Abstract
In spite of a growing literature on pharmaceuticalization, little is known about the pharmaceutical industry’s investments in research and development (R&D). Information about the drugs being developed can provide important context for existing case studies detailing the expanding – and often problematic – role of pharmaceuticals in society. To access the pharmaceutical industry’s pipeline, we constructed a database of drugs for which pharmaceutical companies reported initiating clinical trials over a five-year period (July 2006-June 2011), capturing 2,477 different drugs in 4,182 clinical trials. Comparing drugs in the pipeline that target diseases in high-income and low-income countries, we found that the number of drugs for diseases prevalent in high-income countries was 3.46 times higher than drugs for diseases prevalent in low-income countries. We also found that the plurality of drugs in the pipeline were being developed to treat cancers (26.2%). Interpreting our findings through the lens of pharmaceuticalization, we illustrate how investigating the entire drug development pipeline provides important information about patterns of pharmaceuticalization that are invisible when only marketed drugs are considered.
Keywords: Pharmaceuticals, clinical trials, R&D, pharmaceuticalization, cancer, mental illness, HIV/AIDS, global burden of disease
Introduction
Within discourses about research and development (R&D), the pharmaceutical industry often represents the process as a pipeline, and a leaky one at that. In these depictions, clinical development - the part of R&D in which investigational drugs are tested on humans - is divided into three phases with some drugs falling out of the pipe at each step as they move toward market approval. Phase I studies primarily rely on healthy volunteers to establish safety profiles for investigational drugs and to help establish appropriate doses that can be given to patients in subsequent clinical trials. A “failed” drug at this stage would be one that produces high rates of serious adverse events (i.e., side effects) in participants. Phase II trials enroll a small number of patients with the target illness in a proof-of-concept trial that aims to collect additional data on the safety of the investigational drug as well as preliminary evidence of its efficacy. Drugs that do not exhibit sufficient promise in treating the targeted illness or are not well tolerated by patients are likely to drop out of the pipeline at this stage. Phase III studies are large-scale clinical trials designed to show the investigational drug’s efficacy by comparing the outcomes of several hundred or more patients randomly assigned to receive the drug with a placebo and/or a competitor product. According to industry analysts, the probability that an investigational drug will transition from Phase I to Phase II is 71% and from Phase II to Phase III is 45% (DiMasi et al., 2010). Pharmaceutical companies submit applications to the U.S. Food and Drug Administration (FDA) to market approximately 64% of all drugs that enter Phase III trials (DiMasi et al., 2010) (Figure 1). Although the FDA subsequently approves 93% of all such applications, these represent only 19% of all drugs that began clinical testing (DiMasi et al., 2010). In other words, more than 80% of all investigational drugs that enter the proverbial pipeline are likely to “leak out” and never make it to market.
Figure 1.
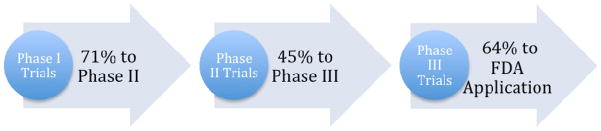
Visualization of the Pharmaceutical Pipeline
The pharmaceutical industry claims that drug development is a high-risk activity, with lengthy and expensive clinical trials on which the success or failure of their products hinge. As part of this framing, the industry lobbying group PhRMA – as well as industry-supported, academic economists – have circulated stunning estimates of costs associated with bringing new drugs to market (DiMasi et al., 2003; PhRMA, 2004, n.d.). DiMasi and colleagues (2003) estimated cost based on a sample of 68 self-originated new molecular entities (i.e., the most expensive drugs to develop) and calculated the average investment in a drug brought to market is $802 million. At the same time, consumer advocacy groups and industry critics – within and outside academia – challenge not only this projected average cost of drug development but also the therapeutic value of many new pharmaceuticals (Angell, 2004; Goozner, 2005; Light & Warburton, 2011). On this latter point, for example, Light, Lexchin, and Darrow (2013) have shown that only 8% of drugs approved by the FDA from 2002 to 2011 offer substantial therapeutic benefit for patients over existing products on the market and 15% were deemed to be more harmful than beneficial.
In spite of diverse groups’ interest in the process and politics of drug development, the pharmaceutical pipeline itself remains relatively black-boxed. In part, the pipeline metaphor works as a marketing tool for the industry, creating the impression that there is an endless supply of new and innovative products in development. In spite of the powerful imagery, there is much evidence to suggest that the number of investigational drugs is on the decline and those that make it to market offer few therapeutic breakthroughs for patients (Angell, 2004; Light & Lexchin, 2012; Light & Warburton, 2011). In addition, the pharmaceutical industry places understandably more emphasis on promoting information about marketed products than those unable to meet FDA safety and efficacy benchmarks. Within social science and biomedical communities, scholarship has also centered on marketed pharmaceuticals, analyzing physicians’ relationships with industry, direct-to-consumer advertising, and industry constructions of illness (e.g., Conrad & Leiter, 2008; Dumit, 2012; Greene, 2007; Kassirer, 2005). This literature often mobilizes the concept of “pharmaceuticalization” to signal the increasing power of the pharmaceutical industry to shape physicians’ and patients’ engagement with health and illness (Abraham, 2010; Bell & Figert, 2012; Busfield, 2010; Williams, Gabe, and Davis, 2008; Williams et al., 2011). Even when this scholarship includes examinations of clinical trials, it often does so retrospectively either for marketed pharmaceuticals or those removed from the market due to safety concerns.
Given the dearth of information about the pharmaceutical pipeline, we constructed a database of drugs for which pharmaceutical companies reported initiating clinical trials over a five-year period (July 2006-June 2011), capturing 2,477 drugs being evaluated in 4,182 clinical trials. Querying these data, we asked the following questions about drugs in the development pipeline: (1) Including Phase I, II, and III clinical trials, what therapeutic areas are targeted?; (2) To what extent does the distribution of disease categories reflect global disease burden?; and (3) What can be inferred about the pharmaceutical industry’s priorities for products they intend to market? Interpreting our findings through the lens of pharmaceuticalization, we argue that much of drug development focuses on illnesses prevalent in Western contexts, where drugs have more potential to generate significant revenue for pharmaceutical companies. We also illustrate how investigating the entire drug development pipeline provides important information about patterns of pharmaceuticalization that are invisible when only marketed drugs are considered.
Pharmaceuticalization and Drug Development
Sociological interest in the role of pharmaceuticals in medicine has emerged from a longer-standing research tradition investigating the medicalization of society (Clarke et al., 2003; Conrad, 2007). This broader area of scholarship has shown how the profession of medicine has encroached on and claimed expertise over routine aspects of life from birth to death (e.g., Howarth, 2007; Starr, 1982; Sullivan & Weitz, 1988). Similarly, scholars have shown how pharmaceuticals have extended medicalization such that aging, sex, and sleep have all become problems requiring chemical intervention (Fishman et al., 2010; Fox & Ward, 2008; Healy, 2012; Marshall, 2002; Williams, Seale, et al., 2008). Williams, Martin, and Gabe (2011) define pharmaceuticalization as “the translation or transformation of human conditions, capabilities and capacities into opportunities for pharmaceutical intervention” (711). They further note that scholars must include in their analyses of pharmaceuticalization “both upstream (macro) level processes concerning the development, testing and regulation of pharmaceuticals and downstream (micro) processes pertaining to the meaning and use of pharmaceuticals in medical practice and everyday life” (711-2). More concretely, increased pharmaceutical use can enable further medicalization, such as the expanded use of drugs developed for depression being used to treat shyness in the form of “social anxiety disorder” and monthly PMS as “premenstrual dysphoric disorder” (Greenslit, 2005; Lane, 2008). In some instances, however, pharmaceuticalization occurs outside of the purview of the medical profession. Examples include increased consumer use of over-the-counter medications and recreational use of prescription drugs for erectile dysfunction and attention deficit hyperactivity disorder (ADHD) drugs for performance enhancement (Abraham, 2010; Loe, 2008; Race, 2009).
Most of the literature frames pharmaceuticalization as a negative trend. By simply watching U.S. television or reading newspapers, it is clear why many scholars are critical. Pharmaceutical companies develop and promote some products that seem to have frivolous uses and unnerving side effects, such as drugs for thickening eyelashes or eastbound travel-induced jet lag (Pollack, 2010; Saint Louis, 2010). Even when the illnesses targeted by pharmaceuticals are relevant to significant morbidity and mortality, aggressive marketing campaigns provide ample fodder for critics to raise concerns about negative social consequences, such as the over-treatment of such conditions (e.g., Applbaum, 2009a; Hart et al., 2006). Feminist scholars have been especially critical of pharmaceutical companies’ mobilization of gender norms and stereotypes in order to market diverse products including drugs for sexual dysfunction, cervical cancer, low testosterone (Low “T”), Alzheimer’s disease, fibromyalgia, and migraines (Asberg & Lum, 2009; Barker, 2011; Casper & Carpenter, 2008; Fishman, 2004; Kempner, 2006; Watkins, 2013). Additionally, the withdrawal of “dangerous” drugs from the market raises scholarly questions about the harms that accompany pharmaceuticalization (Abraham & Davis, 2005; Prosser, 2008). Adverse drug reactions are now the fourth leading cause of death in the U.S. (Light, 2010). Most notable was Merck’s 2004 voluntary withdrawal of Vioxx® from the market when patients taking this arthritis drug experienced severe cardiac side effects, including death. This was a particularly important example of pharmaceuticalization because extensive advertising led to its over-prescription, endangering patients whose arthritis would have benefitted as much or more from over-the-counter naproxen with fewer risks (Biddle, 2007).
In reaction to this focus on the negative aspects of pharmaceuticalization, Williams, Martin, and Gabe (2011) encourage scholars to see the concept as value-neutral in order to also capture positive outcomes. Methodologically, they propose that researchers should view pharmaceuticalization as an uneven process that requires empirical investigation to determine the extent of the phenomenon in specific cases. While this approach opens up the possibility for researchers to analyze societal advantages associated with pharmaceuticalization, the recommendation of a case study approach is unlikely to yield this desired outcome. As the examples from the literature described above suggest, scholars have already primarily engaged pharmaceuticalization through case studies, generally selecting specific drugs or industry-constructions of illnesses because they provide rich examples of the pharmaceutical industry’s negative influence on society. Similarly, a case study approach has the potential to distort the overall picture of the prevalence of certain types of products, such as those often referred to as “lifestyle drugs” because of their spurious therapeutic value or their continued use by healthy individuals as part of preventive maintenance of the body (Dumit, 2012; Williams, Gabe, and Davis, 2008). In examining the literature on pharmaceuticalization, it is unclear how dominant these trends are when put in the broader context of the pharmaceutical industry’s portfolio of products currently on the market or in development.
In contrast, much of the drug development literature examines large-scale trends in the industry, but researchers rarely make explicit empirical or conceptual connections to pharmaceuticalization. For example, some scholars have documented the extent to which industry-funded research generates results from clinical trials that make the drugs look safer and more effective than do independent studies (Healy, 2004; Lexchin et al., 2003). Another area of scholarship regarding drug development has focused on the outsourcing of clinical trials to contract research organizations, private research companies, and for-profit ethics review companies, documenting dramatic changes in how clinical research is conducted both in the U.S. and around the world (Fisher, 2009; Lemmens & Freedman, 2000; Petryna, 2009). These studies have shown the complex, global networks of auxiliary companies that work with the pharmaceutical industry to help bring new drugs to market, but they usually focus on how clinical trials are organized rather than on the products being developed. A final stream of research focuses on specific clinical trials and examines the perspectives of enrolled research participants (e.g., Bishop et al., 2012; Lowton, 2005; Morris & Balmer, 2006). Having some conceptual overlap with literature on pharmaceuticalization, this scholarship engages how research participants make sense of elements of study design (like randomization and placebo use) in ways that often underscore their desire for medical treatment, especially when their access to health care is limited (Stacey et al., 2009; Timmermans & McKay, 2009).
One area of scholarship that brings together pharmaceuticalization and R&D focuses on innovation. Industry and popular media sources have raised the alarm that the pharmaceutical industry is facing an “innovation crisis” in which increasingly fewer promising products are being developed or approved by the FDA (Pammolli et al., 2011). Social scientists, however, interpret the “crisis” differently. For example, Light and Lexchin (2012) show that the decline in new molecules approved since 1996 was only a return from a spike to the long-term mean. They argue that the real innovation crisis is the low number of clinically superior drugs approved each year since the 1970s. Similarly, Applbaum (2009b) has argued that the involvement of marketing teams during drug development instead of after market approval has diminished the scientific and clinical value of R&D, resulting in a much less innovative industry. Innovation itself is a loaded term when analyzing the pharmaceutical industry. Some scholars seem to use it synonymously with R&D (e.g., Williams et al., 2011). Others rely on the FDA categorization of pharmaceuticals as “new molecular entities” as evidence of innovation (e.g., Abraham, 2010; Carpenter, 2004), but even this definition gets challenged when scholars criticize the FDA for being liberal in its determination of what drugs are novel and what drugs offer significant therapeutic advances (Angell, 2004; Davis & Abraham, 2011b).
In spite of the increasing attention to pharmaceuticals by social scientists, there nonetheless remains a void in connecting analyses of pharmaceuticalization with larger trends in R&D. Case studies about specific drugs have created engaging examples of how new pharmaceuticals shape conceptions of health and illness, but it is unclear the extent to which these types of drugs are the exception or the norm. Reading this absence in the current literature led us to ask what products are in the pharmaceutical industry pipeline, how can companies’ drug development investments be characterized, and how could this information be mobilized to provide new, accurate insights into patterns of pharmaceuticalization. We contextualize our findings by further comparing the diseases targeted by drugs in the pharmaceutical pipeline with high mortality diseases in low- versus high-income countries.
Methods
In order to capture a more complete picture of the pharmaceutical industry’s pipeline, we created a database of all industry products reported to be in Phase I, II, and III clinical trials over a five-year period (2006–2011). Phase IV or postmarketing trials were excluded from the database. The database is comprised of information on drug research and development from CenterWatch Weekly (CWW), a leading clearinghouse for industry information. CWW is a weekly, subscription-only newsletter about important trends and updates on industry clinical trials written for professionals working in the pharmaceutical and clinical trials industries. We extracted data about investigational drugs and clinical trials from the “Drug & Device Pipeline News” (DDPN) from each issue of CWW from July 3, 2006 (Volume 10, Issue 27) to June 27, 2011 (Volume 15, Issue 26). While not exhaustive, the 250 weekly reports in this selected five-year timeframe provide a detailed snapshot of the industry.
In order to target only industry-sponsored clinical trials, we opted to build our database from CWW instead of using ClinicalTrials.gov, the U.S. federal registry of publicly and privately sponsored clinical trials. Additionally, the accuracy of ClinicalTrials.gov has been questioned because of a lack of reporting standards and underreporting of trials (Heger, 2012). There are several reasons why companies may be disincentivized to report trials to governing agencies, including a desire to avoid reporting of inconclusive results and concern for increased federal scrutiny. With self-promotion and marketing the aim of CWW rather than regulatory compliance or the reporting of study results, our database presents a more streamlined version of the pipeline and may be more inclusive than those reported to ClinicalTrials.gov.
Using REDCap electronic data capture tools (Harris et al., 2009) hosted at [removed for peer-review], we extracted data, including the pharmaceutical company name, drug name, the therapeutic area designated for each drug, and the phase of the trial (I, II, III). We included all DDPN entries that reported on drugs that were in Phase I-III clinical trials, and we excluded entries that were regulatory updates (e.g., the filing of FDA applications or country-specific market approvals).
To assess the general breakdown of trials across disease categories, we assigned each trial an International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9) code based on the therapeutic area reported in the publication. ICD-9 codes were assigned with the assistance of an online resource for classification (http://www.icd9data.com). Entries not easily identifiable were researched in more detail using the web and consulting with physician colleagues, as needed. Ambiguous diseases and conditions were discussed with a second reviewer to reach consensus on the therapeutic area.
In order to compare the number of trials that target diseases in developed and developing countries, we used the World Health Organization’s (WHO) lists of the ten leading causes of death in low-income countries (LIC) and high-income countries (HIC) (WHO, 2011; for tables of these diseases, see http://www.who.int/mediacentre/factsheets/fs310/en/index1.html). We opted to focus on illnesses with high rates of mortality because measurements of disease prevalence (i.e., morbidity) are notoriously difficult to standardize, particularly across countries with widely varying economic resources (Riley 1993; WHO 2010). Some diseases are leading causes of death worldwide and appear on both lists, so we refined our categorization of these diseases into three subgroups: (1) distinct LIC diseases, (2) distinct HIC diseases, and (3) high-impact diseases. LIC diseases, thus, includes diseases found only on the list of top ten causes of death in low-income countries and excludes those found on both the HIC and LIC lists. These distinct LIC diseases include: HIV/AIDS, diarrhoael diseases, malaria, and tuberculosis. All clinical trials in our database for drugs that targeted these four diseases were coded as 1, otherwise 0, as distinct LIC diseases. Similarly, the HIC category includes only those diseases among the top ten causes of death in high-income countries that do not also appear on the LIC list. Distinct HIC diseases include: chronic obstructive pulmonary disease (COPD), Alzheimer’s disease and other dementias, colorectal cancers, trachea bronchus/lung cancers, breast cancer, hypertensive heart disease, and diabetes mellitus. All clinical trials in our database for drugs that targeted these seven types of diseases were coded as 1, otherwise 0, as distinct HIC diseases. If the DDPN did not specify a cancer type, we excluded these clinical trials or drugs from the HIC category, thereby creating a more conservative measure of trials and drugs targeting distinct HIC diseases. Our use of the term “distinct” here for both LIC and HIC diseases references the appearance of these diseases on the WHO lists of the leading causes of death in these countries, not the incidence or prevalence of these diseases around the world. Cases of these diseases can appear in both types of countries, but they are responsible for a notable share of total deaths in one type or the other.
Three causes of death appear on both HIC and LIC top ten lists and these were combined with the distinct HIC and LIC diseases to form a high-impact diseases category. Overlapping diseases include: lower respiratory infections, ischaemic heart disease, and stroke. Based on the WHO’s estimates, the 14 diseases in the combined high-impact category accounted for roughly 843 deaths per 100,000 in both high-income and low-income countries in 2011. We use the term “high-impact” to note the fact that these diseases account for a large number of deaths in both low-income and high-income countries, not that specific drugs that target these diseases necessarily have a high-impact on treatment. All clinical trials in our database for drugs that targeted a high-impact disease were coded as 1, otherwise 0, as high-impact diseases.
To illustrate our constructed categories, references to dementia, colon or rectal cancer, or hypertension in the DDPN therapeutic description meant that a clinical trial was classified as HIC and High-Impact. Trials that targeted HIV/AIDS, malaria, or tuberculosis were classified as LIC and High-Impact, while trials targeting lower respiratory infections or strokes were classified as only High-Impact. Therapeutic areas that were coded as 0 across all three categories, that is, diseases not considered distinct LIC, HIC, or High-Impact, ranged widely from gastroesophageal reflux, inflammatory bowel disease, and Parkinson’s disease to pain, asthma, and allergies. Raw numbers were calculated along with percentages of the total database and the ratio of clinical trials and drugs targeting distinct LIC therapeutic areas versus those targeting distinct HIC therapeutic areas.
The CWW also reports the companies undertaking the development of specific drugs. To assess the types of drugs, diseases targeted, and stage in the pharmaceutical pipeline that the top 20 highest-grossing companies invest in, we developed a Top 20 Company category. These companies profited from roughly 70% of the market share in worldwide prescription drug sales in 2009 (Parexel, 2011). Companies included in this category were the following: Pfizer, Merck, Novartis, Roche, Sanofi-Aventis, GlaxoSmithKline, Abbott Laboratories, Johnson & Johnson, AstraZeneca, Teva Pharmaceutical, Amgen, Bayer, Eli Lilly, Bristol Myers Squibb, Novo Nordisk, Boehringer Ingelheim, Gilead Sciences, Baxter International, Takeda, and Daiichi Sankyo. As Wyeth and Schering-Plough were acquired during the five-year period by Pfizer and Merck, respectively, these were also included in our Top 20 Company category.
Results
Our five-year snapshot of the pharmaceutical pipeline captured information on 2,477 investigational drugs in 4,182 clinical trials. The number of clinical trials is greater than the number of drugs because our database captured multiple clinical trials and different phases of testing for some drugs in the five-year time period. An initial exploration of how the drugs were distributed across therapeutic areas resulted in marked differences across ICD-9 codes (Table 1). We found that the number of drugs targeting neoplasms (i.e., cancers) as a disease category far outstripped all other therapeutic areas and represented 26.2% of the total pipeline (code #2, n=649). The next most common therapeutic areas were neurological diseases (code #6, n=334, 13.5%), infectious and parasitic diseases (code #1, n=260, 10.5%), and endocrine, metabolic, nutrition and immunity (code #3, n=234, 9.5%). After cancers, the individual diseases that were the most common targets of investigational drugs were diabetes, pain, HIV/AIDS, and hepatitis C (3.5%, 2.5%, 2.4%, and 2.4% of the pipeline, respectively) (see Table 2). In terms of the number of clinical trials reported in each phase, 1,497 were in Phase I, 1,935 in Phase II, and 750 in Phase III. Notably more clinical trials were in Phase II than Phases I or III.
Table 1.
ICD-9-CM Disease Categories and Totals for 2,477 Drugs in the Database
| ICD-9 | Therapeutic Area | Total n | % |
|---|---|---|---|
| 2 | Neoplasm | 649 | 26.20 |
| 6 | Diseases of the Nervous System and Sense Organs | 334 | 13.48 |
| 1 | Infectious and Parasitic Diseases | 260 | 10.50 |
| 3 | Endocrine, Nutritional and Metabolic Diseases, and Immunity Disorders | 234 | 9.45 |
| 8 | Diseases of the Respiratory System | 168 | 6.78 |
| 7 | Diseases of the Circulatory System | 147 | 5.93 |
| 13 | Diseases of the Musculoskeletal System and Connective Tissue | 119 | 4.80 |
| 9 | Diseases of the Digestive System | 100 | 4.04 |
| 5 | Mental Disorders | 102 | 4.12 |
| 12 | Diseases of the Skin and Subcutaneous Tissue | 98 | 3.96 |
| 10 | Diseases of the Genitourinary System | 71 | 2.87 |
| 17 | Injury and Poisoning | 63 | 2.54 |
| 4 | Diseases of the Blood and Blood Forming Organs | 57 | 2.30 |
| 16 | Symptoms, Signs, and Ill-Defined Conditions | 50 | 2.02 |
| Suppl V | Supplementary Classification of Factors Influencing Health Status and Contact with Health Services | 11 | 0.44 |
| Suppl E | Supplementary Classification of External Causes of Injury and Poisoning | 6 | 0.24 |
| 14 | Congenital Anomalies | 6 | 0.24 |
| 11 | Complications of Pregnancy, Childbirth, and the Puerperium | 1 | 0.04 |
| 15 | Certain Conditions Originating in the Perinatal Period | 1 | 0.04 |
Table 2.
Most Common Diseases Represented in the Database
| ICD-9 | Therapeutic Area | Drugs (n) | % of “Pipeline” |
|---|---|---|---|
| 2 | Cancer* | 649 | 26.2 |
| 3 | Diabetes | 87 | 3.5 |
| 6 | Pain | 62 | 2.5 |
| 1 | HIV/AIDS | 59 | 2.4 |
| 1 | Hepatitis C | 59 | 2.4 |
| 8 | Influenza | 55 | 2.2 |
| 13 | Arthritis | 48 | 1.9 |
| 6 | Alzheimer’s | 31 | 1.3 |
| 12 | Psoriasis | 29 | 1.2 |
| 8 | Asthma | 28 | 1.1 |
| 6 | MS | 27 | 1.1 |
| 3 | Diabetes-related | 27 | 1.1 |
| 6 | Parkinson’s | 26 | 1.0 |
| 8 | Allergies | 21 | 0.8 |
| 7 | Hypertension | 19 | 0.8 |
See Table 4 for distribution of clinical trials by cancer type.
Looking at the breakdown of the pipeline along targeted diseases of low-income countries (LIC) and high-income countries (HIC), Table 3 shows that 83 drugs in 121 clinical trials targeted distinct LIC diseases, with 52 in Phase I, 48 in Phase II, and 21 in Phase III (see Table 3). In comparison, 287 drugs in 569 clinical trials targeted distinct HIC diseases. Across phases, 155 HIC trials were in Phase I, 305 in Phase II, and 109 in Phase III. These findings indicate that the number of drugs in the pipeline for distinct HIC diseases outpaced those for distinct LIC diseases by 3.46 to 1. Comparing clinical trials for distinct LIC and HIC diseases, the number of trials for HIC diseases were 4.70 times that of LIC diseases (see Table 3). Because our categorization of distinct LIC and HIC diseases had an uneven number of diseases represented (i.e., 4 and 7 respectively), we also calculated a more conservative figure for the number of clinical trials related to the top four distinct HIC diseases (not shown in the table). For the top four distinct HIC diseases, 262 clinical trials were reported for 125 drugs. Using this more conservative estimate, there were 1.50 times more drugs for the top four HIC diseases in the pipeline than for the four distinct LIC diseases, and clinical trials for those HIC diseases still outpaced those for LIC diseases by 2.17 to 1.
Table 3.
Clinical Trials and Drugs that Target LIC, HIC, and High-Impact Disease Categories
| Category | # of Trials | # of Drugs | % of Total Trials |
|---|---|---|---|
| Distinct LIC* | 121 | 83 | 2.89 |
| Phase I | 52 | ||
| Phase II | 48 | ||
| Phase III | 21 | ||
| Distinct HIC** | 569 | 287 | 13.61 |
| Phase I | 155 | ||
| Phase II | 305 | ||
| Phase III | 109 | ||
| High-Impact*** | 898 | 492 | 21.47 |
| Phase I | 298 | ||
| Phase II | 446 | ||
| Phase III | 154 | ||
| Ratio of HIC to LIC | 4.70:1 | 3.46:1 |
Distinct Low- Income Country Diseases = HIV/AIDS, diarrhoael diseases, malaria, and tuberculosis
Distinct High-Income Country Diseases = chronic obstructive pulmonary disease (COPD), Alzheimer’s disease and other dementias, colorectal cancers, trachea bronchus/lung cancers, breast cancer, hypertensive heart disease, and diabetes mellitus
High-Impact = Combined total of distinct LIC, distinct HIC, plus diseases that overlap both lists of the top ten causes of death (i.e., stroke, ischemic heart disease, and lower respiratory infections, WHO 2011, see http://www.who.int/mediacentre/factsheets/fs310/en/index1.html)
Comparing drugs that target distinct LIC and HIC diseases with the total database, HIC drugs comprised 11.59% of the 2,477 total drugs in the database while LIC drugs comprised 3.35%. In terms of clinical trials, distinct HIC and LIC diseases were respectively the target of 13.61% and 2.89% of the 4,182 total clinical trials in the database. To account for the importance of diseases contributing to the leading causes of death in both LIC and HIC contexts, we calculated high-impact diseases by combining the distinct LIC and HIC diseases with the three additional diseases that are found in WHO’s lists of the top ten causes of death in both high-income and low-income countries (i.e., stroke, ischemic heart disease, and lower respiratory infections). There were 492 drugs in 898 clinical trials targeting these high-impact diseases, or 19.86% of all investigational drugs and 21.47% of clinical trials in the total database (see Table 3). Nearly 80% of all drugs thus targeted diseases of lower-impact in terms of mortality.
Looking more closely at one prevalent disease in the developing world, HIV/AIDS accounted for 1.6 million deaths worldwide in 2011 (WHO, 2011). Only 59 drugs in 88 clinical trials targeted the treatment of HIV/AIDS (2.38% of drugs in the database). Among HIV/AIDS trials, 34 were in Phase I, 39 in Phase II, and 15 in Phase III. These accounted for approximately 23% of drugs and 34% of clinical trials within the infectious and parasitic disease category of the ICD-9 (code #1).
The pipeline includes a large proportion of cancer drugs; 651 drugs (26.2%) and nearly a third of all reported clinical trials targeted cancer (n = 1,355). By examining the breakdown of types of cancers as well as clinical trial phase (shown in Table 4), interesting patterns in how pharmaceutical companies prioritize drug development emerge. Specifically, the largest percentage of oncology clinical trials (25%) included either unspecified or multiple types of cancer listed in the DDPN as the target of intervention. A major trend was that companies reported clinical trials for “solid tumors,” “malignancies,” or simply “cancer.” Examples of multiple types of cancer include clinical trials reportedly underway for “lung and gastrointestinal cancers” and “breast and ovarian cancer.” What is most striking about this trend, however, is that the vast majority of clinical trials (80.6%) for unspecified or multiple cancers were in Phase I and tapered off dramatically at Phase II (16.7%) and Phase III (2.6%).
Table 4.
Total Clinical Trials in Neoplasm/Oncology Subcategories
| Category | % of Neoplasms |
|---|---|
| Cancer - Unspecified | 25.0 |
| Hematological (e.g., leukemias and lymphomas) | 21.0 |
| Lung | 7.8 |
| Prostate | 6.9 |
| Biliary (e.g., pancreas, gall bladder, and liver cancers) | 6.7 |
| Breast | 6.3 |
| Gastrointestinal (e.g., colon, rectal, stomach, and esophageal cancers) | 5.0 |
| Skin | 4.6 |
| Female Reproduction (e.g., cervical, uterine, endometrial, and ovarian cancers) | 4.3 |
| Brain | 4.1 |
| Urinary (e.g., kidney and bladder cancers) | 3.2 |
| Head and Neck | 2.1 |
| Neuroendocrine (e.g., adrenal and thyroid cancers) | 1.7 |
| Metastases | 0.8 |
| Bone | 0.1 |
When examining specific cancers, hematological cancers, including leukemias and lymphomas, predominated in clinical trials (21%). The next most common cancer clinical trials were lung cancers; prostate cancer; biliary cancers such as liver, gall bladder, and pancreatic cancers and breast cancers (see Table 4). Other cancers that had similar percentages of clinical trials were gastrointestinal cancers, skin cancers, cancers of the female reproductive organs, brain cancers, and urinary cancers. The least represented cancers in clinical trials were head and neck cancers, neuroendocrine cancers, metastases, and bone cancers.
Only 4% of the pipeline database included drugs being developed for mental illness. We explored the distribution of these 102 drugs in 162 clinical trials because this ICD-9 category is popular in the literature on pharmaceuticalization. By further classifying mental illnesses, the following categories emerged as aggregate therapeutic areas: addiction, ADHD, anxiety/depression, autism, insomnia, psychosis, and sexual dysfunction. Clinical trials for anxiety/depression (31.5%), addiction (23.5%), and psychosis (23.5%) represented nearly 80% of all studies of drugs to treat mental illness. Of the 21 drugs under development for addiction, 12 were targeting illicit and prescription drug dependence (57.1%), 3 alcohol dependence (14.3%), and 4 nicotine dependence (19%). Additionally, one drug each was being tested for gambling addiction and binge eating. Of the remaining mental illnesses, insomnia, sexual dysfunction, and ADHD drugs made up respectively 6.8%, 6.2%, and 5.6% of the mental illness clinical trials. In the category of sexual dysfunction, two drugs were for male premature ejaculation and three were for female dysfunction. Only two drugs were under development for autism in five clinical trials (3.1%).
The database included 1,148 companies that were developing investigational drugs. Clinical trials reported by the top 20 companies totaled 414 (10% of the total sample), with 294 drugs in development (12% of the total sample). In terms of phases in the pipeline, top 20 companies reported 81 trials in Phase I, 146 in Phase II, and 187 in Phase III. This represents 5.4% of all Phase I, 7.5% of all Phase II, and 25% of all Phase III trials in the database, suggesting that top 20 companies are more likely to invest in the development of drugs at Phase III than at Phases I or II. In terms of distinct LIC and HIC diseases, 14 clinical trials in development by the top 20 companies were for drugs that targeted LIC diseases, and 53 were for drugs that targeted HIC diseases.
Looking at the development pipeline, we can see if and how conditions become established, ceasing to receive the same investment in research and development over time. Within the category of mental illness, clinical trials for drugs related to addiction increased by 200% between 2007 and 2010 (see Table 5). In contrast, clinical trials for psychosis and anxiety/depression decreased by 40% and 38%, respectively. It is possible that investment in drugs for illnesses with well-established pharmacological intervention such as psychosis (i.e., schizophrenia) and anxiety/depression are on the decline as the industry shifts its investment into addiction-drug development. Turning to drug development that targets distinct LIC diseases, trials for drugs that treat tuberculosis and malaria remain stagnant, with 0% change between 2007 and 2010, while clinical trials for HIV/AIDS-related drugs decreased from 22 trials reported in 2007 to 14 in 2010, a decline of 36%.
Table 5.
Percentage Change in Reported Number of Clinical Trials over Time
| Category | 2007 | 2008 | 2009 | 2010 | % Change* |
|---|---|---|---|---|---|
| Mental Disorders | 37 | 35 | 23 | 28 | −24% |
| Psychosis | 10 | 9 | 5 | 6 | −40% |
| Anxiety/Depression | 13 | 8 | 3 | 8 | −38% |
| Addiction | 3 | 9 | 7 | 9 | 200% |
| LIC Diseases | 29 | 29 | 20 | 21 | −28% |
| HIV/AIDS | 22 | 25 | 12 | 14 | −36% |
| Tuberculosis | 3 | 3 | 3 | 3 | 0% |
| Malaria | 2 | 0 | 2 | 2 | 0% |
Percent change comparing figures in 2007 to those reported in 2010. Figures for 2006 and 2011 are excluded because data were collected for only half of each of these years.
Discussion
Examining drugs at various stages of development in the pharmaceutical pipeline from 2006 through 2011 provides important insights into the industry’s R&D priorities. Although scholars of pharmaceuticalization have given limited attention to cancer drugs (e.g., Davis & Abraham, 2011a; Ecks, 2008), these are the biggest class of drugs under development. Much of the pharmaceuticalization literature has instead examined drugs that would be categorized within the ICD-9 code targeting mental disorders, such as anxiety, depression, insomnia, PMDD, and sexual dysfunction. We were surprised to find, however, that drugs targeting these disorders accounted for only 4% of all drugs reportedly under development. Our findings also suggest that more sociological analysis is needed of the effects of research and development, marketing, and use of pharmaceuticals designed to treat neurological diseases; infectious and parasitic diseases; and endocrine, metabolic, nutrition, and immunity disorders. In particular, given the prevalence of drugs in the database, more inquiry should be directed at the development of pharmaceuticals for the treatment of diabetes, pain, HIV/AIDS, and hepatitis C (Table 2). Without further investigation into these drugs, our understanding of pharmaceuticalization as a process remains myopic and fixated on the small portion of drugs that appear frivolous.
Much of the pharmaceuticalization literature has assumed or argued that the pharmaceutical industry’s focus for the development and promotion of new drugs is highly Western-centric (e.g, Busfield, 2003). As part of our analysis of the pipeline, we analyzed this trend by comparing the leading causes of death in low-income countries to those in high-income countries. Delving into these high-impact diseases that accounted for almost a quarter of all reported clinical trials in the database, we found that the number of drugs in the pipeline was roughly 3.5 times higher for distinct HIC diseases than distinct LIC diseases. Even when examining a smaller subset of distinct HIC diseases to all distinct LIC diseases, we found that there were 2.17 times more clinical trials for HIC diseases. This is only one possible metric for assessing the extent to which the pharmaceutical industry prioritizes Western diseases. Nonetheless, it is clear that the pharmaceutical industry’s pipeline privileges Western diseases over those of developing nations.
Turning to specific therapeutic areas, we focused on HIV/AIDS, cancer, and mental illness. We elected to focus on HIV/AIDS and cancer because these were common areas of drug development as well as of particular interest to social scientists, especially in terms of social movements (e.g, Epstein, 1996; King, 2006). With respect to HIV/AIDS, it was one of the more common target diseases for drugs in the pipeline as a whole. After all categorizations of cancers, it was the third most prevalent type of drug. However, putting this in perspective of the entire pipeline, the 59 HIV/AIDS drugs in development represented only 2.4% of all drugs. Moreover, we were surprised to find evidence of possible disinvestment in HIV/AIDS drugs, with the number of reported clinical trials declining by 36% from 2007 to 2010. More attention to the implications of this trend for pharmaceuticalization is needed, especially when considering the market potential of these drugs given the dramatic drop in AIDS mortality rates in Western countries and HIV-positive patients’ dependence on antiretrovirals to remain healthy (Maskovsky, 2005).
Making up more than a quarter of all drugs and a third of all clinical trials, pharmaceuticals targeting cancers were the most common products in the pipeline. Our most striking finding was the number of drugs and clinical trials that either listed multiple types of cancer as the target illness or did not specify any particular cancer at all. Our analyses of cancer types by clinical trial phase provided some explanation to this pattern of investment in generalized cancer therapies. More than 80% of these non-specific cases occurred in Phase I, indicating that pharmaceutical companies may primarily focus on testing the safety and tolerability of these drugs before defining the targeted types of cancer. Nonetheless, this finding provokes a question about how pharmaceutical companies decide which types of cancer become the object of Phase II (and subsequently Phase III) trials. Using the lens of pharmaceuticalization, an answer could come from future investigations into the competing roles of science, markets, and advocacy groups.
In analyzing the pipeline for mental illness drugs, our goal was to situate much of the literature on pharmaceuticalization in the broader context of industry R&D. Psychotropic pharmaceuticals make up only a small percentage of investigational drugs (4%). We found that the majority of mental illness clinical trials targeted anxiety and depression, but that investment in these studies appears to be on the decline given a nearly 40% drop in a four-year period (2007 – 2010). Likewise, the number of drugs was quite limited for contested disorders like sexual dysfunction, ADHD, and insomnia in spite of significant social scientific interest in the emergence of these as medical conditions. On the rise, however, were clinical trials for drugs related to addiction, which experienced a 200% increase in the same time period. Pharmaceuticalization scholarship should account for this rapidly developing area of industry R&D, especially given that this trend appears to be a renewed interest in old drugs (Campbell & Lovell, 2012).
Finally, we analyzed the pipeline according to the size of the pharmaceutical company to determine the share of investigational drugs being developed by the top 20 highest-grossing companies in the world. Although the percentages of drugs in the pipeline for these 20 companies closely reflected the distribution of drugs by therapeutic area of the entire dataset, the overall number of drugs was quite small (approximately 12% of the pipeline). We also found that these large firms were more likely to be involved with products targeting HIC diseases as well as those in Phase III clinical trials. These patterns support industry critics’ perspective that the largest pharmaceutical companies are primarily engaged in less financially risky R&D and focused on Western markets (Angell, 2004). Additional research, however, is needed on collaborations between larger and smaller firms in order to see how and when these product handoffs occur. A key area for studies of pharmaceuticalization should be additional investigations into drugs being developed by small companies that show initial promise in Phase II but fail to move to Phase III because larger companies are not interested in investing in these pharmaceuticals.
Limitations
The results reported here rely on industry-reported data that may or may not fully capture the true scope of companies’ investment in drug development across Phases I - III. Previous research in pharmaceuticalization has focused on drugs already on the market rather than industry’s R&D investment prior to approval. Although the five-year period covered in the data includes 2,477 investigational drugs in 4,182 clinical trials, it provides only an initial snapshot of drug development and cannot fully address long-term trends in the industry. While we believe that this database has distinct advantages over alternatives like ClinicalTrials.gov, it is clear that its self-reported nature limits our ability to draw firm conclusions. Future work on the pharmaceutical pipeline should address these limitations by seeking out more exhaustive data sources on drug development prior to market approval that cover longer periods of time. A second limitation of the current study is our reliance on measures of mortality rather than morbidity. While our decision to use the WHO’s top ten causes of death is based on the difficulties in measuring disease prevalence in low-income countries, future analysis should examine the extent to which drug development may target diseases based on prevalence rather than those that have a high-impact on population mortality.
Conclusion
We constructed a database of drug development reported over a five-year period as a window into the pharmaceutical industry’s R&D priorities. The industry’s investment in “innovation” has been both in research and marketing to meet its need for ever-expanding markets and increasingly profitable new drugs (Angell, 2004; Greene, 2007). With the pharmaceuticalization literature emphasizing disease-mongering and the “transformation of human conditions” across a spectrum of established and newly redefined conditions (Williams et al., 2011: 711), we wanted our database to help establish a context for these case studies. Abraham (2010) previously noted that “sociological debate about pharmaceuticalization and medicalization revolves almost exclusively around psycho-social or ‘lifestyle’ areas of medicine and associated pharmaceuticals to treat sexual activity, sleep disorders, social anxiety, hyperactivity, attention difficulties and depression” (605). Like Abraham, we share the concern that the centrality of these areas within the social sciences reflects the Western-centrism of both the industry and scholarship and simultaneously narrows our understanding of broader trends and processes.
Our findings confirm some of the major critiques made of the pharmaceutical industry, but they also indicate that, taken as a whole, pharmaceuticalization case studies published to date might exaggerate the industry’s investment in drugs for spurious diseases. For example, the Western influences that Busfield (2003) observes in the pharmaceutical industry overall and that seem to be borne out in our data suggest that the ratio of gains and losses varies based on global positioning. Etkin (1992) argues that “too many varieties of pharmaceuticals have been exported to the Third World. The specific categories of drugs selected for export have reflected less the local epidemiologic patterns than they have diseases prevalent in their countries of origin” (99). Moreover, groups like Doctors without Borders claim that the fundamental problem for the developing world is not the development of new drugs but reliable access to essential drugs (Pecoul et al., 1999). Perhaps as Williams, Martin, and Gabe (2011) claim, pharmaceuticalization as a conceptual process is value-neutral, but the implementation of that process through resource allocation in the drug development pipeline privileges drug treatments that target profitable Western illnesses over those in non-Western parts of the world.
Our analysis of the pipeline also underscores the important sociological work still to be done on the plethora of “legitimate,” high-impact diseases that pharmaceutical companies are targeting with investigational drugs. This is especially important when much evidence suggests that the newest pharmaceuticals do not offer much therapeutic value in the treatment of these conditions (Light, Lexchin, and Darrow, 2013). The field needs in-depth case studies of pharmaceuticals being developed to treat cancer, HIV/AIDS, hepatitis C, diabetes, and pain to explore how the development of these products can add new insights into processes of pharmaceuticalization underway in Western societies. Recognizing the prevalence of particular pharmaceuticals in the pipeline can direct scholarship towards the politics of research in important therapeutic areas where less is known about how drugs are marketed to and used by consumers.
Research Highlights.
3.46 times more drugs being developed target diseases of high-income countries.
The majority of pharmaceuticals being developed are cancer treatments.
Only 4% of the pharmaceuticals being developed are for mental illness.
The 20 biggest pharmaceutical companies are developing only 12% of all drugs.
Acknowledgments
The research was supported in part by the U.S. National Institutes of Health, National Cancer Institute (grant 5R21CA131880) with additional institutional support for REDCap provided by the NIH National Center for Research Resources (grant 1UL1RR024975). Additionally, we thank colleagues Jeffrey Bishop and Noelle Robertson for their help in classifying diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Abraham J. Pharmaceuticalization of society in context: Theoretical, empirical and health dimensions. Sociology. 2010;44:603–622. [Google Scholar]
- Abraham J, Davis C. A comparative analysis of drug safety withdrawals in the UK and the US (1971–1992): Implications for current regulatory thinking and policy. Social Science and Medicine. 2005;61:881–892. doi: 10.1016/j.socscimed.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Angell M. The truth about the drug companies: How they deceive us and what to do about it. New York: Random House; 2004. [Google Scholar]
- Applbaum K. Getting to yes: Corporate power and the creation of a psychopharmaceutical blockbuster. Culture, Medicine and Psychiatry. 2009a;33:185–215. doi: 10.1007/s11013-009-9129-3. [DOI] [PubMed] [Google Scholar]
- Applbaum K. Is marketing the enemy of pharmaceutical innovation? Hastings Center Report. 2009b;39:13–17. doi: 10.1353/hcr.0.0157. [DOI] [PubMed] [Google Scholar]
- Asberg C, Lum J. PharmAD-ventures: A feminist analysis of the pharmacological imaginary of Alzheimer’s disease. Body & Society. 2009;15:95–117. [Google Scholar]
- Barker KK. Listening to Lyrica: Contested illnesses and pharmaceutical determinism. Social Science and Medicine. 2011;73:833–842. doi: 10.1016/j.socscimed.2011.05.055. [DOI] [PubMed] [Google Scholar]
- Bell SE, Figert AE. Medicalization and pharmaceuticalization at the intersections: Looking backward, sideways and forward. Social Science and Medicine. 2012;75:775–783. doi: 10.1016/j.socscimed.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Biddle J. Lessons from the Vioxx debacle: What the privatization of science can teach us about social epistemology. Social Epistemology. 2007;21:21–39. [Google Scholar]
- Bishop FL, Jacobson EE, Shaw JR, Kaptchuk TJ. Scientific tools, fake treatments, or triggers for psychological healing: How clinical trial participants conceptualise placebos. Social Science and Medicine. 2012;74:767–774. doi: 10.1016/j.socscimed.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busfield J. Globalization and the pharmaceutical industry revisited. International Journal of Health Services. 2003;33:581–605. doi: 10.2190/262X-56RG-M4T6-1GU5. [DOI] [PubMed] [Google Scholar]
- Busfield J. ‘A pill for every ill’: Explaining the expansion in medicine use. Social Science and Medicine. 2010;70:934–941. doi: 10.1016/j.socscimed.2009.10.068. [DOI] [PubMed] [Google Scholar]
- Campbell ND, Lovell AM. The history of the development of buprenorphine as an addiction therapeutic. Annals of the New York Academy of Sciences. 2012;1248:124–139. doi: 10.1111/j.1749-6632.2011.06352.x. [DOI] [PubMed] [Google Scholar]
- Carpenter DP. The political economy of FDA drug review: Processing, politics, and lessons for policy. Health Affairs. 2004;23:52–63. doi: 10.1377/hlthaff.23.1.52. [DOI] [PubMed] [Google Scholar]
- Casper MJ, Carpenter LM. Sex, drugs, and politics: The HPV vaccine for cervical cancer. Sociology of Health and Illness. 2008;30:886–899. doi: 10.1111/j.1467-9566.2008.01100.x. [DOI] [PubMed] [Google Scholar]
- Clarke AE, Shim JK, Mamo L, Fosket JR, Fishman JR. Biomedicalization: Technoscientific transformations of health, illness, and U.S. biomedicine. American Sociological Review. 2003;68:161–194. [Google Scholar]
- Conrad P. The medicalization of society: On the transformation of human conditions into treatable disorders. Baltimore: Johns Hopkins University Press; 2007. [Google Scholar]
- Conrad P, Leiter V. From Lydia Pinkham to Queen Levitra: Direct-to-consumer advertising and medicalisation. Sociology of Health and Illness. 2008;30:825–838. doi: 10.1111/j.1467-9566.2008.01092.x. [DOI] [PubMed] [Google Scholar]
- Davis C, Abraham J. Desperately seeking cancer drugs: Explaining the emergence and outcomes of accelerated pharmaceutical regulation. Sociology of Health and Illness. 2011a;33:731–747. doi: 10.1111/j.1467-9566.2010.01310.x. [DOI] [PubMed] [Google Scholar]
- Davis C, Abraham J. Rethinking innovation accounting in pharmaceutical regulation: A case study in the deconstruction of therapeutic advance and therapeutic breakthrough. Science, Technology & Human Values. 2011b;36:791–815. [Google Scholar]
- DiMasi JA, Feldman L, Seckler A, Wilson A. Trends in risks associated with new drug development: Success rates for investigational drugs. Clinical Pharmacology and Therapeutics. 2010;87:272–277. doi: 10.1038/clpt.2009.295. [DOI] [PubMed] [Google Scholar]
- DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: New estimates of drug development costs. Journal of Health Economics. 2003;22:151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- Dumit J. Drugs for life: How pharmaceutical companies define our health. Durham: Duke University Press; 2012. [Google Scholar]
- Ecks S. Global pharmaceutical markets and corporate citizenship: The case of Novartis’ anti-cancer drug Glivec. BioSocieties. 2008;3:165–181. [Google Scholar]
- Epstein S. Impure science: AIDS, activism, and the politics of knowledge. Berkeley: University of California Press; 1996. [PubMed] [Google Scholar]
- Etkin NL. “Side effects”: Cultural constructions and reinterpretations of Western pharmaceuticals. Medical Anthropology Quarterly. 1992;6:99–113. [Google Scholar]
- Fisher JA. Medical research for hire: The political economy of pharmaceutical clinical trials. New Brunswick: Rutgers University Press; 2009. [Google Scholar]
- Fishman JR. Manufacturing desire: The commodification of female sexual dysfunction. Social Studies of Science. 2004;34:187–218. doi: 10.1177/0306312704043028. [DOI] [PubMed] [Google Scholar]
- Fishman JR, Settersten RA, Jr, Flatt MA. In the vanguard of biomedicine? The curious and contradictory case of anti-ageing medicine. Sociology of Health and Illness. 2010;32:197–210. doi: 10.1111/j.1467-9566.2009.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NJ, Ward KJ. Pharma in the bedroom … And the kitchen. … The pharmaceuticalisation of daily life. Sociology of Health and Illness. 2008;30:856–868. doi: 10.1111/j.1467-9566.2008.01114.x. [DOI] [PubMed] [Google Scholar]
- Goozner M. The $800 million pill: The truth behind the cost of new drugs. Berkeley: University of California Press; 2005. [Google Scholar]
- Greene JA. Prescribing by numbers: Drugs and the definition of disease. Baltimore: Johns Hopkins University Press; 2007. [Google Scholar]
- Greenslit N. Depression and consumption: Psychopharmaceuticals, branding, and new identity practices. Culture, Medicine, and Psychiatry. 2005;29:477–501. doi: 10.1007/s11013-006-9005-3. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart N, Grand N, Riley K. Making the grade: The gender gap, ADHD, and the medicalization of boyhood. In: Rosenfeld D, Faircloth CA, editors. Medicalized masculinities. Philadelphia: Temple University Press; 2006. pp. 132–164. [Google Scholar]
- Healy D. Let them eat Prozac: The unhealthy relationship between the pharmaceutical industry and depression. New York: New York University Press; 2004. [Google Scholar]
- Healy D. Pharmageddon. Berkeley: University of California Press; 2012. [Google Scholar]
- Heger M. Clinical trials website struggles to serve as research data hub. Nature Medicine. 2012;18:837. doi: 10.1038/nm0612-837. [DOI] [PubMed] [Google Scholar]
- Howarth G. Death and dying: A sociological introduction. Cambridge, UK: Polity; 2007. [Google Scholar]
- Kassirer JP. On the take: How America’s complicity with big business can endanger your health. New York: Oxford University Press; 2005. [Google Scholar]
- Kempner J. Gendering the migraine market: Do representations of illness matter? Social Science and Medicine. 2006;63:1986–1997. doi: 10.1016/j.socscimed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- King S. Pink ribbons, Inc. Breast cancer and the politics of philanthropy. U of Minnesota Press; 2006. [Google Scholar]
- Lane C. Shyness: How normal behavior became a sickness. New Haven: Yale University Press; 2008. [Google Scholar]
- Lemmens T, Freedman B. Ethics review for sale? Conflict of interest and commercial research review boards. Milbank Quarterly. 2000;78:547–584. doi: 10.1111/1468-0009.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexchin J, Bero L, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: Systematic review. British Medical Journal. 2003;326:1167–1170. doi: 10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light D, Lexchin J. Pharmaceutical R&D: What do we get for all that money? British Medical Journal. 2012;345:e4348. doi: 10.1136/bmj.e4348. [DOI] [PubMed] [Google Scholar]
- Light DW, editor. The risks of prescription drugs. New York: Columbia University Press; 2010. [Google Scholar]
- Light DW, Lexchin J, Darrow JJ. Institutional corruption of pharmaceuticals and the myth of safe and effective drugs. Journal of Law, Medicine & Ethics. 2013;41:590–600. doi: 10.1111/jlme.12068. [DOI] [PubMed] [Google Scholar]
- Light DW, Warburton R. Demythologizing the high costs of pharmaceutical research. BioSocieties. 2011;6:34–50. [Google Scholar]
- Loe M. The prescription of a new generation. Contexts. 2008;7:46–49. [Google Scholar]
- Lowton K. Trials and tribulations: Understanding motivations for clinical research participation amongst adults with cystic fibrosis. Social Science and Medicine. 2005;61:1854–1865. doi: 10.1016/j.socscimed.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Marshall BL. “Hard science”: Gendered constructions of sexual dysfunction in the “Viagra age”. Sexualities. 2002;5:131–158. [Google Scholar]
- Maskovsky J. Do people fail drugs, or do drugs fail people?: The discourse of adherence. Transforming Anthropology. 2005;13:136–142. [Google Scholar]
- Morris N, Balmer B. Volunteer human subjects’ understandings of their participation in a biomedical research experiment. Social Science and Medicine. 2006;62:998–1008. doi: 10.1016/j.socscimed.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Pammolli F, Magazzini L, Riccaboni M. The productivity crisis in pharmaceutical R&D. Nature reviews Drug discovery. 2011;10:428–438. doi: 10.1038/nrd3405. [DOI] [PubMed] [Google Scholar]
- Parexel . Pharmaceutical R&D statistical sourcebook 2011/2012. Waltham, MA: Parexel International Corporation; 2011. [Google Scholar]
- Pecoul B, Chirac P, Trouiller P, Pinel J. Access to essential drugs in poor countries: A lost battle? JAMA. 1999;281:361–367. doi: 10.1001/jama.281.4.361. [DOI] [PubMed] [Google Scholar]
- Petryna A. When experiments travel: Clinical trials and the global search for human subjects. Princeton: Princeton University Press; 2009. [Google Scholar]
- PhRMA. Response to Marcia Angell. 2004. Sep 15, [Google Scholar]
- PhRMA; PhRMA, editor. What goes into the cost of prescription drugs? …And other questions about your medicines. Washington, D.C: PhRMA; n.d. [Google Scholar]
- Pollack A. A drug’s second act: Battling jet lag. New York Times; New York: 2010. [Google Scholar]
- Prosser H. Marvelous medicines and dangerous drugs: The representation of prescription medicine in the UK newsprint media. Public Understanding of Science. 2008;19:52–69. doi: 10.1177/0963662508094100. [DOI] [PubMed] [Google Scholar]
- Race K. Pleasure consuming medicine: The queer politics of drugs. Durham: Duke University Press; 2009. [Google Scholar]
- Riley JC. Measuring morbidity and mortality. In: Kiple KF, editor. The Cambridge World History of Human Disease. Cambridge University Press; 1993. [Google Scholar]
- Saint Louis C. New York Times. New York: 2010. Long lashes without prescription, but with risks. [Google Scholar]
- Stacey CL, Henderson S, MacArthur KR, Dohan D. Demanding patient or demanding encounter?: A case study of a cancer clinic. Social Science and Medicine. 2009;69:729–737. doi: 10.1016/j.socscimed.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr P. The social transformation of American medicine: The rise of a sovereign profession and the making of a vast industry. New York, NY: Basic Books; 1982. [Google Scholar]
- Sullivan DA, Weitz R. Labor pains: Modern midwives and home birth. New Haven: Yale University Press; 1988. [Google Scholar]
- Timmermans S, McKay T. Clinical trials as treatment option: Bioethics and health care disparities in substance dependency. Social Science and Medicine. 2009;69:1784–1790. doi: 10.1016/j.socscimed.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Watkins ES. Testosterone and the pharmaceuticalization of male aging. In: Kampf A, Marshall BL, Petersen A, editors. Aging men, masculinities and modern medicine. New York: Routledge; 2013. [Google Scholar]
- WHO – World Health Organization. Global status report on noncommunicable diseases. Geneva: 2010. [Google Scholar]
- WHO – World Health Organization. The top 10 causes of death. 2011. [Google Scholar]
- Williams SJ, Gabe J, Davis P. The sociology of pharmaceuticals: Progress and prospects. Sociology of Health and Illness. 2008;30:813–824. doi: 10.1111/j.1467-9566.2008.01123.x. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Martin P, Gabe J. The pharmaceuticalisation of society? A framework for analysis. Sociology of Health and Illness. 2011;33:710–725. doi: 10.1111/j.1467-9566.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Seale C, Boden S, Lowe P, Steinberg DL. Waking up to sleepiness: Modafinil, the media and the pharmaceuticalisation of everyday/night life. Sociology of Health and Illness. 2008;30:839–855. doi: 10.1111/j.1467-9566.2008.01084.x. [DOI] [PubMed] [Google Scholar]


