Abstract
Cardiac mitochondria are responsible for generating energy in the form of ATP through oxidative phosphorylation and are crucial for cardiac function. Mitochondrial dysfunction is a major contributor to loss of myocytes and development of heart failure. Myocytes have quality control mechanisms in place to ensure a network of functional mitochondria. Damaged mitochondria are degraded by a process called mitochondrial autophagy, or mitophagy, where the organelle is engulfed by an autophagosome and subsequently delivered to a lysosome for degradation. Evidence suggests that mitophagy is important for cellular homeostasis, and reduced mitophagy leads to inadequate removal of dysfunctional mitochondria. In this review, we discuss the regulation of mitophagy and the emerging evidence of the cardioprotective role of mitophagy. We also address the prospect of therapeutically targeting mitophagy to treat patients with cardiovascular disease.
Keywords: Mitophagy, Heart failure, Autophagy, Mitochondria
Introduction
Cardiac mitochondria are responsible for generating energy in the form of ATP through oxidative phosphorylation (OxPhos) and are crucial for cardiac function. Therefore, it is not surprising that mitochondrial dysfunction is considered to be a major contributor to the development of heart failure. Dysfunctional mitochondria are less efficient at generating ATP, produce excessive amounts of reactive oxygen species (ROS), and are more likely to activate cell death [1]. Although mitochondria generate ROS as a by-product of oxidative phosphorylation, they are also highly susceptible to ROS-mediated damage. For example, mitochondrial DNA (mtDNA) is susceptible to ROS due to its proximity to the respiratory chain in the mitochondrial inner membrane and the lack of protective histone-like proteins. Studies have found that cardiac mtDNA accumulates mutations with age, which contributes to mitochondrial dysfunction and development of age-related cardiomyopathy [2, 3].
To ensure a network of functional mitochondria, myocytes have quality control mechanisms in place that act at both the protein and organelle levels. Damaged mitochondrial proteins are degraded by proteases in the mitochondria and the ubiquitin-proteasome system [4]. If the degradation of damaged proteins is insufficient to rescue the mitochondrion, then, the organelle is engulfed by an autophagosome and subsequently delivered to a lysosome for degradation. This selective removal of impaired mitochondria by autophagosomes is known as mitophagy. Both autophagy and mitophagy are critical for cellular homeostasis [5–8] and adapting to acute unfavorable conditions such as starvation or ischemia [9–11]. Unfortunately, both of these processes are reduced with age, which leads to inadequate removal of dysfunctional mitochondria [6, 12]. In this review, we discuss the regulation of mitophagy and the emerging evidence of its role in cardioprotection. We also address the prospect of targeting mitophagy therapeutically to treat patients with cardiovascular disease.
Autophagy in the myocardium
Macroautophagy, hereafter referred to as autophagy, is the process by which cells segregate and degrade cellular proteins and organelles. The process of autophagy involves a double-membraned autophagosome that engulfs cytoplasm and organelles and then fuses with a lysosome for degradation [13]. The activation of a complex composed of BECLIN 1, vacuolar protein sorting (VPS) 34, and VPS15 leads to nucleation of a double-membrane structure called the phagophore, or isolation membrane [14]. Two ubiquitin-conjugating systems—microtubule-associated protein 1 light chain 3 (LC3) [15] and ATG12-ATG5-ATG16 [16]—are involved in elongation of the membrane. LC3 is also responsible for tethering the autophagosome to the cargo [17, 18]. The autophagosome then encloses around the cargo and fuses with the lysosome, where the contents are degraded by lysosomal enzymes [13].
Under normal physiological conditions, basal autophagy maintains homeostasis by degrading long-lived proteins and abnormal organelles. Impaired or dysregulated autophagy in the heart is a major contributor to development and progression of heart failure. For instance, loss of VPS34 in myocytes disrupts the initiation of autophagy and leads to impaired protein turnover, a disorganized mitochondrial network and contractile dysfunction [19]. Similarly, cardiac ATG5-deficient mice are unable to elongate the phagophore to form mature autophagosomes. These mice accumulate dysfunctional mitochondria, have disorganized sarcomeres, and develop cardiac hypertrophy [5]. In addition, the lysosomal-associated membrane protein 2 (LAMP-2) is required for the fusion between autophagosomes and lysosomes, and LAMP-2 deficiency causes accumulation of autophagosomes in myocytes, which leads to a lethal cardiomyopathy [20, 21]. Cathepsin-L (CTSL) is a lysosomal protease that plays a key role in degradation of lysosomal content. CTSL deficiency leads to impaired autophagic flux and development of dilated cardiomyopathy [22]. Collectively, these studies demonstrate that a functional autophagy-lysosomal pathway is critical for cardiac homeostasis.
Myocytes increase autophagic activity during stress to adapt to changes in nutritional and energy demands. Enhancing autophagy helps maintain ATP levels to sustain contractile force of the myocytes. For instance, acute exercise induces autophagy in skeletal and cardiac muscles of fed mice, whereas mutant mice that are deficient in exercise-induced autophagy have reduced endurance during acute exercise [23]. Fasting also leads to a rapid increase in cardiac autophagy and inhibiting autophagy depresses cardiac function in fasting mice [11]. In addition, autophagy is enhanced after a myocardial infarction to preserve cellular ATP levels in myocytes. Inhibiting autophagy reduces ATP levels and exacerbates post-infarct remodeling, whereas enhancing autophagy mitigates remodeling and cardiac dysfunction [24, 25]. It functions to selectively clear cytotoxic protein aggregates and damaged organelles as well. Increased autophagy is also able to attenuate cardiac hypertrophy in a mouse model of desmin-related cardiomyopathy [26].
However, excessive autophagy can be detrimental due to excessive degradation of critical proteins and organelles. Autophagy is rapidly activated in the heart in response to cardiac pressure overload and remains elevated for weeks [27]. In this setting, enhanced autophagy is detrimental to the myocardium. Similarly, chronic and excessive activation of autophagy in myocardial ischemia/reperfusion is also detrimental to the heart [9].
Regulation of mitophagy
Although originally thought to be non-selective, it is now clear that autophagy can selectively target protein aggregates [28] as well as organelles such as peroxisomes [29], endoplasmic reticulum [30], and mitochondria [31]. Mitophagy is the selective sequestration and degradation of mitochondria by autophagosomes and is important in clearing mitochondria both under baseline conditions and in response to stress.
PINK1/Parkin pathway
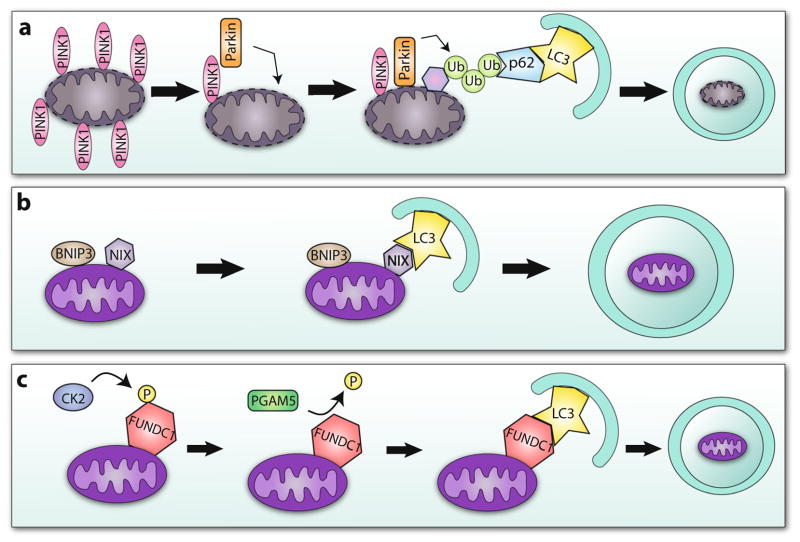
One important pathway involved in regulating mitophagy is the PINK1/Parkin pathway (Fig. 1a). When mitochondria are healthy, the serine/threonine kinase PTEN-inducible kinase 1 (PINK1) is immediately imported into the mitochondrial matrix by the translocase of the outer membrane (TOM) complex [32] where it is cleaved by mitochondrial processing proteinase (MPP) and presenilin-associated rhomboid-like (PARL) [33]. However, upon loss of mitochondrial membrane potential, PINK1 ceases to be imported and instead accumulates on the outer mitochondrial membrane [33]. This leads to recruitment of the E3 ubiquitin ligase, Parkin, from the cytosol to the mitochondrial membrane [34]. Studies have demonstrated that Parkin translocation is dependent on PINK1 [35–37]. At least two PINK1-mediated events are required to initiate Parkin-mediated autophagy: (1) PINK1 must phosphorylate Mitofusin 2 (MFN2) which then becomes a receptor for Parkin at the mitochondria [38] and (2) PINK1 must phosphorylate ubiquitin at Ser65 [35, 36, 39]. To date, only a few mitochondrial Parkin substrates have been identified and characterized, and these include hexokinase 1 [40], VDAC1 [41], MFN1 and MFN2 [42], and MIRO [43]. The functional importance of these substrates in Parkin-mediated mitophagy is still unclear and controversial. Studies indicate that there is a redundancy between substrates and that the loss of one substrate has little or no effect on Parkin-mediated mitophagy. It is not surprising that this redundancy exists in cells because it ensures clearance of mitochondria even if one substrate is downregulated or mutated. Moreover, it is likely that there are many additional Parkin substrates on cardiac mitochondria and future studies should focus on identifying these substrates.
Fig. 1.
Mechanisms of mitophagy. a Upon mitochondrial depolarization, PINK1 accumulates on the surface of the mitochondrion, recruiting Parkin to the outer mitochondrial membrane. Parkin polyubiquitinates mitochondrial membrane proteins, which allows them to be recognized by the adaptor protein p62. The autophagosome then engulfs the mitochondrion. b LC3 directly recognizes mitophagy receptor proteins BNIP3 and NIX resulting in mitochondrial clearance. c CK2 phosphorylates themitophagy receptor FUNDC1 to suppress its interaction with LC3. Hypoxia induces dephosphorylation of FUNDC1 by PGAM5, restoring its ability to interact with LC3 and trigger mitochondrial autophagy
Studies suggest that Parkin-mediated protein ubiquitination serves two purposes to facilitate mitophagy:
Ubiquitination of mitochondrial proteins via Lys63-linkage marks them for degradation by the autophagosome. Adaptor proteins such as p62 and NBR1 bind to LC3 on the autophagosome and to the ubiquitinated mitochondrial proteins, tethering the autophagosome to the mitochondrion [17, 44].
Ubiquitination of mitochondrial fusion proteins MFN1/2 via Lys48-linkage leads to their degradation by the UPS [42]. This causes a shift in the balance between mitochondrial fission and fusion towards fission. Fission increases the probability of the mitochondrial fragment being removed by mitophagy [45, 46].
Mitophagy receptor pathway
The mitophagy receptor pathway is another important but less well-characterized pathway that regulates mitochondrial clearance in cells. There are proteins present on the outer mitochondrial membrane that can function as autophagy receptors by directly binding LC3 on the autophagosomes. This pathway is independent of ubiquitination and adaptor proteins such as p62. BNIP3 [47], NIX [48], and FUNDC1 [49] are the only three mitophagy protein receptors that have been identified to date. Cardiolipin is a phospholipid that has also been found to interact with LC3. Cardiolipin is found exclusively in the inner mitochondrial membrane where it is essential for the function of many enzymes that are involved in mitochondrial energy metabolism [50]. Damage to mitochondria leads to redistribution of cardiolipin to the outer mitochondrial membrane which is associated with activation of apoptosis. However, a recent study demonstrated that the externalized cardiolipin can also interact with LC3 on the autophagosome and that this might prevent the activation of apoptosis [51].
Many studies have focused on the atypical BH3-only proteins NIX/BNIP3L and BNIP3 and their role in promoting cell death. It is now clear that these proteins also function to activate autophagy and mitophagy. NIX was first identified to act as a mitophagy receptor and is required for clearance of mitochondria in maturing erythrocytes [52]. Similar to the adaptor protein p62, BNIP3 and NIX contain LC3-interacting region (LIR) motifs and interact directly with LC3 on the autophagosome (Fig. 1b) [47, 48]. They can also directly activate autophagy by disrupting the interaction between BCL-2 and BECLIN 1 [53]. BCL-2 maintains BECLIN 1 in an inactive state. Release of BCL-2 allows BECLIN 1 to form a complex with VPS34 and VPS15 to initiate formation of the phagophore [54]. Thus, BNIP3 and NIX can both increase the number of autophagosomes in the cells and facilitate selective removal of mitochondria. Unlike the PINK1/Parkin pathway, it is still unclear under what conditions these receptors induce mitophagy. A recent study by Glick et al. found that fasting of mice leads to significant upregulation of BNIP3 in the liver [55]. This raises the possibility that BNIP3 is responsible for selective mitophagy under starvation. Moreover, FUNDC1 is a more recently characterized mitophagy receptor (Fig. 1c). Under pro-survival conditions, Src kinase and CK2 phosphorylate FUNDC1, which suppresses its ability to interact with LC3 [56]. However, during hypoxia, FUNDC1 is dephosphorylated by the mitochondrial phosphatase PGAM5 allowing it to bind to LC3 to mediate mitochondrial clearance [49, 56].
The functional redundancy not only exists in the mitophagy receptor pathway, but also across pathways. Both NIX and BNIP3 overexpression induce translocation of Parkin to mitochondria [57, 58]. In addition, BNIP3-mediated mitophagy is reduced in Parkin-deficient myocytes [57], suggesting that the presence of Parkin is required for efficient clearance of mitochondria by BNIP3. Although the relationship between FUNDC1 and PINK1/Parkin or BNIP3 has not been investigated yet, it is possible that these pathways coordinate to ensure efficient mitochondrial quality control, especially during stress. For instance, a recent study reported that FUNDC1-mediated mitophagy is activated by ULK1 in response to hypoxia or by mitochondrial membrane depolarization by FCCP [59]. Interestingly, BNIP3 is well known to be upregulated by hypoxia and participates in hypoxia-mediated mitophagy [60], whereas Parkin-mediated mitophagy is activated by depolarized mitochondria [34]. Thus, activation of multiple pathways ensures efficient clearance of potentially harmful mitochondria during stress.
Mitochondrial dynamics and mitophagy
Mitochondria are dynamic organelles that undergo fission or fusion in response to changes in the cellular environment. MFN1/2 and OPA1 are involved in regulating mitochondrial fusion, whereas DRP1, FIS1 and MFF regulate fission [61]. Mitochondrial dynamics play an important role in mitochondrial quality control. For instance, mitochondria undergo fusion during starvation to maintain ATP production and protect them from mitophagy [62]. However, mitochondrial fusion is not always protective. Bhandari et al. found that re-fusion of damaged mitochondrial fragments that had escaped mitophagy can be harmful to myocytes [63].
In contrast, other studies have reported that fission is a prerequisite for mitophagy [46, 57, 64]. Mitochondrial fission allows for segregation of dysfunctional mitochondria. When photolabeled and tracked, mitochondria were found to divide asymmetrically, leaving one daughter mitochondrion with a lower membrane potential than the other. After asymmetrical fission, the mitochondrion with the higher membrane potential subsequently fuses with other healthy mitochondria, whereas the mitochondrion with the low membrane potential is less likely to re-fuse, and instead undergoes autophagic clearance [46]. Emerging evidence also indicates that proteins involved in mitophagy are able to stimulate mitochondrial fission. For example, overexpression of FUNDC1 induces mitochondrial fission, whereas knockdown leads to fused mitochondria [49]. Similarly, BNIP3 overexpression induces DRP1-mediated mitochondrial fission [57]. How these mitophagy receptors coordinate with DRP1 to induce fission is currently unknown.
Calcium signaling and autophagy
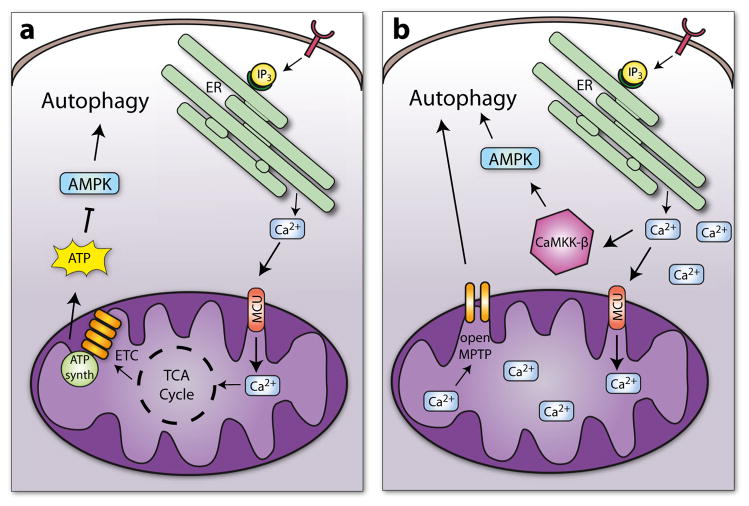
Calcium also regulates autophagic activity in cells. Adenosine monophosphate-activated protein kinase (AMPK) is a cellular energy sensor that is activated when energy levels are low. AMPK, in turn, activates autophagy to ensure survival during the nutrient scarce conditions [65]. Mitochondria and endoplasmic reticulum (ER) are closely associated in cells, and calcium released from the ER is efficiently taken up into the mitochondrial matrix by the mitochondrial calcium uniporter (MCU) [66–69]. Mitochondrial calcium uptake is important for effective oxidative phosphorylation and ATP synthesis [70]. Mitochondrial calcium uptake also regulates AMPK activity and activation of autophagy [68, 70]. For instance, when nutrients are abundant, the presence of growth factors activates cell surface receptors, which leads to generation of IP3. IP3 binds to IP3R on the ER, which induces calcium efflux. Adjacent mitochondria take up the calcium and activate several key enzymes in the mitochondria, including pyruvate dehydrogenase, enzymes in the tricarboxylic acid cycle, and the F0F1 ATPase [71], resulting in increased ATP production. In contrast, when nutrients are scarce, less calcium is released and taken up by the mitochondria resulting in reduced energy production, activation of AMPK, and induction of autophagy (Fig. 2a). Several studies have demonstrated that abrogation of calcium release from ER or inhibition of calcium uptake into mitochondria results in reduced energy production and activation of autophagy under nutrient-rich conditions [68, 70]. Overall, these studies suggest that in the absence of ER-mitochondrial calcium transfer, cells turn on autophagy to sustain survival. However, how calcium levels regulate autophagy in cardiac myocytes still needs to be investigated.
Fig. 2.
Regulation of autophagy by intracellular calcium. a Mitochondrial uptake of calcium by the MCU results in increased ATP production, inhibition of AMPK, and suppression of autophagy. b Excessive levels of intracellular calcium leads to excessive mitochondrial calcium uptake, opening of the MPTP, and disruption of mitochondrial function. The reduced energy levels result in activation of AMPK and autophagy. Excess intracellular calcium also leads to direct activation of AMPK and autophagy via CaMKK-β
Paradoxically, it has been reported that increases in cytosolic calcium also activate autophagy (Fig. 2b). Cytosolic calcium activates the Ca2+/calmodulin-dependent protein kinase kinase-β (CaMKK-β), which in turn activates AMPK [72, 73] and autophagy [74]. Additionally, excess uptake of mitochondrial calcium leads to opening of the mitochondrial permeability transition pore (MPTP) and collapse of the proton gradient [1]. Opening of the MPTP has also been linked to activation of autophagy and mitophagy [31, 75, 76]. Thus, different levels of cytosolic calcium have opposing effects on autophagy. At lower concentrations, calcium is taken up by mitochondria which promotes energy production and inhibits autophagy. In contrast, excess levels of calcium leads to direct activation of AMPK and autophagy via CaMKK-β and indirectly via excess mitochondrial calcium uptake.
Mitophagy in the heart
Mitophagy is a critical mitochondrial quality control mechanism in myocytes, and impaired mitophagy leads to accumulation of aberrant mitochondria and subsequent contractile dysfunction [6–8]. It is well established that loss-of-function mutations in the genes that encode Pink1 or Parkin lead to the development of early onset Parkinson’s disease [77]. More recent studies have demonstrated that a defect in PINK1/Parkin-mediated mitophagy also has negative consequences for the heart (Table 1). For instance, PINK1 deficiency in mice leads to cardiac mitochondrial dysfunction and enhanced oxidative stress [7]. These mice develop cardiac hypertrophy at 2 months of age. Although Parkin is the downstream effector of PINK1, Parkin−/− mice have a different cardiac phenotype. These mice have normal cardiac function when young [8], but accumulate abnormal mitochondria with age [6, 78]. The differences in cardiac phenotypes suggest that other E3 ubiquitin ligases can compensate for the lack of Parkin to some extent or that PINK1 has additional functions in myocytes. Moreover, hearts lacking the Parkin receptor MFN2 have reduced Parkin-mediated mitophagy as well as reduced contractility, increased hypertrophy, and heart failure by 30 weeks of age [79]. MFN2 has other critical functions in cells, including mitochondrial fusion [80] and tethering mitochondria to the ER [81]. This disruption of multiple processes likely accounts for the more severe cardiac phenotype of MFN2-null mice compared to the Parkin- and PINK1-deficient mice.
Table 1.
Mitophagy regulators and cardiac phenotypes
| Model | Mitophagy | Phenotype | Ref |
|---|---|---|---|
| Parkin−/− | Decreased | Increased sensitivity to MI and doxorubicin exposure, accumulation of dysfunctional mitochondria, and oxidative damage with age, reduced life span | [6, 8, 78] |
| Parkin TG | Increased | Increased life span, preserved cardiac function with aging | [6, 97] |
| PINK1−/− | Not assessed | Mitochondrial dysfunction, cardiomyopathy, increased sensitivity to I/R | [7, 90] |
| BNIP3−/− | Not assessed | Decreased apoptosis and cardiac remodeling in response to I/R | [93] |
| BNIP3 TG | Not assessed | Increased sensitivity to MI, increased apoptosis | [93] |
| NIX−/− | Not assessed | Decreased cardiac remodeling and preserved cardiac function in response to pressure overload | [92] |
| NIX TG | Not assessed | Ventricular dilation, reduced cardiac function | [95] |
| BNIP3−/− Nix−/− |
Not assessed | Accumulation of dysfunctional mitochondria, development of cardiac hypertrophy, decreased cardiac function | [82] |
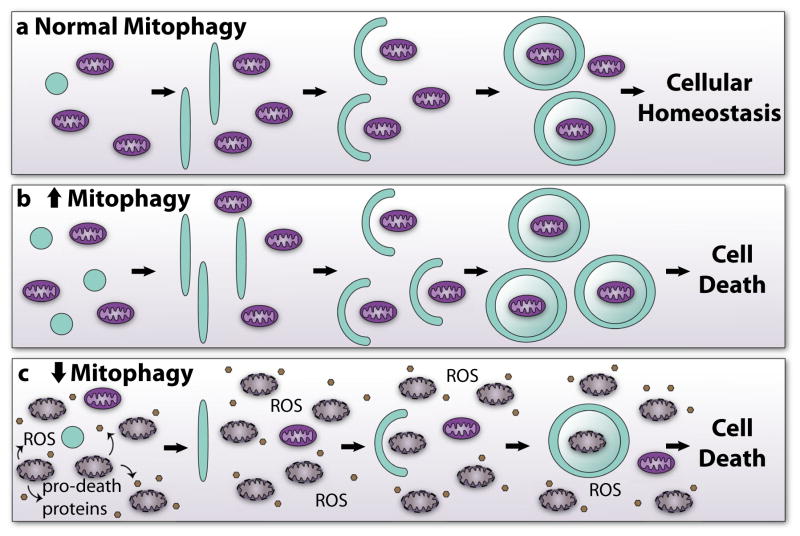
Many studies have demonstrated that overexpression of NIX or BNIP3 activates mitophagy in cells, including myocytes [57, 58]. However, the importance of BNIP3/NIX-mediated mitophagy in cardiac homeostasis was first demonstrated in mice deficient in NIX and NIX/BNIP3 [82]. Germline deletion of NIX in mice leads to development of cardiac hypertrophy and decreased cardiac function with age [82]. Mice deficient in both BNIP3 and NIX accumulate abnormal mitochondria and develop cardiac dysfunction at about twice the rate that NIX-deficient mice do [82]. Thus, BNIP3 and NIX have overlapping functions and play an important role in mitochondrial turnover in the heart. Taken together, these studies demonstrate that mitochondrial maintenance through the PINK1/Parkin and mitophagy receptor pathways is important for cardiac homeostasis and that the dysregulation of mitophagy leads to accumulation of abnormal mitochondria, loss of myocytes, and contractile dysfunction (Fig. 3).
Fig. 3.
Mitophagy and mitochondrial quality control. a Normal mitophagy begins with the initiation and elongation of a double-membraned autophagic vesicle. The vesicle then sequesters and engulfs mitochondria for degradation. Proper regulation of mitophagy leads to mitochondrial quality control and cellular homeostasis. b Increased mitophagy may greatly reduce the pool of functional mitochondria. With too few mitochondria, the cell loses its ability to produce sufficient energy and eventually dies. c A reduction in mitophagy causes accumulation of dysfunctional mitochondria. The dysfunctional mitochondria generate excessive ROS and release pro-death proteins, triggering rapid cell death modification of Parkin and inhibition of mitophagy in the myocardium.
Unfortunately, autophagy has been reported to decrease with age in tissues including the nervous system [83] and the heart [12, 84]. This leads to inadequate removal of dysfunctional mitochondria, which can generate up to tenfold more H2O2 than healthy organelles [85]. Oxidative damage to mitochondrial proteins, lipids, and DNA has been detected in the aged myocardium [86]. Parkin-mediated mitophagy is reduced with age [6] but the underlying mechanisms for this are unknown. Interestingly, Parkin contains several conserved cysteine residues that are vital for preserving its solubility [87]. Modification of these residues can lead to Parkin misfolding and aggregation [88]. A growing body of evidence indicates that misfolding is a major mechanism of Parkin inactivation in neurons [89]. Thus, it is possible that with age, the number of dysfunctional mitochondria exceeds the capacity of Parkin-mediated mitochondrial clearance, resulting in
In addition, mitophagy plays an essential role in adapting to myocardial stress, and inadequate activation of mitophagy contributes to development of heart failure. Several studies have reported that mitophagy deficiency exacerbates cardiac injury in various experimental models of heart failure. For instance, Parkin deficiency leads to accumulation of dysfunctional mitochondria in myocytes, development of heart failure, and increased mortality after myocardial infarction in mice [8]. PINK1−/− mice are more susceptible to ex vivo I/R injury [90] and pressure overload-mediated heart failure [7]. Interestingly, PINK1 levels are reduced in human heart failure patients indicating inefficient mitophagy [7]. However, it is unknown if the reduced PINK1 levels are a cause or consequence of heart failure. Doxorubicin is an effective chemo-therapeutic agent that is associated with cardiotoxicity. It is well established that doxorubicin causes mitochondrial damage in myocytes, which contributes to the loss of myocytes and development of heart failure [91]. A recent study demonstrated that doxorubicin exposure also impairs Parkin-mediated mitophagy [6]. It found that p53 binds and sequesters Parkin in the cytosol, which prevents it from translocating to mitochondria. This results in impaired clearance of dysfunctional mitochondria and subsequent development of cardiac dysfunction [6]. Thus, mitochondria-rich myocytes are particularly sensitive to doxorubicin compared to other cell types since doxorubicin induces mitochondrial damage and inhibits the ability to clear these mitochondria.
Although ablation of NIX and BNIP3 leads to impaired mitochondrial turnover, these proteins can be detrimental during stress. Cardiac-specific NIX-null mice have reduced cardiac fibrosis and preserved contractile function compared to wild type in pressure overload-induced heart failure [92]. BNIP3-deficient mice have reduced apoptosis and cardiac remodeling after myocardial infarction [93]. Similarly, a BNIP3 dominant negative protects against ex vivo I/R injury [94]. Additionally, cardiac-specific overexpression of NIX leads to activation of apoptosis in myocytes and development of heart failure [95]. The above data suggest that activation of BNIP3 and NIX in response to stress can be deleterious. It is possible that under baseline conditions, BNIP3 and NIX promote mitochondrial quality control through mitophagy. However, during certain conditions of cardiac injury, they become pro-death proteins instead. Under what conditions they switch from promoting mitophagy to promoting cell death still needs to be determined.
Conclusion and future directions
Under baseline conditions, reduced or dysfunctional autophagy is sufficient to cause cardiomyopathy [5]. Patients with diseases associated with dysfunctional mitophagy such as Danon’s disease and Parkinson’s disease often also develop heart failure [20, 96]. Because mitophagy levels decrease with age and heart failure is more prevalent in older populations, the possibility of therapeutically targeting mitophagy becomes more relevant as the global population continues to age. During cardiac stress and injury, mitophagy increases to help clear the damaged mitochondria and prevent oxidative damage and cell death [6, 8]. Therefore, it is possible that carefully controlled activation of mitophagy is the panacea for heart disease. Overexpression of Parkin in tissues has been shown to reduce age-associated cardiac dysfunction, increase life span, and preserve mitochondrial function in mice [6, 97]. However, it is important to consider that upregulation of mitophagy may not be beneficial in all circumstances. Mitophagy exists as a mitochondrial quality control mechanism and is important to maintain mitochondrial homeostasis. The beating cardiomyocytes depend on having a consistent pool of healthy mitochondria. With decreased clearance of damaged mitochondria, myocytes accumulate ROS and may die from mitochondrially triggered apoptosis. Increased mitophagy, however, may cause excessive mitochondrial clearance, leaving the myocytes with too few mitochondria to produce sufficient ATP. During acute cardiac injury, such as MI or I/R, a limited increase in mitophagy could be beneficial to clear damaged mitochondria. However, in chronic cardiac diseases, such as heart failure, sustained upregulation of mitophagy may be harmful and lead to excessive mitochondrial clearance. Thus, additional studies are needed to understand the regulation of mitophagy in myocytes and the consequences of short-term versus long-term upregulation of mitophagy on mitochondrial homeostasis, cell viability, and cardiac function.
Acknowledgments
Å.B. Gustafsson is supported by an AHA Established Investigator Award and National Institutes of Health grants R01HL087023, R01HL101217, and P01HL085577. S.E. Shires is supported by T32GM007752.
Footnotes
Disclosure None
References
- 1.Baines CP. The cardiac mitochondrion: nexus of stress. Annu Rev Physiol. 2010;72:61–80. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]
- 2.Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, Ngo CP, Prolla TA, Rabinovitch PS. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell. 2010;9:536–544. doi: 10.1111/j.1474-9726.2010.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 5.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 6.Hoshino A, Mita Y, Okawa Y, Ariyoshi M, Iwai-Kanai E, Ueyama T, Ikeda K, Ogata T, Matoba S. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat Commun. 2013;4:2308. doi: 10.1038/ncomms3308. [DOI] [PubMed] [Google Scholar]
- 7.Billia F, Hauck L, Konecny F, Rao V, Shen J, Mak TW. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci U S A. 2011;108:9572–9577. doi: 10.1073/pnas.1106291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK, Jimenez R, Petrosyan S, Murphy AN, Gustafsson AB. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem. 2013;288:915–926. doi: 10.1074/jbc.M112.411363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 10.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 11.Kanamori H, Takemura G, Maruyama R, Goto K, Tsujimoto A, Ogino A, Li L, Kawamura I, Takeyama T, Kawaguchi T, et al. Functional significance and morphological characterization of starvation-induced autophagy in the adult heart. Am J Pathol. 2009;174:1705–1714. doi: 10.2353/ajpath.2009.080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–612. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;119:259–270. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- 15.Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci. 2004;117:2805–2812. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- 16.Kuma A, Mizushima N, Ishihara N, Ohsumi Y. Formation of the approximately 350-kDa Apg12-Apg5.Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. J Biol Chem. 2002;277:18619–18625. doi: 10.1074/jbc.M111889200. [DOI] [PubMed] [Google Scholar]
- 17.Lamark T, Kirkin V, Dikic I, Johansen T. NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets. Cell Cycle. 2009;8:1986–1990. doi: 10.4161/cc.8.13.8892. [DOI] [PubMed] [Google Scholar]
- 18.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 19.Jaber N, Zong WX. Class III PI3K Vps34: essential roles in autophagy, endocytosis, and heart and liver function. Ann N Y Acad Sci. 2013;1280:48–51. doi: 10.1111/nyas.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishino I, Fu J, Tanji K, Yamada T, Shimojo S, Koori T, Mora M, Riggs JE, Oh SJ, Koga Y, et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- 22.Stypmann J, Glaser K, Roth W, Tobin DJ, Petermann I, Matthias R, Monnig G, Haverkamp W, Breithardt G, Schmahl W, et al. Dilated cardiomyopathy in mice deficient for the lysosomal cysteine peptidase cathepsin L. Proc Natl Acad Sci U S A. 2002;99:6234–6239. doi: 10.1073/pnas.092637699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanamori H, Takemura G, Goto K, Maruyama R, Ono K, Nagao K, Tsujimoto A, Ogino A, Takeyama T, Kawaguchi T, et al. Autophagy limits acute myocardial infarction induced by permanent coronary artery occlusion. Am J Physiol Heart Circ Physiol. 2011;300:H2261–2271. doi: 10.1152/ajpheart.01056.2010. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe T, Takemura G, Kanamori H, Goto K, Tsujimoto A, Okada H, Kawamura I, Ogino A, Takeyama T, Kawaguchi T, et al. Restriction of food intake prevents postinfarction heart failure by enhancing autophagy in the surviving cardiomyocytes. Am J Pathol. 2014;184:1384–1394. doi: 10.1016/j.ajpath.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM, Hill JA, Sadoshima J, Robbins J. Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest. 2013;123:5284–5297. doi: 10.1172/JCI70877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tannous P, Zhu H, Nemchenko A, Berry JM, Johnstone JL, Shelton JM, Miller FJ, Jr, Rothermel BA, Hill JA. Intracellular protein aggregation is a proximal trigger of cardiomyocyte autophagy. Circulation. 2008;117:3070–3078. doi: 10.1161/CIRCULATIONAHA.107.763870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwata J, Ezaki J, Komatsu M, Yokota S, Ueno T, Tanida I, Chiba T, Tanaka K, Kominami E. Excess peroxisomes are degraded by autophagic machinery in mammals. J Biol Chem. 2006;281:4035–4041. doi: 10.1074/jbc.M512283200. [DOI] [PubMed] [Google Scholar]
- 30.Bernales S, Schuck S, Walter P. ER-phagy: selective autophagy of the endoplasmic reticulum. Autophagy. 2007;3:285–287. doi: 10.4161/auto.3930. [DOI] [PubMed] [Google Scholar]
- 31.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 32.Lazarou M, Jin SM, Kane LA, Youle RJ. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell. 2012;22:320–333. doi: 10.1016/j.devcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, Banerjee S, Youle RJ. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol. 2014;205:143–153. doi: 10.1083/jcb.201402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, Kimura Y, Tsuchiya H, Yoshihara H, Hirokawa T, et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510:162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- 37.Okatsu K, Oka T, Iguchi M, Imamura K, Kosako H, Tani N, Kimura M, Go E, Koyano F, Funayama M, et al. PINK1 autophosphorylation upon membrane potential dissipation is essential for Parkin recruitment to damaged mitochondria. Nat Commun. 2012;3:1016. doi: 10.1038/ncomms2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Dorn GW., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kazlauskaite A, Kondapalli C, Gourlay R, Campbell DG, Ritorto MS, Hofmann K, Alessi DR, Knebel A, Trost M, Muqit MM. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem J. 2014;460:127–139. doi: 10.1042/BJ20140334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okatsu K, Iemura S, Koyano F, Go E, Kimura M, Natsume T, Tanaka K, Matsuda N. Mitochondrial hexokinase HKI is a novel substrate of the Parkin ubiquitin ligase. Biochem Biophys Res Commun. 2012;428:197–202. doi: 10.1016/j.bbrc.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 41.Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191:1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirkin V, Lamark T, Sou YS, Bjorkoy G, Nunn JL, Bruun JA, Shvets E, McEwan DG, Clausen TH, Wild P, et al. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol Cell. 2009;33:505–516. doi: 10.1016/j.molcel.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Gomes LC, Scorrano L. High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim Biophys Acta. 2008;1777:860–866. doi: 10.1016/j.bbabio.2008.05.442. [DOI] [PubMed] [Google Scholar]
- 46.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, Gustafsson AB. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J Biol Chem. 2012;287:19094–19104. doi: 10.1074/jbc.M111.322933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Novak I, Kirkin V, McEwan DG, Zhang J, Wild P, Rozenknop A, Rogov V, Lohr F, Popovic D, Occhipinti A, et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, Ma Q, Zhu C, Wang R, Qi W, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14:177–185. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 50.Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65:2493–2506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15:1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Glick D, Zhang W, Beaton M, Marsboom G, Gruber M, Simon MC, Hart J, Dorn GW, 2nd, Brady MJ, Macleod KF. BNip3 regulates mitochondrial function and lipid metabolism in the liver. Mol Cell Biol. 2012;32:2570–2584. doi: 10.1128/MCB.00167-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen G, Han Z, Feng D, Chen Y, Chen L, Wu H, Huang L, Zhou C, Cai X, Fu C, et al. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol Cell. 2014;54:362–377. doi: 10.1016/j.molcel.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 57.Lee Y, Lee HY, Hanna RA, Gustafsson AB. Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2011;301:H1924–1931. doi: 10.1152/ajpheart.00368.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB, Dorn GW, 2nd, Yin XM. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem. 2010;285:27879–27890. doi: 10.1074/jbc.M110.119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu W, Tian W, Hu Z, Chen G, Huang L, Li W, Zhang X, Xue P, Zhou C, Liu L, et al. ULK1 translocates to mitochondria and phosphorylates FUNDC1 to regulate mitophagy. EMBO Rep. 2014;15:566–575. doi: 10.1002/embr.201438501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol. 2007;27:6229–6242. doi: 10.1128/MCB.02246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 62.Gomes LC, di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhandari P, Song M, Chen Y, Burelle Y, Dorn GW., 2nd Mitochondrial contagion induced by Parkin deficiency in Drosophila hearts and its containment by suppressing mitofusin. Circ Res. 2014;114:257–265. doi: 10.1161/CIRCRESAHA.114.302734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 65.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M, et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balaban RS. Domestication of the cardiac mitochondrion for energy conversion. J Mol Cell Cardiol. 2009;46:832–841. doi: 10.1016/j.yjmcc.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 74.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 75.Carreira RS, Lee Y, Ghochani M, Gustafsson AB, Gottlieb RA. Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells. Autophagy. 2010;6:462–472. doi: 10.4161/auto.6.4.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cui T, Fan C, Gu L, Gao H, Liu Q, Zhang T, Qi Z, Zhao C, Zhao H, Cai Q, et al. Silencing of PINK1 induces mitophagy via mitochondrial permeability transition in dopaminergic MN9D cells. Brain Res. 2011;1394:1–13. doi: 10.1016/j.brainres.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 77.Deas E, Wood NW, Plun-Favreau H. Mitophagy and Parkinson’s disease: the PINK1-parkin link. Biochim Biophys Acta. 2011;1813:623–633. doi: 10.1016/j.bbamcr.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kubli DA, Quinsay MN, Gustafsson AB. Parkin deficiency results in accumulation of abnormal mitochondria in aging myocytes. Commun Integr Biol. 2013;6:e24511. doi: 10.4161/cib.24511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song M, Chen Y, Gong G, Murphy E, Rabinovitch PS, Dorn GW., 2nd Super-suppression of mitochondrial reactive oxygen species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circ Res. 2014;115:348–353. doi: 10.1161/CIRCRESAHA.115.304384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114:867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- 81.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 82.Dorn GW., 2nd Mitochondrial pruning by Nix and BNip3: an essential function for cardiac-expressed death factors. J Cardiovasc Transl Res. 2010;3:374–383. doi: 10.1007/s12265-010-9174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCray BA, Taylor JP. The role of autophagy in age-related neurodegeneration. Neuro-Signals. 2008;16:75–84. doi: 10.1159/000109761. [DOI] [PubMed] [Google Scholar]
- 84.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 85.Grivennikova VG, Kareyeva AV, Vinogradov AD. What are the sources of hydrogen peroxide production by heart mitochondria? Biochim Biophys Acta. 2010;1797:939–944. doi: 10.1016/j.bbabio.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 87.Wong ES, Tan JM, Wang C, Zhang Z, Tay SP, Zaiden N, Ko HS, Dawson VL, Dawson TM, Lim KL. Relative sensitivity of parkin and other cysteine-containing enzymes to stress-induced solubility alterations. J Biol Chem. 2007;282:12310–12318. doi: 10.1074/jbc.M609466200. [DOI] [PubMed] [Google Scholar]
- 88.Winklhofer KF, Henn IH, Kay-Jackson PC, Heller U, Tatzelt J. Inactivation of parkin by oxidative stress and C-terminal truncations: a protective role of molecular chaperones. J Biol Chem. 2003;278:47199–47208. doi: 10.1074/jbc.M306769200. [DOI] [PubMed] [Google Scholar]
- 89.Tan JM, Wong ES, Lim KL. Protein misfolding and aggregation in Parkinson’s disease. Antioxid Redox Signal. 2009;11:2119–2134. doi: 10.1089/ars.2009.2490. [DOI] [PubMed] [Google Scholar]
- 90.Siddall HK, Yellon DM, Ong SB, Mukherjee UA, Burke N, Hall AR, Angelova PR, Ludtmann MH, Deas E, Davidson SM, et al. Loss of PINK1 increases the heart’s vulnerability to ischemia-reperfusion injury. PLoS One. 2013;8:e62400. doi: 10.1371/journal.pone.0062400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou S, Starkov A, Froberg MK, Leino RL, Wallace KB. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res. 2001;61:771–777. [PubMed] [Google Scholar]
- 92.Diwan A, Wansapura J, Syed FM, Matkovich SJ, Lorenz JN, Dorn GW., 2nd Nix-mediated apoptosis links myocardial fibrosis, cardiac remodeling, and hypertrophy decompensation. Circulation. 2008;117:396–404. doi: 10.1161/CIRCULATIONAHA.107.727073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, Li H, Kirshenbaum LA, Hahn HS, Robbins J, et al. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest. 2007;117:2825–2833. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA, Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 95.Yussman MG, Toyokawa T, Odley A, Lynch RA, Wu G, Colbert MC, Aronow BJ, Lorenz JN, Dorn GW., 2nd Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat Med. 2002;8:725–730. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- 96.Zesiewicz TA, Strom JA, Borenstein AR, Hauser RA, Cimino CR, Fontanet HL, Cintron GB, Staffetti JF, Dunne PB, Sullivan KL. Heart failure in Parkinson’s disease: analysis of the United States medicare current beneficiary survey. Parkinsonism Relat Disord. 2004;10:417–420. doi: 10.1016/j.parkreldis.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 97.Rana A, Rera M, Walker DW. Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc Natl Acad Sci U S A. 2013;110:8638–8643. doi: 10.1073/pnas.1216197110. [DOI] [PMC free article] [PubMed] [Google Scholar]