Abstract
Background
Prompt recognition of underlying cardiovascular implantable electronic device (CIED) infection in patients presenting with S. aureus bacteremia (SAB) is critical for optimal management of these cases. The goal of this study was to identify clinical predictors of CIED infection in patients presenting with SAB and no signs of pocket infection.
Methods and Results
All cases of SAB in CIED recipients at Mayo Clinic from 2001 to 2011 were retrospectively reviewed. We identified 131 patients with CIED who presented with SAB and had no clinical signs of device pocket infection. Forty-five (34%) of these patients had underlying CIED infection based on clinical and/or echocardiographic criteria. The presence of a permanent pacemaker rather than an implantable cardioverter-defibrillator (OR 3.90, 95% CI 1.65–9.23), P=0.002), >1 device-related procedure (OR 3.30, 95% CI 1.23–8.86, P=0.018), and duration of SAB ≥4 days (OR 5.54, 95% CI 3.32–13.23, P<0.001) were independently associated with an increased risk of CIED infection in a multivariable model. The area under the receiver operating characteristics curve (AUC) for the multivariable model was 0.79, indicating a good discriminatory capacity to distinguish SAB patients with and without CIED infection.
Conclusions
Among patients presenting with SAB and no signs of pocket infection, the risk of underlying CIED infection can be calculated based on the type of device, number of device-related procedures, and duration of SAB. We propose that patients without any of these high-risk features have a very low risk of underlying CIED infection and may be monitored closely without immediate device extraction. Prospective studies are needed to validate this risk prediction model.
Keywords: infection, infective endocarditis, pacemaker, implantable cardioverter-defibrillator, staphylococcus aureus, bacteremia, scoring system
Introduction
Staphylococcus aureus is one of the leading causes of bloodstream infection in both community and healthcare settings and is associated with significant morbidity and mortality.1, 2 S. aureus bacteremia (SAB) in cardiovascular implantable electronic device (CIED) recipients can be due to device infection or a separate source.3 Patients with a non-CIED source of SAB are at risk of hematogenous seeding of CIED leads.4 In earlier publications, the reported rates of underlying CIED infection in patients presenting with SAB range from 30% to 50%.3, 5, 6 Prompt recognition of underlying CIED infection in SAB cases is critical as antimicrobial therapy alone without expedited complete device removal is associated with high mortality and risk of relapse. In one study of SAB in CIED recipients3, patients who were managed with antimicrobial therapy alone were more likely to die (47.6%) versus those who underwent complete device extraction [16.7%]. Treatment failure (death or development of relapsing SAB) in this study was 52% with conservative therapy versus 25% with device extraction.
Diagnosis of CIED infection is obvious in the large majority of patients who present with inflammatory changes (pain, erythema, swelling, drainage) or cellulitis at the generator pocket.7 However, a high index of suspicion is needed in SAB patients without CIED pocket symptoms to secure the diagnosis of underlying CIED infection by obtaining transesophageal echocardiography (TEE). There is limited evidence-based guidance for clinicians to decide which patients with SAB and presence of CIED should undergo further testing with TEE and undergo device extraction if endovascular device infection is confirmed. Earlier studies addressing this issue had relatively smaller cohorts of patients, included patients with generator pocket infection and none had a multivariable analysis to identify independent predictors of CIED infection.3, 5 Consequently, the most recent scientific statement from the American Heart Association/Heart Rhythm Society regarding diagnosis and management of CIED infection8 specifically identifies a need to “Define a scoring system that distinguishes patients with S aureus bacteremia and no other evidence of device infection who prove to have CIED infection from those who do not, so that unnecessary device removal can be avoided”. In current investigation, our goal was to identify clinical predictors of underlying CIED infection in patients presenting with SAB but no signs of pocket infection, using a multivariable logistic regression model, to enable clinicians estimate the risk of CIED infection for individual patients.
Methods
All consecutive patients diagnosed with SAB at Mayo Clinic, Rochester, from January 2001 to December 2011 were retrospectively reviewed. Cases of SAB were identified via computerized database of the microbiology laboratory at Mayo Clinic and electronic medical records. Medical records of all SAB patients were then manually reviewed to identify patients who had a CIED (permanent pacemaker, PPM or implantable cardioverter-defibrillator, ICD) in place at the time of SAB. Patients with clinical evidence of CIED pocket infection were excluded from this study. The Mayo Foundation Institutional Review Board approved the study protocol.
Definitions
CIED infection
Cardiovascular implantable electronic device infection was defined based on clinical, microbiologic and echocardiographic criteria described earlier.5, 7, 8 Clinical evidence of CIED generator pocket infection included local signs of inflammation at the generator pocket (e.g., erythema, warmth, fluctuance, wound dehiscence, tenderness, purulent drainage, or erosion of the generator leads through the skin). CIED-related infective endocarditis (IE) was confirmed if lead vegetations were detected by echocardiography or if the modified Duke criteria for infective endocarditis were met.9–11 Only cases that met Duke criteria for “definite” endocarditis were classified as CIED-IE because patients with SAB, fever, and presence of an intracardiac device would automatically be classified as Duke “possible.” CIED infection was microbiologically confirmed on the basis of positive culture results from the generator pocket, lead(s), or blood (in the presence of local inflammatory signs at the generator pocket). Device infection was rejected if the patient had no clinical evidence of CIED infection at the time of the initial SAB diagnosis, the device was not removed, and there was no evidence of SAB relapse within 12 weeks after discharge.
SAB
Cases SAB were classified as nosocomial, healthcare associated, or community acquired, as defined by Freidman et al.12
Nosocomial bacteremia
Nosocomial bacteremia was defined as positive blood culture result obtained from patients who had been hospitalized for ≥48 hours. If a patient were to be transferred from another hospital, the duration of inpatient stay was calculated from the date of the first hospital admission.
Healthcare-associated bacteremia
Healthcare-associated bacteremia was defined by a positive blood culture result obtained from a patient at the time of hospital admission or within 48 hours of admission if the patient fulfilled any of the following criteria: (A) Received intravenous therapy at home; received wound care or specialized nursing care through a health care agency, family, or friends; or had self-administered intravenous medical therapy in the 30 days before bloodstream infection. Patients whose only home therapy is oxygen use were excluded, B) Attended a hospital or hemodialysis clinic or received intravenous chemotherapy in the 30 days before the blood-stream infection, (C) Was hospitalized in an acute care hospital for 2 days in the 90 days before the bloodstream infection or (D) Resided in a nursing home or long-term care facility.
Community-acquired bacteremia
Community-acquired bacteremia was defined by a positive blood culture result obtained at the time of hospital admission or within 48 hours after hospital admission for patients who did not fit the criteria for a healthcare-associated infection.
Duration of SAB
Duration of SAB was defined from the date when the initial positive blood culture was obtained to date when the first negative blood culture was obtained.
All cases of SAB or CIED infection for which classification was unclear or controversial were reviewed by an experienced investigator (M.R.S. or L.M.B.).
Statistical Methods
Continuous features were summarized with means, medians, and 25th and 75th percentiles; categorical features were summarized with counts and percentages. Associations with CIED infection were evaluated using logistic regression models and summarized with odds ratios and 95% confidence intervals (CIs). A multivariable model was developed using stepwise selection, with the p-value for a feature to enter or leave the model set to 0.05. Model discrimination (how well the features in the model separate patients with and without CIED infection) was summarized using the area under a receiver operating characteristics curve, or AUC. The AUC can range from 0.5 to 1.0, with higher values indicating improved predictive ability or improved discrimination. Model calibration (how well the predicted probabilities of the event estimated by the model agree with the observed event) was summarized using the Hosmer and Lemeshow goodness-of-fit test. A statistically significant p-value from this test would reject the null hypothesis that the features in the model fit the data well. Analyses were performed using the SAS software package (SAS Institute, Inc., Cary, NC). All tests were two-sided and p-values <0.05 were considered statistically significant.
Results
Study Population
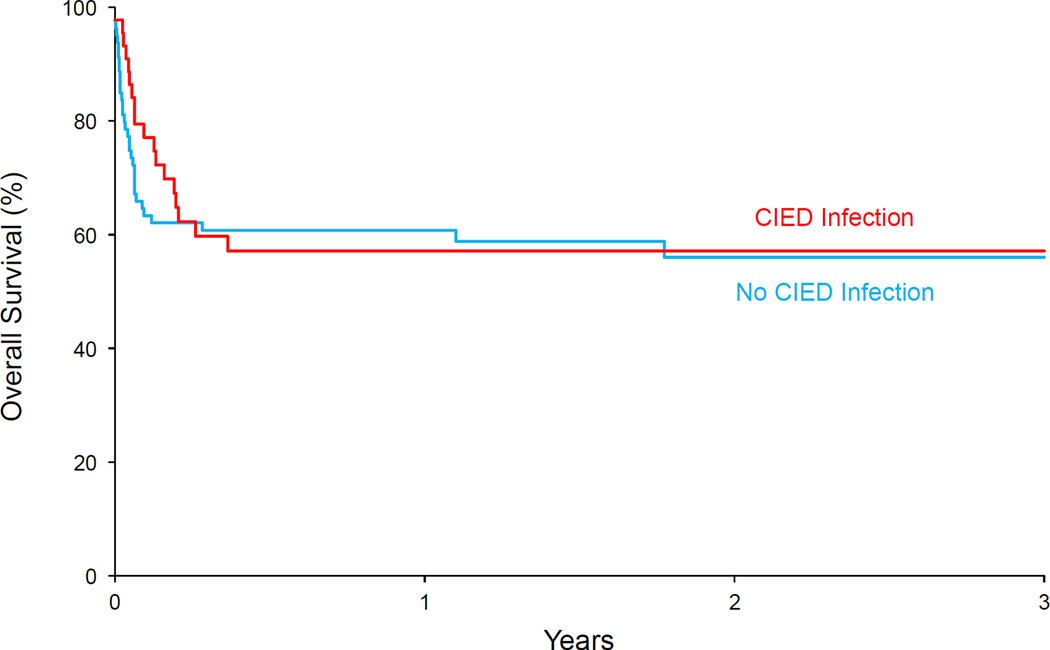
We identified 131 patients with CIEDs who were hospitalized with SAB during the study period and met inclusion criteria. Seventy-seven percent of patients were males and median age of the patient cohort was 73 years The majority (85%) of patients underwent echocardiography. Approximately 64% of patients underwent TEE and 21% had transthoracic echocardiography (TTE) only. Forty-five (34.3%) of patients met the study criteria for CIED infection. All patients who were classified as having CIED infection underwent echocardiography; 91% had TEE and 9% had only TTE. Forty-one cases of CIED infection had intra-cardiac vegetations on TEE. Three patients had negative TTE but vegetations on device lead were observed on subsequent TEE. Clinical presentation, management and outcome of the 45 patients classified as having CIED infection is summarized in Tables 1 and 2. There was no statistically significant difference in overall survival between patients with and without CIED infection (Figure 1).
Table 1.
Clinical presentation of 45 patients with CIED infection
| Clinical Feature | N (%) |
|---|---|
| Type of CIED infection* | |
| Intraoperative signs of pocket infection | 3 (7) |
| CIED lead vegetation | 24 (53) |
| Native valve vegetation | 9 (20) |
| Prosthetic valve vegetation | 8 (18) |
| Location of valvular vegetation (N=15)* | |
| Tricuspid | 8 (53) |
| Pulmonary | 1 (7) |
| Mitral | 8 (53) |
| Aortic | 4 (27) |
Patients can be listed in more than one category.
Abbreviations: CIED = cardiovascular implantable electronic device
Table 2.
Management and outcome of 45 cases of CIED infection
| Antibiotics alone (N=12, 27%) |
Antibiotics + CIED removal (N= 26, 58%) |
Antibiotics + chronic suppression (N=7, 16%) |
|
|---|---|---|---|
| Outcome at discharge | |||
| Cured | 3 | 16 | 2 |
| Relapse | 4 | 1 | 2 |
| Death | 5 | 7 | 2 |
| Lost to follow-up | 0 | 2 | 0 |
| Other | 0 | 0 | 1 |
| Vital status at 3-months | |||
| Alive | 6 | 17 | 3 |
| Dead | 6 | 8 | 4 |
| Unknown | 0 | 1 | 0 |
Figure 1.
Overall survival with or without CIED infection
Model Development
Univariate associations of host and device-related risk factors with CIED infection are summarized in Table 3. Age at onset of SAB (OR 0.97, 95% CI 0.95–1.00), PPM as device type (OR 3.77, 95% CI 1.73–8.18), history of more than one CIED-related procedure (OR 2.37, 95% CI 1.03–5.45), and duration of SAB (OR 1.28 [representing a 1-day increase in the SAB duration], 95% CI 1.12–1.48) were associated with increased odds of having CIED infection. Risk of CIED infection with SAB duration ≥4 days, for instance, was associated with OR of 5.12 (95% CI 2.30–11.40).Of note, number of CIED leads, presence of a central venous catheter, prosthetic heart valve, hemodialysis, presumed source of SAB and onset of SAB (nosocomial vs. healthcare associated vs. community-onset) were not associated with increased risk of CIED infection.
Table 3.
Univariate associations of host and device-related risk factors with CIED infection (N=131)
| No CIED Infection N=86 |
CIED Infection N=45 |
|||
|---|---|---|---|---|
| Clinical Features | Mean (Median; Q1–Q3: Range) | Odds Ratio (95% CI) | P-value | |
| Age at onset of SAB (years) | 75.2 (78; 70–83; 39–92) | 70.2 (71; 64–81; 20–102) | 0.97 (0.95–1.00)* | 0.045 |
| Years from device placement to SAB | 4.0 (3.5; 0.9–5.2; 0.1–14.9) | 4.5 (3.3; 1.0–6.3; 0.0–18.7) | 1.03 (0.94–1.13)* | 0.50 |
| Duration of SAB (days; N=128) | 3.3 (3; 1–4; 1–14) | 8.2 (5; 3–8; 1–60) | 1.28 (1.12–1.48)* | <0.001 |
| Number of leads in place | 1.9 (2; 2-2; 1–4) | 1.8 (2; 1–2; 1–5) | 0.66 (0.34–1.26)* | 0.20 |
| Sex | N (%) | |||
| Female | 19 (22) | 11 (24) | 1.0 (reference) | |
| Male | 67 (78) | 34 (76) | 0.88 (0.38–2.05) | 0.76 |
| Type of device | ||||
| ICD | 52 (60) | 13 (29) | 1.0 (reference) | |
| PPM | 34 (40) | 32 (71) | 3.77 (1.73–8.18) | <0.001 |
| >1 Device-related procedure | 15 (17) | 15 (33) | 2.37 (1.03–5.45) | 0.043 |
| <1 Year from device placement to SAB | 22 (26) | 12 (27) | 1.06 (0.47–2.40) | 0.89 |
| <3 Months from revision/upgrade to SAB | 4 (5) | 3 (7) | 1.46 (0.31–6.85) | 0.63 |
| Diabetes | 41 (48) | 15 (33) | 0.55 (0.26–1.16) | 0.12 |
| Hemodialysis | 22 (26) | 8 (18) | 0.63 (0.26–1.56) | 0.32 |
| Active malignancy | 11 (13) | 4 (9) | 0.67 (0.20–2.22) | 0.51 |
| Prosthetic heart valve | 19 (22) | 10 (22) | 1.01 (0.42–2.40) | 0.99 |
| Central venous catheter | 28 (33) | 13 (29) | 0.84 (0.38–1.85) | 0.67 |
| Immunocompromised | 12 (14) | 4 (9) | 0.60 (0.18–1.99) | 0.40 |
| HIV | 0 | 0 | NA | NA |
| Heart failure | 60 (70) | 35 (78) | 1.52 (0.66–3.51) | 0.33 |
| Coronary artery disease | 58 (67) | 30 (67) | 0.97 (0.45–2.08) | 0.93 |
| Atrial fibrillation | 48 (56) | 24 (53) | 0.91 (0.44–1.87) | 0.79 |
| Onset of SAB | ||||
| Nosocomial | 21 (24) | 13 (29) | 1.0 (reference) | |
| Health care-associated | 43 (50) | 14 (31) | 0.53 (0.21–1.32) | 0.17 |
| Community | 22 (26) | 18 (40) | 1.32 (0.52–3.35) | 0.56 |
| Causative pathogen | ||||
| MSSA | 51 (59) | 28 (62) | 1.0 (reference) | |
| MRSA | 35 (41) | 17 (38) | 0.89 (0.42–1.86) | 0.75 |
| Presumed source of SAB† | ||||
| Catheter-related | 19 (22) | 7 (16) | 0.65 (0.25–1.69) | 0.38 |
| Deep tissue abscess/skin/cellulitis | 22 (26) | 13 (29) | 1.18 (0.53–2.65) | 0.68 |
| Other | 25 (29) | 8 (18) | 0.53 (0.22–1.29) | 0.16 |
| Unknown (no clear portal of entry) | 29 (34) | 22 (49) | 1.88 (0.90–3.92) | 0.093 |
| Duration of SAB ≥2 days (N=128) | 58 (70) | 42 (93) | 6.03 (1.71–21.31) | 0.005 |
| Duration of SAB ≥3 days (N=128) | 47 (57) | 36 (80) | 3.06 (1.31–7.17) | 0.010 |
| Duration of SAB ≥4 days (N=128) | 29 (35) | 33 (73) | 5.12 (2.30–11.40) | <0.001 |
Odds ratio represents a 1-unit increase in the feature listed.
Patient can be listed in more than one category.
(Sample sizes for clinical features with missing data are indicated in italics in parentheses)
Abbreviations: CIED = cardiovascular implantable electronic device, ICD = implantable cardioverter-defibrillator, MRSA = Methicillin-resistant S. aureus, MSSA = Methicillin-susceptible S. aureus NA = not applicable, PPM = permanent pacemaker, SAB = S. aureus bacteremia
A multivariable model was developed using stepwise selection, with the p-value for a feature to enter or leave the model set to 0.05. Significant associations identified in the multivariable model are summarized in Table 4. Presence of PPM as cardiac device (OR 3.90, 95% CI 1.65–9.23), history of >1 device-related procedure (OR 3.30, 95% CI 1.23–8.86), and SAB duration ≥4 days (OR 5.54, 95% CI 3.32–13.23) were independently associated with increased risk of CIED infection. The AUC for this multivariable model was 0.79 and the p-value from the Hosmer and Lemeshow goodness-of-fit test was 0.90, indicating that the features in the model fit the data well.
Table 4.
Multivariable model to predict CIED infection in SAB cases (N=128)
| Clinical Feature | Odds Ratio (95% CI) | P-value |
|---|---|---|
| Type of device | ||
| ICD | 1.0 (reference) | |
| PPM | 3.90 (1.65–9.23) | 0.002 |
| >1 Device-related procedure | 3.30 (1.23–8.86) | 0.018 |
| Duration of SAB ≥4 days | 5.54 (3.32–13.23) | <0.001 |
A total of 128 patients had non-missing data for these four features.
Abbreviations: CIED = cardiovascular implantable electronic device, ICD = implantable cardioverter-defibrillator, PPM = permanent pacemaker, SAB = S. aureus bacteremia
The equation derived from this logistic regression model can be used to calculate the predicted probability that a patient will experience CIED infection based on the three features listed above. The equation of the model is as follows:
x = −2.5956 + 1.3602 (if PPM as device type) + 1.1926 (if history of >1 device-related procedure) + 1.7128 (if SAB duration is ≥4 days)with the predicted probability of CIED infection = ex/(1 + ex).
For example, if a patient does not have any of the features listed above, x = −2.5956 and the predicted probability of CIED infection is 7% (e−2.5956/[1 + e−2.5956] = 0.07). At the other end of the spectrum, if a patient has all three features listed above, x = 1.6700 and the predicted probability of CIED infection is 84%.
Scoring System
A scoring algorithm was created using the coefficients from the multivariable logistic regression model. The coefficient for each feature was divided by the coefficient for duration of SAB ≥4 days (i.e., the highest coefficient) and multiplied by a constant, in this case 5, resulting in 4 points assigned for PPM as device type, 3.5 points assigned for history of >1 device-related procedure, 5 points assigned for duration of SAB duration ≥4 days, and 0 otherwise. The calculated scores ranged from a low of 0 to a high of 12.5 (mean 5.2; median 5; Q1–Q3 3.5–9). If the score is placed in a logistic regression model, each 1-unit increase in the score was associated with a 41% increased odds of CIED infection (odds ratio 1.41; 95% CI 1.23–1.61; p<0.001), with a c-index of 0.79.
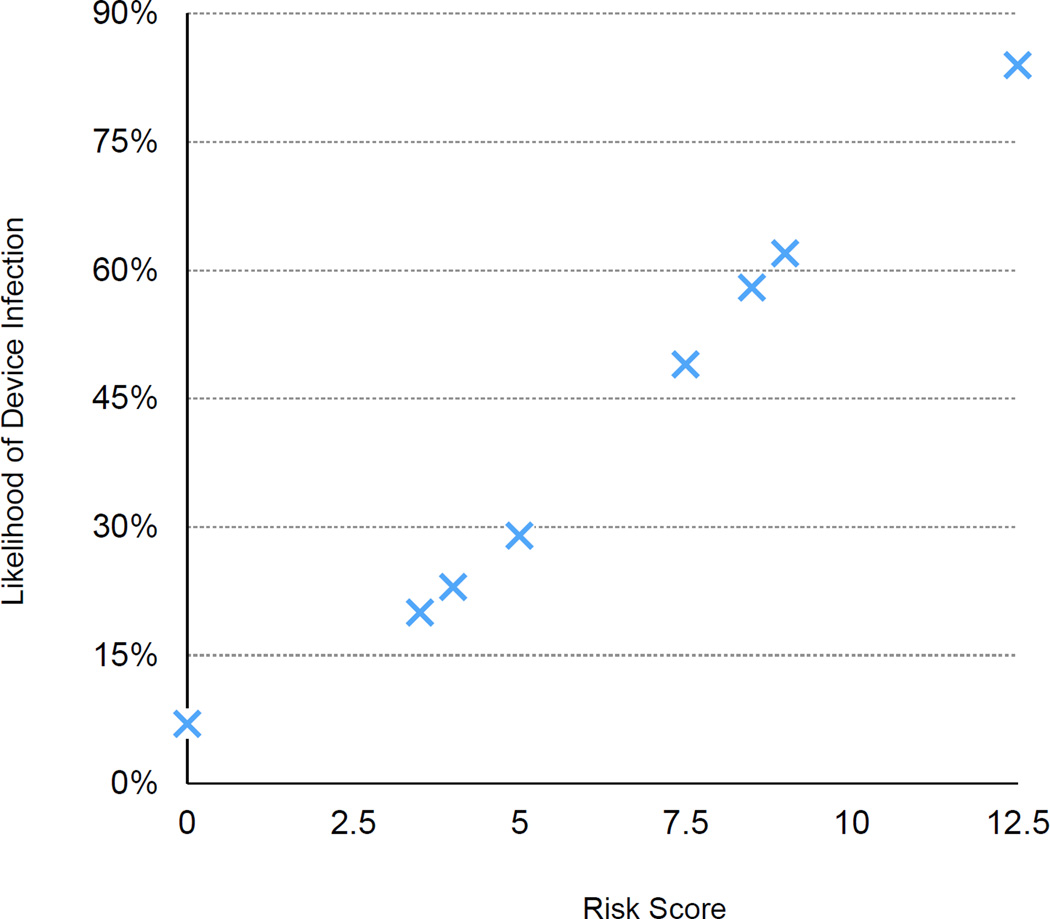
The combinations of three risk factors identified in the multivariable model, the calculated risk score, and the corresponding predicted probabilities of CIED infection are summarized in Table 5 and Figure 2.
Table 5.
Combinations of clinical features and predicted probabilities of CIED infection (N=128)
| Device Type | >1 Device- related Procedure |
SAB ≥4 days |
Risk Score | # SAB cases |
# Infected cases |
Predicted Probability |
|---|---|---|---|---|---|---|
| ICD | No | No | 0 | 29 | 1 | 0.07 |
| ICD | No | Yes | 5 | 21 | 7 | 0.29 |
| ICD | Yes | No | 3.5 | 7 | 2 | 0.20 |
| ICD | Yes | Yes | 8.5 | 6 | 3 | 0.58 |
| PPM | No | No | 4 | 23 | 5 | 0.23 |
| PPM | No | Yes | 9 | 27 | 17 | 0.62 |
| PPM | Yes | No | 7.5 | 7 | 4 | 0.49 |
| PPM | Yes | Yes | 12.5 | 8 | 6 | 0.84 |
Abbreviations: CIED = cardiovascular implantable electronic device, ICD = implantable cardioverter-defibrillator, PPM = permanent pacemaker, SAB: S. aureus bacteremia, # SAB cases = patients in current study with combination of features listed, # Infected Cases = number of patients with CIED infection in our cohort, PP = predicted probability of CIED infection from multivariable model.
Risk Scores: We assigned 4 points for PPM device, 3.5 points assigned for >1 device-related procedure, 5 points assigned for duration of SAB ≥4 days, and 0 otherwise
Figure 2.
Risk scores and predicted probability of CIED infection
Four points were assigned for permanent pacemaker, 3.5 points were assigned for >1 device-related procedure, and 5 points were assigned for duration of SAB ≥4 days.
Discussion
Our study is the first to include a multivariable analysis and a prediction model to assess risk of underlying CIED infection in device recipients who present with SAB. Underlying CIED infection should always be a concern in device recipients who present with SAB. However, CIED infection may not be clinically evident if signs or symptoms of generator pocket infection are absent. Among our study cohort of SAB cases in CIED recipients, only 11 (19.6%) of 56 had clinical evidence of generator pocket infection (and these patients were excluded from the risk factor analysis). Undiagnosed CIED infection in SAB cases and management of bloodstream with antimicrobial therapy alone in these cases is associated with high morbidity and mortality.3, 13 In an earlier cohort of CIED patients who presented with SAB,3 CIED-infection related mortality was 47% if infected device was not removed versus 16% in patients managed with complete device extraction. In the current investigation, conservative management of known CIED infection cases with antimicrobial therapy alone resulted in adverse outcome (relapse or death) in 75% (9 of 12) of the cases. Therefore, prompt recognition of underlying device infection in SAB cases and expedited device removal is critical for optimal management of these cases. On the other hand, unnecessary device extraction in low risk patients is neither advisable nor desirable. Currently, there is limited guidance3, 5 on which patients presenting with SAB are at increased risk of underlying device infection and should undergo expedited TEE for further diagnostic evaluation.
In our multivariable analysis, PPM as device type, >1 device-related procedure and duration of SAB ≥4 days were independent predictor CIED infection in SAB cases. Presence of one or more of these risk factors was associated with higher likelihood of underlying CIED infection. Based on the findings of our multivariable analysis, we were able to devise a prediction model and scoring system to predict the probability of CIED infection in patients presenting with SAB (Table 4 & 5 and Figure 2). Only one patient in our study cohort had none of these high-risk features but had echocardiographic evidence of device lead infection. We believe that this prediction model and the scoring system can be helpful for clinicians to risk stratify their patients presenting with SAB and make informed decision regarding additional testing and need for CIED extraction.
Prolonged duration of SAB has been identified as risk factor for complicated bloodstream infection in earlier investigations. In a large prospective, observational study of 724 patients14, sustained SAB (positive follow-up blood culture results at 48–96 hours) was the strongest predictor (OR 5.58, 95% CI 3.93–7.95) of complicated SAB. Similarly, in an earlier smaller study15 of 55 patients with catheter-related SAB, sustained bacteremia beyond 3 days was associated with higher likelihood of complications (endocarditis or osteomyelitis). In our multivariable prediction model, a SAB duration ≥4 day was one of the strongest predictors of underlying CIED infection (OR 5.54, 95% CI 3.32–13.23). Patients with signs of pocket infection were excluded to address the question of hematogenous seeding of CIED. Among patients who present with no signs of pocket infection and SAB, the duration of bacteremia is the strongest predictor of underlying CIED infection. It is not clear why the presence of a PPM rather than ICD is associated with a higher risk of CIED infection. In our analysis, presence of prosthetic valve or hemodialysis was not associated with a higher risk of CIED infection. However, regardless of cardiac device type or host factors, prolonged SAB is associated with a higher risk of CIED infection and evaluation with TEE should be performed.
Patients who have signs and symptoms suggestive of endocarditis (such as new murmur or worsening heart murmur, conjunctival hemorrhages, or embolic lesions), those who have additional risk factors for CIED infection identified in our analysis (pacemaker as CIED type or prior history of device revisions), and those that are clinically unstable (hypotension, cardiac arrhythmia, EKG changes), should undergo TEE soon after admission. However, for cases where the only indication for performing TEE are positive blood cultures for S. aureus, we recommend waiting until blood cultures are negative and then use the duration of SAB (>4 days or less) as a criteria for deciding whether conservative management is appropriate in a given case. Clinical judgment and consultation with an Infectious Diseases expert in recommended in cases in whom patients do not have high-risk criteria identified but clinical suspicion for CIED infection is high.
CIED revision procedures (generator change, upgrade, lead addition or revision) can result in contamination of the pocket tissues, fibrous capsule around the generator or device surface and has been associated with increased odds of CIED infection.16–21 In our prediction model, patients with history of more than one device-related procedure, without any additional risk factors, had 20% predicted probability of CIED infection. Interestingly, PPM as device type was also an independent predictor of CIED infection in our multivariable analysis. The precise reasons for this association are unclear. We analyzed the time from the last device procedure to SAB, age of the patient at presentation, the number of device leads and presence of comorbid conditions and did not find any significant differences to explain this finding.
Only two prior publications have explored the association between SAB and risk of CIED infection.3, 5 First report by Chamis et al.3 included 33 patients with CIEDs and SAB over a 6-year time period. The overall incidence of CIED infection was 45.4% (15 of 33 SAB cases). In 60% of the patients (9 of 15) with confirmed device infection, patients had no local signs or symptoms suggesting generator pocket infection. In our analysis, we excluded patients with clinical signs of pocket infection as diagnosis of CIED infection is self-evident and use of a clinical prediction tool is unnecessary. Due to a small sample size, the report by Chamis et al.3 did not include a statistical analysis to identify risk factors associated with the development of CIED infection. The second report from our institution5 included an earlier cohort of 62 SAB cases in CIED recipients from 2001 to 2006. Twenty-two patients (35.5%) had a diagnosis of CIED infection. The majority of CIED infections cases in this cohort were device-related infective endocarditis (12 of 22, 55%). Similarly, due to smaller sample size, a multivariable analysis to identify risk factors for CIED infection in SAB cases could not be performed.
Our study has certain limitations, including selection, referral, and cases ascertainment biases primarily due to its retrospective design. We have attempted to minimize these biases by relying only on objective data such as blood culture results and by using a standardized (and reproducible) definition for device infection.5, 7, 8 As the decision to obtain TEE in SAB cases and its timing was at the discretion of the attending physician, it was not possible to definitely exclude lead or valvular vegetations in all patients. Therefore, it is conceivable that we may have missed lead vegetation in few patients who did not undergo TEE testing. Therefore, it is possible, though unlikely, that prolonged antimicrobial therapy without CIED extraction may have cured some patients with unknown CIED infection. However, our rate of CIED infection in SAB cases is similar to earlier investigations and therefore we do not believe that many cases of CIED infection were missed. Although we included clinical follow-up data for 12-weeks after completion of antimicrobial therapy for SAB, patients can potentially develop relapsing bacteremia months after completion of antimicrobial course. Without long-term clinical follow-up data, it is possible that late relapses may have been missed. The presence of a mobile mass on CIED lead on echocardiography, in the setting of SAB, was used as a diagnostic criterion for CIED infection in our study. However, it is possible that some of these masses were sterile thrombi and not infected vegetations.22 Ideally the study sample should have been split, with one group of cases used to develop the prediction model and the other group to validate the model. However, due to the sample size in our cohort, this was not feasible.
Conclusions
The presence of a PPM rather than ICD, history of multiple device revisions, and SAB duration ≥4 days are independent predictors of CIED infection. Clinicians can estimate an individual patient’s risk of underlying CIED infection based on the presence or absence of these risk factors. Patients who do not have any of these high-risk features and no evidence of generator pocket infection on physical examination have very low risk of underlying CIED infection and may be managed without device extraction. However, prospective validation of our proposed model is necessary before it can be advocated for use in routine clinical practice. Close monitoring and follow-up is recommended in all cases to detect any relapse of SAB.
Acknowledgments
Funding Source: This study was supported by a research grant from the American Heart Association (AHA 12CRP12080058) to Dr. Sohail (principal investigator). Resources of Mayo Clinic Center for Clinical and Translational Science (CCaTS), funded by National Institutes of Health (NIH Clinical and Translational Science Awards grant UL1 RR024150), were used for data analysis and manuscript preparation. The study database was created and maintained using REDCap (grant UL1 TR000135).
Dr. Friedman reports honoraria/consultant fees from Medtronic, Guidant, Astra Zeneca; research grant from Medtronic, Astra Zeneca via Beth Israel, Guidant, St. Jude, Bard; and intellectual property rights with Bard EP, Hewlett Packard, Medical Positioning, Inc. Dr. Baddour reports royalty payments (authorship) from UpToDate, Inc. [<$20,000] and Editor-in-Chief payments from Massachusetts Medical Society (Journal Watch Infectious Diseases) [<$20,000]. Dr. Sohail reports receiving funds from TYRX Inc. and Medtronic for prior research unrelated to this study administered according to a sponsored research agreement (SRA) between Mayo Clinic and study sponsor that prospectively defined the scope of the research effort and corresponding budget; and honoraria/consulting fees from Medtronic, Spectranetics. Dr. Uslan reports receiving honoraria from Medtronic, BIOTRONIK, and consultant/advisory Board for Medtronic/TYRX, Inc. Dr. Hayes reports honoraria/consultant fees from Medtronic, Boston-Scientific, Sorin Medical, Biotronik, St. Jude Medical and royalties from Up To Date and Wiley Publishing.
Footnotes
Abstract presenting preliminary results from this study was presented at the 2014 American Heart Association (AHA) Scientific Sessions, Chicago, IL.
Conflict of Interest Disclosures: All others have none.
References
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Hill PC, Birch M, Chambers S, Drinkovic D, Ellis-Pegler RB, Everts R, Murdoch D, Pottumarthy S, Roberts SA, Swager C, Taylor SL, Thomas MG, Wong CG, Morris AJ. Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Intern Med J. 2001;31:97–103. [PubMed] [Google Scholar]
- 3.Chamis AL, Peterson GE, Cabell CH, Corey GR, Sorrentino RA, Greenfield RA, Ryan T, Reller LB, Fowler VG., Jr Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation. 2001;104:1029–1033. doi: 10.1161/hc3401.095097. [DOI] [PubMed] [Google Scholar]
- 4.Greenspon AJ, Rhim ES, Mark G, Desimone J, Ho RT. Lead-associated endocarditis: the important role of methicillin-resistant Staphylococcus aureus. Pacing Clin Electrophysiol. 2008;31:548–553. doi: 10.1111/j.1540-8159.2008.01039.x. [DOI] [PubMed] [Google Scholar]
- 5.Uslan DZ, Dowsley TF, Sohail MR, Hayes DL, Friedman PA, Wilson WR, Steckelberg JM, Baddour LM. Cardiovascular implantable electronic device infection in patients with Staphylococcus aureus bacteremia. Pacing Clin Electrophysiol. 2010;33:407–413. doi: 10.1111/j.1540-8159.2009.02565.x. [DOI] [PubMed] [Google Scholar]
- 6.Camus C, Leport C, Raffi F, Michelet C, Cartier F, Vilde JL. Sustained bacteremia in 26 patients with a permanent endocardial pacemaker: assessment of wire removal. Clin Infect Dis. 1993;17:46–55. doi: 10.1093/clinids/17.1.46. [DOI] [PubMed] [Google Scholar]
- 7.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, Stoner S, Baddour LM. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49:1851–1859. doi: 10.1016/j.jacc.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 8.Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, Masoudi FA, Okum EJ, Wilson WR, Beerman LB, Bolger AF, Estes NAM, III, Gewitz M, Newburger JW, Schron EB, Taubert KA on behalf of the American Heart Association Rheumatic Fever E, Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Y, Council on Cardiovascular S, Anesthesia, Council on Cardiovascular N and Council o. Update on Cardiovascular Implantable Electronic Device Infections and Their Management. A Scientific Statement From the American Heart Association. Circulation. 2010;121:458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 9.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 10.Greenspon AJ, Prutkin JM, Sohail MR, Vikram HR, Baddour LM, Danik SB, Peacock J, Falces C, Miro JM, Blank E, Naber C, Carrillo RG, Tseng CH, Uslan DZ. Timing of the most recent device procedure influences the clinical outcome of lead-associated endocarditis results of the MEDIC (Multicenter Electrophysiologic Device Infection Cohort) J Am Coll Cardio.l. 2012;59:681–687. doi: 10.1016/j.jacc.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, Jenkins SM, Baddour LM. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clin Proc. 2008;83:46–53. doi: 10.4065/83.1.46. [DOI] [PubMed] [Google Scholar]
- 12.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 13.Cacoub P, Leprince P, Nataf P, Hausfater P, Dorent R, Wechsler B, Bors V, Pavie A, Piette JC, Gandjbakhch I. Pacemaker infective endocarditis. Am J Cardiol. 1998;82:480–484. doi: 10.1016/s0002-9149(98)00365-8. [DOI] [PubMed] [Google Scholar]
- 14.Fowler VG, Jr, Olsen MK, Corey GR, Woods CW, Cabell CH, Reller LB, Cheng AC, Dudley T, Oddone EZ. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003;163:2066–2072. doi: 10.1001/archinte.163.17.2066. [DOI] [PubMed] [Google Scholar]
- 15.Raad II, Sabbagh MF. Optimal duration of therapy for catheter-related Staphylococcus aureus bacteremia: a study of 55 cases and review. Clin Infect Dis. 1992;14:75–82. doi: 10.1093/clinids/14.1.75. [DOI] [PubMed] [Google Scholar]
- 16.Borleffs CJ, Thijssen J, de Bie MK, van Rees JB, van Welsenes GH, van Erven L, Bax JJ, Cannegieter SC, Schalij MJ. Recurrent implantable cardioverter-defibrillator replacement is associated with an increasing risk of pocket-related complications. Pacing Clin Electrophysiol. 2010;33:1013–1019. doi: 10.1111/j.1540-8159.2010.02780.x. [DOI] [PubMed] [Google Scholar]
- 17.Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R, Gottipaty V, Shinn T, Dan D, Feldman LA, Seide H, Winston SA, Gallagher JJ, Langberg JJ, Mitchell K, Holcomb R REPLACE registry Investigators. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122:1553–1561. doi: 10.1161/CIRCULATIONAHA.110.976076. [DOI] [PubMed] [Google Scholar]
- 18.Bloom H, Heeke B, Leon A, Mera F, Delurgio D, Beshai J, Langberg J. Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. Pacing Clin Electrophysiol. 2006;29:142–145. doi: 10.1111/j.1540-8159.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- 19.Klug D, Balde M, Pavin D, Hidden-Lucet F, Clementy J, Sadoul N, Rey JL, Lande G, Lazarus A, Victor J, Barnay C, Grandbastien B, Kacet S. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116:1349–1355. doi: 10.1161/CIRCULATIONAHA.106.678664. [DOI] [PubMed] [Google Scholar]
- 20.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, Stoner SM, Baddour LM. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis. 2007;45:166–1673. doi: 10.1086/518889. [DOI] [PubMed] [Google Scholar]
- 21.Lekkerkerker JC, van Nieuwkoop C, Trines SA, van der Bom JG, Bernards A, van de Velde ET, Bootsma M, Zeppenfeld K, Jukema JW, Borleffs JW, Schalij MJ, van Erven L. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart. 2009;95:715–720. doi: 10.1136/hrt.2008.151985. [DOI] [PubMed] [Google Scholar]
- 22.Supple GE, Ren JF, Zado ES, Marchlinski FE. Mobile thrombus on device leads in patients undergoing ablation: identification, incidence, location, and association with increased pulmonary artery systolic pressure. Circulation. 2011;124:772–778. doi: 10.1161/CIRCULATIONAHA.111.028647. [DOI] [PubMed] [Google Scholar]