Abstract
Background
Preclinical and epidemiologic studies suggest chemopreventive effects of green tea (GT) and black tea (BT) in prostate cancer. In the current study we determined the effect of GT and BT consumption on biomarkers related to prostate cancer development and progression.
Methods
In this exploratory, open label, phase II trial 113 men diagnosed with prostate cancer were randomized to consume six cups daily of brewed GT, BT or water (control) prior to radical prostatectomy (RP). The primary endpoint was prostate tumor markers of cancer development and progression determined by tissue immunostaining of proliferation (Ki67), apoptosis (Bcl-2, Bax, Tunel), inflammation [nuclear and cytoplasmic nuclear factor kappa B (NFκB)] and oxidation [8-hydroxydeoxy- guanosine (8OHdG)]. Secondary endpoints of urinary oxidation, tea polyphenol uptake in prostate tissue, and serum prostate specific antigen (PSA) were evaluated by high performance liquid chromatography and ELISA analysis.
Results
Ninety three patients completed the intervention. There was no significant difference in markers of proliferation, apoptosis and oxidation in RP tissue comparing GT and BT to water control. Nuclear staining of NFkB was significantly decreased in RP tissue of men consuming GT (p=0.013) but not BT (p=0.931) compared to water control. Tea polyphenols were detected in prostate tissue from 32 of 34 men consuming GT but not in the other groups. Evidence of a systemic antioxidant effect was observed (reduced urinary 8OHdG) only with GT consumption (p=0.03). GT, but not BT or water, also led to a small but statistically significant decrease in serum prostate-specific antigen (PSA) levels (p=0.04).
Conclusion
Given the GT-induced changes in NFkB and systemic oxidation, and uptake of GT polyphenols in prostate tissue, future longer-term studies are warranted to further examine the role of GT for prostate cancer prevention and treatment, and possibly for other prostate conditions such as prostatitis.
Keywords: Bcl-2, bax, nuclear factor kappa B, phase II clinical intervention study
Introduction
Both green tea (GT) and black tea (BT) are derived from the leaves of the Camellia sinensis plant. However, post-harvest processing preserves the polyphenol composition of GT, while during the production of BT auto-oxidation/ fermentation takes place. As a result, the concentrations and types of polyphenols found in GT and BT are different. The polyphenols present in GT are in monomeric forms including: (−)-epigallocatechin-3-gallate (EGCG); (−)-epigallocatechin (EGC); (−)-epicatechin (EC); and (−)-epicatechin-3-gallate (ECG) [1]. During the production of BT monomeric tea polyphenols undergo polymerization to theaflavins and thearubigins, while only small amounts of monomeric polyphenols remain [1]. Metabolites from larger polyphenols in BT have antiproliferative effects in a number of malignancies including prostate cancer, and have anti-inflammatory effects as demonstrated in vitro and animal studies [2–5]. Evidence from cell culture and animal studies also suggest that tea polyphenols inhibit proliferation and cell cycle events and induce apoptosis through multiple mechanisms including antioxidant and anti-inflammatory activity [6,7]. Evidence of the chemopreventive effects of GT and BT for delaying the development of prostate cancer from human population studies is inconsistent [5]. One recent meta-analysis of the effect of GT and BT consumption on prostate cancer risk demonstrated a borderline significant decreased risk of prostate cancer in Asian men consuming the highest versus none/lowest GT consumption, whereas another meta-analysis did not show any significant decrease in risk [8,9]. The majority of recent clinical intervention studies have been performed using GT extracts [5,10–12]. For example, a placebo-controlled study in men diagnosed with high grade prostate intraepithelial neoplasia (PIN) demonstrated that consuming 600 mg of GT extract daily for one year decreased progression from PIN to adenocarcinoma [10]. Another single arm intervention study without a control arm showed a significant decrease in serum PSA after 3–6 weeks administration of 800 mg Polyphenon E, a purified GT extract with high content of EGCG [12]. A third placebo-controlled intervention study of 800 mg of Polyphenon E supplementation daily did not show a significant decrease in serum PSA [11]. Two intervention studies in castration resistant prostate cancer did not demonstrate beneficial effects [13,14].
The aim of this preprostatectomy trial was to examine the effects of brewed GT and BT consumption compared to water on biomarkers involved in prostate cancer development and progression. Based on the outcome of preclinical studies, we investigated the effect of GT or BT on markers of proliferation, apoptosis, inflammation and oxidation [5,7,15,16]. Secondary endpoints examined in this trial were uptake of tea polyphenols in prostate tissue, urinary 8-hydroxydeoxyguanosine (8OHdG) as a marker of systemic oxidative DNA damage, and serum PSA levels. The dose of green tea (6 cups per day) was selected to match the tea polyphenol content of previously published clinical trials with polyphenon E [2, 5–7]. Although there are very few preclinical studies of black tea and prostate cancer, we included a black tea arm since 78% of the worlds tea consumption is in the form of BT [1]. The present trial was designed to establish whether consuming green or black tea alters biomarkers related to prostate cancer development and progression, and may therefore support the conduct of large-scale prospective trials incorporating tea consumption.
Materials and Methods
Patients
Participants were recruited from the urology clinics at the Veterans Administration Greater Los Angeles Healthcare System, UCLA, and the UCLA Santa Monica Medical Center from 2008 to 2012. Participants (40–70 y) had a diagnosis of clinically localized prostate adenocarcinoma and were scheduled to undergo radical prostatectomy at least three weeks after study entry. Participants were ineligible if they had a history of hepatitis, alcohol abuse and other significant medical or psychiatric condition or took 5-alpha reductase inhibitors, antiandrogens, or luteinizing hormone-releasing hormone agonists. Subjects were instructed to abstain from all teas and tea containing products other than the study tea, and stop nutritional supplements and herbal therapies (i.e., lycopene, selenium, vitamin E, fish oil, and saw palmetto). The study was approved by the UCLA and Veterans Administration Institutional Review Boards.
Clinical Trial Design and Tea Interventions
This was a prospective randomized, open label, three arm phase II intervention trial. The trial was registered with ClinicalTrial.gov (NCT00685516). All subjects signed informed consent documents prior to study entry. Men were randomized to six cups daily of GT, BT, or water for three to eight weeks prior to RP. The dose was chosen based on two prior prostatectomy trials using Polyphenon E, a GT extract [11,12]. Both studies used a daily dose of 1300 mg GT polyphenol (GTP) including 800 mg of EGCG. In the current study the 6 cups of GT provided a total of 1010 mg of GT polyphenols including 562 mg of EGCG and 6 cups of BT provided 80 mg of tea polyphenols including 28 mg of EGCG and 35 mg theaflavins in addition to 348 mg gallic acid. The thearubigin content was not analyzed. Study subjects were randomized in permuted random block design with blocks of 12 for GT, BT and water control. GT bags (authentic green tea) were provided by Celestial Seasonings (Boulder, CO) and BT bags (Decaffeinated English Breakfast Tea) were purchased from Twinings (Clifton, NJ). Subjects kept a tea consumption diary to record compliance (minimum of 75% consumption of the six cups of tea). A morning first voided urine sample was collected at baseline, two times during the intervention, and at the end of the intervention. Fasting blood was collected before the intervention and within 5 days of the surgery to measure liver function tests and prostate-specific antigen (PSA) concentration. Liver function tests (aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase) were measured by the UCLA clinical laboratory. Serum PSA concentration was analyzed by ELISA assay (GenWay Biotech Inc., San Diego, CA) according to the manufacturer’s instruction. Prostate tissue was processed according to standard protocol of the UCLA and VA pathology departments and paraffin embedded blocks were archived. Aliquots of fresh prostate tissue obtained after the prostatectomy were wrapped in foil, frozen in liquid nitrogen, and stored at −70 °C.
Outcomes
The primary endpoint of this exploratory study was focused on the evaluation of markers of cancer development and progression in malignant RP tissue by immunostaining. The objective was to determine the effect of GT and BT consumption on proliferation (Ki67), apoptosis (TUNEL, Bax, Bcl-2), inflammation (nuclear and cytoplasmic NFkB) and oxidation (8OHdG) in malignant RP tissue compared to water control using immunohistochemistry. Secondary endpoints were levels of tea polyphenols and methylated tea polyphenol metabolites in fresh frozen radical prostatectomy tissue and urine, urinary oxidative DNA damage (8OHdG) and serum prostate-specific antigen (PSA) levels.
Polyphenol analysis of brewed GT and BT
The GT and BT polyphenol composition of brewed tea (one bag in 240 mL of boiling water for 5 min) was determined by HPLC with CoulArray electrochemical detection (ESA, Chelmsford, MA) as previously described (GT: mg/L: 855.0±11.8 total phenolics, 396.8 ± 28.3 EGCG, 202.3 ± 10.9 EGC, 52.1 ± 4.7 EC, 62.1 ± 4.6 ECG, 11.8±2.3 gallic acid and 0 THE and BT: mg/L: 661.5±5.8 total phenolics, 28.1±0.8 EGCG, 5.6±0.5 EGC, 8.4±0.2 EC, 15.5±0.3 ECG, 242.1±3.6 gallic acid and 24.6±2.5 THE) [17]. All HPLC grade solvents were purchased from Fisher Scientific (Pittsburgh, PA). EGCG, EC, ECG, EGC and black tea extract (mixture of theaflavin, theaflavin-3-monogallate, theaflavin-3’-monogallate and theaflavin-3,3’-digallate) were purchased from Sigma-Aldrich (St.Louis, MO), 4'-O-methyl EGC (4'-MeEGC) and 4"-O-methyl EGCG (4"-MeEGCG) were purchased from Nacalai USA Inc. (San Diego, CA) and theaflavin-3,3’-digallate and theaflavin-3-gallate from Wako Chemicals USA (Richmond VI).
Tea polyphenol analysis in prostate tissue and urine
Total unconjugated tea polyphenols were analyzed in prostate tissue as previously described [17]. 300 mg of fresh frozen prostate tissue was homogenized and treated with 1,000 units of β-glucuronidase (G7896, Sigma Chemicals, St Louis, MO) and 40 units of sulfatase (S-9754, Sigma Chemicals) at 37°C for 45 min. Solid phase extraction (SPE) was performed using preconditioned HLB cartridges (Waters Corporation, Milford, MA) and tea polyphenols quantified by HPLC-CoulArray electrochemical detection (ESA, Chelmsford, MA) as previously described [18]. The detection limit was 0.2 pmol/g tissue. The method for urinary total tea polyphenol detection was described previously and used with minor modifications [17]. The prostate tissue samples used for polyphenol measurements were not analyzed for the presence of malignancy.
Urine 8-hydroxydeoxyguanosine (8OHdG)
Urinary concentration of 8OHdG was analyzed using HPLC with coularray electrochemical detection as previously published [19]. Briefly, 8OHdG was extracted from 1 ml urine with the Oasis-HLB 3 cc (60 mg) cartridge (Waters Corporation) following the manufacturer’s instructions. The eluents were dried under ultra-pure N2 stream and reconstituted in buffer (10 mM ammonium acetate in 2% MeOH, pH 4.3) for analysis with the same HPLC-ECD system.
Immunohistochemical Analysis
Paraffin embedded tumor tissue was selected that matched the patient’s radical prostatectomy Gleason grade and had the largest tumor area. One section was stained with H&E and tumor areas were identified and circled by the pathologist (J.W.S.). Additional sections were probed with primary antibodies against the following biomarkers (nuclear Ki67, cytoplasmic Bcl-2 and Bax; nuclear and cytoplasmic NFκB p65 and nuclear 8OHdG) as previously described [20]. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 10 min. Heat-induced antigen retrieval (HIER) was carried out for all sections in 0.01M sodium citrate buffer, pH = 6.00 (for Ki67, NFκB) or 0.001M EDTA, pH = 8.00 (for Bcl-2) or 0.05 Tris, pH = 9.00 (for Bax) using either a vegetable steamer at 95°C for 25 min or pressure cooker at 115°C for 2–3 min. Proteolytic enzyme induced epitope retrieval (PIER) was used for 8OHdG, using proteinase K at 37°C for 10 min. Mouse monoclonal antibodies to Ki67, Bcl-2 (DakoCytomation, Carpinteria, USA), NFκB p65, Bax (Santa Cruz Biotechnology, Santa Cruz, USA) and 8OHdG from Oxis International, Foster city, CA, USA) were applied at a dilution of 1:100, 1:100, 1:100, 1:20 and 1:50 respectively for 45 min (Bcl-2), or 1 hour (Ki67) or 2 hour (8OHdG) at room temperature or overnight (Bax, NFκB p65) at 4°C. The signal was detected using the mouse DAKO horseradish peroxidase EnVision kit (DAKO) or Mach2 mouse HRP polymer (Biocare Medical) for Ki67 and visualized with the diaminobenzidine reaction. The sections were counterstained with hematoxylin. For measurements of apoptosis, the ApopTag® Plus Peroxidase In Situ Apoptosis Kit was used, which is based on the TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) reaction (Millipore, Temecula, CA, USA). Slides were digitally scanned using the Ariol SL-50 high-throughput scanning system (Applied Imaging, San Jose, CA). Five 20× fields of the cancerous glands within the area of representative adenocarcinoma were analyzed to quantify the amount of positive staining in epithelial cells.
Statistical analyses
A sample size of 60 subjects per group was calculated to provide 88% power to detect a 57% difference in urinary 8OHdG between the GT intervention and control groups. This power calculation was based on a published green tea intervention trial that reported significant changes in urinary 8OHdG concentrations [19] since no prostate tissue data from comparable trials was available.
Primary Analysis
After over one third of the study participants (n= 74 patients) completed the trial, we conducted an unplanned interim conditional power analysis which demonstrated that with completion of enrollment to the trial, there was a negligible chance (maximum power = 4%) of finding a significant difference in urinary 8OHdG (original outcome marker for power calculation) and tissue bcl-2 between the BT and the control group. Therefore the BT group was closed to recruitment at 26 subjects. After approximately 2/3 of participants completed the trial (n=113), a second interim analysis was performed. There were significant differences in urinary 8OHdG and tissue NFκB staining between the GT and control group. The trial was terminated at this point due to lack of funding resources and the final analyses were carried out.
Final Analysis
Comparisons of baseline clinical and demographic variables between GT, BT, and controls was conducted using the ANOVA or Kruskal-Wallis tests for continuous variables and Fisher's exact test or chi-square tests for categorical variables. Outcomes were either evaluated at surgery (tumor-immunostaining), or as changes from baseline to surgery (serum and urinary markers). Distributions for each study measure were plotted and evaluated for normality. If a statistically significant overall group effect was found, follow-up analysis was performed using pairwise t-tests or the Mann Whitney U tests to investigate which pairs of groups were significantly different.
To assess intervention effects on serum PSA and urinary 8OHdG we utilized analysis of variance (ANOVA) to compare changes in these measures from baseline to the post intervention assessment. The proportions of participants with polyphenols and their methyl-metabolites in urine or prostate at the end of the intervention were compared between GT vs control and BT vs control using the Fisher’s exact test. The study had multiple primary endpoints and we assessed the potential for finding false positives using the false discovery rate (q-values). To control for multiple testing, the false discovery rate for the overall set of hypotheses for the 7 outcomes (80HdG, Ki67, Tunel, BCL2, BAX, NFkB cytoplasmic and NFkB nuclear) was computed. The rate was estimated using the bootstrap method in the Q-values package in R 3.0.2 (Vienna, Austria, www.R-project.org). Data management, variable transformations, and other statistical analyses were conducted using SAS 9.2 (Statistical Analysis System, Cary, NC, 2008). P-values <0.05 were considered statistically significant.
Results
Clinical characteristics and compliance
113 men were enrolled and 93 completed the intervention (Control: 33, GT: 34, BT: 26). Four participants in the water group withdrew early: three for personal reasons and one due to surgery cancellation. Nine participants from the GT group withdrew for the following reasons: caffeine reactions (2), surgery postponed (2), unrelated death (1), tea volume too large (3), and stomach distress (1). Seven participants from the BT group withdrew for the following reasons: personal reasons (3), surgery cancelled (2), and caffeine reactions (2). At baseline there were no significant differences in demographics and clinical characteristics between the GT, BT and control groups in regards to age, body composition, race, ethnicity, mean biopsy Gleason score and baseline PSA levels (Table 1). The mean duration of the intervention in the GT, BT and the control group was 33, 31 and 29 days, respectively, with an average compliance of 95, 92 and 93% for the GT, BT and water control groups as calculated from the diary entries. There were no serious adverse events related to the interventions, and there was no liver toxicity as measured by pre vs. post-intervention serum levels of alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase.
Table 1.
Baseline characteristics of study population (mean±std).
| Gleason Biopsy | ||||
|---|---|---|---|---|
| Watera | GT | BT | p-value | |
| N | 33 | 34 | 26 | |
| Age | 62.8±6.2 | 62.1±6.9 | 61.4±7.4 | 0.93 |
| Weight (kg) | 86.6±14.3 | 85.7±12.2 | 89.9±13.4 | 0.47 |
| Height (cm) | 175±22 | 176±21 | 175±22 | 0.98 |
| BMI (kg/m2) | 27.4±4.9 | 27.2±3.8 | 27.4±3.6 | 0.98 |
| Intervention (days) | 29±7.9 | 33±23 | 31±10 | 0.60 |
| Compliance (%) | 93±12 | 95±10 | 92±13 | 0.75 |
| Biopsy Gleason Score (%) | ||||
| 6 | 14 (42) | 18 (53) | 13 (50) | 0.74 |
| 7 (3+4) | 15 (45) | 11 (32) | 9 (35) | |
| 7 (4+3) | 3 (9) | 3 (9) | 2 (8) | |
| ≥8 | 1 (3) | 2 (6) | 2 (8) | |
| Race/Ethnicity (%) | 0.14 | |||
| Asian | 0 (0) | 1 (3) | 3 (12) | |
| Black | 6 (18) | 5 (15) | 6 (23) | |
| Hispanic | 3 (9) | 1 (3) | 4 (15) | |
| White | 23 (70) | 27 (80) | 13 (50) | |
| Other | 1 (3) | 0 (0) | 0 (0) | |
| Serum PSA (ng/mL) | 9.9±8.5 | 9.6±5.2 | 9.2±4.3 | 0.93 |
green tea (GT), black tea (BT), body mass index (BMI), prostate-specific antigen (PSA)
Proliferation, apoptosis, NFkB and oxidation
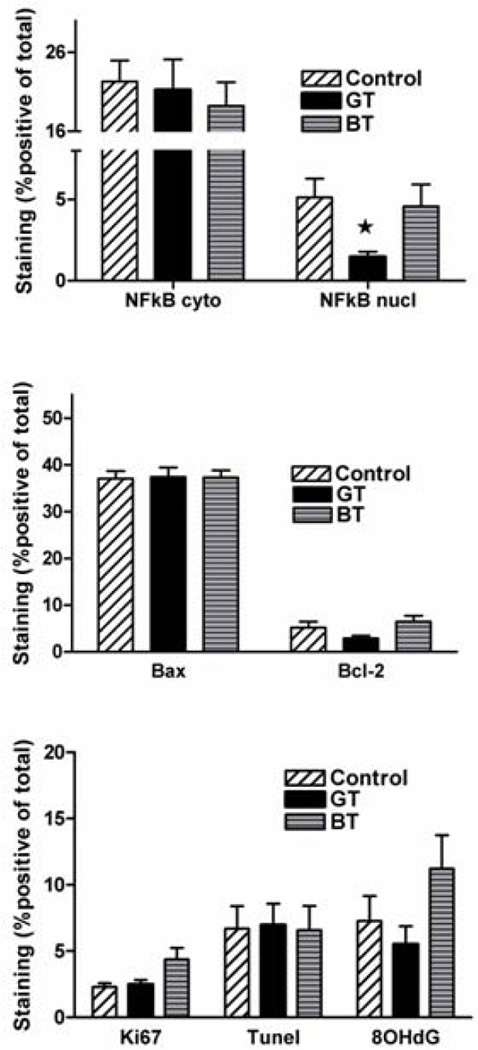
There were no significant differences in RP staining for proliferation (Ki67), apoptosis (TUNEL, Bcl-2, Bax) or oxidative DNA damage (8OhdG) comparing GT and BT to control (Figure 1). Nuclear NFκB staining in malignant prostate tissue from men in the GT group (p=0.013) was significantly lower compared to control whereas there was no significant difference when comparing BT (p=0.931) to control (Figure 1). Representative photomicrographs of the immunohistochemical staining are shown in Figure 2. When accounting for multiple variables in the primary endpoint, the probability of a false positive result (false discovery rate) was 10.8%.
Figure 1.
Immunostaining of A) Ki67, TUNEL and 8OHdG; B) Bcl-2 and Bax; C) nuclear and cytoplasmic NFκB in radical prostatectomy malignant epithelium from men consuming GT (29), BT (24) or water (30); mean±std; * p<0.05.
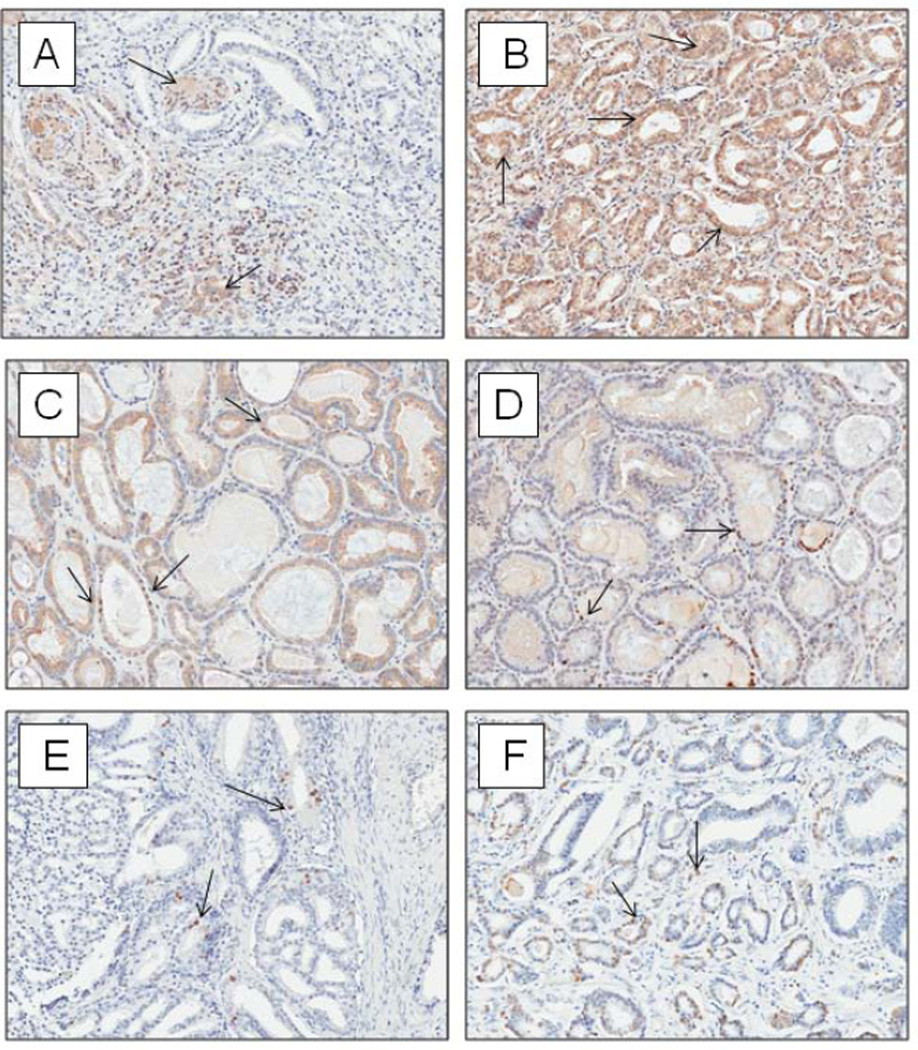
Figure 2.
Photomicrographs of immunohistochemical staining for A) cytoplasmic Bcl-2, B) cytoplasmic Bax, C) nuclear NFκB, D) nuclear 8OHdG, E) nuclear Ki67 and F) TUNEL in radical prostatectomy malignant epithelium (20× magnification).
GT and BT polyphenol analysis in prostate tissue and urine
EGCG was the most abundant polyphenol in the brewed GT preparations used in this clinical trial. Although the total phenolic content of brewed BT measured as gallic acid equivalent (662±6 mg GAE) was 77% of the total phenolic content of GT (855±12 mg GAE), the content of monomeric tea polyphenols was significantly lower in brewed BT compared to GT. Brewed BT, however, contained theaflavin and 20-fold higher concentration of gallic acid compared to GT. In addition BT contains thearubigins which were not analyzed due to the lack of commercial standards. Among participants consuming GT, tea polyphenols were found in 32 out of 34 prostate tissue specimens, but with considerable individual variation in the concentrations (Table 2). An average of 48% of total EGCG was found in methylated form (4"-MeEGCG) in the prostate of subjects in the GT group (Table 2). No tea polyphenols were detected in prostate tissue from the BT or control group. EGC, EC and methyl EGC were found in early morning urine through the course of the study in all participants in the GT group and in significantly lower concentrations in the BT group (Table 2). No polyphenols were found in urine from the control group or in baseline urine specimens from all 3 groups. In the post-intervention urine samples 47% and 30% of EGC was found in methylated forms after GT and BT intervention, respectively (Table 2). No BT theaflavins were found in either prostate tissue or urine from men consuming the BT (Table 2).
Table 2.
Concentration of tea polyphenols and methyl-metabolites in prostate tissue and urine collected before and after the consumption of GT and BT.
| Prostate Tissue Concentration (pmol/g tissue)‡a | |||
|---|---|---|---|
| Water | GT | BT | |
| EGCG | ‡ | 16.7 ± 12.7* | ‡ |
| ECG | ‡ | 7.6 ± 5.1* | ‡ |
| 4"-MeEGCG | ‡ | 15.8 ± 10.1* | ‡ |
| Theaflavin | ‡ | ‡ | ‡ |
| Urine Concentration (µmol/g creatinine)‡a,b | ||||
|---|---|---|---|---|
| GT-Pre | GT-Post | BT-Pre | BT-Post | |
| EGC | ‡ | 9.2 ± 16* | ‡ | 0.4 ± 0.4* |
| EC | ‡ | 4.8 ± 6.1* | ‡ | 0.3 ± 0.3* |
| 4'-MeEGC | ‡ | 8.0 ± 18* | ‡ | 0.2 ± 0.3* |
| Theaflavins | ‡ | ‡ | ‡ | ‡ |
Compared with the control group, p< 0.01. N= 34 (GT), 26 (BT), 33 (control), mean ± std.
Below detection limit.
(−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin-3-gallate (ECG), (−)-epigallocatechin (EGC), (−)-epicatechin (EC), 4'-O-methylEGC (4'-MeEGC), 4"-O-methylEGCG (4"-O-methylEGCG).
Polyphenols were not detected in urine after water consumption.
Urinary oxidative DNA damage
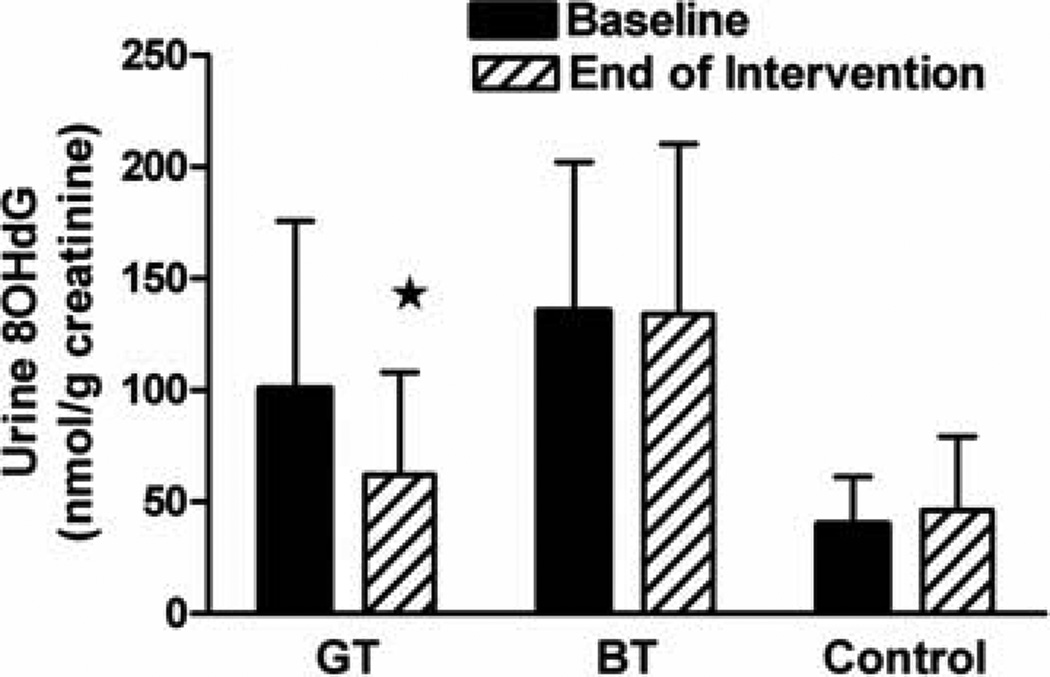
There was a significant decrease (pre vs. post-intervention) in urinary 8OHdG in men in the GT (−19.4±13%) group compared to control group, whereas there was no change in the BT group (−3.1±6.3%) relative to control (+23.6±19%) (Figure 3).
Figure 3.
Change in urinary concentration of oxidative DNA damage marker 8OHdG in urine collected at baseline and post-intervention (mean±SEM; GT N=14; BT N=16; Water N=14).
Serum prostate-specific antigen
There was a small but statistically significant decrease in serum PSA levels in the GT group compared to the control group whereas this was not the case when comparing the BT and control group (Table 3).
Table 3.
Prostate specific antigen concentrations in serum collected from men consuming GT, BT or water control collected at baseline and on the morning of radical prostatectomy (post-intervention).
| Serum PSA (ng/mL)a | |||
|---|---|---|---|
| Water | GT | BT | |
| PSA baseline | 9.9±8.5 | 9.6±5.2 | 9.2±4.3 |
| PSA post-intervention | 10.0±9.0 | 8.4±4.3* | 9.6±6.0 |
PSA changes from pre to post were compared between the 3 groups using Analysis of Variance with pairwise contrasts, p< 0.05.
Data are presented as mean ± std; N=30 (control), 30 (GT) and 23 (BT).
green tea (GT), black tea (BT), prostate specific antigen (PSA)
Discussion
The rationale for selecting the primary endpoint was based on various mouse studies demonstrating a decrease in proliferation and NFkB expression, decrease in urinary DNA oxidation and increase in apoptosis through decreased concentration of Bcl-2 and increased concentration of Bax protein expression in mice treated with green tea [7,21,22]. In addition it has been shown that NFkB and Bcl-2 are overexpressed in human malignancies [23]. The major finding from the RP tissue staining experiments was a significant decrease in nuclear NFkB staining in RP tissue from men consuming GT compared to water, whereas this was not the case in the BT group. In addition we found no significant change in malignant prostate tissue proliferation, apoptosis and oxidation in either the GT or BT groups relative to control.
The transcription factor NFkB, has an important role mediating inflammatory processes and is involved in regulating proliferation and apoptosis [24]. NFkB has been shown to be constitutively activated in numerous cancers [25–28]. NFκB is located in the cytoplasm bound to the inhibitor IκB. Upon activation, IκB is released and degraded by the proteasome and NFkB relocates to the nucleus to induce gene expression. Nuclear localization of NFκB has been suggested as a prognostic marker of prostate cancer progression [29]. In preclinical studies, green tea polyphenols were found to decrease NFκB activity either by deactivating IKkinase (IKK) or by inhibiting the proteasome [7,21]. In the present trial GT consumption led to a significant decrease in nuclear immunostaining of NFκB in RP tissue compared to water control, which may potentially decrease proinflammatory cytokines such as IL-6 or TNF-α [24]. NFkB is also involved in the regulation of apoptosis, and in mouse studies green tea intake increased tumor apoptosis [30]. This may be due to higher green tea dosing leading to a two- fold higher EGCG and 4”-MeEGCG concentration in mouse xenograft tumors compared to the human prostate tissue in the present study [30]. Higher green tea dosing or longer exposure in humans may be found to increase apoptosis in future trials.
The consumption of six cups of BT was not associated with any significant effect on inflammation, proliferation, apoptosis, or oxidation. In line with our findings are several prospective cohort studies evaluating the association of BT consumption to prostate cancer risk and showing inconclusive results [31–33]. Two studies found an increase in prostate cancer risk with highest versus none/lowest intake of BT [31,32] and one study found a decrease in prostate cancer risk associated with BT consumption [33].
Chronic inflammation is associated with oxidative stress and the generation of reactive oxygen species (ROS), which may contribute to prostate carcinogenesis [34]. ROS are integral components of cell signaling pathways and have been shown to regulate cell transformation, survival, proliferation, invasion, angiogenesis, and metastasis [35,36]. ROS at low levels stimulate prostate cancer cell proliferation, but in higher amounts lead to cell cycle arrest and tumor cell death as observed with anticancer medications and radiation therapy known to markedly increase oxidant damage [36]. GT polyphenols are commonly known for their antioxidant activity. However, as an active redox compound under certain circumstances they also exhibit pro-oxidant activity [37]. Little is known as to whether GT polyphenols act as pro- or antioxidants in human tissue [20,37]. In the present study, we did not observe any significant change in oxidative DNA damage measured in RP tissue by immunohistochemistry. However the urinary concentration of 8OHdG, a marker of systemic oxidative DNA damage [38], was significantly decreased in urine from men drinking GT compared to control. Other studies also demonstrated a decrease in urinary 8OHdG associated with consumption of GT extracts [19,39] but none of the previous clinical GT studies examined tissue 8OHdG. It may be that other antioxidant defenses including enzymes in the antioxidant network are upregulated (e.g. superoxide dismutase) so that the impact of GT on oxidation in tumor tissue is difficult to detect [40].
In 32 out of 34 men daily consumption of 6 cups of GT led to detectable levels of EGCG, 4"-MeEGCG and ECG in prostate tissue. Nguyen et al. also analyzed tea polyphenols in RP tissue after consumption of 4 capsules daily of Polyphenon E (800 mg EGCG) and found EGCG and 4"-MeEGCG in the prostate of one participant only, and ECG in 5 out of 15 participants [11]. Higher prostate tissue uptake in the present trial may be due to consumption of the brewed GT throughout the day, the composition of tea polyphenols in the brewed GT, or possibly due to other components in brewed GT not present in the Polyphenon E capsule.
In men consuming BT no polyphenols or theaflavins were detectable in the prostate tissue, and significantly lower concentrations of EGC, EC and 4'-MeEGC were found in urine compared to men consuming GT. The presence of tea polyphenols in tissue depends on the composition of the tea or GT supplement since in a previous study tea polyphenols were found in the prostate tissue of men consuming a different brewed BT (Darjeeling), which contained higher levels of the EGCG, ECG and EGC than the BT (English Breakfast) used in the present trial [41].
One limitation of the present trial was that it was not blinded. Due to the varying flavors of brewed tea it was not possible to use a blinded trial design. One potential benefit of the intervention in the present trial was that subjects consumed the six cups of brewed tea throughout the day. This may be the reason that in the present trial GT polyphenols were found in prostate tissue in 94% of participants in the GT group compared to 30% in another clinical trial in which GT extract was given once per day [11]. It remains to be determined if green tea extracts or Polyphenon E consumed in multiple small doses throughout the day will result in improved prostate tissue polyphenol levels. Statistical limitations of the study are that the study design was revised during the trial with the BT intervention group discontinued due to the conditional power calculation showing futility for the BT group. Further, the study was exploratory in nature and therefore had multiple endpoints and the overall false discovery rate was 10.8%.
In summary, daily consumption of six cups of brewed GT resulted in uptake of tea polyphenols in the prostate gland, a significant decrease in nuclear NFκB, and a decrease in systemic antioxidant activity as measured by urinary 8OHdG. Given the GT-induced changes in nuclear staining of NFkB and oxidation in this exploratory study, future studies are warranted examining the role of GT for prostate cancer prevention and treatment and possibly for other prostate conditions such as prostatitis.
Acknowledgement
We would like to thank Sheryl Jeffery for her assistance with the clinical study coordination. In addition we would like to thank Clara Magyar, Ngan Doan, Brandon Castor for their assistance with immunohistochemistry and Max Deng with data entry.
Funding Sources: NIH Grant RO1 CA116242 (S.M. Henning), NIH P50CA092131 (W.J. Aronson). This material is the result of work supported with resources and the use of facilities at the Veterans Administration Medical Center West Los Angeles. Statistical analyses were funded by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Sharma V, Rao LJ. A thought on the biological activities of black tea. Crit Rev Food Sci Nutr. 2009;49:379–404. doi: 10.1080/10408390802068066. [DOI] [PubMed] [Google Scholar]
- 2.Huang MT, Liu Y, Ramji D, Lo CY, Ghai G, Dushenkov S, Ho CT. Inhibitory effects of black tea theaflavin derivatives on 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and arachidonic acid metabolism in mouse ears. Mol Nutr Food Res. 2006;50:115–122. doi: 10.1002/mnfr.200500101. [DOI] [PubMed] [Google Scholar]
- 3.Sun S, Pan S, Miao A, Ling C, Pang S, Tang J, Chen D, Zhao C. Active extracts of black tea (Camellia Sinensis) induce apoptosis of PC-3 prostate cancer cells via mitochondrial dysfunction. Oncol Rep. 2013;30:763–772. doi: 10.3892/or.2013.2504. [DOI] [PubMed] [Google Scholar]
- 4.Henning SM, Wang P, Abgaryan N, Vicinanza R, de Oliveira DM, Zhang Y, Lee RP, Carpenter CL, Aronson WJ, Heber D. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol Nutr Food Res. 2013 doi: 10.1002/mnfr.201200646. 1-14-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henning SM, Wang P, Heber D. Chemopreventive effects of tea in prostate cancer: Green tea versus black tea. Mol Nutr Food Res. 2011;55:1–16. doi: 10.1002/mnfr.201000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang CS, Wang H. Mechanistic issues concerning cancer prevention by tea catechins. Mol Nutr Food Res. 2011;55:819–831. doi: 10.1002/mnfr.201100036. [DOI] [PubMed] [Google Scholar]
- 7.Connors SK, Chornokur G, Kumar NB. New insights into the mechanisms of green tea catechins in the chemoprevention of prostate cancer. Nutr Cancer. 2012;64:4–22. doi: 10.1080/01635581.2012.630158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng J, Yang B, Huang T, Yu Y, Yang J, Li D. Green tea and black tea consumption and prostate cancer risk: an exploratory meta-analysis of observational studies. Nutr Cancer. 2011;63:663–672. doi: 10.1080/01635581.2011.570895. [DOI] [PubMed] [Google Scholar]
- 9.Lin YW, Hu ZH, Wang X, Mao QQ, Qin J, Zheng XY, Xie LP. Tea consumption and prostate cancer: an updated meta-analysis. World J Surg Oncol. 2014;12:38. doi: 10.1186/1477-7819-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen MM, Ahmann FR, Nagle RB, Hsu CH, Tangrea JA, Parnes HL, Sokoloff MH, Gretzer MB, Chow HH. Randomized, Double-Blind, Placebo-Controlled Trial of Polyphenon E in Prostate Cancer Patients before Prostatectomy: Evaluation of Potential Chemopreventive Activities. Cancer Prev Res. 2012;5:290–298. doi: 10.1158/1940-6207.CAPR-11-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli J. Tea Polyphenols Decrease Serum Levels of Prostate-Specific Antigen, Hepatocyte Growth Factor, and Vascular Endothelial Growth Factor in Prostate Cancer Patients and Inhibit Production of Hepatocyte Growth Factor and Vascular Endothelial Growth Factor In vitro. Cancer Prev Res. 2009;2:673–682. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 13.Choan E, Segal R, Jonker D, Malone S, Reaume N, Eapen L, Gallant VA. A prospective clinical trial of green tea for hormone refractory prostate cancer: an evaluation of the complementary/alternative therapy approach. Urol Oncol. 2005;23:108–113. doi: 10.1016/j.urolonc.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Jatoi A, Ellison N, Burch PA, Sloan JA, Dakhil SR, Novotny P, Tan W, Fitch TR, Rowland KM, Young CY, Flynn PJ. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97:1442–1446. doi: 10.1002/cncr.11200. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui IA, Zaman N, Aziz MH, Reagan-Shaw SR, Sarfaraz S, Adhami VM, Ahmad N, Raisuddin S, Mukhtar H. Inhibition of CWR22Rnu1 tumor growth and PSA secretion in athymic nude mice by green and black teas. Carcinogenesis. 2006;27:833–839. doi: 10.1093/carcin/bgi323. [DOI] [PubMed] [Google Scholar]
- 16.Hsu A, Bruno RS, Lohr CV, Taylor AW, Dashwood RH, Bray TM, Ho E. Dietary soy and tea mitigate chronic inflammation and prostate cancer via NFkappaB pathway in the Noble rat model. J Nutr Biochem. 2010;22:502–510. doi: 10.1016/j.jnutbio.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang P, Aronson WJ, Huang M, Zhang Y, Lee RP, Heber D, Henning Green tea polyphenols and metabolites in prostatectomy tissue: implications for cancer prevention. Cancer Prev Res. 2010;3:985–993. doi: 10.1158/1940-6207.CAPR-09-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henning SM, Niu Y, Lee NH, Thames GD, Minutti RR, Wang H, Go VL, Heber D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am J Clin Nutr. 2004;80:1558–1564. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]
- 19.Luo H, Tang L, Tang M, Billam M, Huang T, Yu J, Wei Z, Liang Y, Wang K, Zhang ZQ, Zhang L, Wang JS. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis. 2006;27:262–268. doi: 10.1093/carcin/bgi147. [DOI] [PubMed] [Google Scholar]
- 20.Henning SM, Wang P, Said J, Magyar C, Castor B, Doan N, Tosity C, Moro A, Gao K, Li L, Heber D. Polyphenols in brewed green tea inhibit prostate tumor xenograft growth by localizing to the tumor and decreasing oxidative stress and angiogenesis. J Nutr Biochem. 2012;23:1537–1542. doi: 10.1016/j.jnutbio.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqui IA, Shukla Y, Adhami VM, Sarfaraz S, Asim M, Hafeez BB, Mukhtar H. Suppression of NFkappaB and its regulated gene products by oral administration of green tea polyphenols in an autochthonous mouse prostate cancer model. Pharm Res. 2008;25:2135–2142. doi: 10.1007/s11095-008-9553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S, Ahmad N, Nieminen AL, Mukhtar H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (−)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol Appl Pharmacol. 2000;164:82–90. doi: 10.1006/taap.1999.8885. [DOI] [PubMed] [Google Scholar]
- 23.Fahy BN, Schlieman MG, Mortenson MM, Virudachalam S, Bold RJ. Targeting BCL-2 overexpression in various human malignancies through NF-kappaB inhibition by the proteasome inhibitor bortezomib. Cancer Chemother Pharmacol. 2005;56:46–54. doi: 10.1007/s00280-004-0944-5. [DOI] [PubMed] [Google Scholar]
- 24.Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross JS, Kallakury BV, Sheehan CE, Fisher HA, Kaufman RP, Jr, Kaur P, Gray K, Stringer B. Expression of nuclear factor-kappa B and I kappa B alpha proteins in prostatic adenocarcinomas: correlation of nuclear factor-kappa B immunoreactivity with disease recurrence. Clin Cancer Res. 2004;10:2466–2472. doi: 10.1158/1078-0432.ccr-0543-3. [DOI] [PubMed] [Google Scholar]
- 26.Suh J, Payvandi F, Edelstein LC, Amenta PS, Zong WX, Gélinas C, Rabson AB. Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate. 2002;52:183–200. doi: 10.1002/pros.10082. [DOI] [PubMed] [Google Scholar]
- 27.Sweeney C, Li L, Shanmugam R, Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, Gardner T, Smith M, Nakshatri H, Cheng L. Nuclear factor-kappaB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res. 2004;10:5501–5507. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- 28.Jin RJ, Lho Y, Connelly L, Wang Y, Yu X, Saint JL, Case TC, Ellwood-Yen K, Sawyers CL, Bhowmick NA, Blackwell TS, Yull FE, Matusik RJ. The nuclear factor-kappaB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res. 2008;68:6762–6769. doi: 10.1158/0008-5472.CAN-08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lessard L, Mes-Masson AM, Lamarre L, Wall L, Lattouf JB, Saad F. NF-kappa B nuclear localization and its prognostic significance in prostate cancer. BJU Int. 2003;91:417–420. doi: 10.1046/j.1464-410x.2003.04104.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang P, Vadgama JV, Said JW, Magyar CE, Doan N, Heber D, Henning SM. Enhanced inhibition of prostate cancer xenograft tumor growth by combining quercetin and green tea. J Nutr Biochem. 2014;25:73–80. doi: 10.1016/j.jnutbio.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shafique K, McLoone P, Qureshi K, Leung H, Hart C, Morrison DS. Tea consumption and the risk of overall and grade specific prostate cancer: a large prospective cohort study of Scottish men. Nutr Cancer. 2012;64:790–797. doi: 10.1080/01635581.2012.690063. [DOI] [PubMed] [Google Scholar]
- 32.Montague JA, Butler LM, Wu AH, Genkinger JM, Koh WP, Wong AS, Wang R, Yuan JM, Yu MC. Green and black tea intake in relation to prostate cancer risk among Singapore Chinese. Cancer Causes Control. 2012;23:1635–1641. doi: 10.1007/s10552-012-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geybels MS, Verhage BA, Arts IC, van Schooten FJ, Goldbohm RA, van den Brandt PA. Dietary flavonoid intake, black tea consumption, and risk of overall and advanced stage prostate cancer. Am J Epidemiol. 2013;177:1388–1398. doi: 10.1093/aje/kws419. [DOI] [PubMed] [Google Scholar]
- 34.Bardia A, Platz EA, Yegnasubramanian S, De Marzo AM, Nelson WG. Anti-inflammatory drugs, antioxidants, and prostate cancer prevention. Curr Opin Pharmacol. 2009;9:419–426. doi: 10.1016/j.coph.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BB. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal. 2012;16:1295–1322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li GX, Chen YK, Hou Z, Xiao H, Jin H, Lu G, Lee MJ, Liu B, Guan F, Yang Z, Yu A, Yang CS. Pro-oxidative Activities and Dose-response Relationship of (−)-Epigallocatechin-3-gallate in the Inhibition of Lung Cancer Cell Growth: A Comparative Study in vivo and in vitro. Carcinogenesis. 2010;31:902–910. doi: 10.1093/carcin/bgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooke MS, Olinski R, Loft S. Measurement and meaning of oxidatively modified DNA lesions in urine. Cancer Epidemiol Biomarkers Prev. 2008;17:3–14. doi: 10.1158/1055-9965.EPI-07-0751. [DOI] [PubMed] [Google Scholar]
- 39.Hakim IA, Harris RB, Chow HH, Dean M, Brown S, Ali IU. Effect of a 4-month tea intervention on oxidative DNA damage among heavy smokers: role of glutathione S-transferase genotypes. Cancer Epidemiol Biomarkers Prev. 2004;13:242–249. doi: 10.1158/1055-9965.epi-03-0193. [DOI] [PubMed] [Google Scholar]
- 40.Floriano-Sanchez E, Castro-Marin M, Cardenas-Rodriguez N. Molecular markers associated with prostate cancer: 3-nitrotyrosine and genetic and proteic expression of Mn-superoxide dismutasa (Mn-SOD) Arch. Esp. Urol. 2009;62:702–711. doi: 10.4321/s0004-06142009000900003. [DOI] [PubMed] [Google Scholar]
- 41.Henning SM, Aronson W, Niu Y, Conde F, Lee NH, Seeram NP, Lee RP, Lu J, Harris DM. Tea polyphenols and theaflavins are found in prostate tissue of humans and mice after green and black tea consumption. J Nutr. 2006;136 doi: 10.1093/jn/136.7.1839. 1839-143. [DOI] [PubMed] [Google Scholar]