Abstract
Purpose
This study aimed to detect cell-surface vimentin (CSV) on the surface of epithelial-mesenchymal transitioned (EMT) circulating tumor cells (CTCs) from blood of patients with epithelial cancers.
Experimental Design
In this study, 101 patients undergoing post-surgery adjuvant chemotherapy for metastatic colon cancer were recruited. EMT CTCs were detected from blood of patients using 84-1 monoclonal antibody against CSV as a marker. EMT CTCs isolated were characterized further using EMT-specific markers, fluorescent in situ hybridization and single cell mutation analysis.
Results
Using 84-1 antibody, we detected CSV exclusively on EMT CTCs from a variety of tumor types but not in the surrounding normal cells in the blood. The antibody exhibited very high specificity and sensitivity towards different epithelial cancer cells. With this antibody, we detected and enumerated EMT CTCs from patients. From our observations, we defined a cutoff of < five or ≥ five EMT CTCs as optimal threshold with respect to therapeutic response using ROC curves. Using this defined threshold, the presence of ≥ five EMT CTCs was associated with progressive disease, while patients with less than five EMT CTCs showed therapeutic response.
Conclusion
Taken together, number of EMT CTCs detected correlated with the therapeutic outcome of the disease. These results establish cell-surface vimentin as a universal marker for EMT CTCs from a wide variety of tumor types and thus provide the foundation for emerging CTC detection technologies and for studying the molecular regulation of these EMT CTCs.
Introduction
Metastasis is the main cause for cancer-related deaths worldwide and circulating tumor cells (CTCs) are considered to be the roots of metastases (1). These cells are emerging as a novel target for early detection of metastasis and for monitoring the therapeutic efficacy of anti-cancer drugs (2). Current CTC technology relies on the capture of these cells with antibodies against the epithelial phenotype-specific markers EpCAM and cytokeratins (2). A major drawback with these markers is their inability to detect CTCs that no longer express EpCAM after undergoing epithelial-mesenchymal transition (EMT) (i.e., EMT CTCs), a cellular process in which epithelial cells acquire a mesenchymal phenotype and thus become more aggressive and invasive (3). These EMT CTCs are considered the key cell subtype that causes metastasis (4). Although EMT CTCs have been gaining attention, the absence of a cell-surface mesenchyme-specific marker hampers research in the field of CTC detection.
EMT in cancer cells has been associated with an increasingly invasive, chemo-resistant, and metastatic phenotype in a wide variety of cancer types. The EMT process is associated mainly with overexpression of vimentin (5), and single-cell profiling of CTCs isolated from cancer patients has indicated overexpression of vimentin transcript compared with established cancer cell lines (6), indicating a mesenchymal phenotype in these CTCs. However, intracellular expression of vimentin in normal mesenchymal cells, including most white blood cells, limits the use of this protein as a CTC marker. We and others have previously reported the detection of vimentin on the surface of cancer cells (5, 7-9). Unlike intracellular vimentin, the expression of cell-surface vimentin (CSV) is mainly associated with cancer cells only. We therefore hypothesized that CSV can serve as a marker for EMT CTCs.
Sieuwerts et al. previously showed that the CellSearch detection method does not identify cells that have undergone EMT (3). Although a few researchers have reported detecting transitioned CTCs with a panel of markers (4, 10) or individual markers (11, 12), the uncertainty regarding their ability to detect these cells from a wide variety of solid tumors using the existing technologies or markers calls for the discovery of novel single and specific markers for EMT CTCs. Moreover, those few reported EMT CTC markers have not been used to test the correlation between EMT CTCs and disease progression. Here, we report the discovery of cancer cell CSV as a marker of EMT CTCs with a monoclonal antibody we developed that shows high specificity and sensitivity towards different cancer types, thus making it a universal marker for EMT CTCs. Using our antibody, we were able to correlate counts of EMT CTCs with disease status by using blood samples from colorectal cancer patients and other independent clinical diagnostic methods.
Methods
Cell culture
All cell lines used in this study were obtained from American Type Culture Collection (Manassas, VA, USA) and were grown according to the supplier’s recommendations. All cell lines were cultured within three passages from the time of purchase. Cell lines with no particular culture recommendations were grown in DMEM-F12 medium (Sigma Aldrich) with 10% fetal bovine serum, 1% L-glutamine, and 0.1% penicillin/streptomycin (Gibco, Invitrogen). NCM-356, normal human colonic cell line was used as control. Primary cultures obtained from human colon (HPC), liver (HLM: primary colon cancer metastasized to liver), and lung (CPM: primary colon cancer metastasized to lung) cancers were obtained after cell dissociation from the primary tumors and cultured in DMEM/F-12 medium with 10% heat-inactivated fetal bovine serum, 1% L-glutamine, 100 μg/mL Primocin (Invivogen), and 0.1% penicillin/streptomycin. Medium was changed once every 4 days. All cells were maintained at 37°C in an incubator with a 5% CO2.
Geltrex thin layer method
HPC-1 cells were grown on Geltrex reduced growth factor basement membrane matrix (Invitrogen). This matrix is a soluble form of basement membrane purified from Engelbreth-Holm-Swarm tumor, which gels at 37°C to form a reconstituted basement membrane that provides the matrix for the culture of cells. Major components of Geltrex include various growth factors and laminin, collagen IV, and entactin. According to the manufacturer’s recommendation, Geltrex was thawed on ice and 100 μL was used to coat the Lab-Tek chamber slides (Thermo) 1 h before the cells were plated. Later, 1000 HPC-1 cells in 100 μL of cold serum-free DMEM/F-12 medium with 2% Geltrex were plated on the chamber slides with a thin gel coating. The cells were grown at 37°C in a humidified atmosphere of 5% CO2 in air and were observed through a bright field microscope for the formation of spheres. Sphere-containing chamber slides were then processed for immunofluorescence staining.
Study cohort
Patients of any age with metastatic colorectal cancer refractory to 5-fluorouracil who were undergoing palliative chemotherapy at The University of Texas MD Anderson Cancer Center were eligible for this study. Patients were at different stages of treatment with different therapeutic regiments as listed in the Supplementary Table 1. Routine diagnostic workup included diagnostic imaging, chest X-rays, bone scan, blood sampling, and clinical examination. Study age matched blood samples from healthy blood donors were obtained from the Gulf Coast Blood Center in Houston, TX. Nine patients had died during the course of this study. For preliminary analysis only, blood samples from breast, bladder and liver cancer patients were collected.
Blood collection and processing
Human blood samples for CTC analysis were obtained after informed consent had been obtained from the patient or blood donor, per the Institutional Review Board protocol at the MD Anderson Cancer Center. CTC detection was conducted as and when possible; no attempt made to reach a defined statistical power. At any given blood draw, a maximum of 7.5 mL of blood was obtained using CPT Vacutainer tubes (BD Bioscience). Single nucleated cells were isolated within 48 h of blood collection, per the manufacturer’s recommendation. Cells were then washed in phosphate-buffered saline (PBS) and used for further analysis. Neither patients nor clinicians were informed of the results from the CTC analysis.
84-1+ Cell selection
Method of cell isolation, confirmation for positive selection and validation steps were described in (9). Briefly, first CD45+ cells were depleted using an EasySep human CD45 depletion kit (Stem Cell Technologies) according to the manufacturer’s recommendation. To minimize nonspecific binding, antibody against human Fc receptor (Miltenyi Biotec) was added to the cocktail. Second, the CD45− cell fraction was subjected to 84-1+ selection. Cells were labeled with the 84-1 anti-vimentin antibody, and later mouse IgG-binding microbeads (Miltenyi Biotec) were added to the mixture. 84-1+ Cells were then extracted using the magnetic column according to the manufacturer’s recommendation (Miltenyi Biotec). The cells thus obtained were 84-1+ and CD45− and ready for further analysis.
Antibodies
Antibodies against the specific markers EpCAM (D1B3) Rabbit mAb #2626, SLUG (C19G7) Rabbit mAb #9585, E-Cadherin (24E10) Rabbit mAb #3195, β-catenin (D10A8) XP® Rabbit mAb #8480 and c-myc (D84C12) XP® Rabbit mAb #5605 were obtained from Cell Signaling. Antibodies against the specific markers FOXC2 (AF5044), TWIST-1 (AF6230), and SNAIL (AF3639) were obtained from R&D Systems.
Flow cytometry
A total of 5 × 105 cells was detached with a non-enzymatic dissociation buffer, washed, and stained for 20 min on ice in the dark. For CSV analysis, cells were stained with the 84-1 monoclonal antibody we developed (1:100); mouse primary antibody (Invitrogen) was used as an isotype control. Later, cells were rinsed twice in PBS and labeled for secondary antibody using Alexa Fluor-405, -488 or -555 secondary antibody (Invitrogen). Cells were then washed twice in PBS and immediately used for data acquisition using an Attune flow cytometer (Applied Biosystems). Fifty thousand cells were counted for the analysis. The data were analyzed using FlowJo software (Treestar).
Spiking assay
To demonstrate the precision and reproducibility of CTC capture by the 84-1 antibody, cultured cancer cells were spiked into blood collected from healthy donors. For the sensitivity assay, ~2, ~5, 10, or 25 Calcein AM-labeled (EMD Bioscience) HLM-3 cells were spiked into a sample containing 1 × 106 peripheral blood mononuclear cells. To demonstrate specificity, ~5 Calcein AM-labeled HLM-3 cells were spiked into samples containing 1 × 106, 2 × 106, or 2.5 × 106 peripheral blood mononuclear cells. All cells used for spiking experiments were subjected to 84-1+ selection a day before the spiking analysis to increase the fraction of 84-1+ cells. For cell counting, cells were harvested in culture medium and then serially diluted to achieve the required counts, which were then confirmed in a series of 5-μL spots under a microscope. If lower or higher numbers of cells were observed, we calculated the necessary counts of cells required to be spiked. Spiking experiments were performed in triplicate to ensure the sensitivity and specificity of the method. For negative controls, CSV− HEK293T and NCM-356 cells were spiked into blood and analyzed for 84-1+ selection. Also, as an additional control mouse IgG was used for the isolation procedure and probed with anti-mouse secondary antibody.
Statistical analysis
All statistical analyses were performed using GraphPad Prism software, where P<0.05 was considered significant. Differences in baseline characteristics between treatment responders and non-responders were analyzed using Fisher's exact and t tests. Diagnostic performance of CTC count was assessed by constructing a receiver operating characteristic (ROC) curve, and was further evaluated by calculating the area under each ROC curve (AUC-ROC) (13). An AUC-ROC of value 1 denotes that the test method is able to discriminate perfectly, while an AUC-ROC of value 0.5 would denote a worst discrimination of the test. P value was calculated for the difference between each AUC-ROC.
Microscopy image capture and analysis: is included in the supplemental methods section.
RESULTS
CSV expression is restricted to cancer cells
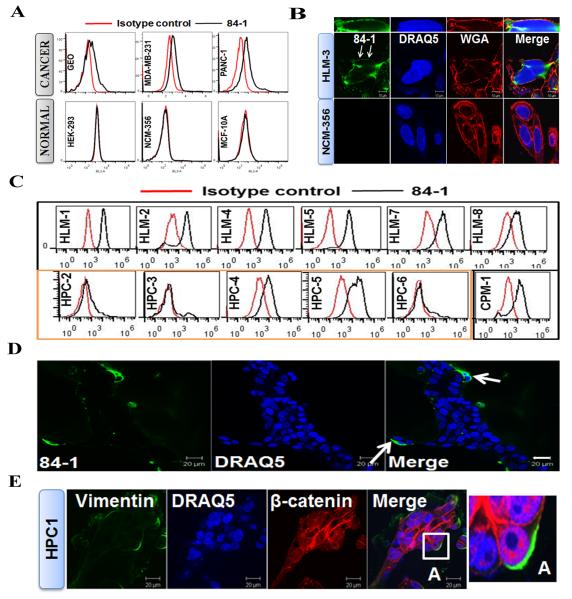
Since commercial antibodies against CSV are not available, we generated a CSV-specific monoclonal antibody, called 84-1, using a differential expression screening strategy described in (9). Briefly, to screen for CSV-specific antibodies produced against full-length vimentin, we used the cell line LM7 (human metastatic osteosarcoma cells) to represent CSV-positive cells and the cell lines NCM-356 (human colon epithelial cells) and hFOB (human fetal osteoblasts) to represent CSV-negative cells. The monoclonal antibody had very high affinity for cancer cell CSV and did not bind to normal epithelial or mesenchymal cells. Screening for established cancer (GEO [colonic], MDA-MB-231 [breast], and PANC-1 [pancreatic]) and normal (HEK-293, NCM-356, and MCF-10A) cell lines indicated the presence of vimentin only on the surface of cancer cells (Fig. 1A). It is evident from these results that only a fraction of cells were positive for CSV. This fraction may be strongly associated with metastasis. Furthermore, our 84-1 antibody was specific to cancer cells and did not exhibit binding affinity towards any of the subsets of white blood cells. We have also analyzed several different established cancer cell lines (breast, liver, colon, brain, bladder and pancreas) for CSV expression using flow cytometry and our results indicate that majority of these cancer cell lines are positive for CSV (Table 1). These results thus prove CSV to be an excellent universal marker for any given tumor type.
Figure 1. Cell surface vimentin expression is limited to cancer cells.
(A) Immunological assessment of CSV expression in cancer (top) and normal (bottom) cell lines using flow cytometry. CSV was detected by the 84-1 antibody in only the cancer cell lines. Isotype controls were used as negative controls. (B) Cell surface staining analysis for CSV in liver cancer cell line HLM-3 and normal colon cell line NCM-356 using confocal microscopy. Cells were stained for CSV (with the 84-1 antibody; green), nuclei (with DRAQ5; blue), and the cell-surface marker WGA (red). Arrows, cell surface vimentin co-localized with WGA. Scale bar, 10 μm. (C) Immunological assessment of CSV in human primary colon cancer (HPC) cells, human colon cells metastasized to the liver (HLM), and human colon cells metastasized to the lung (CPM-1). Metastatic cancers are outlined in black; primary tumors are outlined in orange. (D) CSV detection in HPC-1-derived spheres on thin Geltrex-coated wells using confocal microscopy. CSV (green) was detectable in a few cells at the periphery of the sphere (arrows). DRAQ5 was used to demarcate the cells. Scale bar, 20 μm. (E) Vimentin and β-catenin expression in HPC-1-derived spheres on thin Geltrex-coated wells using confocal microscopy. Total vimentin (green) was detectable in a few cells at the periphery of the sphere, which correlated with the increased nuclear accumulation of β-catenin (red). DRAQ5 was used to demarcate the cells. Scale bar, 20 μm. Inset A in “Merge” image is enlarged at right.
Table 1.
| Cell Line | Cell-surface Vimentin |
Cell Line | Cell-surface Vimentin |
|---|---|---|---|
| Breast | Colon | ||
| MCF-7 (H) | + | DLD-1 (H) | ++ |
| SKBR3 (H) | + | GEO (H) | ++ |
| MDA-MB-231 (H) | + | OS-187 (H) | ++ |
| MDA-MB-453 (H) | + | SW620 (H) | + |
| MDA-MB-458 (H) | ++ | SW480 (H) | + |
| 4T1 (M) | + | HCT-116 (H) | + |
| Liver | HT-29 (H) | ++ | |
| AMC14 (M) | ++ | Caco-2 (H) | + |
| CT-26 (M) | + | ||
| Brain | Bladder | ||
| SKNAS (H) | ++ | RT4V6 (H) | + |
| SKNBE2 (H) | +++ | T24 (H) | ++ |
| NGP (H) | + | Pancreas | |
| SH-SY5Y (H) | ++ | PANC-1 (H) | ++ |
| LAN5 (H) | ++ | MiaPACA-2 (H) | + |
| KCN (H) | + | ||
| DBT (M) | + | ||
| U251 (H) | + |
(H): Human, (M): Mouse. Cell-surface vimentin was scored using flow cytometric analysis by measuring mean fluorescence intensity of CSV.
“-”: Not detectable, “+, ++, +++”: <2, <4, >4 fold presence compared to isotype control.
To confirm the surface expression of vimentin, we performed immunocytochemical analysis of human liver metastatic cells (HLM-3) and normal human colon cells (NCM-356). The results indicated that vimentin was present on the surface of the cancer cells (Fig. 1B) but not on the surface of the normal cells. In addition, vimentin co-localized with the cell-surface marker wheat germ agglutinin (WGA). Permeabilizing these cells revealed the presence of cytoplasmic vimentin in only the cancer cells and not in the normal cells (Supplementary Fig. 1A). These results confirmed that the surface expression of vimentin was restricted to cancer cells.
To analyze whether CSV-expressing cancer cells are metastatic, we used flow cytometry to analyze CSV expression in primary cancer cells isolated from human colon (HPC), human colon cells metastasized to the liver (HLM), and human colon cells metastasized to the lung (CPM). The results indicated over-expression of CSV in metastatic tumors compared with primary tumors (Fig. 1C), suggesting that CSV expression is mainly associated with metastasis and could serve as a potential biomarker for metastasis. This result also suggested that a subpopulation of primary colon cancer cells that express CSV have an invasive or metastatic phenotype and are shed into the blood circulation for spreading to distant organs. This possibility was supported by the observed presence of CSV+ positive cells at the periphery of the sphere in a three-dimensional HPC-derived sphere model (Fig. 1D). Cells at the periphery were more aggressive and invasive, which is supported by the increasing nuclear accumulation of β-catenin that denotes the invasive phenotype of these cells (14) (Fig. 1E). From these observations, it was evident that CSV could serve as a specific marker for detecting metastatic cancer cells.
84-1 Antibody detects spiked cancer cells with high sensitivity and specificity
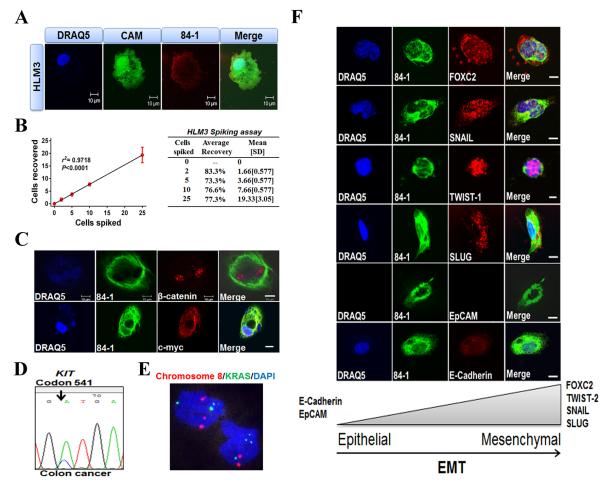
Based on our detection of CSV in a range of metastatic tumors, we postulated that CSV could serve as a biomarker to detect metastatic CTCs from epithelial cancers. We verified the detection of 84-1+ HLM-3 cells, which were labeled with Calcein-AM tracking dye, and spiked different numbers of cells into 7.5 mL of normal human blood. After CD45 depletion and 84-1+ selection, the cells recovered were subjected to immunofluorescence staining. From fluorescence microscopy micrographs, it was evident that even a single labeled cell could be isolated from whole blood by using the 84-1 antibody (Fig. 2A). Because the sensitivity (limit of detection) and specificity (no background/unwanted cells) of detection are important parameters for using an antibody for CTC enumeration, we evaluated these parameters in our spiking assay using 84-1+ HLM-3 cells. Linear regression of the number of detected tumor cells versus the number of tumor cells spiked yielded a correlation coefficient (R2) of 0.971 (P <0.001) (Fig. 2B) with ~100% specificity. Normal cells spiked in the blood were undetectable by 84-1 antibody and tumor cells were undetectable using mouse IgG used as a control. Taken together, these results indicated a very high specificity and sensitivity of the antibody to detect spiked cells at various concentrations.
Figure 2. Spiking assay and detection of CTCs from human colorectal cancer patients.
(A) Detection of HLM-3 cells labeled with tracker dye Calcein-AM (CAM) (green) that were spiked into 7.5 mL of blood using fluorescence microscopy. Cells were also stained for nuclei (with DRAQ5; blue) and vimentin (with the 84-1 antibody; red). (B) Regression analysis of capture efficiency for up to 25 HLM-3 cells spiked in human blood. (C) Analysis of 84-1+ CD45− CTCs from human colon cancer samples that were isolated and stained for nuclei (with DRAQ; blue), total vimentin (with 84-1; green), β-catenin (red), and c-myc (red). The results indicated complete nuclear localization of β-catenin and c-myc. Scale bar, 10 μm. (D) Mutational analysis of KIT in colon cancer-derived CTC. (E) Dual-probe FISH with a centromere probe for chromosome 8 (red) and region-specific probe for KRAS (green). Three chromosome 8 signals and three KRAS signals were detected in the cell at top, while two chromosome 8 signals and four KRAS signals were detected in the cell at bottom. Nuclei were counterstained with DAPI (blue). (F) Analysis of colon cancer-derived 84-1+ CD45− CTCs from patient samples for specific molecular EMT markers. CTCs were stained for vimentin (with 84-1 antibody) and for FOXC2, SNAIL, TWIST-1, SLUG, EpCAM, and E-Cadherin with the respective antibodies. Scale bar, 5 μm.
84-1 Antibody detects EMT CTCs that express EMT-specific markers
Because our spiking assays showed very high specificity for spiked cancer cells, we used the above-described method to detect CTCs from colorectal cancer patients as well as several methods to confirm the cancerous phenotype of these isolated CTCs. β-Catenin and c-myc proteins are over-expressed in colorectal cancers (15), and in our study we detected and isolated 84-1+ CD45− CTCs from blood of colorectal cancer patients and confirmed the over-expression of these proteins in these cells (Fig. 2C). The localization of these proteins in the nucleus indicated that these were active transitioned cells (16). For further validation of 84-1+ CD45− CTCs isolated from all cancers, we subjected these cells to cancer-specific mutation analysis. A representative colon cancer sample was positive for mutations in KIT, KRAS and APC; however, CTCs isolated from this patient harbored a mutation in KIT only (Fig. 2D). Mutational analysis from other patients is listed in Supplementary Table 1. Heterogeneity among CTCs from the same patient have been reported (17), and from our observations it was interesting to note that CTCs harbored only specific mutations.
As a second step for validation, we used fluorescent in situ hybridization (FISH) to detect amplification of the KRAS gene and chromosome 8 signals in EMT CTCs from a representative sample (Fig. 2E). The FISH data indicated a heterogeneous amplification of targets, again highlighting the heterogeneous nature of these CTCs. Taken together, our results indicated that the cells isolated from the blood of cancer patients were of cancerous origin and were heterogeneous.
Because we were able to detect CSV+ CTCs in cancers of epithelial origin and establish vimentin as a marker for EMT, we hypothesized that 84-1+ CD45− CTCs had undergone EMT. An analysis of vimentin positive CTCs with known EMT-specific markers (18) revealed increased expression of FOXC2, SNAIL, TWIST-1, and SLUG and markedly decreased expression of epithelium-specific markers E-Cadherin and EpCAM (Fig. 2F), thus confirming the mesenchymal nature of 84-1+ CD45− CTCs. Expression of EMT-inducing transcription factors, including the same four proteins, has been associated with tumor invasion and metastasis (18), and TWIST-1 (19) and SLUG (20) overexpression has been shown to be independent prognostic parameters for poor survival in colorectal carcinoma patients. Thus, “metastatic-like CTCs” might be detected using CSV as a marker.
Moreover, we detected EMT CTCs from other solid tumors, including those of the breast, bladder, and liver (Fig. 3A), which suggests that CSV has a universal capacity for detecting EMT CTCs. With the use of CSV as a marker, further characterization of transitioned CTCs and better understanding of their contribution to the metastatic spread of a wide variety of cancer types are now possible.
Figure 3.
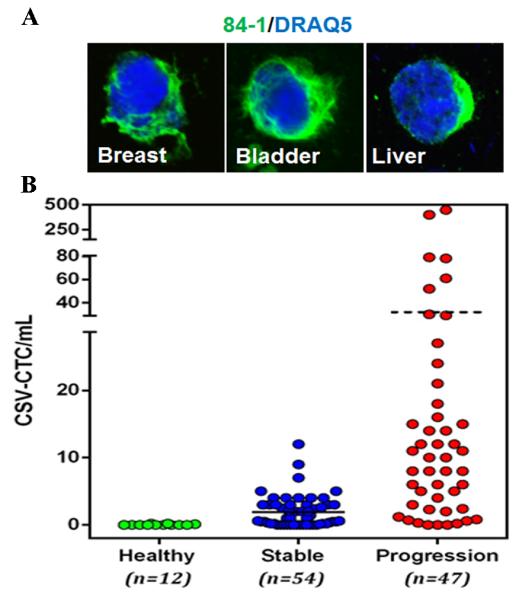
(A) EMT CTCs isolated from breast, bladder, and liver cancers. CTCs were stained for vimentin (green) and nuclei (DRAQ5). (B) Enumeration of CSV+ CTCs from blood specimens from healthy volunteers and from patients with metastatic colorectal cancer. Patients were categorized based on whether the disease was stable or progressive. Horizontal bar in the graph represents the mean value of the CTC.
84-1+ CTCs isolated from colorectal cancer patients correlate with disease status
Because we were able to confirm that the CTCs detected with our anti-CSV antibody were cancerous and had transitioned to the mesenchymal phenotype, we next tested human blood samples from 12 healthy blood donors and from 101 patients with metastatic colorectal cancer for 84-1+ CD45− CTCs. In this study, fluorescent images were reviewed independently by researchers blinded to treatment and outcome. No adverse events or complications had been reported during the blood collections. Blood samples from the healthy donors were analyzed under the same conditions. None of the healthy donors had detectable CTCs, indicating a high specificity of the 84-1 antibody. CTCs were detectable in 85 of the 101 (84%) metastatic colorectal cancer patient samples. The number of CTCs isolated from these patients ranged from 0 to 450 mL−1 (Fig. 3B). We divided the patient population into two groups based on RECIST guidelines: a) stable or responding disease, b) progressive disease. Of these 101 patients, 54 (53%) exhibited radiographic response or stable disease, and 47 (46%) had progressive disease (Table 2). We observed a significant difference in response to therapy by CTC count: patients with ≥5 mL−1 were more likely to resist treatment and exhibit progressive disease, whereas patients with <5 mL−1 were more likely to be sensitive to treatment and exhibit stable disease (P=0.0195). This threshold cut-off range of 5CTCs/ mL was calculated using receiver operating characteristic curves (Supplementary Fig. 2 and Supplementary Table 2). Fourteen patients (14%) with a count of <5 mL−1 showed progressive disease, whereas 5 patients (5%) with a count of ≥5 mL−1 had stable disease. This discrepancy might be attributable to the clinical stage of the disease and the treatment type used for each patient. From the analysis it was observed that CTC are better prognostic markers for evaluating therapeutic responses.
Table 2.
Patient Characteristics
| Characteristic | No. (%) of patients |
CTC Count mL−1 | ||
|---|---|---|---|---|
| 0 | <5 | ≥5 | ||
| Age | ||||
| ≤50 years | 34 (34%) | 3 | 16 | 15 |
| >50 years | 67 (66%) | 13 | 31 | 23 |
| Sex | ||||
| Male | 59 (58%) | 13 | 29 | 17 |
| Female | 42 (42%) | 3 | 18 | 21 |
| Disease status | ||||
| Stable | 54 (53%) | 13 | 36 | 5 |
| Progressive | 47 (47%) | 3 | 11 | 33 |
| Overall | 101 (100%) | 16 | 47 | 38 |
Sensitivity, specificity, and positive predictive value
From our study data, we determined that enumerating EMT CTCs is highly specific (91%) in identifying the progressive cell population and has a positive predictive value of 87% for the therapeutic outcome of the disease.
Discussion
Many approaches have been implemented to detect CTCs from blood samples, including flow cytometry, size-based separation, and optical imaging-based technology (21). Although these techniques are relatively new, antibody-based separation of CTCs has several advantages that are already making those technologies obsolete. One of the most successful antibody-dependent technologies is the CellSearch (22) method by Veridex, which is the only FDA-approved CTC detection platform available for detecting CTCs from patients. CellSearch technology is dependent on anti-EpCAM antibody for isolating CTCs and on staining with cytokeratins from the blood of patients with epithelial cancers. Although this platform has gained much attention, in recent years it has been shown that by using EpCAM, cytokeratins, or both as a CTC target, CellSearch and other technologies have overlooked CTCs that have undergone EMT and have lost the ability to express EpCAM (3) and other epithelial markers. EMT CTCs have been shown to correlate with disease progression (4) or relapse and their presence could be a key determinant of metastasis and poor prognosis.
The main goal of our lab was to identify EMT CTCs from blood of patients by using a single, specific marker instead of a multitude of markers and probes. As a first step towards this goal, we analyzed the proteins that are transported to the cell surface of cancer cells during EMT. We previously demonstrated that vimentin is an EMT marker and is localized on the surface of cancer cells (5), so it served as an interesting target for us. However, due to the lack of a specific antibody that can bind to vimentin on the cell surface (due, perhaps, to a structural change in the protein as it is transported to the surface); we were unable to detect this antigen on the cell surface of cancer cells. Therefore, we created the specific monoclonal antibody 84-1 to fill this gap in technology. This antibody showed very high specificity towards cancer cells and had very high sensitivity as assessed from spiking assays (see Fig. 2B). These characteristics thus fulfilled the basic requirements for establishing a CTC marker: sensitivity, specificity, and reproducibility. Moreover, this technique of isolating CTCs provides an opportunity to study the morphological and other characteristics of these CTCs and to further utilize a wide variety of markers, including protein markers and FISH probes (Fig. 2E, F).
EMT involves a functional transition of epithelial cells into mobile mesenchymal cells (23). The common mesenchymal cell markers used to identify EMT cells include FOXC2 (24), TWIST1 (19), SNAIL (25), SLUG (20) and vimentin (5). Although negative staining results for epithelial markers suggests EMT, researchers mainly rely on mesenchymal EMT markers. In this study, we showed that EMT CTCs are in fact associated with multiple EMT markers. However, it remains unclear whether the expression of multiple EMT markers in different CTCs is heterogeneous. Also, co-localization of epithelial and EMT-specific markers may define an intermediate phenotype of EMT cells (4), i.e., cells that are in transition. In our study, we observed that most EMT CTCs had lost the ability to express EpCAM and just a few of the EMT CTCs still retained the epithelial phenotype. Not much is known about the function or role of intermediate EMT cells.
Cytoplasmic vimentin is overexpressed during EMT and is associated with metastasis, invasion, and proliferation (5). It is still unclear what the role of vimentin transported to the cell surface is. In this study, we observed a positive correlation between CSV+ EMT CTCs and disease progression, which suggests a role for CSV in promoting disease progression. Since vimentin is an intermediate filament protein and is known to form vimentin adhesion networks (5), CSV could be involved in routing CTCs to the metastatic site and assisting in the invasion process to re-seed the metastatic niche, thus promoting tumor progression . Also, CSV+ EMT CTCs might represent a unique subset of CTCs that are chemo resistant and thus do not respond to chemotherapeutic regimens. In addition, CSV could be associated with cancer stem-like cells, which have the propensity to form stable colonies. These possibilities need to be tested to confirm the role of CSV in cancer.
CTCs have been used in several studies to monitor response to chemotherapy (26). However, an exclusive correlation between EMT CTC count and disease progression has never been reported. To our knowledge, we are the first to report a correlation between therapeutic outcome in metastatic colorectal cancer patients and EMT CTC count. Because our cohort consisted of only patients undergoing chemotherapy for metastatic colorectal cancer, the results suggested that the CTCs detected in the bloodstream were released mainly from the metastatic sites. Also, from our study it is evident that these CTCs were heterogeneous, so these cells should be assessed in regards to their propensity to successfully form metastases or re-seed the primary tumor. Capturing and culturing these CTCs and determining their propensities would allow characterization their phenotypes using in vitro and in vivo modeling, studies of which are currently in progress.
Our study had a few limitations. For example, we retrospectively analyzed information on colorectal cancer patients treated with different chemotherapeutic protocols. Because this was a pilot study to assess the therapeutic outcome based on EMT CTC counts, we recruited post-surgery patients who were undergoing chemotherapy. It would be interesting to follow up with a large group of patients at different stages of treatment (before surgery, pre-neoadjuvant chemotherapy, post-neoadjuvant chemotherapy, and after surgery). Also, long-term follow-up would be essential to understanding how EMT CTCs could be used to predict relapse. Technical limitations of the study included the optimization of assays, positive selection of cancer cells a day before spiking experiments, timing of blood collection, and preservation of the integrity of the CTCs in blood. In addition, because several types of host cells in the blood are mesenchymal and express vimentin, the CTCs isolated must be tested for EMT-specific markers that are absent in host cells. The protein Plastin3 (11) has been used to identify EMT CTCs from blood samples of colon cancer patients, but it has not been tested with other types of solid tumors. We presented here, for the first time, evidence that EMT CTCs can be detected in multiple solid tumor types by using a single, specific marker.
In conclusion, we demonstrated that CSV is a marker for EMT CTCs and that by using the 84-1 anti-vimentin antibody we developed; these CTCs can be isolated from the blood of cancer patients with metastatic colorectal cancer. A key protein that is overexpressed during EMT, vimentin has been shown to be overexpressed in other types of cancers that undergo EMT, thereby making it a universal EMT CTC marker. Also, by using this antibody we can not only isolate or detect EMT CTCs but also characterize the nature of these cells by using other specific markers. Therefore, isolating CSV+ CTCs will help us understand the metastatic precursor cell subpopulation and will help in developing novel diagnostic, treatment, and prognostic options based on therapeutic monitoring.
Supplementary Material
Translational Relevance.
In the present study epithelial-mesenchymal transitioned (EMT) circulating tumor cells (CTCs) were detected and isolated from colon cancer patients using cell-surface vimentin (CSV) as a newly developed mesenchymal CTC marker. Patients with progressive disease showed increasing number of EMT CTC in comparison with patients that are responding/ have stable disease. This is of potential clinical importance, as it provides evidence that EMT CTC are critical in detecting patients with tumor progression and are missed using the conventional EpCAM based CTC isolation technologies. These results help provide more convincing evidence to using EMT CTC detection in future clinical practice.
Acknowledgements
Work in the authors’ laboratory was supported by a grant from the National Institutes of Health to Dr. Shulin Li (NIH RO1CA120895). We thank the Cancer Center Support Grant (CCSG), Hybridoma, Flow Cytometry and DNA Analysis Core facilities at MD Anderson Cancer Center (MDACC) for their assistance.
Footnotes
Conflict of Interest: None
References
- 1.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–56. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 2.Parkinson DR, Dracopoli N, Petty BG, Compton C, Cristofanilli M, Deisseroth A, et al. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med. 2012;10:138. doi: 10.1186/1479-5876-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Schutte M, et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst. 2009;101:61–6. doi: 10.1093/jnci/djn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–4. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68:3033–46. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell AA, Talasaz AH, Zhang H, Coram MA, Reddy A, Deng G, et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS One. 2012;7:e33788. doi: 10.1371/journal.pone.0033788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutrera J, Dibra D, Xia X, Hasan A, Reed S, Li S. Discovery of a linear peptide for improving tumor targeting of gene products and treatment of distal tumors by IL-12 gene therapy. Mol Ther. 2011;19:1468–77. doi: 10.1038/mt.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huet D, Bagot M, Loyaux D, Capdevielle J, Conraux L, Ferrara P, et al. SC5 mAb represents a unique tool for the detection of extracellular vimentin as a specific marker of Sezary cells. J Immunol. 2006;176:652–9. doi: 10.4049/jimmunol.176.1.652. [DOI] [PubMed] [Google Scholar]
- 9.Satelli A, Mitra A, Cutrera JJ, Devarie M, Xia X, Ingram DR, et al. Universal marker and detection tool for human sarcoma circulating tumor cells. Cancer research. 2014;74:1645–50. doi: 10.1158/0008-5472.CAN-13-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pecot CV, Bischoff FZ, Mayer JA, Wong KL, Pham T, Bottsford-Miller J, et al. A novel platform for detection of CK+ and CK- CTCs. Cancer discovery. 2011;1:580–6. doi: 10.1158/2159-8290.CD-11-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokobori T, Iinuma H, Shimamura T, Imoto S, Sugimachi K, Ishii H, et al. Plastin3 is a novel marker for circulating tumor cells undergoing the epithelial-mesenchymal transition and is associated with colorectal cancer prognosis. Cancer research. 2013;73:2059–69. doi: 10.1158/0008-5472.CAN-12-0326. [DOI] [PubMed] [Google Scholar]
- 12.Bitting RL, Boominathan R, Rao C, Kemeny G, Foulk B, Garcia-Blanco MA, et al. Development of a method to isolate circulating tumor cells using mesenchymal-based capture. Methods. 2013 doi: 10.1016/j.ymeth.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 14.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–9. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 15.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–20. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 16.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–61. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasch C, Bauernhofer T, Pichler M, Langer-Freitag S, Reeh M, Seifert AM, et al. Heterogeneity of Epidermal Growth Factor Receptor Status and Mutations of KRAS/PIK3CA in Circulating Tumor Cells of Patients with Colorectal Cancer. Clin Chem. 2012 doi: 10.1373/clinchem.2012.188557. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–29. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Gomez I, Pena C, Herrera M, Munoz C, Larriba MJ, Garcia V, et al. TWIST1 is expressed in colorectal carcinomas and predicts patient survival. PLoS One. 2011;6:e18023. doi: 10.1371/journal.pone.0018023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shioiri M, Shida T, Koda K, Oda K, Seike K, Nishimura M, et al. Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients. Br J Cancer. 2006;94:1816–22. doi: 10.1038/sj.bjc.6603193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danova M, Torchio M, Mazzini G. Isolation of rare circulating tumor cells in cancer patients: technical aspects and clinical implications. Expert review of molecular diagnostics. 2011;11:473–85. doi: 10.1586/erm.11.33. [DOI] [PubMed] [Google Scholar]
- 22.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. The New England journal of medicine. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 23.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci U S A. 2007;104:10069–74. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan F, Samuel S, Evans KW, Lu J, Xia L, Zhou Y, et al. Overexpression of snail induces epithelial-mesenchymal transition and a cancer stem cell-like phenotype in human colorectal cancer cells. Cancer medicine. 2012;1:5–16. doi: 10.1002/cam4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierga JY, Bidard FC, Mathiot C, Brain E, Delaloge S, Giachetti S, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:7004–10. doi: 10.1158/1078-0432.CCR-08-0030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.