Abstract
The endoplasmic reticulum (ER) is a highly specialized organelle that provides an oxidizing, pro-folding environment for protein synthesis and maturation. The ER also hosts a dynamic signaling network that can sense and respond to physiological changes that impact its environment, thereby influencing overall cell fate. Limitation of nutrients and oxygen have a direct effect on the efficiency of protein folding in the ER, and are classical inducers of the ER resident signaling pathway, the Unfolded Protein Response (UPR). Not only does the UPR regulate ER homeostasis in normal cells experiencing such stress, but strong evidence also suggests that tumor cells can co-opt the cytoprotective aspects of this response in order to survive the hypoxic, nutrient-restricted conditions of the tumor microenvironment.
Background
Signal transduction in response to ER stress
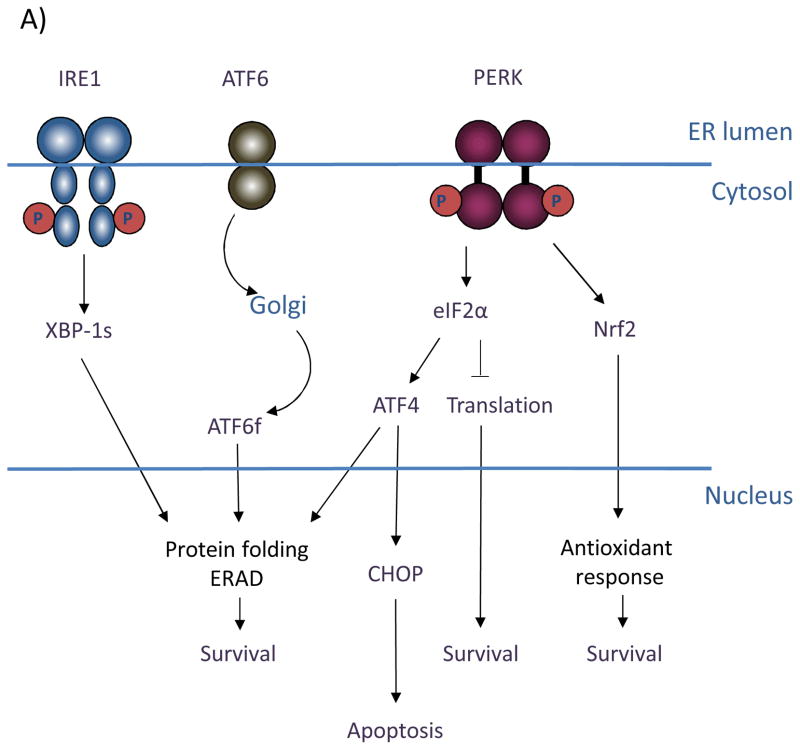
The UPR is mediated by three primary sensors/signal transducers: (PKR)-like ER kinase (PERK), inositol-requiring gene 1 (IRE1), and activating transcription factor 6 (ATF6), all of which span the ER membrane (Figure 1). Under homeostatic conditions, the ER chaperone GRP78/BiP associates with the luminal domain of each of these three effectors thereby inhibiting their activation (1, 2). Upon ER stress can be triggered by low oxygen or restrictions in key nutrients such as glucose, disrupts protein maturation and folding in the ER through limitations in 6 carbon sugar units or reducing equivalents thereby increasing the need for chaperone activity and thus GRP78/BiP titration. Upon GRP78/BiP titration, PERK and IRE1 are released permitting oligomerization and trans-autophosphorylation (1). Release of GRP78/BiP from ATF6 exposes an ATF6 Golgi localization signal, leading to its translocation and activation by proteolytic cleavage (2).
Figure 1. (A) Signaling through the three branches of the Unfolded Protein Response.
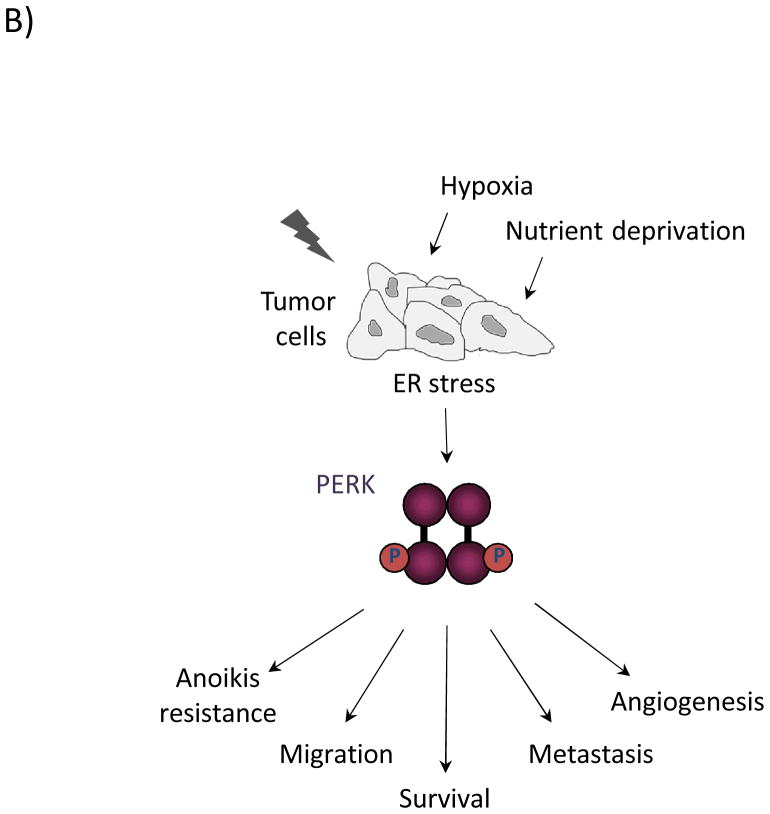
The ER stress sensors inositol-requiring gene 1 (IRE1), activating transcription factor 6 (ATF6), and (PKR)-like ER kinase (PERK) span the ER membrane. In response to conditions that perturb ER homeostasis, IRE1 is activated through oligomerization and trans-autophosphorylation. IRE1 RNase activity cleaves the mRNA of XBP-1 to generate an active transcription factor (XBP-1s) that targets genes involved in protein folding and ER-associated degradation to facilitate ER stress resolution and ultimately promote cell survival. Activation of ATF6 in response to ER stress involves its migration to the trans-Golgi, where it is proteolytically processed. This cleavage event releases the cytosolic bZIP domain, which translocates to the nucleus to activate the expression of ER chaperones and ERAD components. PERK activation occurs through oligomerization and trans-autophosphorylation, leading to phosphorylation of the translation initiation factor, eIF2α, and the antioxidant response factor, Nrf2. Phosphorylation of eIF2α leads to general inhibition of translation, contributing to overall cell survival. Phosphorylation of Nrf2 activates transcription of antioxidant factors. Selective upregulation of the bZIP transcription factor ATF4 through eIF2α targets both pro-survival genes as well as the pro-apoptotic factor GADD153/CHOP. (B) Oncogenic functions of PERK. Restricted nutrient and oxygen conditions in the tumor microenvironment triggers UPR signaling in cancer cells. The cell adaptive nature of PERK signaling enables enhanced cell survival, increased migratory and metastatic capacity, resistance to anoikis in ECM-detached cells, and increased pro-angiogenic potential to support tumor growth.
Current evidence supports a model wherein the immediate effects of UPR activation are cytoprotective and PERK is pivotal for cell adaptation to ER stress. PERK phosphorylates the eukaryotic translation initiation factor eIF2α, which inhibits general protein synthesis and lowers the protein load (3, 4). Also important for ER stress resolution is the PERK-dependent downregulation of cyclin D1 through eIF2α. Inhibition of cyclin D1 synthesis triggers a G1 cell cycle arrest, thereby reducing cellular biosynthetic needs and providing a window during which to re-establish ER homeostasis (5). In addition to limiting protein influx through eIF2α, PERK directly phosphorylates the transcription factor Nrf2, which contributes to cell survival through maintaining redox homeostasis. In unstressed cells, Nrf2 is held in an inactive state through binding the cytoskeletal anchor protein Keap1. With ER stress, PERK phosphorylates Nrf2, triggering its release from Keap1. This facilitates translocation of Nrf2 to the nucleus, where to it regulates the expression of detoxifying enzymes and thereby protects cells from oxidative damage (6). Through PERK, there is also a selective upregulation of certain factors, notably the bZIP transcription factor ATF4. ATF4 induces expression of pro-survival genes involved in protein folding, redox homeostasis, and amino acid metabolism (7, 8). Following prolonged or acute ER stress, ATF4 also targets the pro-apoptotic transcription factor GADD153/CHOP (9). CHOP expression leads to cell death, suggesting a unique role for PERK in cell fate determination.
IRE1 provides important adaptive signals through activation of the X-box protein 1 transcription factor (XBP-1). IRE1 endoribonuclease activity is responsible for processing XBP-1 via a splicing mechanism that shifts the reading frame to encode a stable, active transcription factor (XBP-1s) (10–12). XBP-1 target genes include key factors involved in protein folding, ER-associated degradation (ERAD), and ER expansion under stress (13, 14). IRE1 RNase activity also contributes to ER stress resolution through regulated IRE1-dependent decay (RIDD) of mRNA (15). This pathway in conjunction with PERK-dependent translational repression may serve to reduce the influx of ER-bound proteins during ER stress.
Contributing to the adaptive transcriptional program, ATF6 transduces signals from the endoplasmic reticulum to the nucleus via its cytosolic bZIP domain. Following proteolytic processing in the trans-Golgi, the cleaved form of ATF6 (ATF6f) is released to translocate to the nucleus where it targets ERAD components as well as XBP-1 itself (12, 16).
PERK in tumor cell survival and proliferation
Provided the cytoprotective effects of the UPR during stress, it is not surprising that cancer cells might co-opt the UPR for tumor perpetuation. As tumor cells begin to proliferate and expand into surrounding tissue, there is an ever-increasing demand for nutrients and oxygen. This quickly exceeds the capacity of existing tissue vasculature to support such demand, creating an environment of glucose and oxygen restriction that challenges tumor expansion. These conditions impinge on the proper folding and maturation of secreted proteins in the ER, which is immediately sensed by the three ER stress sensors. The ensuing response enables tumor cell adaptation and survival.
Consistent with the idea that UPR signaling supports tumorigenesis, major UPR mediators are often upregulated in cancer and have been implicated in critical stages of cancer progression (8, 17). The overexpression of IRE1 and ATF6, as well as of the ER chaperones GRP78/BiP, GRP94, and GRP170 in a variety of cancer types offers a case in point (18–20). These studies correlated UPR activation to a more advanced tumor grade. Functionally, UPR signaling contributes to a broad spectrum of cancer-related processes including cell survival, migration, metastasis, autophagy, angiogenesis, and chemotherapeutic resistance. The importance of this response has been demonstrated through genetic and chemical-based manipulation of UPR components in tumor models in vivo. Recent work exploring the effect of XBP-1 and PERK deletion are prime examples of the requirement for ER stress signaling in tumor growth (21–23). Paradoxically, both PERK and IRE1 have also been suggested to contribute to tumor suppression (24–29). These observations underscore the need for more clearly defining UPR signaling branches and their underlying molecular mechanisms.
As one of the master regulators of the ER stress response and a key pro-survival effector, PERK has received considerable attention in the context of tumor initiation/progression, and significant efforts have been recently been focused on developing specific and potent small molecule regulators of PERK activity. Deletion of PERK, ATF4, or Nrf2, or mutation of the PERK-mediated eIF2α phospho-site were demonstrated to have deleterious effects on cell survival following chronic ER stress in cell culture (6, 21, 30, 31). Consistent with these observations, tumor growth was significantly impaired with PERK excision in ectopic and orthotopic tumor models (21, 22). Furthermore, mammary gland-specific PERK knockout in the MMTV-Neu breast cancer model delayed tumor onset and reduced metastatic lesions. In this study, PERK knockdown triggered oxidative DNA damage and activated the DNA damage checkpoint in breast cancer cells and orthotopic tumors, suggesting a mechanism whereby tumor cell proliferation and survival are attenuated through PERK loss (22). Recent work has also demonstrated a significant pro-survival effect of PERK on ECM-detached mammary epithelial cells; PERK is activated upon cell detachment and induces autophagy via AMPK/mTORC1 regulation, thus protecting cells from anoikis (32, 33). Additional pathways through which PERK likely contributes to cell survival are the PI3K-Akt and NFκB networks; however, these mechanisms have not yet been fully elucidated.
PERK contributes to metastatic progression
Metastasis of primary tumor cells to a distant site requires multiple steps that challenge a cell’s ability to navigate harsh conditions. Cells must detach from the primary site, migrate through surrounding tissue, enter and survive blood stream circulation, and finally, extravasate to colonize a secondary site. Successful completion of these steps requires altered cell-cell and cell-substratum contacts and acquisition of a more migratory, invasive phenotype. While previous work suggested a PERK-dependent effect on metastasis, it is not until very recently that the details of its pro-metastatic influence are beginning to become clear. These lines of investigation have centered around regulation of a previously uncharacterized metastasis-associated gene, LAMP3. LAMP3 is transcriptionally upregulated in several tumor types, as well as in response to hypoxic conditions in various cancer cell lines. This response is PERK-eIF2α-ATF4 dependent, though direct regulation by ATF4 has not yet been shown (34). Furthermore, depletion of PERK, ATF4, or LAMP3 inhibits migration in breast cancer cell lines (35), with subsequent studies demonstrating an inhibitory effect on invasion and metastasis in vivo (36).
A connection between PERK signaling and the epithelial-mesenchymal transition (EMT) has also been proposed (37). EMT is a transition from epithelial, cuboidal morphology with tight cell-cell junctions to a more motile, invasive, mesenchymal cell type. EMT contributes to normal development and to oncogenic transformation; in the latter context, it is thought to facilitate metastatic progression. Agents that induce ER stress can induce an EMT-like transition (38). Supporting these observations, recent work demonstrated specific activation of the PERK-eIF2α-ATF4 branch of the UPR in cells undergoing EMT, as well as a positive correlation between ATF4 expression and EMT genes in primary human tumors. Moreover, PERK signaling was required for the migratory and invasive properties of these cells, as well as the metastatic capability of 4T1 cells in vivo (37).
Clinical-Translational Advances
Targeting PERK in cancer: small molecule inhibitors
The multifaceted participation of PERK in tumorigenesis makes it an attractive therapeutic target in treating cancer. This is reflected in current efforts to develop small molecule PERK inhibitors, several of which have shown high potency and as well as selectivity in vitro and in vivo (39–41). Prior to crystallization of the PERK catalytic domain, initial attempts to identify ATP-competitive PERK inhibitors used homology modeling based upon the eIF2α kinase, GCN2. Docking and loop sampling studies revealed 14 compounds that inhibited PERK activity in vitro, with a maximal potency of 1μM. Selectivity of these compounds was not tested (42).
More recently, a screen of proprietary compounds for inhibitors of PERK catalytic activity toward eIF2α was performed (40). Lead optimization resulted in the identification of compounds with in vitro IC50s in the nanomolar range. Of these, 8 compounds exhibited activity in cells. Importantly, these compounds displayed at least 100-fold selectivity for PERK over the closely-related eIF2α kinases HRI and PKR. One of the most potent, selective compounds (GSK2606414) was further assessed for its in vivo efficacy in a human tumor xenograft model. At its highest dose, GSK2606414 inhibited pancreatic tumor burden by 59%. A subsequent study of another compound identified in this screen (GSK2656157) demonstrated inhibitor-dependent restriction not only of tumor growth but also of blood vessel density and vascular perfusion (39). PERK has been shown to upregulate VEGF, therefore, the inhibitory effect of this compound on angiogenesis is not unexpected, and is further evidence of PERK inhibition. In addition, an independent high-throughput screening of approximately 80,000 compounds resulted in the identification of two lead compounds that inhibit PERK catalytic function (41). Unique to this study is the fact that both lead compounds are non-competitive PERK inhibitors. The utility of these compounds in vivo remains to be established.
Therapeutic considerations and alternatives
Despite initial promising results, the small molecule inhibitors tested in vivo have had deleterious effects on pancreatic function. The requirement for PERK in pancreatic beta cell fitness and survival has previously been shown through genetic mouse models in which PERK was either conventionally excised (43), or excised postnatally (44). PERK excision resulted in apoptotic loss of insulin-secreting beta cells and acinar tissue (43, 44); beta cell loss was presumably triggered by an accumulation of misfolded proinsulin and mediated through activation of the remaining UPR branches (44). PERK-deficient mice experienced compromised glucose homeostasis that quickly led to hyperglycemia. These symptoms occurred regardless of age at PERK excision (44), suggesting that PERK function is not only required during early beta cell development but also for adult tissue homeostasis.
Consistent with these reports, PERK inhibition via small molecule inhibitors also resulted in aberrant insulin maturation in Min6 beta cells as well as rat pancreatic islets (45), and led to degeneration of both islet and acinar cells accompanied by a 50% decrease in pancreas weight in mice (39). Though this certainly does not preclude the use of PERK inhibitors in treating neoplastic disease, it does highlight the fact that extreme caution should be used when considering treatment options. It may be possible to titrate the drug to a level of PERK inhibition that would restrict tumor burden while retaining enough activity to support pancreatic homeostasis. Transient inhibition may also lessen toxic effects on the pancreas. In addition, experimental evidence supports an approach wherein pancreatic function could be preserved through insulin supplementation (44). Administering exogenous insulin relieved the demand for insulin synthesis and secretion, which resulted in partial rescue of beta cell death. Finally, PERK inhibition may present a viable option for those with compromised pancreatic function, e.g. patients suffering from pancreatic cancer.
Though the prevailing view of PERK is as an oncogenic effector, PERK has also been proposed to play a role in tumor suppression in certain contexts. This adds an additional layer of complexity to its study not only from a purely biological standpoint but also from a therapeutic perspective. Early studies demonstrated oncogenic transformation of NIH-3T3 cells with inhibition of eIF2α phosphorylation (25), as well as transformation of primary human kidney cells with expression of an eIF2α phospho-mutant (26). Moreover, expression of a dominant-negative PERK mutant in mammary epithelial cells resulted in hyper-proliferation, and orthotopic implantation of these cells promoted mammary tumor formation (28), while PERK activation inhibited tumor growth in a colon cancer model (27). Collectively, these studies suggest a role for PERK in cancer is highly context-dependent, underscoring the need for further definition of its mechanisms of action and downstream target activation under varying conditions, environments, and stages of cancer progression.
In summary, PERK is involved in multiple stages of tumor initiation, progression, and metastasis, aspects of which are just beginning to become clear. Its contribution to the oncogenic process continues to make it a viable target for therapeutic intervention, however, its essential function in pancreatic homeostasis and its potential for dual function warrant further consideration from a clinical perspective. Alternatives to PERK inhibition that are currently being explored target eIF2α (46), IRE1α (47, 48), GRP78/BiP (49–53), proteasomal degradation, and ERAD, among others (8, 54, 55). These may offer the advantage of reduced organ toxicity. The coming years will also undoubtedly see further clarification of PERK signaling mechanisms. Identification of additional interactors and downstream targets should provide the opportunity for more selectively targeting the oncogenic potential of PERK, while preserving its vital functions in cellular homeostasis.
Acknowledgments
This work was supported by NIH grant P01 CA165997 (to J.A. Diehl).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 2.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–4. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 5.Brewer JW, Hendershot LM, Sherr CJ, Diehl JA. Mammalian unfolded protein response inhibits cyclin D1 translation and cell-cycle progression. Proc Natl Acad Sci U S A. 1999;96:8505–10. doi: 10.1073/pnas.96.15.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 8.Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12:703–19. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 9.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 11.Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002;16:452–66. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–91. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 13.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–59. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, et al. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell. 2007;27:53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–7. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–76. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–77. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 18.Tsukamoto Y, Kuwabara K, Hirota S, Kawano K, Yoshikawa K, Ozawa K, et al. Expression of the 150-kd oxygen-regulated protein in human breast cancer. Lab Invest. 1998;78:699–706. [PubMed] [Google Scholar]
- 19.Fernandez PM, Tabbara SO, Jacobs LK, Manning FC, Tsangaris TN, Schwartz AM, et al. Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res Treat. 2000;59:15–26. doi: 10.1023/a:1006332011207. [DOI] [PubMed] [Google Scholar]
- 20.Shuda M, Kondoh N, Imazeki N, Tanaka K, Okada T, Mori K, et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol. 2003;38:605–14. doi: 10.1016/s0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 21.Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24:3470–81. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobrovnikova-Marjon E, Grigoriadou C, Pytel D, Zhang F, Ye J, Koumenis C, et al. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29:3881–95. doi: 10.1038/onc.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64:5943–7. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- 24.Denoyelle C, Abou-Rjaily G, Bezrookove V, Verhaegen M, Johnson TM, Fullen DR, et al. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat Cell Biol. 2006;8:1053–63. doi: 10.1038/ncb1471. [DOI] [PubMed] [Google Scholar]
- 25.Donze O, Jagus R, Koromilas AE, Hershey JW, Sonenberg N. Abrogation of translation initiation factor eIF-2 phosphorylation causes malignant transformation of NIH 3T3 cells. EMBO J. 1995;14:3828–34. doi: 10.1002/j.1460-2075.1995.tb00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkins DJ, Barber GN. Defects in translational regulation mediated by the alpha subunit of eukaryotic initiation factor 2 inhibit antiviral activity and facilitate the malignant transformation of human fibroblasts. Mol Cell Biol. 2004;24:2025–40. doi: 10.1128/MCB.24.5.2025-2040.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranganathan AC, Ojha S, Kourtidis A, Conklin DS, Aguirre-Ghiso JA. Dual function of pancreatic endoplasmic reticulum kinase in tumor cell growth arrest and survival. Cancer Res. 2008;68:3260–8. doi: 10.1158/0008-5472.CAN-07-6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sequeira SJ, Ranganathan AC, Adam AP, Iglesias BV, Farias EF, Aguirre-Ghiso JA. Inhibition of proliferation by PERK regulates mammary acinar morphogenesis and tumor formation. PLoS One. 2007;2:e615. doi: 10.1371/journal.pone.0000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auf G, Jabouille A, Guerit S, Pineau R, Delugin M, Bouchecareilh M, et al. Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Proc Natl Acad Sci U S A. 2010;107:15553–8. doi: 10.1073/pnas.0914072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 31.Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082–96. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avivar-Valderas A, Bobrovnikova-Marjon E, Alan Diehl J, Bardeesy N, Debnath J, Aguirre-Ghiso JA. Regulation of autophagy during ECM detachment is linked to a selective inhibition of mTORC1 by PERK. Oncogene. 2013;32:4932–40. doi: 10.1038/onc.2012.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, et al. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol. 2011;31:3616–29. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mujcic H, Rzymski T, Rouschop KM, Koritzinsky M, Milani M, Harris AL, et al. Hypoxic activation of the unfolded protein response (UPR) induces expression of the metastasis-associated gene LAMP3. Radiother Oncol. 2009;92:450–9. doi: 10.1016/j.radonc.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Nagelkerke A, Bussink J, Mujcic H, Wouters BG, Lehmann S, Sweep FC, et al. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res. 2013;15:R2. doi: 10.1186/bcr3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mujcic H, Nagelkerke A, Rouschop KM, Chung S, Chaudary N, Span PN, et al. Hypoxic activation of the PERK/eIF2alpha arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clin Cancer Res. 2013;19:6126–37. doi: 10.1158/1078-0432.CCR-13-0526. [DOI] [PubMed] [Google Scholar]
- 37.Feng Y, Sokol ES, Del Vecchio CA, Sanduja S, Claessen JH, Proia TA, et al. Epithelial-to-mesenchymal transition activates PERK-eIF2a and sensitizes cells to endoplasmic reticulum stress. Cancer Discov. 2014;4:702–15. doi: 10.1158/2159-8290.CD-13-0945. [DOI] [PubMed] [Google Scholar]
- 38.Ulianich L, Garbi C, Treglia AS, Punzi D, Miele C, Raciti GA, et al. ER stress is associated with dedifferentiation and an epithelial-to-mesenchymal transition-like phenotype in PC Cl3 thyroid cells. J Cell Sci. 2008;121:477–86. doi: 10.1242/jcs.017202. [DOI] [PubMed] [Google Scholar]
- 39.Atkins C, Liu Q, Minthorn E, Zhang SY, Figueroa DJ, Moss K, et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73:1993–2002. doi: 10.1158/0008-5472.CAN-12-3109. [DOI] [PubMed] [Google Scholar]
- 40.Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) J Med Chem. 2012;55:7193–207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 41.Pytel D, Seyb K, Liu M, Ray SS, Concannon J, Huang M, et al. Enzymatic characterization of ER stress-dependent kinase, PERK, and development of a high-throughput assay for identification of PERK inhibitors. J Biomol Screen. 2014;19:1024–34. doi: 10.1177/1087057114525853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Blais J, Ron D, Cardozo T. Structural determinants of PERK inhibitor potency and selectivity. Chem Biol Drug Des. 2010;76:480–95. doi: 10.1111/j.1747-0285.2010.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–74. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Y, Sartori DJ, Li C, Yu QC, Kushner JA, Simon MC, et al. PERK is required in the adult pancreas and is essential for maintenance of glucose homeostasis. Mol Cell Biol. 2012;32:5129–39. doi: 10.1128/MCB.01009-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harding HP, Zyryanova AF, Ron D. Uncoupling proteostasis and development in vitro with a small molecule inhibitor of the pancreatic endoplasmic reticulum kinase, PERK. J Biol Chem. 2012;287:44338–44. doi: 10.1074/jbc.M112.428987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sidrauski C, Acosta-Alvear D, Khoutorsky A, Vedantham P, Hearn BR, Li H, et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife. 2013;2:e00498. doi: 10.7554/eLife.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–4. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mimura N, Fulciniti M, Gorgun G, Tai YT, Cirstea D, Santo L, et al. Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood. 2012;119:5772–81. doi: 10.1182/blood-2011-07-366633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo T, Wang J, Yin Y, Hua H, Jing J, Sun X, et al. (-)-Epigallocatechin gallate sensitizes breast cancer cells to paclitaxel in a murine model of breast carcinoma. Breast Cancer Res. 2010;12:R8. doi: 10.1186/bcr2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuo J, Tsukumo Y, Sakurai J, Tsukahara S, Park HR, Shinya K, et al. Preventing the unfolded protein response via aberrant activation of 4E-binding protein 1 by versipelostatin. Cancer Sci. 2009;100:327–33. doi: 10.1111/j.1349-7006.2008.01036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cha MR, Yoon MY, Son ES, Park HR. Selective cytotoxicity of Ponciri Fructus against glucose-deprived PANC-1 human pancreatic cancer cells via blocking activation of GRP78. Biosci Biotechnol Biochem. 2009;73:2167–71. doi: 10.1271/bbb.90235. [DOI] [PubMed] [Google Scholar]
- 52.Martin S, Lamb HK, Brady C, Lefkove B, Bonner MY, Thompson P, et al. Inducing apoptosis of cancer cells using small-molecule plant compounds that bind to GRP78. Br J Cancer. 2013;109:433–43. doi: 10.1038/bjc.2013.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–16. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 54.Clarke HJ, Chambers JE, Liniker E, Marciniak SJ. Endoplasmic Reticulum Stress in Malignancy. Cancer Cell. 2014;25:563–73. doi: 10.1016/j.ccr.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Li X, Zhang K, Li Z. Unfolded protein response in cancer: the physician’s perspective. J Hematol Oncol. 2011;4:8. doi: 10.1186/1756-8722-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]