Abstract
Hypertrophic scar contraction (HSc) is caused by granulation tissue contraction propagated by myofibroblast and fibroblast migration and contractility. Identifying the stimulants that promote migration and contractility are key to mitigating HSc. AngiotensinII (AngII) promotes migration and contractility of heart, liver, and lung fibroblasts; thus, we investigated the mechanisms of AngII in HSc. Human scar and unwounded dermis were immunostained for AngII receptors AT1-receptor and AT2-receptor, and analyzed for AT1-receptor expression using western blot. In-vitro assays of fibroblast contraction and migration under AngII stimulation were conducted with AT1-receptor, AT2-receptor, p38, JNK, MEK, and ALK5 antagonism. Excisional wounds were created on AT1-receptor KO and WT mice treated with AngII ± Losartan, and ALK5 and JNK inhibitors SB-431542 and SP-600125 respectively. Granulation tissue contraction was quantified and wounds analyzed by immunohistochemistry. AT1-receptor expression was increased in scar, but not unwounded tissue. AngII induced fibroblast contraction and migration through AT1-receptor. Cell migration was inhibited by ALK5 and JNK, but not p38 or MEK blockade. In-vivo experiments determined that absence of AT1-receptor and chemical AT1-receptor antagonism diminished granulation tissue contraction while AngII stimulated wound contraction. AngII granulation tissue contraction was diminished by ALK5 inhibition, but not JNK. AngII, promotes granulation tissue contraction through AT1-receptor and downstream canonical TGFβ signaling pathway; ALK5. Further understanding the pathogenesis of HSc as an integrated signaling mechanism could improve our approach to establishing effective therapeutic interventions.
Introduction
Disability due to hypertrophic scar contraction (HSc) following burn wounds results in approximately $80.2 billion in lost wages worldwide annually [1]. The additional impact of medical expenses, social costs, and emotional trauma due to disfigurement is immeasurable. HSc leads to contractures which are inelastic, thickened scars that fail to regress [2, 3]. These fixed lesions cause pain, deformity, profound itching, and severe disability of joints [3–5]. Contractures as little as a 10% reduction in joint motion is clinically significant [6]. Current anti-HSc therapies are ineffective [7]. Thus, there remains an urgent need to understand the pathogenesis of HSc and identify targets to prevent this disabling process.
Over the last half century, it has been established that HSc is mediated by myofibroblast and fibroblast contractility and migration. While contractility and migration are known to be caused by intracellular focal adhesion complex formation, cytoskeletal protein activation, and upregulation included but not limited to α-smooth muscle actin (ASMA) [8], vimentin [7], non-muscle myosin (NM IIA) [9], and the regulatory proteins RhoA and Rho Kinase (ROCK) [9], it is unknown, which extracellular soluble substances activate these pathways. Angiotensin II (AngII) is one soluble mediator that has been implicated in stimulating pro-fibrotic processes in heart, liver, kidney, and the lung [10–13]. There is also accumulating evidence that AngII is a key effector in promoting dermal wound healing and fibrosis [14–17].
AngII signals through two receptors: angiotensin type 1 receptor (AT1-receptor) and angiotensin type 2 receptor (AT2-receptor). It has been hypothesized that the balance between AT1-receptor vs. AT2-receptor activation determines healing versus fibrosis, but this hypothesis has not been conclusively tested [18, 19]. AngII has been linked to upregulation of TGFβ production and activation of the TGFβ signaling pathways; canonical and non-canonical. In the canonical pathway, TGFβ binds to receptor activin receptor like kinase 5 (ALK5) which activates the phosphorylation of Smad2/3. Activated Smad2/3 proteins recruit Smad4, and cause nuclear translocation of the Smad2/4 or Smad3/4 for activation of pro-contractile mRNA transcription [20]. The TGFβ non-canonical signaling pathway involves activation of mitogen activated protein kinase (MAPK) pathways including, extracellular signal-regulated kinase (ERK), Jun N-terminal kinase (JNK), and p38 [21].
Here we investigate the AngII signaling mechanism involved in dermal HSc using a combination of experiments on human tissue, human and murine cell lines, and murine granulation tissue contraction models.
Materials and Methods
Animals
All protocols were approved by Duke University Medical Center Institutional Animal Care and Use Committee. Two strains of 10–12 week -old mice weighing 18–20g were used; (1) C57BL/6J from Jackson Laboratory, and (2) AT1 KO mice from Thomas Coffman, MD, Duke.
Dermal excisional wounding
C57BL/6J mice were randomly divided into control (n=12), Losartan (Sigma Aldrich, St. Louis, MI) treated group (n=12) fed 50mg/kg/day Losartan by 0.4 ml saline gavage, and AngII (Calbiochem, San Diego, CA) treated group (n=36) implanted with sub-dermal osmotic pumps (ALZET, Cupertino, CA) to administer 2200ng/kg/min AngII dissolved in PBS. AngII group had three subgroups (n=8) each administered either ALK-inhibitor on day 3 (10mg/kg of 5mg/0.25ml SB431542 (Tocris Bioscience, Bristol, UK) in DMSO by intraperitoneal (IP) injection), JNK-inhibitor for 14 days (16mg/kg/day of SP600125 (Sigma-Aldrich) in 45% w/v 2-hydroxypropyl-B cyclodextrin IP), or equivalent amounts of PBS. All mice, including AT1 KO (n=5), were halothane-anaesthetized and 8-mm full-thickness excisional wounds created on the dorsum. The wound area was measured using gravitational planimetry and expressed as a percentage of original wound size. On days 3, 7, and 14, animals (n=5 per time point per group) were euthanized, and the wound bed was excised with a 5mm margin of unwounded skin for histological and immunohistochemical (IHC) staining.
Histology
To determine epidermal thickness, collagen deposition, and mast cell infiltration, histological analysis was performed on excised samples by staining with hematoxylin and eosin, Masson’s Trichrome, and Toluidine blue respectively.
Immunohistochemistry
Samples of human studies were obtained after receiving patient consent and approval of the Xiangya Hospital ethics committee. Human hypertrophic scar (HS) between 10 months and 1 year in maturity were sampled from 12 patients (4 female, 8 male) aged 1 to 45 years old, who had sustained thermal injury (scald, burn) to areas of joint mobility (finger, neck, knee, eyelid). HS and adjacent unwounded skin were retrieved as described by Morihara et al [14]. Samples underwent IHC detection of AT1-receptor and AT2-receptor by polyclonal rabbit anti-human (N-10, sc-1173 and H-143, sc-9046, Santa Cruz Biotechnology, Dallas, TX). Mouse specimen underwent detection of Ki-67 (rabbit anti-mouse, 1:400 dilution, Thermo scientific, Fremont, CA), and F4/80 for macrophages (rat anti-mouse, 1:1500 dilution, eBioscience, San Diego, CA). AT1-receptor, AT2-receptor, and Ki67 binding were recognized with goat anti rabbit (1:200 dilution, Vector Laboratories, Burlingame, CA). F4/80 binding was recognized with rabbit anti rat (1:200 dilution, Vector). Tissue staining was visualized using the avidin biotinylated enzyme complex system (Vectastain Elite ABC, Vector) and 3,3'-Diaminobenzidine substrate chromogen solution (Dako, Carpinteria, CA). Labeled cells were visualized with a Nikon eclipse E600 microscope and images were captured with a Nikon DXM 1200 digital camera under the same settings. Positively stained cells were counted in 5 high power fields (HPF) using ImageJ (NIH, Bethesda, MD).
Cell culture
Human cell cultures were exempt by Duke University Medical Center Institutional Review Board (DUMC IRB). HS and adjacent unwounded tissue were excised from the following patients; (1)21 year old female, 6 month old shoulder scar, (2)42 year old female, 1.5 year old chest scar, (3)39 year old female, 3 month old neck scar (4)58 year old female, 2 month old breast scar. HSF and fibroblasts from unwounded tissue samples were explanted from samples as described by Liu et al. Eight human fibroblast cell lines were established as described by Bond et al [22]. Western blot, fibroblast populated collagen lattice (FPLC), and cell migration experiments were conducted with these primary cell cultures when cells became 80–90% confluent between passages 2–6.
Stress fiber staining
Formation of filamentous actin (F-actin) in 3T3 fibroblasts was analyzed. Cells treated with AngII (0.01, 0.1, 1, and 10μM) and 5ng/mL TGFβ (Invitrogen, Carlsbad, CA) were fixed in 4% paraformaldehyde/1% gluteraldehyde/PBS, permeabalized in 0.2% Triton X-100/PBS, blocked with sodium borohydride (0.5mg/ml) in PBS, and stained with fluorescein phalloidin (0.1μg/ml, Invitrogen) in PBS.
Cell migration assays
Chemokinetic migration of fibroblasts was assayed by using modified Boyden chamber and cell scratch assays. Boyden chamber assay was performed as described by Bond et al [22] using 3T3 treated with 0.01μM AngII or 5ng/ml TGFβ and cell scratch was performed using Human Scar Fibroblasts (HSF) treated in 1μM AngII or 20ng/mL TGFβ as these substrate concentrations were found to elicit the most significant response for the respective cell types and experimental conditions. To conduct the cell scratch assay, HSF were plated onto 24-well chambers, 3 chambers per treatment group. At 80–90% confluence, a scratch was made with a plastic P20 pipet tip, chambers were rinsed, and incubated with inhibitors (ALK5-20μΜ SB431542, MEK-10μM U0126 (Cell Signaling, Danvers, MA), p38-10μM SB203580 (Cell Signaling), JNK-10μM SP600125), 10μM Losartan, or DMSO control in DMEM for 1h prior to TGFβ or AngII treatment. Images were taken of scratch area at 0h, 24h, and 48h using 4× air objective, measured using ImageJ freehand measure tool, and area of scratch closure calculated as a fraction of initial size.
FPCL
A mixture of 1.0×105 fibroblasts/mL, 1.28mg/mL purified collagen, (Nutragen, Advanced Biomatrix, Fremont, CA, USA) and 1% FBS in DMEM (growth media) was added in 400μl volumes to a 24-well chamber and incubated for 60 min to allow the collagen to polymerize before adding 500μl of 0.01μM AngII alone, or AngII and Losartan or AngII and PD123319 (Sigma-Aldrich). Lattices were incubated for 24h, sizes were recorded using a digital scanner 5h after release, and areas measured using ImageJ.
RT-PCR
3T3 fibroblasts were grown in 0.1μM AngII for 48h. Rho, ROCK, and NM IIA mRNA levels were analyzed by semi-quantitative RT-PCR using Qiagen RNeasy Universal Plus Mini Kit (Valencia, CA) and Stratagene Mx3005P qPCR system (Santa Clara, CA) per manufacturer’s protocol. Results were analyzed using MxPro QPCR software (Agilent Technologies Inc., Santa Clara, CA). Relative gene expression was calculated as a ratio to housekeeping gene, S9.
Western blot analysis
HSF and fibroblasts from unwounded human tissue were used to determine AT1-receptor and AT2-receptor expression. Primary antibodies used were rabbit anti-AT1-receptor (1μg/ml Santa Cruz Biotechnology), rabbit anti-AT2-receptor (1μg/ml LifeSpan BioSciences, Inc., Seattle, WA). 3T3 fibroblasts incubated in 0.1μM AngII for 4d prior to experiment were used to determine NM IIA, Vimentin, ASMA, and ROCK expression. Primary antibodies used were rabbit anti-NM IIA (1:1000 dilution Abcam, Cambridge, MA), goat anti-vimentin (1:200 dilution, eBioscience), rabbit anti-ASMA (1:200 dilution Abcam), rabbit anti-ROCK (1:200 dilution, Abcam). WB analysis was performed as described by Bond et al [22].
Statistics
The statistical analysis was performed with IBM Statistical Package for the Social Sciences Statistics 17 software program. All quantitative data are presented as the mean and standard error of the mean (SEM) of three independent experiments, performed in triplicate for each condition, using each of the three mice. Statistical analysis was performed by using either analysis of variance (ANOVA) or two-sided Student t test where appropriate. Difference was considered significant at p≤0.05.
Results
AT1-receptor expression is increased in scar tissue compared to unwounded tissue, AT2-receptor is not
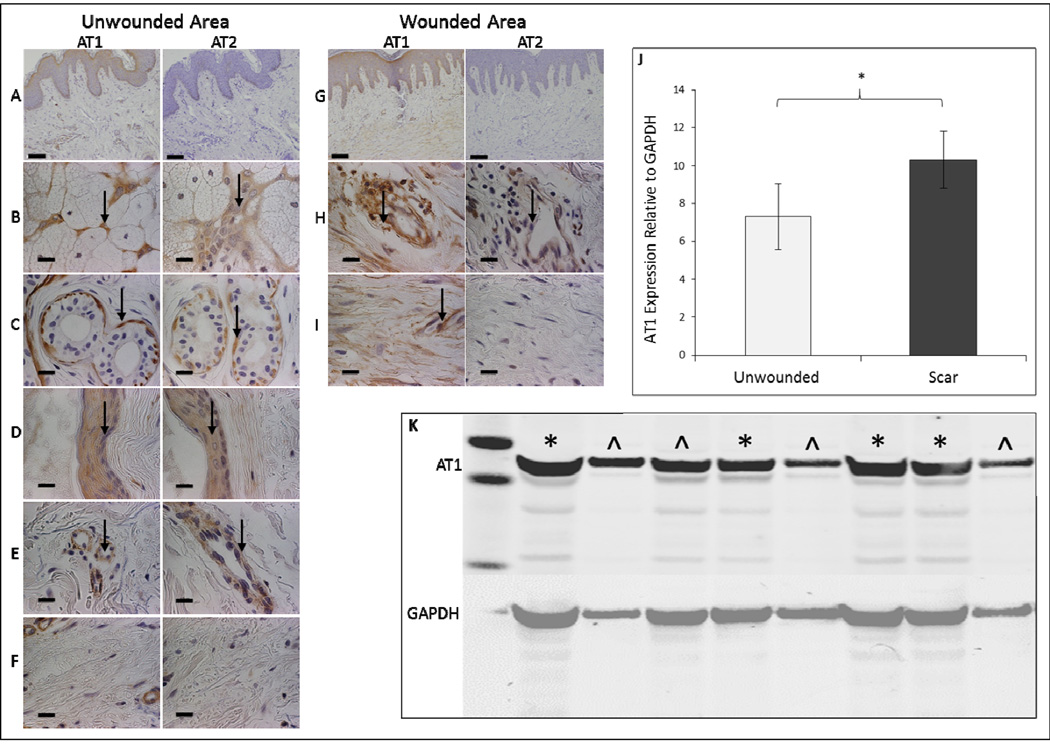
We investigated the involvement of AT1-receptor and AT2-receptor in HSc using immunohistochemical analyses of receptor expression in human HS and surrounding unwounded skin. In unwounded skin, both receptors were expressed in eccrine sweat glands (Fig. 1B), myoepithelial cells (Fig. 1C), arrector pili muscle (Fig. 1D), and vascular endothelial cells (Fig. 1E), but absent in dermal fibroblasts (Fig. 1F). However, in HS, while both AT1-receptor and AT2-receptor were expressed in vascular endothelial cells (Fig. 1H), only AT1-receptor was expressed in HSF (Fig. 1I). Western blot quantification demonstrated significantly increased expression of AT1-receptor in HSF compared to unwounded fibroblasts (p<0.05, Fig. 1J–K). Western blotting of AT2-receptor in HSF did not identify any receptor expression (not shown).
Figure 1. AT1-receptor expression is increased in human scar fibroblasts compared to unwounded dermal tissue.
Representative histological sections of human unwounded skin and wounded skin immunostained for AT1-receptor and AT2-receptor at 10X magnification (A, G – scale bars represent 200μm). Panel B-F, H-I are at 100X magnification (scale bars represent 20μm), arrows indicate positive staining. In normal dermal tissue AT1-receptor and AT2-receptor are expressed in eccrine sweat glands (B), myoepithelial cells (C), arrector pili muscle (D), and vascular endothelial cells (E), but absent in fibroblasts (F). In scar dermal tissue AT1-receptor and AT2-receptor are expressed in vascular endothelial cells (H). AT1-receptor alone is expressed in fibroblasts (I). Western blot analysis of AT1-receptor expression in fibroblasts of unwounded and wounded human samples with quantitative analysis relative to GAPDH (J) There is significantly greater AT1-receptor in fibroblasts of wounded samples *p<0.01 (J). Data shown is mean ± SEM. Statistical significance was determined by student paired t test. Western blot analysis of AT1 expression in 4 normal (^) and scar (*) fibroblast cell lines (K).
AngII stimulates expression of the contractile proteins
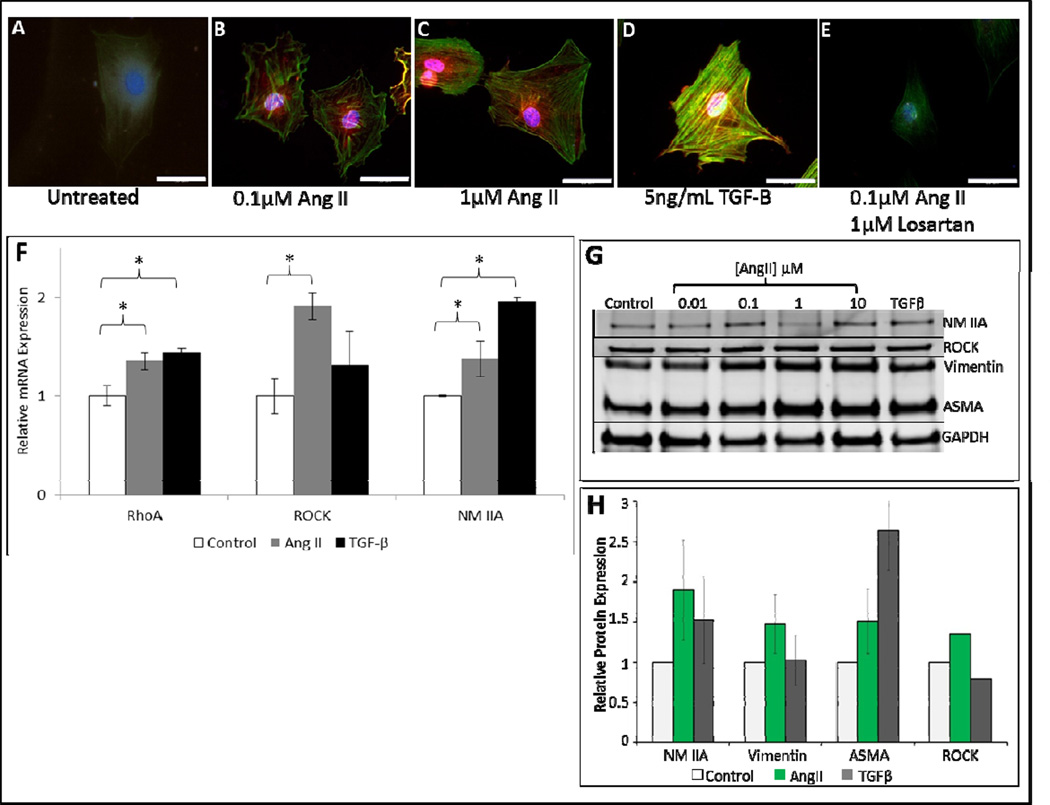
To determine the effects of AngII on the cytoskeleton, fibroblasts were stimulated with AngII and stained for F-actin expression with FITC labeled phalloidin (Fig. 2A–E). Compared to control, F-actin expression was significantly increased in AngII treated cells, and in a dose-dependent manner (Fig. 2A–C). Cells treated with 0.1μM AngII sparsely expressed F-actin (Fig. 2B), whereas, cells treated with 1μM AngII had condensed F-actin throughout the cytoplasm (Fig. 2C). As expected, the effects of AngII were abrogated by the AT1-receptor inhibitor, Losartan (Fig. 2E). AngII also increased the mRNA expression of the pro-contractile and migration mediators RhoA, ROCK, and NM IIA (Fig. 2F). While TGFβ treatment also induced expression of RhoA, ROCK, and NM IIA, in the case of ROCK, AngII was more effective. Similarly, at the protein level, AngII increased the expression of ROCK, NM IIA, vimentin and ASMA (Fig. 2G–H). These findings support a role for AngII in stimulating expression of cytoskeletal proteins responsible for migratory and contractile properties in fibroblasts.
Figure 2. Angiotensin II upregulates expression of fibroblast contractile proteins.
Representative 200× images (scale bars represent 10μm), of filamentous actin phalloidin (FITC-phalloidin) and nuclei (DAPI) staining in 3T3s incubated in DMEM alone (untreated) (A), treated with 0.1μM AngII (B), 1.0μM AngII (C), positive control 5ng/ml TGFβ (D), and 0.1μM AngII + 1μM Losartan (E). Stress fiber formation is increased in AngII treated groups relative to groups incubated in DMEM alone (untreated). Losartan blocks AngII stimulation of stress fibers. qRT-PCR of control 3T3s (untreated), 3T3s treated with 0.1μM AngII, and 3T3s treated with 5ng/ml TGFβ as positive control (n=3) for 48 hours (F). Increased mRNA expression is seen in pro-contractile proteins RhoA, ROCK, and NM IIA of cells treated with AngII. Increased mRNA expression is seen RhoA and NM IIA of cells treated with TGFβ. Western blot analysis of NM IIA, Vimentin, αSMA, and ROCK in 3T3s treated with AngII (G). Quantitative analysis of western blot data for 0.1μM AngII treated cells demonstrating statistically significant increase expression of contractile proteins relative to control (H). Data shown is mean ±SEM, *p<0.05. Statistical significance was determined by ANOVA.
AngII stimulates fibroblast migration and contraction through AT1-receptor
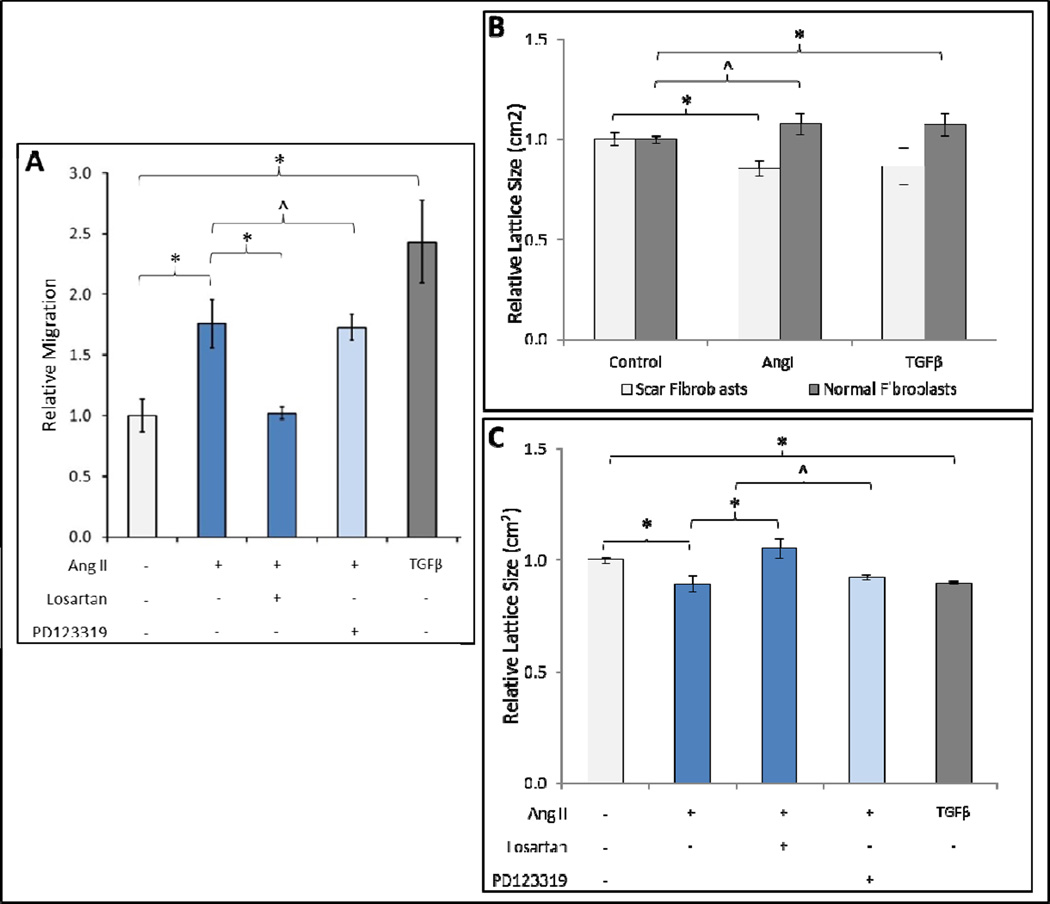
We investigated the AT1-receptor specific effects of AngII on cellular migration and contractility. AngII stimulated fibroblasts had significantly increased migration relative to untreated cells (Fig. 3A). Inhibition of AT1-receptor with Losartan significantly decreased AngII induced migration. In contrast, inhibition of AT2-receptor with PD123319 did not alter AngII induced migration. We found similar results regarding fibroblast contraction wherein AngII induced FPCL contraction was increased in scar fibroblasts (Fig. 3B), an effect that was significantly diminished by AT1-receptor blockade (Fig. 3C), but not AT2-receptor blockade. Taken together, these results suggest that AngII stimulates scar fibroblast migration and contraction through AT1-receptor.
Figure 3. Angiotensin II induces fibroblast migration and contraction through AT1-receptor.
Boyden chamber assay demonstrating increased migration in 3T3 fibroblasts incubated in DMEM with 0.01μM AngII for 4 days prior to experiment relative to control (A). AT1-receptor inhibition with Losartan decreases AngII stimulated migration whereas AT2-receptor inhibition with PD123319 does not. Untreated control group was incubated in DMEM alone; TGFβ (5ng/mL) is positive control. Data are mean ± SEM n=6. *p<0.05; ^p>0.05. HSF populated collagen lattices (FPCL) incubated for 4 days with 0.01μM AngII in 1% FBS demonstrate significantly decreased lattice size compared to untreated control group (1% FBS alone) 5 hours after lattice release (B). AngII treated normal fibroblast collagen lattices demonstrate no significant change in lattice size compared to the untreated group. Data are mean ± SEM n=9. ANOVA scar fibroblast *p<0.05, normal fibroblasts ^p>0.05. Two way ANOVA, p<0.05. AT1-receptor inhibition with Losartan decreases 0.01μM AngII stimulated contraction (C) whereas AT2-receptor inhibition with PD123319 does not. Data are mean ±SEM n=6. *p<0.05; ^p>0.05.
AngII stimulates fibroblast migration through ALK5- and JNK-mediated signaling
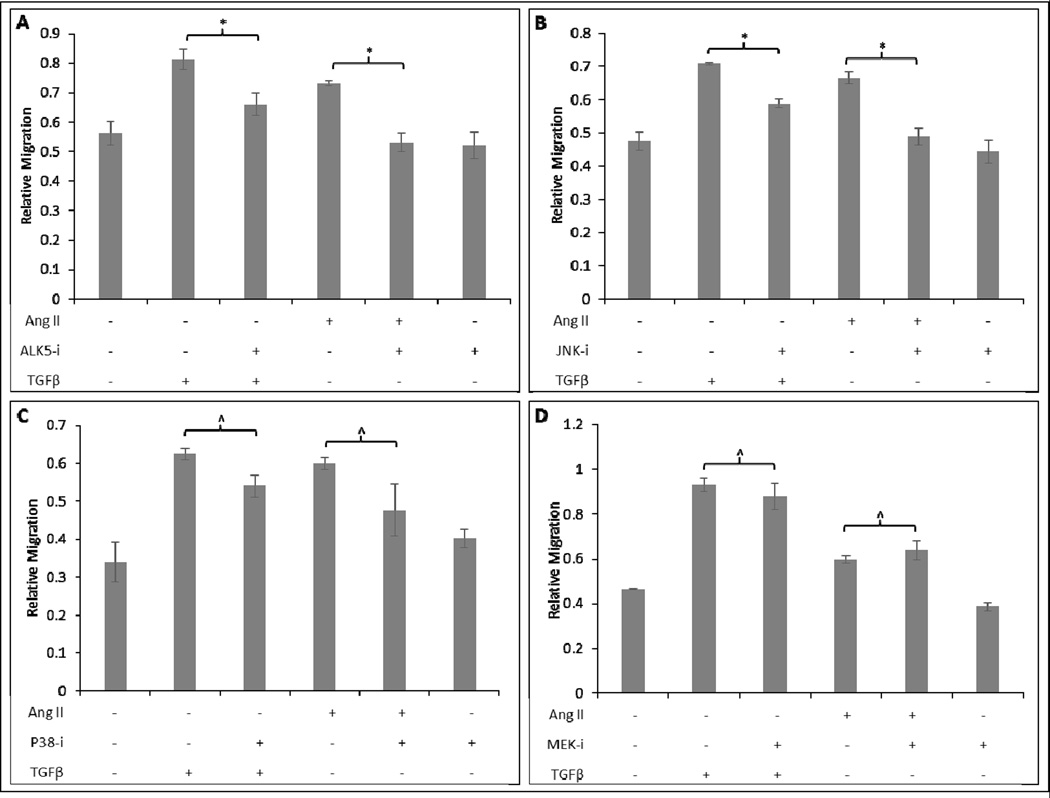
To gain insight into how AngII stimulates fibroblast migration, we investigated the role of the canonical and non-canonical TGFβ signaling pathways using small molecule inhibitors. At 48h after AngII or TGFβ stimulation, ALK5 or JNK inhibition resulted in a statistically significant decrease in migration by 24% (*p<0.02) and 14% (*p<0.007) respectively (Fig. 4A–B). p38 or MEK inhibition yielded 23% (p<0.2) and 14% (p<0.42) decrease in migration but these were not statistically significant effects (Fig. 4C–D). These results suggest that the TGFβ receptor, ALK5, and TGFβ non-canonical signaling mediator, JNK, are involved in AngII induced cellular migration.
Figure 4. AngII stimulated fibroblast migration is diminished by Alk5 and JNK inhibition, but not p38 or MEK inhibition.
Human scar fibroblasts were pretreated for 1h with inhibitor followed by 1μM AngII or 20ng/mL TGF-β. Cells pretreated with ALK5 inhibitor (SB-431542) and stimulated with TGFβ or AngII exhibited significantly diminished migration compared to cells treated with TGFβ or AngII alone (A). Cells pretreated with 10μM JNK inhibitor (SP-600125) and stimulated with TGFβ or AngII exhibited significantly diminished migration compared to cells treated with TGFβ or AngII alone (B). Cells pretreated with 10μM p38 inhibitor (SB-203580) and stimulated with TGFβ or AngII or did not exhibit significantly diminished migration compared to cells treated with TGFβ or AngII alone (C). Cells pretreated with 10μM MEK inhibitor (SB-203580) and stimulated with TGFβ or AngII did not exhibit significantly diminished migration compared to cells treated with TGFβ or AngII alone (D) *p<0.05; ^p>0.05. Note that AngII and TGβ treated fibroblasts had increased migration relative to control in panels A–D (bars indicating significance not shown)
AngII stimulates wound contraction through AT1-receptor using an ALK5-mediated mechanism
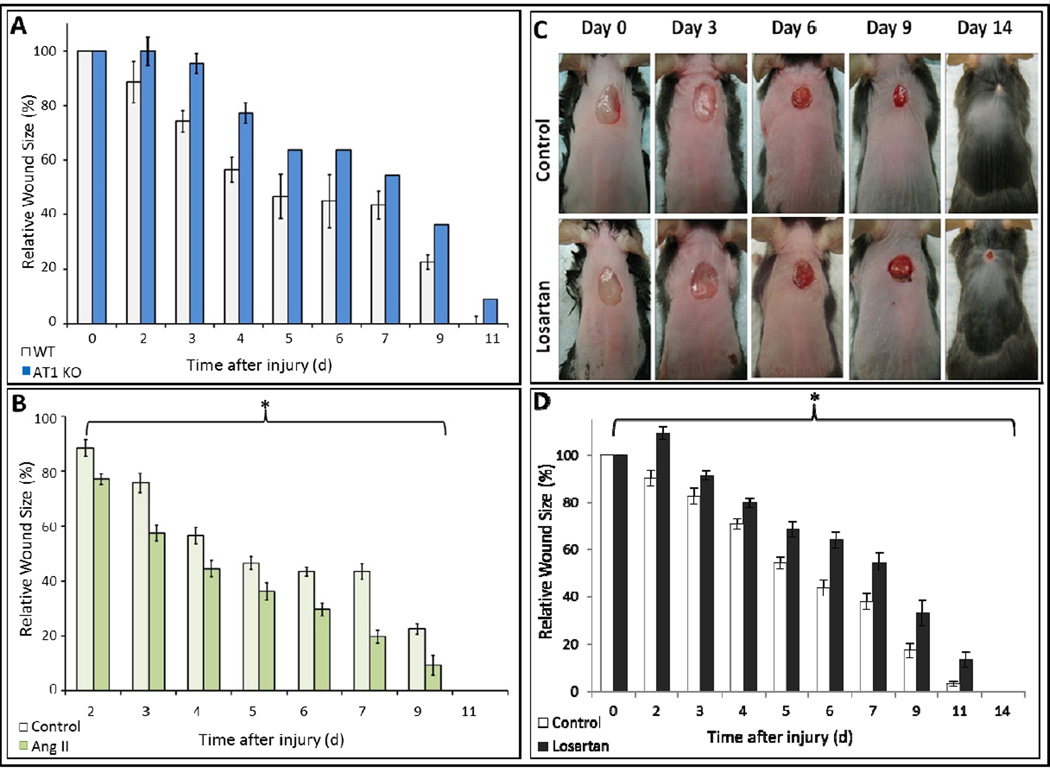
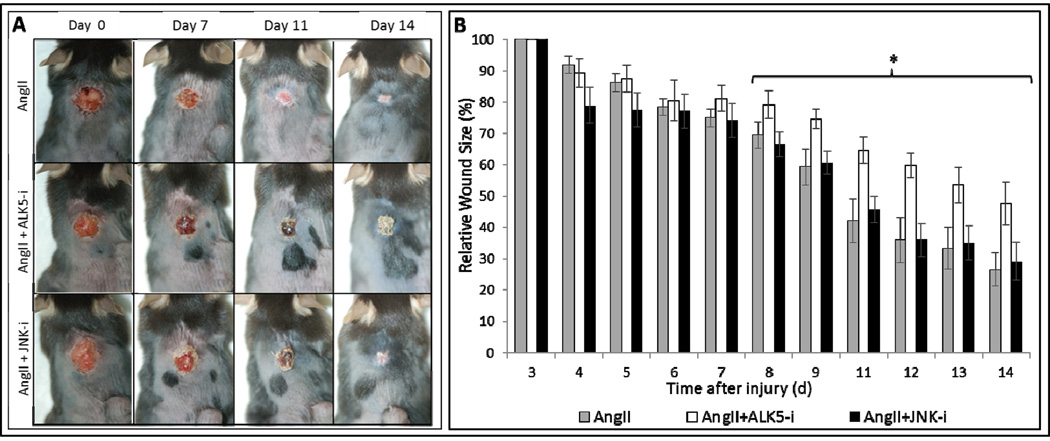
We studied the role of AngII, ALK5, and JNK signaling in granulation tissue contraction using dermal excisional wounds in C57BL/6J WT and AT1 KO mice treated with AngII ± Losartan, ALK5 or JNK inhibition. Granulation tissue contraction was significantly delayed in the KO mice compared to WT mice on day 3, 4, and 5 (Fig. 5A), demonstrating the importance of AT1-receptor in tissue contraction. At each time point, contraction rates in AngII treated WT mice were significantly increased compared to control (Fig. 5B). Conversely, Losartan significantly delayed contraction compared to control (Fig. 5C–D). As of day 9 after injury, contraction rates in AngII/ SB431542, but not SP600125, treated mice were significantly diminished (Fig. 6A–B). At day 14, average wound size decrease in AngII/SB431542 treated groups was 52%, but greater and comparable in AngII alone and AngII/SP600125 treated groups with respective 73% and 71% decrease. These data demonstrate that AngII stimulated granulation tissue contraction is mediated by an AT1-receptor signaling pathway to which ALK5 is an integral component.
Figure 5. AngII increases granulation tissue contraction in-vivo through AT1-receptor activation.
AT1-receptor knockout mice had delayed wound contraction compared to wildtype mice (A). Wildtype mice treated with AngII (2200ng/kg/min) delivered by osmotic pump had increased contraction in compared to wildtype mice treated with saline (B). Wildtype mice treated with Losartan administered daily by oral gavage had delayed wound contraction in comparison to control wildtype mice (C). Quantification demonstrated Losartan inhibited contraction by >40% *p<0.05 (D).
Figure 6. Ang II stimulated granulation tissue contraction is diminished by ALK5 inhibition, but not by JNK inhibition.
Representative images of excisional wounds on mice treated with AngII alone, AngII + Alk5 inhibitor (SB431425), and AngII + JNK inhibitor (SP600125), at 0, 3, 6, 9, and 14 days after injury (A). As of 8 days after injury, contraction of excisional wounds of AngII + ALK5 inhibitor treated mice treated was significantly delayed compared to those of mice treated with AngII + JNK inhibitor. By day 14, contraction is inhibited by 20% *p<0.05 (B).
AT1-receptor, ALK5, and JNK blockade do not significantly alter re-epithelialization, collagen deposition and cell proliferation
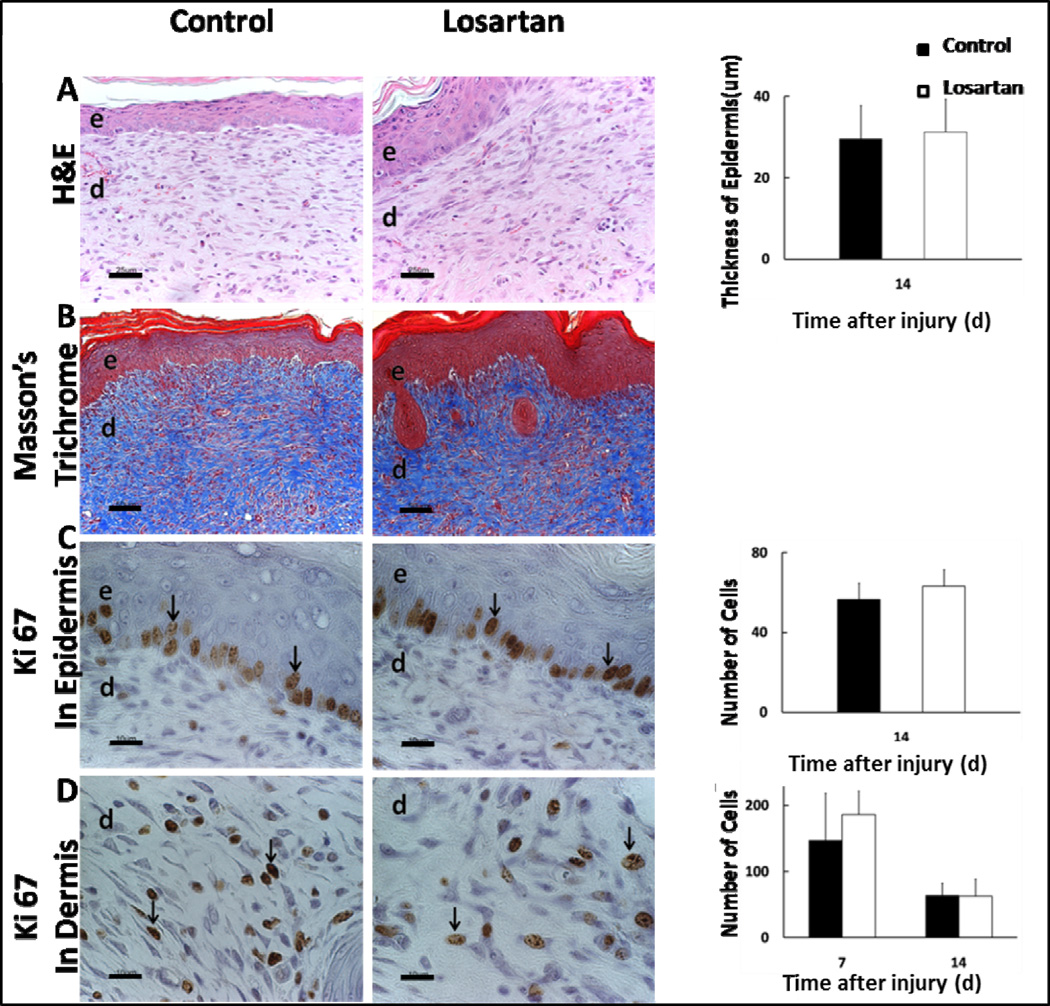
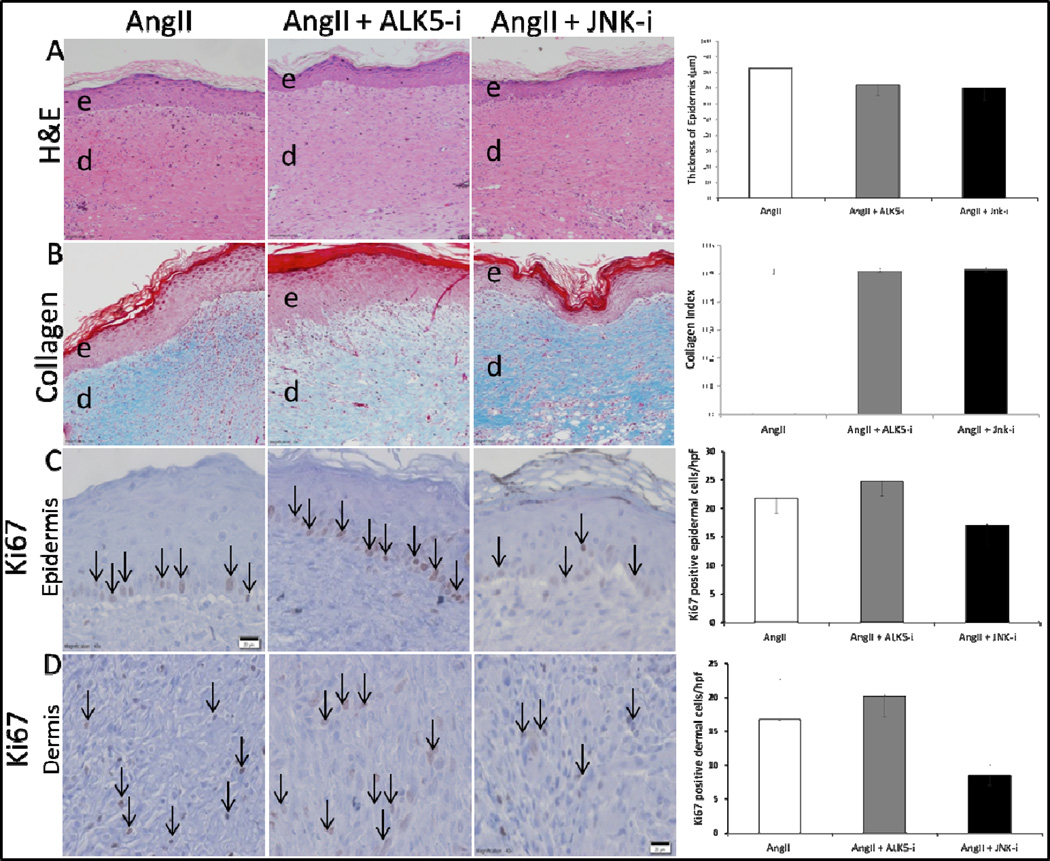
We assessed the effects of AT1-receptor, ALK5, and JNK blockade on wound epithelial regeneration, collagen formation, and cell proliferation by performing H&E, Masson’s Trichrome, and Ki67 staining were performed on tissue samples from respective treatment groups. No significant difference was observed on the gross histological analysis of epithelial regeneration, collagen deposition, or epidermal and dermal cell proliferation between Losartan treated and control groups (Fig. 7A). With respect to ALK5 and JNK inhibition, there was no statistical difference between these groups in re-epithelialization, collagen deposition, or cell proliferation (Fig. 8A–D).
Figure 7. AT1-receptor blockade with Losartan does not alter wound re-epithelialization, collagen deposition, or epidermal/dermal cell proliferation.
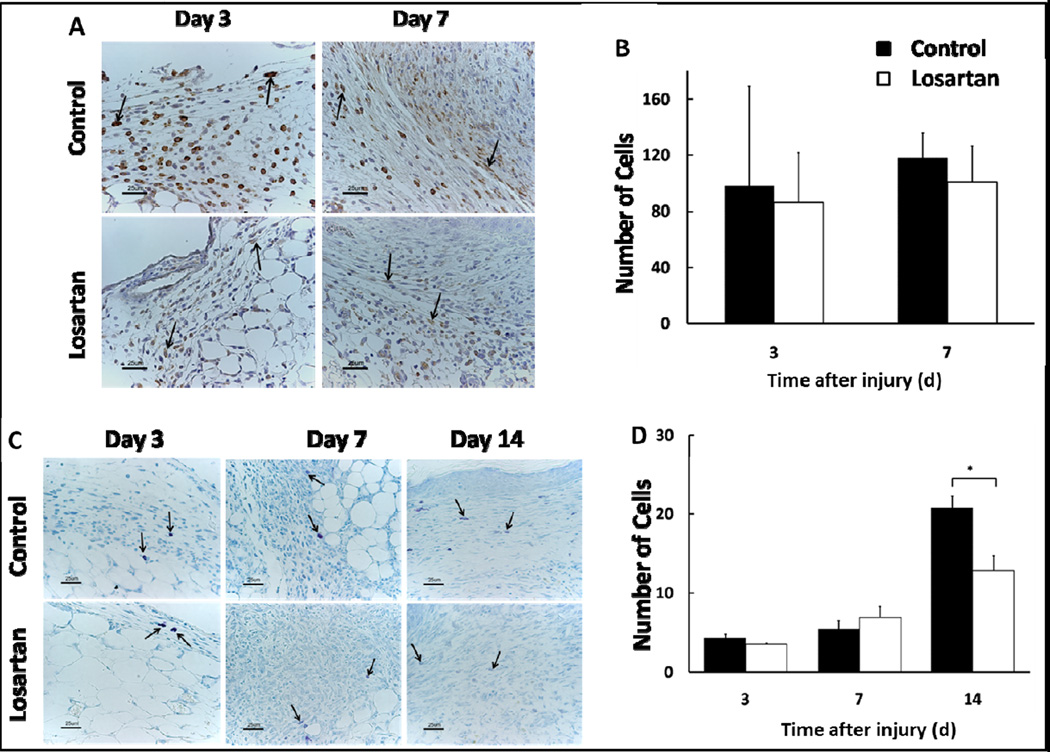
Effects of Losartan on epithelial regeneration and dermal repair in-vivo (A). Representative images of H&E (20× - scale bars represent 100μm), Masson’s Trichrome (20× - scale bars represent 100μm), and Ki67 (40× scale bars represent 50μm) immunohistochemical staining of the wound beds of Losartan treated mice. Quantification of neo-epidermal thickness in control vs. Losartan treated mice on post-operative day 14 demonstrating comparable epithelial thickness in both groups (B). Quantification of Ki67 positive cells (black arrows) per 40× magnification field on post-operative day 7 and 14 in the epidermis (C), and dermis (D) demonstrating an overall decrease in cell proliferation between day 7 and 14, with no significant difference between groups. Each data point represents mean ±SEM, n=5.
Figure 8. Neither ALK5 nor JNK inhibition significantly affected epithelialization, collagen deposition, or cell proliferation.
Representative images of H&E (20× - scale bars represent 100μm), Masson’s Trichrome (20× - scale bars represent 100μm), and Ki67 (40× - scale bars represent 50μm) at 14 days after injury. Masson’s Trichrome staining was quantified by the average collagen index of 5 HPF images for each time point. The collagen index value, ranging from 0 for extremely red objects to 1 for completely blue-green objects, was determined by colorimetric analysis where collagen index = (B+G)/(2R+B+G) for each pixel within the image (R, G, and B represent the red, blue, and green pixel values respectively). There was no statistically significant difference between AngII alone, AngII + ALK5 inhibitor, or AngII + JNK inhibitor in wound bed epithelialization (A), collagen deposition (B), or cell proliferation in the epidermis (C), and dermis (D). Black arrows indicate representative Ki-67 positive cells. Each data point represents mean ±SEM, n=5.
AT1-receptor and ALK5 blockade diminish mast cell and macrophage infiltration respectively
Inflammation is a well characterized modulator of wound healing. To determine the effects of AT1-receptor, ALK5, and JNK blockade on macrophage and mast cell infiltration, we immunostained wound samples with F4/80 antibody and toluidine blue. In both Losartan treated and control groups on day 3, there was marked infiltration of F4/80 positive macrophages in the dermis, particularly the superficial dermis, with only a few localized to the connective tissue (Fig. 9A). However, no significant difference in the number of macrophages per HPF was noted between Losartan treated and control groups at any time point (Fig. 9B). There was no significant difference between the control and Losartan treated groups on days 3 and 7, but on day 14, the control group had significantly higher number of mast cells than the Losartan treated group (21±2 vs. 10±2, Fig. 9D). ALK5 inhibited samples exhibited significantly lower macrophage recruitment compared to the other groups (Fig. 10A). Neither ALK5 nor JNK inhibition conferred significant differences in mast cell infiltration compared to control (Fig. 10A–B).
Figure 9. AT1-receptor blockade with Losartan decreases mast cell infiltration to wound bed.
Representative 40× images (scale bars represent 50μm) of F4/80 immunohistochemical staining on day 3 and 7 (A). Quantitative analysis of macrophages demonstrates no significant difference in the number of F4/80 positive cells per 40× field between Losartan treated and control groups (B). Representative 20× images (scale bars represent 100μm) of toluidine blue staining day 3, 7, and 14 (C). Mast cells are sparse and primarily localized to the normal connective tissue and superficial region of granulation tissue with few noted in the wound bed. Both the total number of mast cells and ratio of degranulated mast cells to total mast cells progressively increased over time in the treated as well as control groups such that by day 14, the majority of mast cells in the wound bed were degranulated. The mast cell per HPF (40×) is significantly greater in the control group on day 14 (D). Each data point represents the mean ± SEM, n=5.
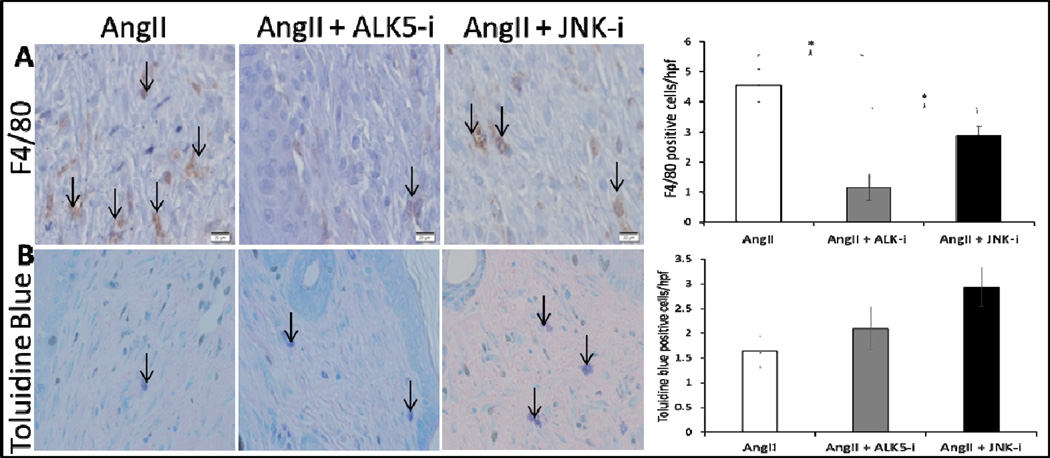
Figure 10. ALK5 inhibition decreases macrophage recruitment to the wound bed.
Representative 40× images (scale bars represent 50μm) of F4/80 (A) and toluidine blue (B) positive cells from wound samples of AngII only, AngII + ALK5 inhibitor, and AngII + JNK inhibitor treated mice on day 14 after wounding. Black arrows indicate representative positively stained cells. Quantitative analysis of F4/80 positive cells demonstrates a statistically significant lower density of macrophages in AngII + ALK5 inhibitor compared to other groups (*p<0.05). Quantitative analysis of Toluidine blue stained cells demonstrates no statistically significant difference between groups.
Discussion
Recent studies have shown that AngII plays an important role in dermal scarring [14, 15, 18]. As reported by Morihara et al, there is increased expression of ACE by human keloid and HSc fibroblasts as compared to unwounded fibroblasts [14]. AngII has been reported as one of the key molecules upregulated in the matrix of deep fibroblasts from stiffened, pathologic scar [16]. Of the two widely studied AngII receptors, AT1-receptor is characterized as the primary regulator for AngII mediated dermal fibrosis. In one study on the effects of thermal skin injury on the Renin-Angiotensin-System in a murine model, the data indicated that AT1-receptor expression was increased in the fibroblasts of injured mice but absent in the fibroblasts of unwounded skin [23]. Similarly, AT1-receptor, as compared to AT2-receptor, was found to be more prominently expressed in the dermis of wounded rats [24]. In HSF, AngII has been shown to stimulate pro-fibrotic pathway, PI 3-K/Akt, in an AT1-receptor but not in an AT2-receptor dependent manner [18]. One mechanism by which AngII accelerates wound closure may be through AT1-receptor mediated activation of proteins that promote cellular contraction and migration [25]. Previously published studies have suggested that AngII stimulates components of the cytoskeleton, including RhoA, ROCK, ASMA, and NM IIA [22, 26]. RhoA is the small GTPase activator of actin stabilizing kinase, ROCK1, which is the calcium independent activator of myosin regulatory light chain [26]. Myosin regulatory light chain stimulates NM IIA activity and promotes actin filament sliding, including ASMA [27].
Normal dermal wound healing includes the processes of 1)re-epithelialization, 2)connective tissue matrix deposition, and 3)contraction, which are dependent on keratinocyte migration and proliferation, collagen deposition, and cellular contraction along the extracellular matrix respectively [28, 29]. Losartan did not alter re-epithelialization, collagen architecture, or cell proliferation in granulation tissue in the first 14 days. Therefore, Losartan most likely inhibited wound healing by diminishing fibroblast contraction and migration. Decreased mast cell recruitment in the Losartan group is another possible explanation for delayed wound contraction observed here. Increased numbers of mast cells are present in cutaneous and systemic fibrotic conditions [30]. Mast cell degranulation releases growth factors and cytokines in the wound bed that stimulate cell proliferation, angiogenesis, and extracellular matrix formation that facilitates wound contraction and closure [30, 31]. One study reported that chymase, a protease released from mast cells, may be responsible for ACE-independent generation of AngII in wounds [31]. This may explain why inhibition of mast cell degranulation significantly decreases AngII in thermally injured hamster wounds [31]. Based on these data, there appears to be a positive feedback loop between AngII, AT1-receptor, and mast cells.
In our investigation of HSF migration using cell scratch assay, ALK5 and JNK inhibitor treatment groups had decreased AngII stimulated HSF migration. However, in-vivo findings revealed only ALK5 inhibition significantly diminished contraction. This could be explained by the fact that AngII upregulates TGFβ and thus, ALK5 signaling is an important component of AngII effects in promoting contraction [32]. AngII and TGFβ form a concerted signaling network in propagating fibrosis. AngII upregulates TGFβ expression, and in turn TGFβ increases AT1-receptor expression. This is supported by other studies demonstrating that inhibition of ALK5 significantly diminishes fibrotic changes in hepatic and pulmonary models of fibrosis [33, 34]. The applicability of these conclusions is limited by the experimental models used in the study. 3T3 cells were used to determine effects of AngII on the cytoskeleton, whereas human tissue was used for the AngII receptor staining, and human fibroblasts were used in the functional assays. For in-vivo experiments, mice were studied for the effects of AngII on granulation tissue contraction rate and immunohistochemistry. Alternative HSc models could further validate the results. In a model of HSc using a rabbit ear model, Uzun et al found that animals treated with the angiotensin converting enzyme (ACE) inhibitor, enalapril, had decreased fibroblast density, capillary density, and scar elevation, as compared to groups treated with intra-lesion steroids – the current standard of care treatment for HSc [15]. The authors hypothesized that the mechanism through which enalapril diminished hypertrophic scarring was prevention of AngII-AT1-receptor stimulation of TGFβ. This is similar to our findings which implicate AngII as a pro-scarring soluble factor that activates AT1-receptor and promotes granulation tissue contraction by potentiating the canonical signaling mechanisms of TGFβ.
Dermal scar contracture is a large unmet clinical need. Presently no effective therapies exist [35]. An effective therapy would mitigate the over-exuberant wound healing response that leads to HSC, but also allow adequate extracellular matrix deposition and granulation tissue to prevent the opposite problem of non-healing. As such, an anti-HSc agent would be detrimental to a non-healing wound (not fully epithelialized) but beneficial to a HSc (epithelialized wound that is contracting). Our findings demonstrated that AngII accelerates HSF migration in-vitro and wound contraction in-vivo and that these effects were prevented by AT1-receptor and ALK5 blockade. Thus, our data suggests AT1-receptor or ALK5 blockade would be beneficial in mitigating HSc. Of course, this would mean that an AT1-receptor or ALK5 antagonist should be applied after the wound has healed and before the hypertrophic scar has developed. Of note, Iannello et al reported two observational case studies where matured (greater than 4 months old) post-surgical HSc and keloid scars significantly improved after 6 months of treatment with 10mg of enalapril daily [17].
In conclusion, we have demonstrated that AngII promotes granulation tissue contraction through AT1-receptor dependent cellular migration and contractility. AT1-receptor stimulates the shared ALK5 and JNK non-canonical TGFβ signaling pathway in-vitro but only ALK5 is relevant in-vivo.
Key Message.
AT1-receptor expression is increased in scar tissue compared to unwounded tissue.
AngII stimulates expression of proteins that confer cell migration and contraction.
AngII stimulates fibroblast migration and contraction through AT1-receptor, ALK5, and JNK.
AngII stimulated in-vivo granulation tissue contraction is AT1-receptor and ALK5 dependent.
Acknowledgments
This study was supported by a grant from the National Institutes of Health; 5 K08GM085562-05, PI Levinson, Howard. The authors wish to thank Bruce Klitzman, PhD, for his supervision of animal experiments, and Dr. Zuowei Su for his technical assistance with immunohistochemical staining. The authors are also grateful to Thomas M. Coffman, MD for providing the AT1 KO mice. We would like to acknowledge Maragatha Kuchibhatla, Ph.D. for providing her expertise in statistical analysis.
Footnotes
Disclosure Statement
The authors have no financial or personal conflict of interest to disclose in connection with this submitted material.
Author Contribution
Tosan Ehanire, MHS: Experimental design and planning, western blot analysis, fibroblast populated collagen lattice assays, manuscript and figure preparation.
Licheng Ren, MD: In-vivo and immunohistology studies with Losartan treated mice, manuscript preparation.
Jennifer Bond, PhD: Experimental design and planning, manuscript preparation.
Manuel Medina, MD: In-vivo studies with ALK5/JNK signaling blockade, immunohistochemistry, manuscript preparation.
George Li, BSc: Cell scratch migration and assays, manuscript preparation.
Latif Bashirov, MD: In-vivo studies with ALK5/JNK signaling blockade, immunohistochemistry, manuscript preparation.
Lei Chen, MD: Immunohistology of human scar and normal fibroblast, manuscript preparation.
George Kokosis, MD: In-vivo studies with AT1 KO mice, manuscript preparation.
Angelica Selim, MD: Immunohistology, manuscript preparation.
Gerard C. Blobe, MD, PhD: Experimental design, manuscript preparation.
Howard Levinson, MD: Experimental design, manuscript preparation.
References
- 1.ReSurge. The Forgotten Global Health Crisis of Burns. 2009. [Google Scholar]
- 2.Wang R, Ghahary A, Shen Q, Scott PG, Roy K, Tredget EE. Hypertrophic scar tissues and fibroblasts produce more transforming growth factor-beta1 mRNA and protein than normal skin and cells. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2000;8:128–137. doi: 10.1046/j.1524-475x.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Z, Ding J, Shankowsky HA, Tredget EE. The molecular mechanism of hypertrophic scar. Journal of cell communication and signaling. 2013 doi: 10.1007/s12079-013-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clayton JL, Edkins R, Cairns BA, Hultman CS. Handle With Care: Incidence and Management of Adverse Events After the Use of Laser Therapies for the Treatment of Hypertrophic Burn Scars. Annals of plastic surgery. 2013 doi: 10.1097/SAP.0b013e31827eac79. [DOI] [PubMed] [Google Scholar]
- 5.Aarabi S, Longaker MT, Gurtner GC. Hypertrophic scar formation following burns and trauma: new approaches to treatment. PLoS Med. 2007;4:e234. doi: 10.1371/journal.pmed.0040234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fearmonti RM, Bond JE, Erdmann D, Levin LS, Pizzo SV, Levinson H. The modified Patient and Observer Scar Assessment Scale: a novel approach to defining pathologic and nonpathologic scarring. Plast Reconstr Surg. 2011;127:242–247. doi: 10.1097/PRS.0b013e3181f959e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Washio H, Fukuda N, Matsuda H, Nagase H, Watanabe T, Matsumoto Y, Terui T. Transcriptional inhibition of hypertrophic scars by a gene silencer, pyrrole-imidazole polyamide, targeting the TGF-beta1 promoter. The Journal of investigative dermatology. 2011;131:1987–1995. doi: 10.1038/jid.2011.150. [DOI] [PubMed] [Google Scholar]
- 8.Kondo S, Kagami S, Urushihara M, Kitamura A, Shimizu M, Strutz F, Muller GA, Kuroda Y. Transforming growth factor-beta1 stimulates collagen matrix remodeling through increased adhesive and contractive potential by human renal fibroblasts. Biochimica et biophysica acta. 2004;1693:91–100. doi: 10.1016/j.bbamcr.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nature medicine. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Fan D, Wang C, Wang JY, Cui XB, Wu D, Zhou Y, Wu LL. Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-beta1 pathways in cardiac fibroblasts. Cardiovasc Res. 2011;91:80–89. doi: 10.1093/cvr/cvr067. [DOI] [PubMed] [Google Scholar]
- 12.Xia CY, Li L, Liu HM, Cong WM. High expression of angiotensin-converting enzyme and angiotensin-converting enzyme 2 in preservation injury after liver transplantation in rats. Hepatology research : the official journal of the Japan Society of Hepatology. 2009;39:1118–1124. doi: 10.1111/j.1872-034X.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Qiu HB, Yang Y, Wang L, Ding HM, Li HP. Losartan, an antagonist of AT1 receptor for angiotensin II, attenuates lipopolysaccharide-induced acute lung injury in rat. Archives of biochemistry and biophysics. 2009;481:131–136. doi: 10.1016/j.abb.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Morihara K, Takai S, Takenaka H, Sakaguchi M, Okamoto Y, Morihara T, Miyazaki M, Kishimoto S. Cutaneous tissue angiotensin-converting enzyme may participate in pathologic scar formation in human skin. Journal of the American Academy of Dermatology. 2006;54:251–257. doi: 10.1016/j.jaad.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Uzun H, Bitik O, Hekimoglu R, Atilla P, Kayikcioglu AU. Angiotensin-converting enzyme inhibitor enalapril reduces formation of hypertrophic scars in a rabbit ear wounding model. Plast Reconstr Surg. 2013;132:361e–371e. doi: 10.1097/PRS.0b013e31829acf0a. [DOI] [PubMed] [Google Scholar]
- 16.Varkey M, Ding J, Tredget EE. Differential collagen-glycosaminoglycan matrix remodeling by superficial and deep dermal fibroblasts: potential therapeutic targets for hypertrophic scar. Biomaterials. 2011;32:7581–7591. doi: 10.1016/j.biomaterials.2011.06.070. [DOI] [PubMed] [Google Scholar]
- 17.Iannello S, Milazzo P, Bordonaro F, Belfiore F. Low-dose enalapril in the treatment of surgical cutaneous hypertrophic scar and keloid--two case reports and literature review. MedGenMed : Medscape general medicine. 2006;8:60. [PMC free article] [PubMed] [Google Scholar]
- 18.Liu HW, Cheng B, Yu WL, Sun RX, Zeng D, Wang J, Liao YX, Fu XB. Angiotensin II regulates phosphoinositide 3 kinase/Akt cascade via a negative crosstalk between AT1 and AT2 receptors in skin fibroblasts of human hypertrophic scars. Life sciences. 2006;79:475–483. doi: 10.1016/j.lfs.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Steckelings UM, Henz BM, Wiehstutz S, Unger T, Artuc M. Differential expression of angiotensin receptors in human cutaneous wound healing. The British journal of dermatology. 2005;153:887–893. doi: 10.1111/j.1365-2133.2005.06806.x. [DOI] [PubMed] [Google Scholar]
- 20.de la Cruz-Merino L, Henao-Carrasco F, Garcia-Manrique T, Fernandez-Salguero PM, Codes-Manuel de Villena M. Role of transforming growth factor beta in cancer microenvironment. Clin Transl Oncol. 2009;11:715–720. doi: 10.1007/s12094-009-0433-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bond JE, Bergeron A, Thurlow P, Selim MA, Bowers EV, Kuang A, Levinson H. Angiotensin-II mediates nonmuscle myosin II activation and expression and contributes to human keloid disease progression. Molecular medicine. 2011;17:1196–1203. doi: 10.2119/molmed.2010.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jadhav SS, Sharma N, Meeks CJ, Mordwinkin NM, Espinoza TB, Roda NR, Dizerega GS, Hill CK, Louie SG, Rodgers KE. Effects of combined radiation and burn injury on the renin-angiotensin system. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013;21:131–140. doi: 10.1111/j.1524-475X.2012.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda H, Katagata Y, Hozumi Y, Kondo S. Effects of angiotensin II receptor signaling during skin wound healing. The American journal of pathology. 2004;165:1653–1662. doi: 10.1016/S0002-9440(10)63422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrlich HP, Rajaratnam JB. Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue & cell. 1990;22:407–417. doi: 10.1016/0040-8166(90)90070-p. [DOI] [PubMed] [Google Scholar]
- 26.Tomasek JJ, Vaughan MB, Kropp BP, Gabbiani G, Martin MD, Haaksma CJ, Hinz B. Contraction of myofibroblasts in granulation tissue is dependent on Rho/Rho kinase/myosin light chain phosphatase activity. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2006;14:313–320. doi: 10.1111/j.1743-6109.2006.00126.x. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandran A, Gangopadhyay SS, Krishnan R, Ranpura SA, Rajendran K, Ram-Mohan S, Mulone M, Gong EM, Adam RM. JunB mediates basal- and TGFbeta1-induced smooth muscle cell contractility. PloS one. 2013;8:e53430. doi: 10.1371/journal.pone.0053430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 29.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 30.Trabucchi E, Radaelli E, Marazzi M, Foschi D, Musazzi M, Veronesi AM, Montorsi W. The role of mast cells in wound healing. Int J Tissue React. 1988;10:367–372. [PubMed] [Google Scholar]
- 31.Dong X, Geng Z, Zhao Y, Chen J, Cen Y. Involvement of mast cell chymase in burn wound healing in hamsters. Experimental and therapeutic medicine. 2013;5:643–647. doi: 10.3892/etm.2012.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin MM, Buckenberger JA, Jiang J, Malana GE, Knoell DL, Feldman DS, Elton TS. TGF-beta1 stimulates human AT1 receptor expression in lung fibroblasts by cross talk between the Smad, p38 MAPK, JNK, and PI3K signaling pathways. American journal of physiology Lung cellular and molecular physiology. 2007;293:L790–L799. doi: 10.1152/ajplung.00099.2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.van Beuge MM, Prakash J, Lacombe M, Post E, Reker-Smit C, Beljaars L, Poelstra K. Enhanced effectivity of an ALK5-inhibitor after cell-specific delivery to hepatic stellate cells in mice with liver injury. PloS one. 2013;8:e56442. doi: 10.1371/journal.pone.0056442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higashiyama H, Yoshimoto D, Kaise T, Matsubara S, Fujiwara M, Kikkawa H, Asano S, Kinoshita M. Inhibition of activin receptor-like kinase 5 attenuates bleomycin-induced pulmonary fibrosis. Experimental and molecular pathology. 2007;83:39–46. doi: 10.1016/j.yexmp.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Reish RG, Eriksson E. Scar treatments: preclinical and clinical studies. J Am Coll Surg. 2008;206:719–730. doi: 10.1016/j.jamcollsurg.2007.11.022. [DOI] [PubMed] [Google Scholar]