Abstract
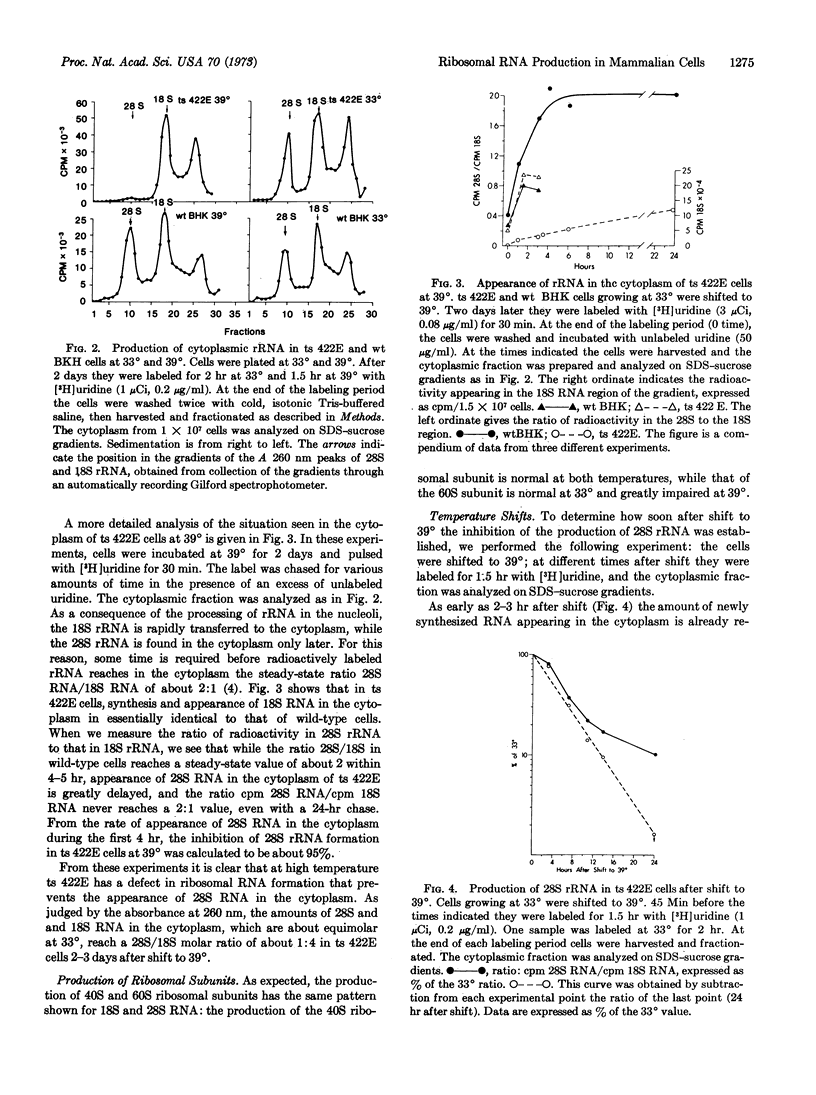
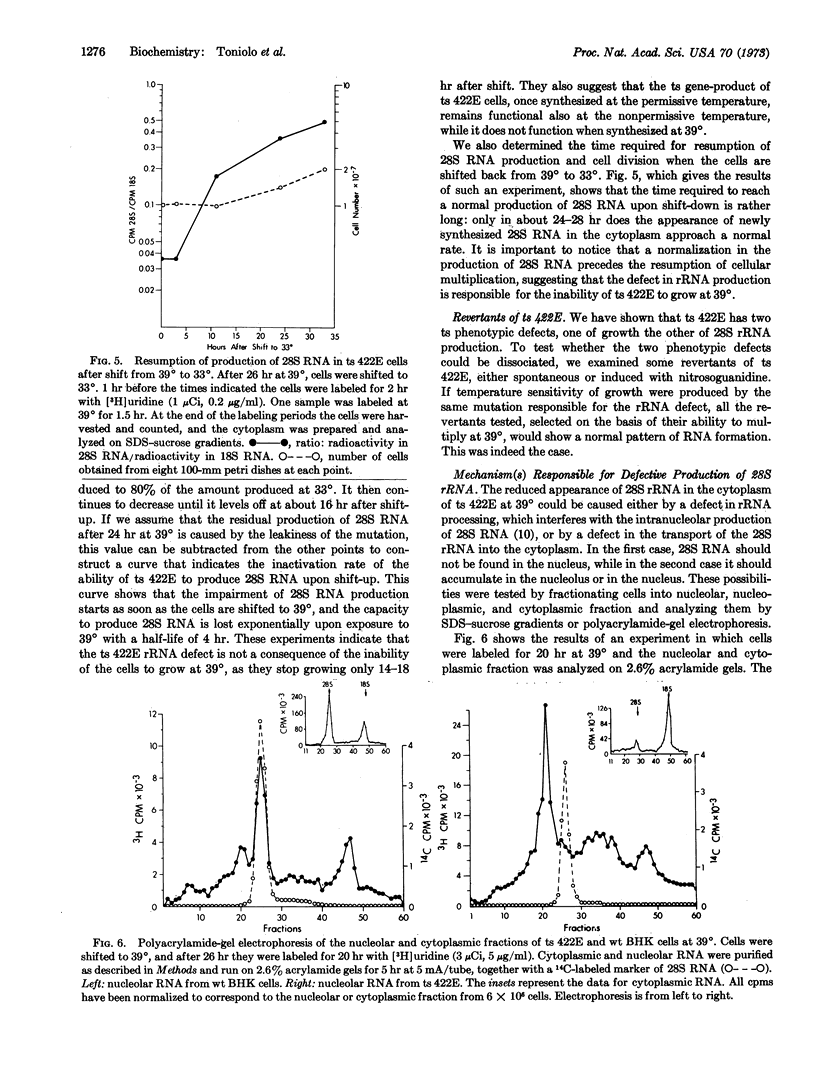
We have characterized a temperature-sensitive (ts) mutant of the hamster cell line BHK 21 that appears to have a defect in the processing of ribosomal RNA precursors at 39°. Mutant ts 422E grows at a normal rate at 33°, but upon shift to 39° growth stops after about one cell doubling. The appearance of 28S rRNA and large ribosomal subunits in the cytoplasm of ts 422E at 39° is inhibited by about 95%, when compared to wild-type BHK cells. Production of 18S rRNA and small ribosomal subunits is unaffected. Shift-up experiments show that the defect in 28S rRNA production can be detected as early as 2-3 hr after the shift to 39°. Synthesis of the larger rRNA precursor is normal at high temperature, but the processing appears to be arrested after the formation of 32S rRNA. 32S rRNA accumulates to some extent in the nucleoli of ts 422E. ts 422E cells appear to have a single mutation, directly affecting the conversion of 32S to 28S rRNA. The reduced amount of 28S rRNA in the cytoplasm of ts 422E cells at 39° seems therefore responsible for their inability to grow at this temperature.
Keywords: somatic cell genetics, BHK 21 hamster cells, rRNA processing
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Amaldi F. Structure and synthesis of ribosomal RNA. Annu Rev Biochem. 1970;39:183–226. doi: 10.1146/annurev.bi.39.070170.001151. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Meiss H. K., Basilico C. Temperature sensitive mutants of BHK 21 cells. Nat New Biol. 1972 Sep 20;239(90):66–68. doi: 10.1038/newbio239066a0. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Penman S., Vesco C., Weinberg R., Zylber E. The RNA metabolism of nucleoli and mitochondria in mammalian cells. Cold Spring Harb Symp Quant Biol. 1969;34:535–546. doi: 10.1101/sqb.1969.034.01.061. [DOI] [PubMed] [Google Scholar]
- Perry R. P. THE CELLULAR SITES OF SYNTHESIS OF RIBOSOMAL AND 4S RNA. Proc Natl Acad Sci U S A. 1962 Dec;48(12):2179–2186. doi: 10.1073/pnas.48.12.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Vesco C., Penman S. The fractionation of nuclei and the integrity of purified nucleoli in HeLa cells. Biochim Biophys Acta. 1968 Nov 20;169(1):188–195. doi: 10.1016/0005-2787(68)90019-1. [DOI] [PubMed] [Google Scholar]
- Warner J. R. The assembly of ribosomes in HeLa cells. J Mol Biol. 1966 Aug;19(2):383–398. doi: 10.1016/s0022-2836(66)80012-8. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Udem S. A. Temperature sensitive mutations affecting ribosome synthesis in Saccharomyces cerevisiae. J Mol Biol. 1972 Mar 28;65(2):243–257. doi: 10.1016/0022-2836(72)90280-x. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Kumar A., Warner J. R. Ribosome formation is blocked by camptothecin, a reversible inhibitor of RNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3009–3014. doi: 10.1073/pnas.68.12.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylber E. A., Penman S. Products of RNA polymerases in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2861–2865. doi: 10.1073/pnas.68.11.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]




