Abstract
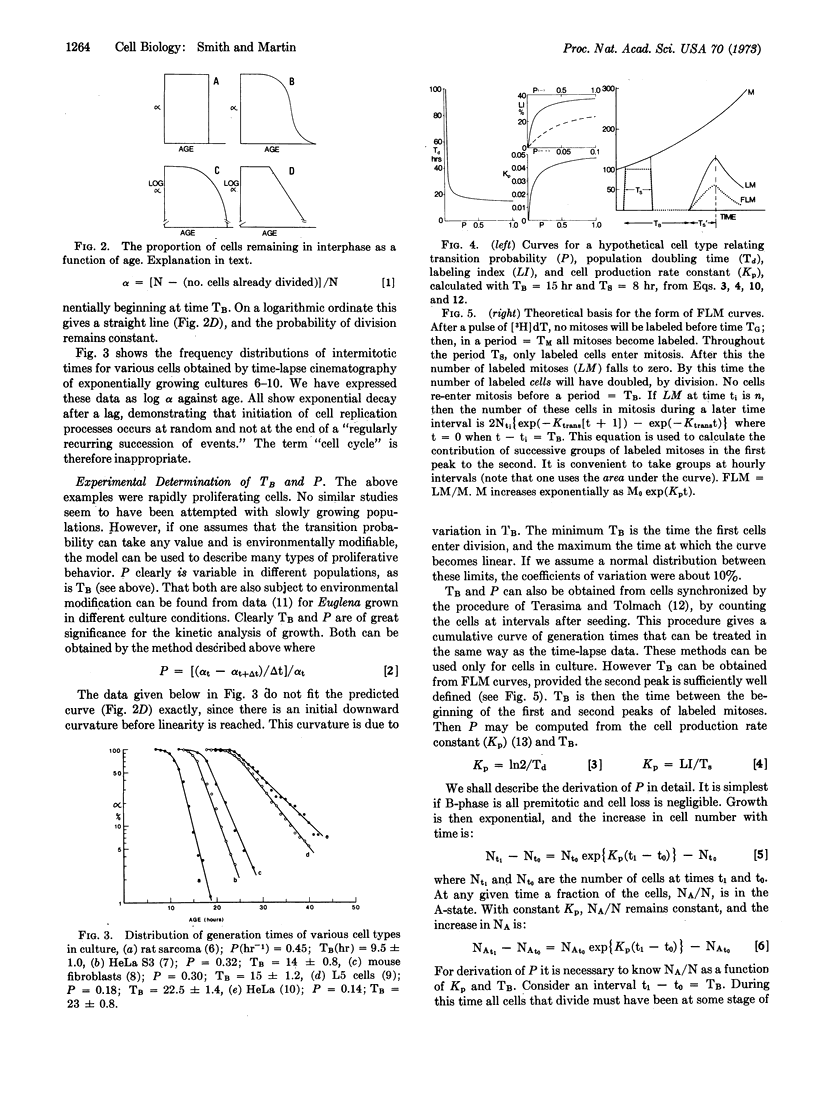
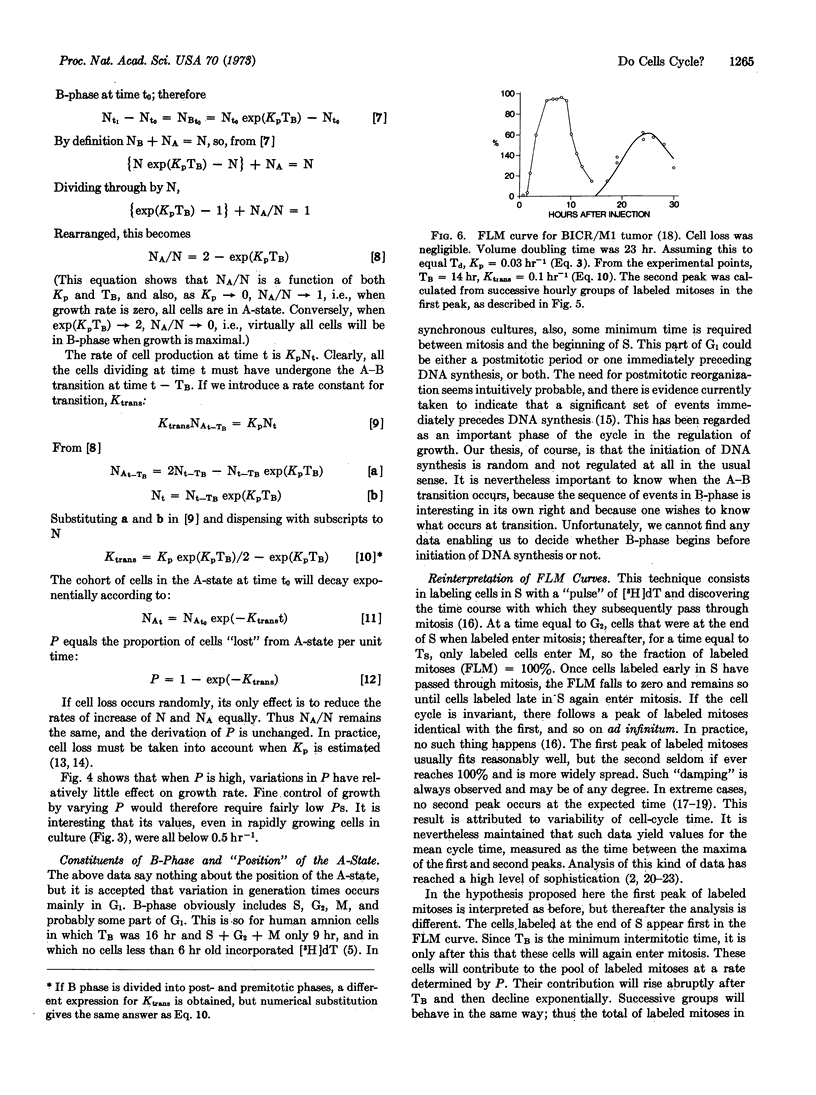
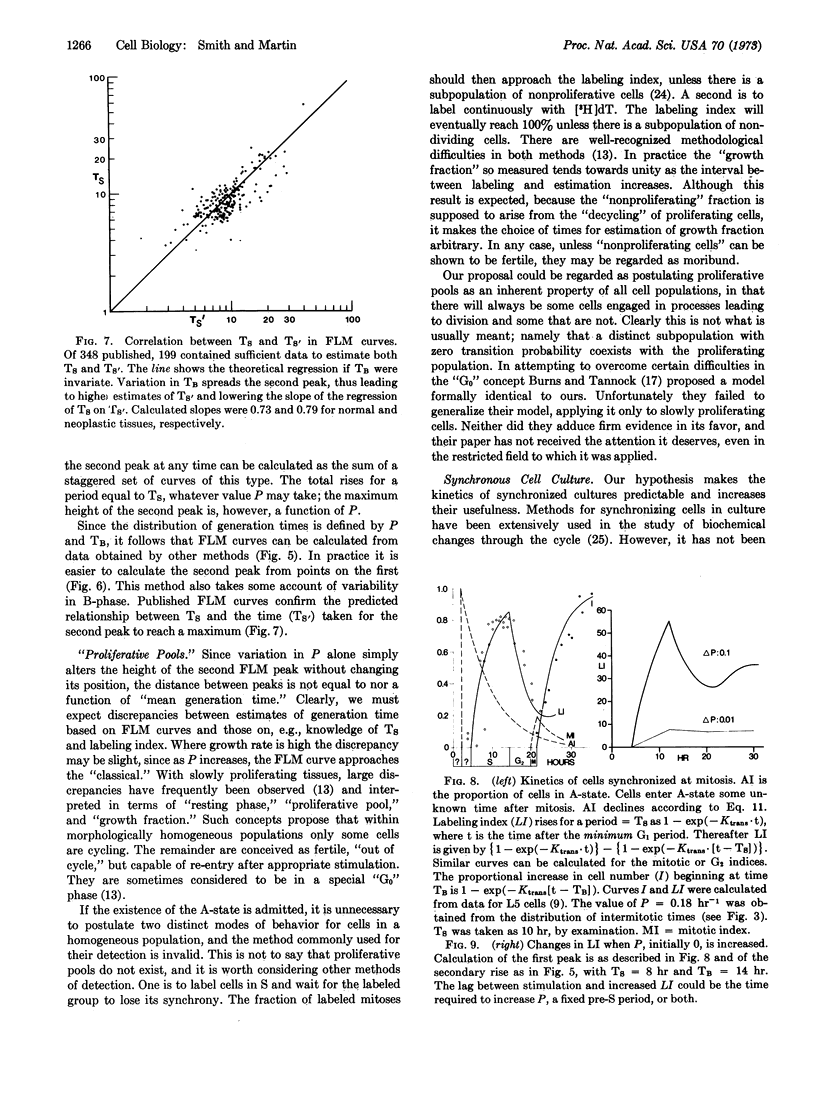
We propose that a cell's life is divided into two fundamentally different parts. Some time after mitosis all cells enter a state (A) in which their activity is not directed towards replication. A cell may remain in the A-state for any lenght of time, throughout which its probability of leaving A-state remains constant. On leaving A-state, cells enter B-phase in which their activities are deterministic, and directed towards replication. Initiation of cell replication processes is thus random, in the sense that radioactive decay is random. Cell population growth rates are determined by the probability with which cells leave the A-state, the duration of the B-phase, and the rate of cell death. Knowledge of these parameters permits precise calculation of the distribution of intermitotic times within populations, the behavior of synchronized cell cultures, and the shape of labeled mitosis curves.
Keywords: cell kinetics, control of growth, DNA replication, cell culture
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKA T. INDUCED CELL PROLIFERATION: THE EFFECT OF ISOPROTERENOL. Exp Cell Res. 1965 Mar;37:662–679. doi: 10.1016/0014-4827(65)90214-4. [DOI] [PubMed] [Google Scholar]
- Barrett J. C. A mathematical model of the mitotic cycle and its application to the interpretation of percentage labeled mitoses data. J Natl Cancer Inst. 1966 Oct;37(4):443–450. [PubMed] [Google Scholar]
- Burns F. J., Tannock I. F. On the existence of a G 0 -phase in the cell cycle. Cell Tissue Kinet. 1970 Oct;3(4):321–334. doi: 10.1111/j.1365-2184.1970.tb00340.x. [DOI] [PubMed] [Google Scholar]
- COOK J. R., COOK B. Effect of nutrients on the variation of individual generation times. Exp Cell Res. 1962 Dec;28:524–530. doi: 10.1016/0014-4827(62)90257-4. [DOI] [PubMed] [Google Scholar]
- DAWSON K. B., MADOC-JONES H., FIELD E. O. VARIATIONS IN THE GENERATION TIMES OF A STRAIN OF RAT SARCOMA CELLS IN CULTURE. Exp Cell Res. 1965 Apr;38:75–84. doi: 10.1016/0014-4827(65)90429-5. [DOI] [PubMed] [Google Scholar]
- Epifanova O. I. Mitotic cycles in estrogen-treated mice: a radioautographic study. Exp Cell Res. 1966 Jun;42(3):562–577. doi: 10.1016/0014-4827(66)90269-2. [DOI] [PubMed] [Google Scholar]
- Fox T. O., Pardee A. B. Animal cells: noncorrelation of length of G1 phase with size after mitosis. Science. 1970 Jan 2;167(3914):80–82. doi: 10.1126/science.167.3914.80. [DOI] [PubMed] [Google Scholar]
- Gibbs S. J., Casarett G. W. Influences of a circadian rhythm and mitotic delay from tritiated thymidine on cytokinetic studies in hamster cheek pouch epithelium. Radiat Res. 1969 Dec;40(3):588–600. [PubMed] [Google Scholar]
- HSU T. C. Generation time of HeLa cells determined from cine records. Tex Rep Biol Med. 1960;18:31–33. [PubMed] [Google Scholar]
- KILLANDER D., ZETTERBERG A. QUANTITATIVE CYTOCHEMICAL STUDIES ON INTERPHASE GROWTH. I. DETERMINATION OF DNA, RNA AND MASS CONTENT OF AGE DETERMINED MOUSE FIBROBLASTS IN VITRO AND OF INTERCELLULAR VARIATION IN GENERATION TIME. Exp Cell Res. 1965 May;38:272–284. doi: 10.1016/0014-4827(65)90403-9. [DOI] [PubMed] [Google Scholar]
- LaBella F. S., Sanwal M. Isolation of nerve endings from the posterior pituitary gland. Electron microscopy of fractions obtained by centrifugation. J Cell Biol. 1965 Jun;25(3 Suppl):179–193. doi: 10.1083/jcb.25.3.179. [DOI] [PubMed] [Google Scholar]
- MENDELSOHN M. L., DOHAN F. C., Jr, MOORE H. A., Jr Autoradiographic analysis of cell proliferation in spontaneous breast cancer of C3H mouse. I. Typical cell cycle and timing of DNA synthesis. J Natl Cancer Inst. 1960 Sep;25:477–484. [PubMed] [Google Scholar]
- Marin G., Bender M. A. Radiation-induced mammalian cell death: lapse-time cinemicrographic observations. Exp Cell Res. 1966 Sep;43(2):413–423. doi: 10.1016/0014-4827(66)90068-1. [DOI] [PubMed] [Google Scholar]
- Martin L., Finn C. A., Trinder G. Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. J Endocrinol. 1973 Jan;56(1):133–144. doi: 10.1677/joe.0.0560133. [DOI] [PubMed] [Google Scholar]
- PETERSEN D. F., ANDERSON E. C. QUANTITY PRODUCTION OF SYNCHRONIZED MAMMALIAN CELLS IN SUSPENSION CULTURE. Nature. 1964 Aug 8;203:642–643. doi: 10.1038/203642a0. [DOI] [PubMed] [Google Scholar]
- Prescott D. M. Regulation of cell reproduction. Cancer Res. 1968 Sep;28(9):1815–1820. [PubMed] [Google Scholar]
- QUASTLER H., SHERMAN F. G. Cell population kinetics in the intestinal epithelium of the mouse. Exp Cell Res. 1959 Jun;17(3):420–438. doi: 10.1016/0014-4827(59)90063-1. [DOI] [PubMed] [Google Scholar]
- Smith J. A., King R. J. Effects of steroids on growth of an androgen-dependent mouse mammary carcinoma in cell culture. Exp Cell Res. 1972 Aug;73(2):351–359. doi: 10.1016/0014-4827(72)90058-4. [DOI] [PubMed] [Google Scholar]
- Steel G. G., Adams K., Barrett J. C. Analysis of the cell population kinetics of transplanted tumours of widely-differing growth rate. Br J Cancer. 1966 Dec;20(4):784–800. doi: 10.1038/bjc.1966.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel G. G., Hanes S. The technique of labelled mitoses: analysis by automatic curve-fitting. Cell Tissue Kinet. 1971 Jan;4(1):93–105. doi: 10.1111/j.1365-2184.1971.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Steel G. G. The cell cycle in tumours: an examination of data gained by the technique of labelled mitoses. Cell Tissue Kinet. 1972 Jan;5(1):87–100. doi: 10.1111/j.1365-2184.1972.tb00358.x. [DOI] [PubMed] [Google Scholar]
- TERASIMA T., TOLMACH L. J. Growth and nucleic acid synthesis in synchronously dividing populations of HeLa cells. Exp Cell Res. 1963 Apr;30:344–362. doi: 10.1016/0014-4827(63)90306-9. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Control of multiplication of uninfected rat cells and rat cells converted by murine sarcoma virus. J Cell Physiol. 1970 Feb;75(1):107–119. doi: 10.1002/jcp.1040750113. [DOI] [PubMed] [Google Scholar]



