 Jul 2;9(7):e100539. doi: 10.1371/journal.pone.0100539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan MR, Bennett DE, Bertagnolio S, et al. World Health Organization surveys to monitor HIV drug resistance prevention and associated factors in sentinel antiretroviral treatment sites. Antivir Ther. 2008;13:15–23. [PubMed] [Google Scholar]
- 12.Pillay V, Ledwaba J, Hunt G, et al. Antiretroviral drug resistance surveillance among drug-naive HIV-1-infected individuals in Gauteng Province, South Africa in 2002 and 2004. Antiviral Therapy. 2008;13(Suppl 2):101–107. [PubMed] [Google Scholar]
- 13.Liu TF, Shafer RW. Web Resources for HIV type 1 Genotypic-Resistance Test Interpretation. Clin Infect Dis. 2006;42(11):1608–18. doi: 10.1086/503914. Epub 2006 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett DE, Camacho RJ, Otelea D, et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One. 2009;4(3):e4724. doi: 10.1371/journal.pone.0004724. Epub 2009 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahon JH, Jordan MR, Kelley K, et al. Pharmacy adherence measures to assess adherence to antiretroviral therapy: review of the literature and implications for treatment monitoring. Clin Infect Dis. 2011;15;52(4):493–506. doi: 10.1093/cid/ciq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong SY, Jerger L, Jonas A, et al. Medication Possession Ratio Associated with Short-Term Virologic Response in Individuals Initiating Antiretroviral Therapy in Namibia. PLoS ONE. 2013;8(2):e56307. doi: 10.1371/journal.pone.0056307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Meeting Report on Assessment of World Health Organization HIV Drug Resistance Early Warning Indicators: Report of the Early Warning Indicator Advisory Panel Meeting; 11–12 August 2011; Geneva, Switzerland. [Accessed 4 August 2014]. http://apps.who.int/iris/bitstream/10665/75186/1/9789241503945_eng.pdf. [Google Scholar]
- 18.World Health Organization. [Accessed 4 August 2014];WHO HIV drug resistance report. 2012 http://www.who.int/hiv/pub/drugresistance/report2012/en/
- 19.World Health Organization. [Accessed 4 August 2014];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. http://www.who.int/hiv/pub/guidelines/arv2013/download/en/
- 20.Erali M, Page S, Reimer LG, et al. Human immunodeficiency virus type 1 drug resistance testing: a comparison of three sequence-based methods. J Clin Microbiol. 2001 Jun;39(6):2157–65. doi: 10.1128/JCM.39.6.2157-2165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eshleman SH, Crutcher G, Petrauskene O, et al. Sensitivity and specificity of the ViroSeq human immunodeficiency virus type 1 (HIV-1) genotyping system for detection of HIV-1 drug resistance mutations by use of an ABI PRISM 3100 genetic analyzer. J Clin Microbiol. 2005 Feb;43(2):813–7. doi: 10.1128/JCM.43.2.813-817.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecossier D, Shulman NS, Morand-Joubert L, et al. Detection of minority populations of HIV-1 expressing the K103N resistance mutation in patients failing nevirapine. J Acquir Immune Defic Syndr. 2005;38:37–42. doi: 10.1097/00126334-200501010-00007. [DOI] [PubMed] [Google Scholar]
- 23.Dykes C, Najjar J, Bosch RJ, et al. Detection of drug-resistant minority variants of HIV-1 during virologic failure of indinavir, lamivudine, and zidovudine. J Infect Dis. 2004;189:1091–1096. doi: 10.1086/382033. [DOI] [PubMed] [Google Scholar]
- 24.Halvas EK, Wiegand A, Boltz VF, et al. Low frequency nonnucleoside reverse-transcriptase inhibitor-resistant variants contribute to failure of efavirenz-containing regiment in treatment-experienced patients. J Infect Dis. 2010;201:672–680. doi: 10.1086/650542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parienti JJ, Massari V, Descamps D, et al. Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis. 2004;38(9):1311–1316. doi: 10.1086/383572. [DOI] [PubMed] [Google Scholar]
- 26.Oyugi JH, Byakika-Tusiime J, Ragland K, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in kampala, uganda. AIDS. 2007;21(8):965–971. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- 27.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014 Apr;14(4):281–90. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawn SD, Myer L, Harling G, et al. Determinants of Mortality and Nondeath Losses from an Antiretroviral Treatment Service in South Africa: Implications for Program Evaluation. Clin Infect Dis. 206;43(6):770–6. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. [Accessed 4 August 2014];Surveillance of HIV drug resistance in adults initiating antiretroviral therapy (pre-treatment HIV drug resistance) http://www.who.int/hiv/pub/drugresistance/pretreatment_drugresistance/en/
- 30.World Health Organization. [Accessed 4 August 2014];Surveillance of HIV drug resistance in adults receiving ART. http://www.who.int/hiv/pub/drugresistance/acquired_drugresistance/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.