Abstract
Chronic alcohol consumption changes gene expression, likely causing persistent remodeling of synaptic structures via altered translation of mRNAs within synaptic compartments of the cell. We profiled the transcriptome from synaptoneurosomes (SNs) and paired total homogenates (THs) from mouse amygdala following chronic voluntary alcohol consumption. In SN, both the number of alcohol-responsive mRNAs and the magnitude of fold-change were greater than in THs, including many GABA-related mRNAs upregulated in SNs. Furthermore, SN gene co-expression analysis revealed a highly connected network, demonstrating coordinated patterns of gene expression and highlighting alcohol-responsive biological pathways, such as long-term potentiation, long-term depression, glutamate signaling, RNA processing and upregulation of alcohol-responsive genes within neuroimmune modules. Alterations in these pathways have also been observed in the amygdala of human alcoholics. SNs offer an ideal model for detecting intricate networks of coordinated synaptic gene expression and may provide a unique system for investigating therapeutic targets for the treatment of alcoholism.
INTRODUCTION
Alcohol dependence is a severe and widespread disease. Over 17 million Americans suffer from alcohol-related problems; total cost estimates of substance abuse in the United States exceed $600 billion annually, with 39% of that cost related to alcohol.1,2 The pharmacotherapies available today are significantly limited due to side effects and failure to relieve drug craving, leading to high relapse rates.
Chronic alcohol use produces long-term neuroadaptations in synaptic structure and function, which are likely caused by persistent changes in gene expression.3–6 This leads to a remodeling of neural circuitry7–9 and is one of the main features of addiction.10–12 Synaptic translation of mRNA is a cardinal process underlying normal function,13–16 and perturbation by alcohol represents a mechanism contributing to synaptic neuroadaptations.17 The composition of specific mRNAs in the synaptic compartment may give insight into the neurobiology of different states of addiction and is an unexplored avenue of research.
Given the role of synaptic plasticity in alcohol dependence, selecting a biologically relevant model system for analysis of the synaptic transcriptome is of critical importance. Although total homogenate (TH) preparations have been used for mRNA and alcohol studies in the past, this method limits identification of regional mRNAs and likely underestimates the number and magnitude of alcohol-responsive transcripts in the synapse. Synaptoneurosomes (SNs) contain membrane vesicles of presynaptic and postsynaptic compartments composed of primarily neurons as well as astrocytes and microglia. SNs have been used to study local translation of mRNAs in the synapse15,16 and may prove to be a superior model system for alcohol effects confined to synaptic regions of the cell.
In order to measure discrete changes within the synaptic transcriptome following chronic alcohol consumption, we profiled mRNAs from SN15,16,18,19 and TH samples from mouse amygdala, a brain region known to be involved with the negative reinforcement of alcohol and other drugs of abuse.20 The present findings reveal greater expression of alcohol-responsive mRNAs in SN compared with TH. Using gene expression patterns to generate biological networks, the SN preparation appears ideally suited for detecting alcohol-responsive groups of genes that have been shown to be important in human alcoholism. The gene clusters isolated in SN could prove useful in developing targets for the future treatment of alcoholism.
MATERIALS AND METHODS
Animal housing and alcohol self-administration
Adult (2-month old) C57BL/6 J female mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and were maintained at the University of Texas at Austin Animal Research Center. Mice were given a 1-week acclimation period in combined housing and another week to acclimate to the bottle position in individual housing. Food and water were provided ad libitum and monitored daily, as were the temperature and light/dark cycles. Mice underwent a 30-day two-bottle choice paradigm with continuous (24 h) access to one bottle of 20% ethanol and one bottle of water, similar to that described previously21 (n = 8 alcohol group, n = 13 control group). Bottle weights were recorded daily, and the amount of alcohol consumed throughout the 30 days was calculated as g kg−1 (Supplementary Figure S1). Bottle positions were changed daily to control for position preferences, and mice were weighed every 4 days. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin and adhere to NIH Guidelines for the ethical care and use of animals in research.
SN preparation and RNA extraction
Mice were euthanized by cervical dislocation and then decapitated. Brains were removed and washed for 1 min with 1ml of ice-cold Homogenizing Buffer (HB) containing 20 mm Hepes, 1 mm EDTA (pH 7.4), 40 U ml−1 RNAseOut (Invitrogen, Carlsbad, CA, USA), phosphatase inhibitor cocktail 3 (Sigma, St Louis, MO, USA) and protease inhibitors ‘Complete’ (Roche, Indianapolis, IN, USA). Brains were then placed in a coronal Zivic mouse brain slicer with a 0.5 mm resolution (Zivic Instruments, Pittsburgh, PA, USA) and sliced in the following coordinates in order to isolate extended amygdala (two coronal slices were made for greater ease of dissection): coronal level 56–66 (Bregma (−0.18)–(−1.155)) and 66–80 (Bregma (−1.155)–(−2.55)). The extended amygdala was dissected, placed in ice-cold HB (250 µl) and homogenized for 1 min using a VWR homogenizer and pestle (VWR, Radnor, PA, USA). To minimize homogenate loss, pestles were washed with 50 µl HB after use, and the wash was collected and added to the sample. Ten percent of the homogenate (30 µl) was snapfrozen in liquid nitrogen and stored at −80 °C for subsequent RNA TH analysis.
Paired SNs18 were isolated from the rest of the homogenate (270 µl) in a manner similar to that described previously.15 Briefly, homogenates were filtered through a 100-µm pore filter and subsequently through a 5-µm pore filter (Millipore, Billerica, MA, USA); filters were washed with HB before use for protection from RNAse. To maximize yield, the filters were washed with 50 µl HB after use, and the wash was collected and added to the homogenate. The homogenate was then centrifuged at 14 000 g for 20 min at 4 °C in order to pellet the cell fraction containing SNs.15,19,22 The supernatant was removed and the pellet snap-frozen and stored at −80 °C for SN RNA analysis. Microscopy was used to further characterize the SN preparation (see Supplementary Methods).
Total RNA was extracted from 21 SN and 21 paired TH samples with the Direct-Zol RNA extraction kit (Zymo Research Corporation, Irvine, CA, USA), using IC columns according to the manufacturer’s instructions. The RNA was quantified using NanoDrop1000 (Thermo Fisher Scientific Inc., Rockford, IL, USA) and assayed for quality using Agilent 2100Tape-Station (Agilent Technologies, Santa Clara, CA, USA). The cutoff criteria were set on 280/260 > 1.7, RIN > 6.5 and amount of total RNA > 500 ng.
Microarray hybridization, data quality assessments and analysis
RNA samples were processed at the University of Texas Southwestern Medical Center microarray facility in Dallas. mRNA was amplified and biotin-labeled using the Illumina TotalPrep RNA Amplification kit (Ambion, Austin, TX, USA) and hybridized to Mouse WG-6 v2.0 Expression BeadChips (Illumina, San Diego, CA, USA). Each array contained SN and paired TH samples from control and alcohol-treated mice. These were assigned randomly to each array. The array data were analyzed using R environment and Bioconductor packages, similar to our published studies.21,23 The ‘Lumi’ package24,25 was used to preprocess the data using variance stabilization transformation (variance within array),26,27 quantile normalization (variance between arrays) and background subtraction.24,25 Quality measures were taken before and after preprocessing using the arrayQualityMetrics package28,29 to remove outliers determined by at least two out of the three tests in the package, and care was taken that the normalization did not skew the data (two TH samples out of the 42 failed to pass the cutoff and were therefore removed from the analysis). This package was also used to generate the principal component analysis. Transcripts significantly detected on 80% of the arrays were used in the analysis (detection probability < 0.05). The data presented in this publication have been deposited in NCBI's Gene Expression Omnibus30 and are accessible through GEO Series accession number GSE51730.
The ‘Limma’ package31 was used for differential expression analysis between SN and paired TH samples (paired/dependent t-test) and between the alcohol and control samples in SN and TH (two independent t-tests). A list of alcohol-responsive mRNAs was compiled from the list of genes differentially expressed between alcohol and control samples. A weighted gene correlation (co-expression) network analysis32 was generated for the combined control and alcohol data, using the weighted gene correlation (co-expression) network analysis (WGCNA) package.32 Alcohol-responsive mRNA enrichment analysis was performed for each module using an over-representation (hypergeometric) test with a cutoff P-value < 0.05. To determine alcohol-responsive SN and TH modules, we used the ‘alcohol-responsive mRNAs’ lists from our data. For details on the WGCNA parameters, see Supplementary Methods. We evaluated whether the correlation between alcohol consumption and TH modules would increase depending on WGCNA parameters. We generated another TH WGCNA network and optimized for the highest correlation of modules with consumption (top 10% of the modules). Enrichment and clustering analyses were performed using KEGG pathways, Wikipathways, gene ontologies and protein interactions, part of the Database for Annotation Visualization and Integrated Discovery (DAVID),33 WEB-based GEne SeT AnaLysis Toolkit (Webgestalt)34,35 and Ingenuity Pathway Analysis (IPA; Qiagen, Valencia, CA, USA). All P-values from these analyses were adjusted using the Benjamini–Hochberg method (BH). Synaptic mRNA enrichment was assessed using a list of mRNAs enriched in the synaptic neuropil36 and in process-localized mRNAs.36 For alcohol-responsive mRNA enrichment, we used a human alcoholic mRNA data set23 from the amygdala, quantitative trait loci mRNA list37 and a list of mRNAs from prefrontal cortex of C57BL6 after a two-bottle choice paradigm.21 For cell types and immune response enrichment, we used the following lists of genes: neuronal, astrocytic and oligodendrocyte,38 microglial,39 glutamate/GABA40 and lipopolysaccharide (LPS)-regulated mRNAs.21
RESULTS
The SN transcriptome is composed of synaptic mRNAs and is distinct from the TH
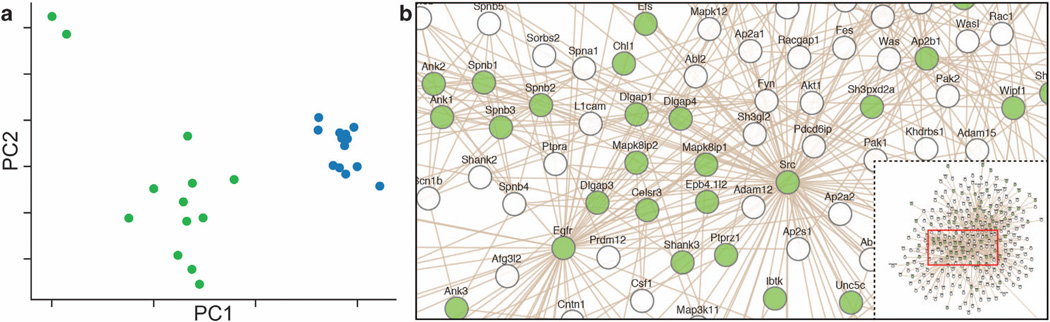
We compared SN and TH transcriptomes from mouse amygdala and detected 17 514 and 18 318 transcripts in the SN and TH microarrays, respectively, with a high overlap of detected transcripts (17 265). We studied the expression levels using principal component analysis and found a distinct clustering of the two types of preparations, while showing a homogenous sample population within each preparation (Figure 1a). The clustering was evident along the first principal component, indicating that the largest variation stems from distinct expression levels in the two preparations. We identified 4539 differentially expressed unique mRNAs (BH, P < 0.05), with 2119 mRNAs enriched in the SN (Supplementary Table S1) and 2420 mRNAs enriched in the TH (Supplementary Table S2). We used DAVID to compare the SN- and TH-enriched cellular components and found that the SN contained fewer somatic and intracellular components, while preserving and enriching the synaptic mRNAs (Table 1). Among the SN-enriched transcripts, many known synaptic mRNAs were over-represented in this preparation (Table 2). Most of these synaptic functional groups were not detected in the TH, indicating that enriched synaptic mRNAs are more readily detected in the SN. Webgestalt was used to investigate known pathways (KEGG and Wikipathways; Supplementary Table S3) and to generate a network of known protein interactions enriched in the SN (Figure 1b). The network was associated with axon guidance and cell leading edge and highlighted the Dlg family (also known as postsynaptic density proteins or PSDs). SN (but not TH) transcripts were also over-represented with synaptic mRNAs in a high-resolution study exploring the synaptic neuropil.36
Figure 1.
(a) Principal component analysis of expression profiles from paired synaptoneurosome (SN) and total homogenate (TH) samples are shown in green and blue, respectively. Preparation difference is the first principal component and explains 17% of the variance. (b) SN-enriched mRNA network illustrating known protein interactions between the SN-enriched mRNAs. The bottom right portion of the figure is an overview of the entire network, and the highlighted portion of this network has been enlarged. The green nodes represent the mRNAs enriched in the SN compared with the TH preparation (fold-change threshold of > 10%, Benjamini–Hochberg method P < 0.05). Many known synaptic mRNAs are found in the center of this network, emphasizing enrichment of the synaptic components in the SN preparation.
Table 1.
Comparison of the DAVID enrichment scores of synaptoneurosome (SN)- and total homogenate (TH)-enriched cellular components
| DAVID enrichment clustering | SN Score | TH Score |
|---|---|---|
| Intracellular | 20 (1370) | 32.4 (1528) |
| Organelle | 6.2 (438) | 29.7 (621) |
| Organelle membrane | 3.5 (137) | 13.2 (179) |
| Synapse | 2.9 (64) | 1.3 (NS) (21) |
Abbreviations: DAVID, Database for Annotation Visualization and Integrated Discovery; NS, non-significant.
The table illustrates reduction of somatic and intracellular components and preservation/enrichment of synaptic mRNAs in SN (the number of genes detected in a cluster are shown in parenthesis). All scores for enrichment clustering are significant with a DAVID Benjamini–Hochberg method P < 0.05. A NS score was defined as a cluster containing only one significant group out of five.
Table 2.
Functional clustering of synaptoneurosome (SN)-enriched mRNAs
| Annotation cluster |
DAVID enrichment score |
Number of mRNAs |
P-value | BH P-value |
Gene symbols |
|---|---|---|---|---|---|
| Regulation of system process | 4.1 | 18 | 8.60E-06 | 1.60E-02 | KCNMA1, MYO6, GNAI2, GRIK5, CTNND2, MECP2, GJA1, ATP1A2, CSPG5, ADORA1, RIMS1, PTPN11, HDAC4, SLC1A3, NTRK2, HOPX, DLG4, CAMK2A. |
| Synapse | 4.0 | 26 | 4.00E-07 | 1.20E-04 | GRIK5, TIMP4, RIMS1, ADORA1, SLC1A2, GP1BB, SNPH, DLG4, CAMK2A, DLG2, MT3, KCNMA1, PHACTR1, ARC, MYO6, DLGAP3, SPARCL1, PSD3, SSPN, SHANK3, PPP1R9B, HDAC4, NTRK2, VAMP3, UNC13C, SNTA1. |
| PDZ/DHR/GLGF | 3.8 | 14 | 4.10E-05 | 3.40E-02 | SNX27, PREX1, PDLIM4, PDLIM2, MPP6, SLC9A3R1, RIMS1, SHANK3, PPP1R9B, MAST2, SIPA1L1, DLG4, DLG2, SNTA1. |
| Cytoskeleton | 3.8 | 51 | 3.30E-05 | 2.00E-03 | KIF23, KIFC2, GFAP, TUBB2B, AIF1, FERMT2, PDLIM2, ADORA1, CTNNB1, NDE1, EVI5, DLG4, DLG2, ARC, MYO6, INPPL1, KIF5A, KIF5C, PSD3, SPIRE1, MID1IP1, TBCEL, RB1, DNAIC1, CTNNA1, FMN2, KIF1A, MAST2, KIF1B, PDE4DIP, ADD3, CAPZB, LLGL1, KLC1, GP1BB, STRBP, CDC42EP4, ACTB, DLGAP3, CKAP5, CSRP1, COTL1, SIRT2, SHANK3, PTPN11, EPB4.1L2, PPP1R9B, HDAC4, EPB4.1L1, NTRK2, SNTA1. |
| Transmission of nerve impulse | 3.0 | 18 | 3.90E-05 | 2.40E-02 | KCNMA1, SCD2, MYO6, ALDH5A1, GRIK5, MECP2, TIMP4, ATP1A2, ADORA1, CTNNB1, MBP, ATXN1, KIF1B, ABAT, LGI4, UNC13C, NCAN, DLG2. |
Four hundred forty-six mRNAs were enriched in the SN and there were 163 functional clusters. In order to find the most synaptically-enriched pathways, we used a higher threshold fold-change of 25%. The top 5 clusters are shown (BH, P < 0.05). Gene symbols are shown for each cluster.
The greater resolution of SN preparation captures the molecular effects of alcohol
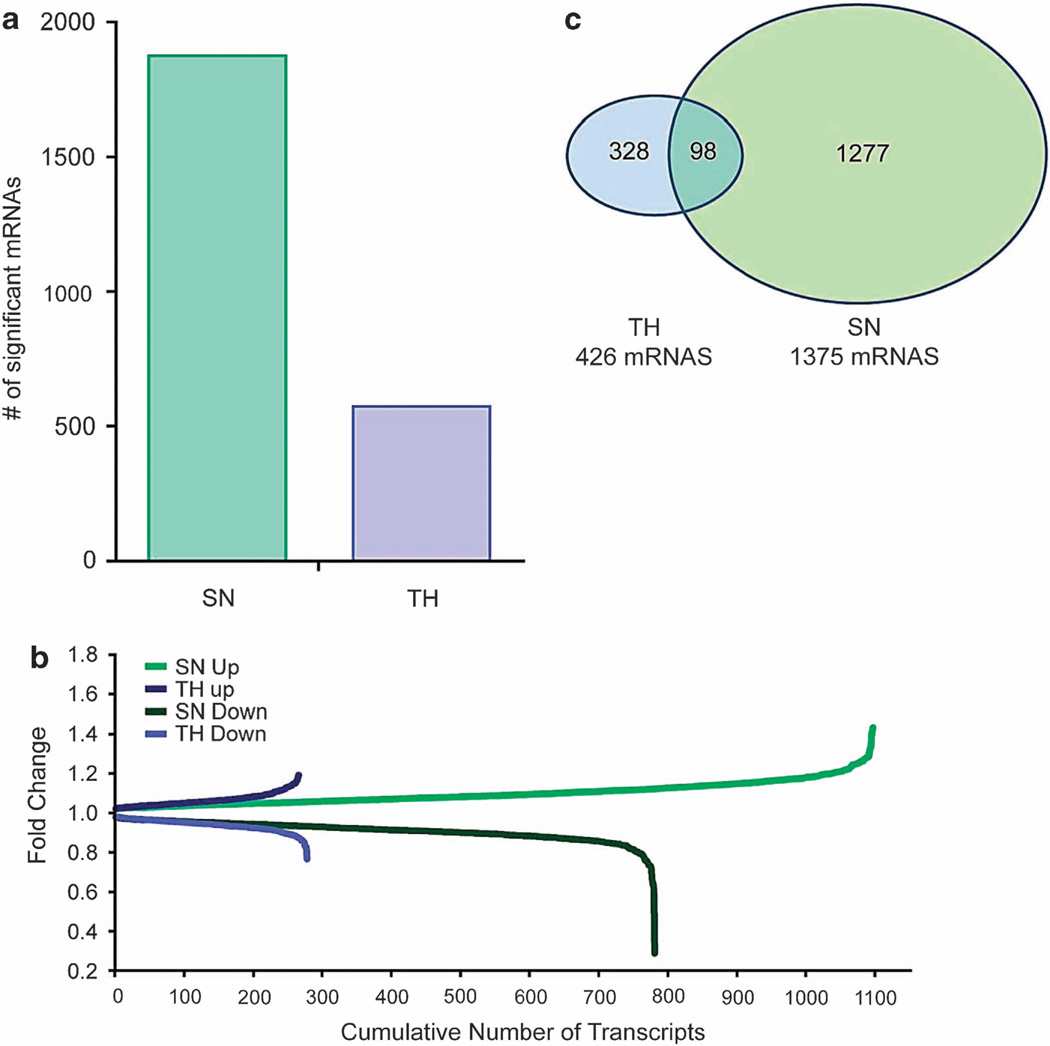
We next identified mRNAs from the amygdala of 8 alcohol-treated and 13 control mice, for a total of 21 SN and paired TH samples. In SNs, 1531 alcohol-responsive mRNAs were identified, compared with 462 in THs (Figure 2a). Examples of alcohol-responsive mRNAs, fold-changes and P-values are shown in Table 3, and the full list is shown in Supplementary Table S4. We examined the number and magnitude of fold-changes (Figure 2b) as a potential means for identifying the most important mRNAs involved in alcohol-induced changes. SNs had three times more alcohol-responsive mRNAs and larger fold-changes than THs. Twenty-three percent of the TH alcohol-responsive mRNAs were also detected in SN, compared with only 7% of SN alcohol-responsive mRNAs that were detected in THs (Figure 2c). A functional annotation of the top alcohol-responsive mRNAs revealed a higher enrichment score for synaptic mRNAs in SNs than in THs. IPA was used to study the molecular and cellular functions of SN alcohol-responsive mRNAs, highlighting the following top five pathways: molecular transport, protein trafficking, RNA posttranscriptional modification, cell morphology, and DNA replication, recombination, and repair. Importantly, the alcohol-responsive mRNA list from SNs was strikingly similar to the mRNA list obtained from human alcoholics (Table 4), suggesting that the alcohol-drinking paradigm used in this study induced similar mRNA changes in mouse amygdala to that observed in human amygdala23 and also demonstrating that SNs are ideal for characterizing transcriptome changes in chronic ethanol-treated mice that are relevant in human alcoholics. The SN alcohol-responsive mRNAs were highly enriched for neuron process-localized mRNAs36 and contained many alcohol-consumption quantitative trait locus genes found in mice,37 further highlighting this preparation as a tool to detect alcohol-responsive mRNAs related to synaptic function and structure.
Figure 2.
(a) The number of alcohol-responsive annotated mRNAs identified in paired synaptoneurosome (SN) and total homogenate (TH) samples for P < 0.05 are shown (n = 8 for alcohol and n = 13 for control). (b) Fold-change produced by alcohol consumption is shown as a function of the cumulative number of transcripts. Alcohol-induced changes in the number of transcripts and magnitude of fold-changes. (c) Venn diagram showing the overlap in alcohol-responsive unique mRNAs between the SN and TH preparations (P < 0.05).
Table 3.
Thirty alcohol-responsive mRNAs in synaptoneurosomes (SN) that were not significantly changed in total homogenates (TH).
| Illumina ID | Gene symbol |
Gene info | SN correlation with alcohol consumption |
SN fold change |
SN P-value |
TH Fold change |
TH P-value |
|---|---|---|---|---|---|---|---|
| ILMN_1229256 | Bzrap1 | Benzodiazepine receptor associated protein 1 | − 0.48 | 0.80 | 2.00E-02 | 0.99 | 8.72E-01 |
| ILMN_1222167 | Gria2 | Glutamate receptor, ionotropic, AMPA2 (alpha 2) | − 0.47 | 0.81 | 2.37E-02 | 1.04 | 2.74E-01 |
| ILMN_2483253 | Dicer1 | Dicer 1, ribonuclease type III | − 0.54 | 0.86 | 5.71E-03 | 1.01 | 8.13E-01 |
| ILMN_1240346 | Socs5 | Suppressor of cytokine signaling 5 | − 0.62 | 0.86 | 1.14E-03 | 0.97 | 5.75E-01 |
| ILMN_2644632 | Stxbp1 | Syntaxin binding protein 1 | − 0.66 | 0.86 | 5.84E-04 | 1.01 | 8.89E-01 |
| ILMN_1231506 | Cnrip1 | Cannabinoid receptor interacting protein 1 | − 0.67 | 0.87 | 5.71E-04 | 1.00 | 9.94E-01 |
| ILMN_3105417 | Bdnf | Brain derived neurotrophic factor | − 0.60 | 0.88 | 2.98E-03 | 0.95 | 2.52E-01 |
| ILMN_2622817 | Kcnq2 | Potassium voltage-gated channel, subfamily Q, member 2 | − 0.46 | 0.88 | 2.77E-02 | 1.04 | 2.74E-01 |
| ILMN_3061460 | Ntrk2 | Neurotrophic tyrosine kinase, receptor, type 2 | − 0.42 | 0.88 | 4.04E-02 | 0.98 | 5.22E-01 |
| ILMN_2724044 | Sncb | Synuclein, beta | − 0.49 | 0.89 | 3.41E-02 | 1.02 | 6.21E-01 |
| ILMN_2760927 | Kcna6 | Potassium voltage-gated channel, shakerrelated, subfamily, member 6 | − 0.47 | 0.90 | 2.25E-02 | 0.97 | 5.43E-01 |
| ILMN_2713841 | Hspd1 | Heat shock protein 1 (chaperonin) | 0.47 | 1.00 | 4.01E-02 | 0.97 | 5.63E-01 |
| ILMN_1217180 | Ifitm1 | Interferon induced transmembrane protein 1 | 0.46 | 1.11 | 2.96E-02 | 1.09 | 1.41E-01 |
| ILMN_1242178 | Adh5 | Alcohol dehydrogenase 5 (class III), chi polypeptide | 0.46 | 1.15 | 2.65E-02 | 0.99 | 7.31E-01 |
| ILMN_2733179 | Aldh2 | Aldehyde dehydrogenase 2, mitochondrial | 0.46 | 1.15 | 2.76E-02 | 0.96 | 5.25E-01 |
| ILMN_1214715 | Gfap | Glial fibrillary acidic protein | 0.49 | 1.16 | 1.31E-02 | 1.03 | 4.81E-01 |
| ILMN_1231625 | Cyp4f14 | Cytochrome P450, family 4, subfamily f, polypeptide 14 | 0.62 | 1.17 | 2.82E-03 | 1.01 | 6.59E-01 |
| ILMN_2771956 | Calu | Calumenin | 0.60 | 1.17 | 3.00E-03 | 1.03 | 1.21E-01 |
| ILMN_2881480 | Vamp3 | Vesicle-associated membrane protein 3 | 0.54 | 1.17 | 1.16E-02 | 0.95 | 8.43E-02 |
| ILMN_2945095 | Tnfrsf10b | Tumor necrosis factor receptor superfamily, member 10b | 0.49 | 1.18 | 1.63E-02 | 1.03 | 5.29E-01 |
| ILMN_1229720 | Tollip | Toll interacting protein | 0.44 | 1.18 | 2.09E-02 | 1.07 | 9.35E-02 |
| ILMN_2719908 | Cyp2j9 | Cytochrome P450, family 2, subfamily j, polypeptide 9 | 0.49 | 1.18 | 2.03E-02 | 1.06 | 6.38E-02 |
| ILMN_2705777 | Gstm5 | Glutathione S-transferase, mu 5 | 0.61 | 1.20 | 2.32E-03 | 1.02 | 7.91E-01 |
| ILMN_1255438 | Cpped1 | Calcineurin-like phosphoesterase domain containing 1 | 0.71 | 1.20 | 2.65E-04 | 1.00 | 9.80E-01 |
| ILMN_2760800 | Cxcl14 | Chemokine (C-X-C motif) ligand 14 | 0.53 | 1.20 | 9.84E-03 | 1.03 | 6.64E-01 |
| ILMN_2680745 | Gabbr1 | Gamma-aminobutyric acid (GABA) B receptor, 1 | 0.40 | 1.20 | 3.23E-02 | 1.00 | 9.82E-01 |
| ILMN_2776008 | Gstk1 | Glutathione S-transferase kappa 1 | 0.52 | 1.21 | 1.36E-02 | 0.99 | 7.63E-01 |
| ILMN_2619620 | C1qb | Complement component 1, q subcomponent, beta polypeptide | 0.45 | 1.21 | 3.30E-02 | 0.99 | 8.93E-01 |
| ILMN_3149251 | Glud1 | Glutamate dehydrogenase 1 | 0.40 | 1.23 | 3.84E-02 | 1.07 | 2.70E-01 |
| ILMN_1239110 | Eef2 | Eukaryotic translation elongation factor 2 | 0.47 | 1.42 | 1.48E-02 | 1.08 | 2.69E-01 |
SN mRNA correlation with alcohol consumption, SN and TH treatment fold-change, and p-values are shown. Fold-change >1 indicates an increase in expression and fold-change < 1 indicates a reduction.
Table 4.
Over-representation of human alcoholic mRNA23, process mRNAs 36 and QTL genes 37 affected by alcohol are shown for the alcohol-responsive synaptoneurosome (SN) and total homogenate (TH) mRNAs
| Human alcohol-responsive genes | Cell process mRNAs | Alcohol QTL genes | ||||
|---|---|---|---|---|---|---|
| Number of mRNAs | P-value | Number of mRNAs | P-value | Number of mRNAs | P-value | |
| SN alcohol-responsive mRNAs | 327 | 1.39E-04 | 1107 | 3.07E-03 | 358 | 8.74E-02 |
| TH alcohol-responsive mRNAs | 83 | 7.59E-01 | 358 | 7.54E-02 | 114 | 2.77E-01 |
Abbreviation: QTL, quantitative trait loci. The significant over-representations are highlighted in bold.
Alcohol affects the transcriptome in a coordinated manner
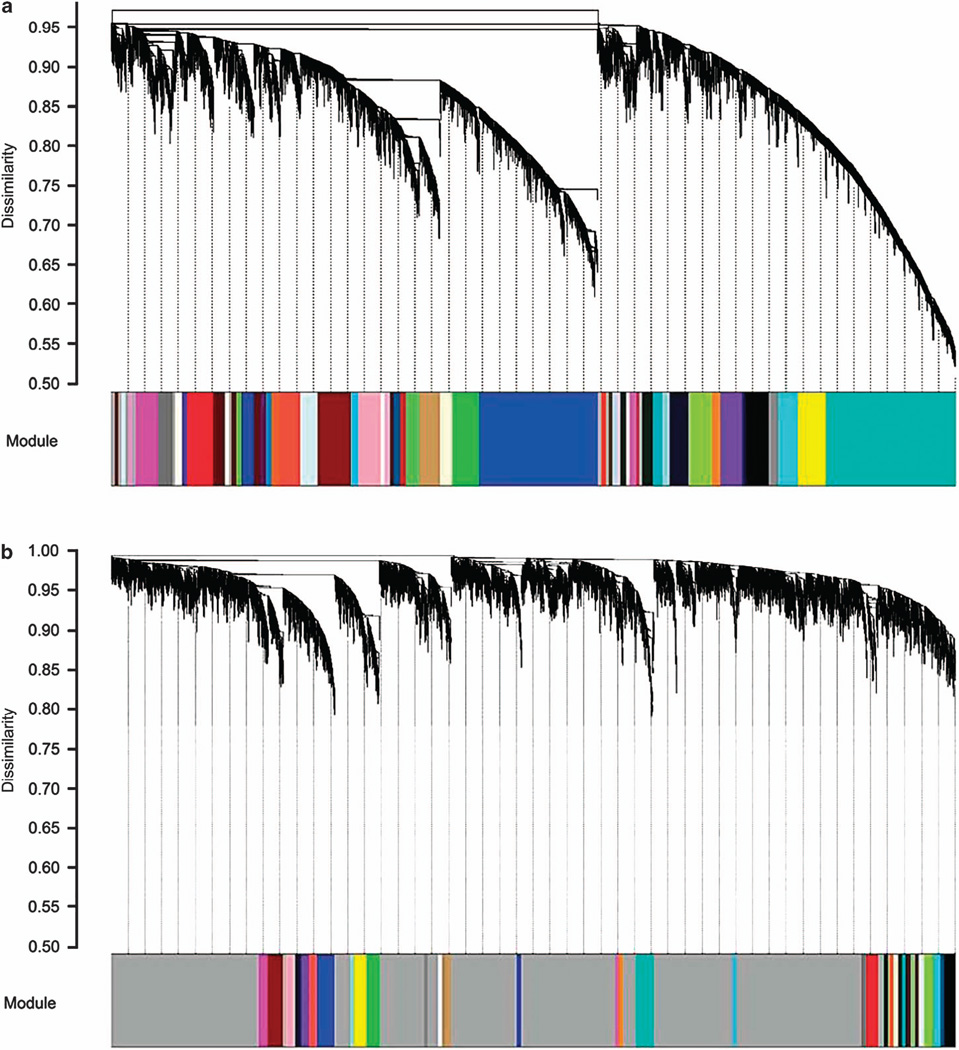
We used WGCNA32 to group genes into modules that have strong co-varying (similar) patterns of expression across the sample set. Hierarchical clustering showed that the SN preparation contains groups of mRNAs that have highly coordinated patterns of expression (Figure 3a). When using the same parameters for THs, the majority of mRNAs showed dissimilar levels of expression (marked by the gray color) (Figure 3b). We adjusted the TH network parameters to optimizing module correlation with alcohol consumption. Allowing for less similarity between the mRNAs would enable greater detection of group-clustered modules in THs (Supplementary Figure S3a). However, the change in parameters did not significantly affect the correlation between the TH modules and alcohol consumption (Supplementary Figure S3b). In SNs, 40% of the modules were significantly correlated with alcohol consumption (average r = 0.6, P < 0.05) (Supplementary Figure S3c). We then determined the modules that were over-represented with alcohol-responsive mRNAs (referred to as ‘alcohol-responsive modules’). In SNs, 10 out of the 54 alcohol-responsive modules were detected (8 were upregulated and 2 were downregulated; Supplementary Table S5).
Figure 3.
(a) Results of a dendrogram-hierarchical cluster of mRNAs from synaptoneurosomes (SNs) (n = 21). In the cluster, each end point represents a gene, and the genes are arranged by similarity in covariance. Genes under the same branch of the dendrogram are more similar than those outside of the branch, and their dissimilarity is represented by the y axis. Gray represents genes unrelated to others. SN preparation contains groups of mRNAs that have highly coordinated patterns of expression as seen by the low dissimilarity values. (b) Results of a dendrogram-hierarchial cluster of mRNAs from total homogenate (TH; n = 21). High dissimilarity values (marked by the gray color) indicate that the TH contains fewer mRNAs with similar patterns of expression.
These modules were significantly associated with biological pathways such as those associated with long-term potentiation and depression and RNA processing and contained many mRNAs associated with potassium channels, glutamate and GABA systems. Upregulated mRNAs include Camkk2, Camta1, Capn2, Ntrk2, Ntsr2, Stx18, Stx8, Stxbp4, Syap1, Synj2bp, Prkcdbp and Grk6. Downregulated mRNAs include brain-derived neurotrophic factor (BDNF), Camsap3, Capn6, Negr1, Nptn, Ntrk2, Unc5c, Stx3, Stxbp1, Stxbp2, Syncrip, Sst, Sstr2, Sncb and Timp4. The potassium channel family was also highly responsive to alcohol and includes the voltage-gated potassium channels (Kcna6 and Kcnq2, downregulated), calcium-activated Kcnu1 (SLO-3-slowpoke3, downregulated) and inwardly rectifying potassium channels (Kcnj1 and Kctd20, upregulated). The following glutamate- and GABA-related transcripts were upregulated: Grk6, Glud1, Slc1a2, Slc1a3, Gabbr1, and Gabrb2, whereas the following were downregulated: Grina, Gria2, Grip1, VGlut2 (Slc17a6), Grm7, and Narg2.
As mentioned, RNA processing machinery was a highly over-represented biological pathway associated with alcohol-responsive mRNAs (Figure 4). These mRNAs include RNA transcriptional, translational, spliceosomal and editing machineries, as well as many mRNAs for ribosomal proteins, suggesting that chronic alcohol affects translational mechanisms in the synapse.
Figure 4.
Alcohol regulates RNA processing and translational machinery in the synapse. The figure illustrates examples of alcohol-responsive mRNAs related to known RNA processing pathways. The cartoon represents the postsynaptic compartment, which was enriched in the synaptoneurosome preparation.
SN alcohol-responsive effects are cell-type specific
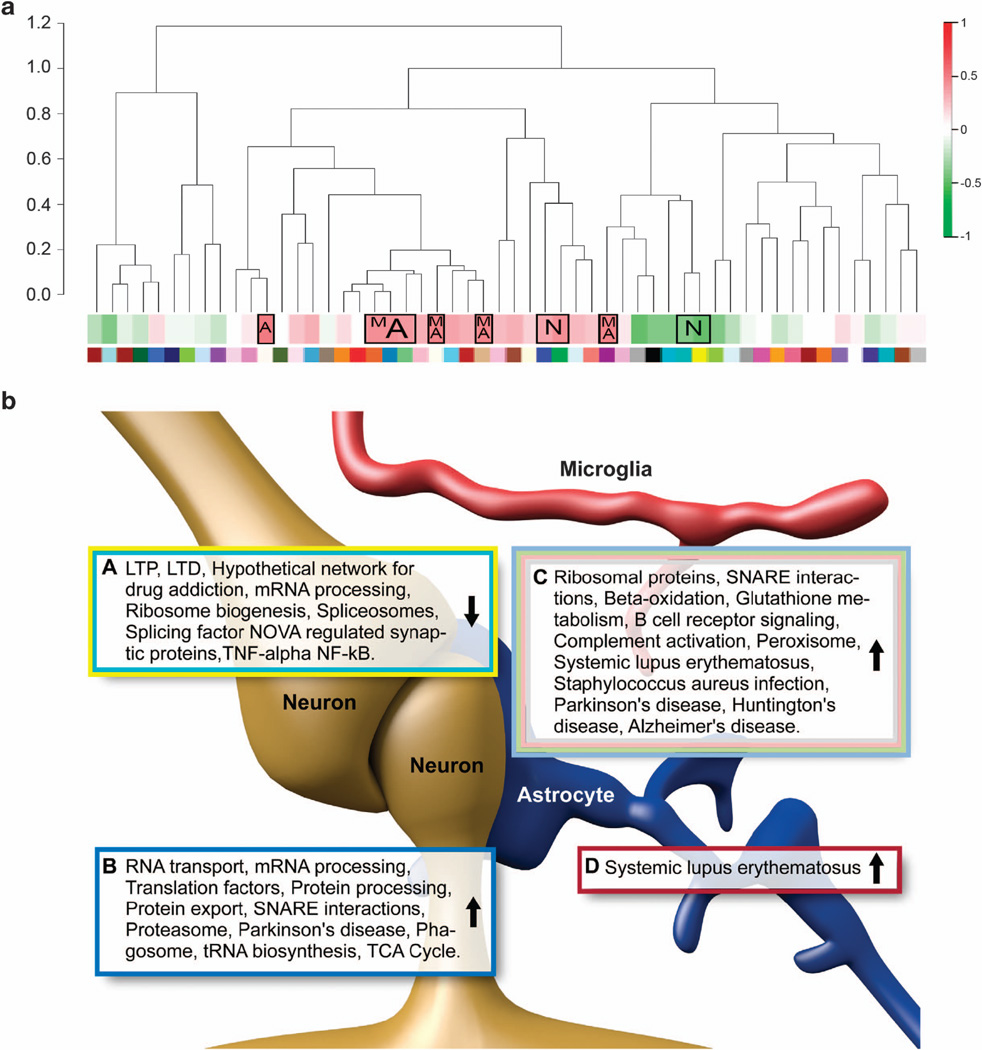
A cell-type-specific enrichment analysis was performed for the alcohol-responsive mRNAs using neuronal, astrocytic, oligodendrocyte, microglial, GABA and glutamate gene lists (see Methods for details). The upregulated alcohol-responsive mRNAs in SNs were enriched with microglial, astrocytic and GABAergic cell types, while the downregulated mRNAs were enriched in neuronal cell types (Table 5). This trend of upregulation of microglial cell types was also true for the SN modules in the WGCNA network (Figure 5a). Of the eight alcohol-responsive upregulated modules in SN, six were also positively correlated with alcohol consumption and enriched with microglia and astrocyte mRNAs (Table 6). The two downregulated modules were negatively correlated with alcohol consumption and enriched in neuronal mRNAs. DAVID and Webgestalt were used to find the KEGG and Wikipathways over-represented in each of the modules (Figure 5b). In the THs, only three modules were found to be cell specific (two astrocytic/microglial and one neuronal).
Table 5.
Over-representation of the cell types for the alcohol-responsive mRNAs in synaptoneurosomes (SN) and total homogenates (TH)
| Data set | Microglia | P-value | Astrocytes | P-value | Neuron | P-value | Oligodendrocytes | P-value | Glutamate | P-value | GABA | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SN enriched | 28 | 5.02E − 04 | 250 | 1.03E − 27 | 39 | 8.68E − 01 | 45 | 6.10E − 05 | 102 | 1.13E − 03 | 152 | 4.71E − 22 |
| TH enriched | 15 | 5.97E − 01 | 43 | 1.00E+00 | 101 | 2.55E − 13 | 24 | 7.34E − 01 | 166 | 1.71E − 20 | 74 | 4.88E − 01 |
| Alcohol’s effect on SN | 25 | 5.40E − 02 | 115 | 5.33E − 05 | 55 | 5.87E − 01 | 19 | 9.94E − 01 | 99 | 4.10E − 01 | 99 | 1.30E − 01 |
| Alcohol’s effect on TH | 7 | 3.94E − 01 | 26 | 4.95E − 01 | 18 | 4.92E − 01 | 7 | 8.60E − 01 | 32 | 4.53E − 01 | 27 | 6.46E − 01 |
| Alcohol-responsive upregulated SN | 20 | 5.81E − 03 | 91 | 2.51E − 09 | 8 | 1.00E+00 | 14 | 8.94E − 01 | 60 | 4.36E − 01 | 70 | 1.27E − 02 |
| Alcohol-responsive downregulated SN | 5 | 8.52E − 01 | 24 | 9.52E − 01 | 47 | 5.12E − 07 | 5 | 9.95E − 01 | 39 | 4.88E − 01 | 29 | 8.94E − 1 |
The number of overlapping mRNAs and over-representation P-values are shown for microglia, astrocyte, neuron, oligodendrocyte, glutamate and GABA mRNA lists. The significant values (P < 0.05) are bolded. Fold changes above 10% were used for the SN- and TH-enriched lists.
Figure 5.
(a) Alcohol-responsive synaptic modules, correlation with alcohol consumption and cell specificity. The dendrogram shows the hierarchical relationship between the gene modules for the synaptoneurosome (SN) mRNA network. Below the dendrogram, a heatmap shows the module correlation to amount of alcohol consumed; red and green indicate strong positive and negative correlations, respectively. The boxed correlations represent the significant alcohol-responsive modules (over-representation-hypergeometric test), and the letters inside the boxes represent the cell type over-represented in the modules: N = neuron, M = microglia, and A = astrocyte. The different color under the dendrogram correspond to the different modules, and these match with the colors in Figure 3a. (b) Alcohol-responsive modules, cell specificity and biological pathways. The KEGG and Wikipathways and biological functions identified as alcohol-responsive are shown for each of the modules in SN, illustrating the most critical biological functions in groups of co-expressed mRNAs (DAVID (Database for Annotation Visualization and Integrated Discovery) and Webgestalt, BH (Benjamini–Hochberg method) corrected P < 0.05). Pathways from modules with similar expression were grouped together for each of the over-represented cell types. The arrow in each box represents the direction of alcohol response (that is, upregulation or downregulation by alcohol treatment). The text-box border colors correspond to the module colors. Boxes A and B are enriched for neurons; Box C is enriched for microglia and astrocytes; Box D is enriched for astrocytes. Cell type illustrations were modified from Kettenman et al., 2013.73
Table 6.
Over-representation of cell type specific mRNA in the synaptoneurosome (SN) modules based on a hypergeometric test with microglia, astrocyte, neuron and oligodendrocyte mRNA lists
| Module | No. of significant mRNAs in module |
P-value | Average fold change |
Microglia | P-value | Astrocytes | P-value | Neuron | P-value | Oligodendrocytes | P-value | Correlation | P-value | KEGG pathways |
Wikipathways |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tan | 237 | 4.27E − 64 | 1.08 | 11 | 6.52E − 04 | 27 | 1.71E − 05 | 3 | 1.00E+00 | 7 | 4.47E − 01 | 0.05 | 8.30E − 01 | Oxidative phosphorylation, metabolic pathways, Parkinson's disease, systemic lupus erythematosus, peroxisome, Huntington's disease, Alzheimer's disease, valine, leucine and isoleucine degradation, pyrimidine metabolism, ribosome, Staphylococcus aureus infection, purine metabolism, proteasome |
Electron transport chain, complement
activation, classical pathway, oxidative phosphorylation, mitochondrial LC-fatty acid beta-oxidation |
| Ivory | 39 | 1.88E − 08 | 1.07 | 4 | 8.82E − 03 | 15 | 3.95E − 08 | 1 | 9.83E − 01 | 3 | 1.90E − 01 | 0.50 | 2.23E − 02 | Keap1-Nrf2, glutathione metabolism, glutathione and one-carbon metabolism |
Glutathione metabolism, B-cell receptor
signaling pathway |
| Blue | 753 | 1.42E − 49 | 1.06 | 18 | 4.59E − 01 | 50 | 8.05E − 01 | 110 | 2.29E − 03 | 23 | 9.68E − 01 | − 0.75 | 9.88E − 05 | Metabolic pathways, proteasome,
oxidative phosphorylation, protein processing in endoplasmic reticulum, phagosome, aminoacyl-tRNA biosynthesis, protein export, lysosome, ubiquitin-mediated proteolysis, Parkinson's disease, collecting duct acid secretion, glycolysis/ gluconeogenesis, RNA transport, nucleotide excision repair, SNARE interactions in vesicular transport, vasopressin-regulated water reabsorption |
Proteasome degradation, translation factors,
mRNA processing, oxidative phosphorylation, electron transport chain, TCA cycle |
| Sienna3 | 42 | 6.84E − 06 | 1.06 | 2 | 2.60E − 01 | 11 | 3.40E − 04 | 0 | 1.00E+00 | 0 | 1.00E+00 | 0.50 | 1.98E − 02 | Systemic lupus erythematosus | NA |
| Lightgreen | 82 | 9.00E − 06 | 1.06 | 10 | 5.29E − 05 | 24 | 8.66E − 08 | 2 | 1.00E+00 | 2 | 9.06E − 01 | 0.52 | 1.55E − 02 | Ribosome, oxidative
phosphorylation, Huntington's disease, Parkinson's disease, Alzheimer's disease, SNARE interactions in vesicular transport, metabolic pathways, peroxisome |
Electron transport chain, cytoplasmic
ribosomal proteins, oxidative phosphorylation |
| Steelblue | 40 | 3.51E − 03 | 1.05 | 5 | 5.75E − 03 | 14 | 1.78E − 05 | 1 | 9.97E − 01 | 1 | 8.83E − 01 | 0.38 | 9.06E − 02 | NA | NA |
| Green | 141 | 1.41E − 04 | 1.03 | 4 | 6.96E − 01 | 26 | 5.56E − 03 | 33 | 2.54E − 02 | 5 | 9.40E − 01 | − 0.57 | 7.16E − 03 | NA | NA |
| Floralwhite | 24 | 2.08E − 02 | 1.03 | 0 | 1.00E+00 | 6 | 2.21E − 02 | 0 | 1.00E+00 | 0 | 1.00E+00 | 0.52 | 1.51E − 02 | Amino-acid metabolism, mRNA processing, TCA cycle |
Spliceosome, alanine, aspartate and
glutamate metabolism |
| Yellow | 149 | 1.07E − 04 | 0.96 | 0 | 1.00E+00 | 4 | 1.00E+00 | 49 | 3.63E − 07 | 6 | 8.64E − 01 | 0.32 | 1.57E − 01 | NA | Hypothetical network for drug addiction,
TNF-alpha NF-kB signaling pathway, PluriNetWork, mRNA processing, splicing factor NOVA-regulated synpatic proteins |
| Turquoise | 747 | 4.62E − 33 | 0.94 | 5 | 9.98E − 01 | 15 | 1.00E+00 | 101 | 2.04E − 05 | 22 | 7.90E − 01 | − 0.05 | 8.17E − 01 | Long-term potentiation, long-term depression |
mRNA processing, hypothetical network for
drug addiction |
The significant values (P < 0.05) are bolded. Average fold-change < 1 indicates an increase in gene expression in a module and fold-changeo1 indicates reduction in gene expression.
The immune system, specifically the neuroimmune system, has been recently implicated in alcohol dependence,41 and LPS treatment, which activates an immune response, enhances alcohol consumption in mice.42 An over-representation analysis of the SN alcohol-responsive mRNAs with a list of LPS-regulated mRNAs showed a significant representation of LPS mRNAs (P < 0.05) and highlighted 55 common mRNAs found in chronic alcohol and chronic LPS treatment. Many astrocyte and microglial transcripts related to neuroimmune signaling were upregulated by chronic alcohol consumption. Key immune/inflammatory genes, including Tnfaip8l2, Tnfrsf10b, Traf4 and Tollip, were all upregulated by alcohol. In addition, chemokine and complement-related transcripts were upregulated, including the following: Ccr5, C1qa, C1qb, Ccrn4l, CCR4, Cxcl14, Gfap and Gbas. The gluthathione and peroxisome pathways were altered by alcohol, almost all of which were upregulated (Gstk1, Gstm5, Gstm6, Gpt2, Gpx4, Gpx7, Pex5, Pex6, Prdx3).
DISCUSSION
We profiled mRNAs from SNs and paired TH preparations from the amygdala of ethanol-treated mice, using a within-subject comparison, and found a robust difference between the SN and TH alcohol-responsive mRNAs. A greater number of alcohol-responsive mRNAs with larger fold-changes were detected in the SNs as well as a greater enrichment of synaptic mRNAs. Our results suggest that the SN is a useful preparation for studying synaptic (both neuronal and glial) molecular changes associated with chronic alcohol consumption.
Although there are other reports of RNA composition in synaptic preparations,36,43–47 there have been no direct comparisons of synaptic vs paired TH. The SN preparation has been used to identify synaptic networks related to neurodegenerative disorders,48 mental retardation,13 schizophrenia49 and cocaine addiction.50 However, this is the first alcohol study utilizing SN preparations. We used a model of alcohol consumption that produces intoxicating blood ethanol concentrations51 and induces mRNA expression changes in the prefrontal cortex of mice,21 as well as functional changes in the nucleus accumbens.51 Our results, showing that the THs from amygdala contained 500 differentially expressed mRNAs, are consistent with previous findings.21 A key finding is that the chronic alcohol paradigm used here in mice induces changes in the transcriptome of the amygdala that are similar to those observed in the amygdala of human alcoholics (Table 4).23 Our current results also highlight the utility of SNs compared with THs in studying alcohol’s molecular effects, given that the overlapping expression changes between mouse and human were observed using SNs but not in our previous studies using THs.21
There are two possible explanations for the difference between the SN and TH preparations. First, restricting gene expression profiling to the synaptic compartments (preventing dilution with the somatic transcriptome) should facilitate detection of specific mRNAs that are localized to the synapse. Our results from the weighted gene co-expression networks showed many mRNAs with similar or overlapping patterns of expression in SNs, while the TH network contained few overlapping networks. The similarity in functional gene networks in SNs facilitates the detection of alcohol actions that are specific to the synaptic region. Second, alcohol could selectively target synaptic mRNAs, ultimately changing gene expression in the synapse. This is supported by the finding that RNA processing machinery was responsive to alcohol. For example, RNA transcriptional, translational, spliceosomal and editing machineries, as well as many ribosomal proteins, were over-represented in the alcohol-responsive mRNAs and the different modules, suggesting that chronic alcohol use affects translation in the synapse. Studies of synaptic compartments show involvement of microRNAs in regulating synaptic translation of mRNAs.15,16,52 Furthermore, microRNAs, their precursors and processing enzymes show synaptic localization, suggesting a well-orchestrated microRNA regulation in the synapse.53–55 In fact, our data show that synaptic microRNA enzymes such as Dicer1 and Eif2c3 are alcohol sensitive. Previous studies from our group anticipated that Dicer would be a predicted target of microRNAs in human alcoholic brain samples.56 It is appealing to propose that alcohol affects synaptic microRNA machinery, allowing for targeted regulation of gene expression in the synapse.
We found that BDNF and its receptor TrkB as well as potassium channels were all altered by alcohol, corroborating well-documented alcohol-induced changes in these receptors. BDNF was downregulated, whereas one TrkB transcript was downregulated and one was upregulated. These results might be due to probe hybridization differences stemming from different splice variants. BDNF is involved in synaptic plasticity,57,58 obesity59 and addiction,60 and potassium channels have been associated with increased sensitivity and tolerance to the sedative effects of ethanol,61 seizure susceptibility,62 neonatal familial convulsions and epilepsy63 and may be important in the withdrawal-induced seizures caused by chronic alcohol consumption in humans.
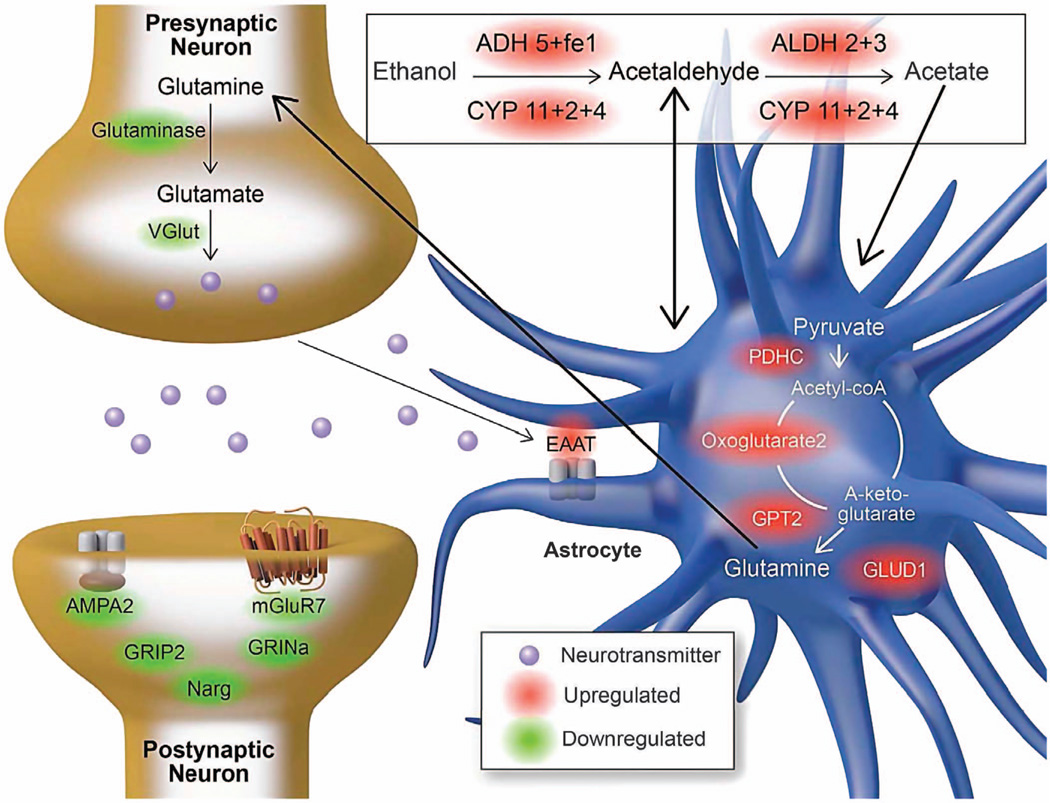
Chronic alcohol use might result in increased metabolism of alcohol or acetate in the brain.64 A study in human alcoholics showed an increase in glutamate–glutamine and GABA labeling in heavy compared with light drinkers.64 In our study, we found many alcohol-metabolizing enzymes that were upregulated by alcohol consumption, in agreement with a previous study.65 Furthermore, alcohol-responsive glutamate and GABA mRNAs were detected in SNs (GABA-related mRNAs were associated with the upregulated alcohol-responsive mRNAs, see Table 5). Figure 6 illustrates how the alcohol-responsive mRNAs can participate in alcohol degradation to produce metabolites that can enter the TCA cycle and be converted into glutamate, which may contribute to the dysregulation in the glutamate system seen in alcoholics.
Figure 6.
Theoretical model for metabolism of alcohol in the brain. Alcohol-responsive mRNAs associated with alcohol metabolism and the glutamate metabolic pathway in synaptoneurosomes. Upregulated mRNAs are shown in red, and downregulated mRNAs are in green.
The sensitivity of the SN preparation compared with the TH also allowed for improved cell-type enrichment analysis, enabling the detection of a high positive correlation of astrocyte and microglial mRNAs with alcohol consumption in the SNs. All of the alcohol-responsive genes in these astrocyte/microglia modules were upregulated. Given that these cell types are generally associated with neuroinflammation, a potential consequence of chronic alcohol use is activation of neuroimmune signaling. The adaptation of the neuroimmune system is consistent with data from the amygdala of human alcoholics23 and supports the emerging concept that there is a neuroimmune response to chronic alcohol use.41 In addition, astrocytes might have role in regulating synaptic plasticity by altering the levels of glutamate, GABA and tumor necrosis factor-alpha available in the synapse.66 We found that all three of these systems were sensitive to alcohol, including glutamate and GABA metabolizing enzymes, receptors and transporters and tumor necrosis factor-alpha receptors and their interacting proteins. This suggests that the upregulated astrocyte-specific genes could induce a wide range of effects following chronic alcohol consumption, ranging from neuroimmune to plasticity responses. The SN preparation also enriches for perisynaptic microglial and astroglial processes.67,68 As microglia and astrocytes can actively engage in synaptic function68 and have been associated with alcoholism,23 the SN preparation may be useful in investigating alcohol’s effects on the neuroimmune system. Because the cell-type specificity in our SN preparation is not known, we used a comprehensive bioinformatics approach similar to Ponomarev et al.,23 including identifying mRNAs which are co-expressed with known astro-glial markers and examining the astro-glia mRNAs altered by alcohol consumption.
Extra-nuclear splicing has been discovered as a process involved in synaptic structure and function,69 and this process may explain why nuclear mRNAs were found in the SN preparation. Alternatively, there could be some nuclear contamination. The estimate of nuclear contamination in our SN preparation is based on two main measurements: (1) DAPI (4',6-diamidino-2-phenylindole) staining of nuclear DNA, showing no detected staining in the SNs (Supplementary Figure S2).70 (2) Neun (a nuclear protein) western blots, showing the SN preparation decreases 75% of the Neun found in the TH.70
Although there is evidence for synaptic translation in postsynaptic neuronal compartments,71 translation might also take place in the presynaptic compartment (axonal terminal).72 The SN preparation enriches for both the presynaptic and the postsynaptic fractions, and further research is warranted to determine whether alcohol differentially affects these two compartments.
In summary, we identified coordinated changes in mRNA expression in SNs and THs following chronic alcohol consumption. The expression changes in SNs from mouse amygdala corroborate that seen in the amygdala of human alcoholics and include overlapping changes in GABA, glutamate and neuroimmune pathways. Our results demonstrate that (1) the mouse chronic-drinking paradigm used in this study is sufficient to produce the same expression changes previously seen in human alcoholics and (2) the parallel changes are evident for the SN but not TH mouse transcriptome. Our results highlight the advantage of the mouse SN preparation for identifying therapeutic gene targets for alcohol dependence that are relevant in humans and for studying synaptic plasticity under normal and disease conditions.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kimberly Raab-Graham for valuable instruction in the isolation of synaptoneurosomes and for providing the PSD-95 antibody. We thank Jill Benavidez and Kathryn Ondricek for help with the behavioral paradigm and synaptoneurosome preparation. We thank Joseph Corey for the generous assistance with computational analysis, Marianna Grenadier for help with the illustrations and Jody Mayfield for helpful scientific editing. This work was supported by the National Institute of Health Grants 1F31-AA022557-01, AA-UO1-13520, AA012404, RC2AA019382 and AA020683.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website (http://www.nature.com/tpj)
REFERENCES
- 1.Wang Y, Kim JD, Saetre R, Pyysalo S, Tsujii J. Investigating heterogeneous protein annotations toward cross-corpora utilization. BMC Bioinformatics. 2009;10:403. doi: 10.1186/1471-2105-10-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 3.Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- 4.Arlinde C, Sommer W, Bjork K, Reimers M, Hyytia P, Kiianmaa K, et al. A cluster of differentially expressed signal transduction genes identified by microarray analysis in a rat genetic model of alcoholism. Pharmacogenomics J. 2004;4:208–218. doi: 10.1038/sj.tpj.6500243. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Lewohl JM, Harris RA, Iyer VR, Dodd PR, Randall PK, et al. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology. 2006;31:1574–1582. doi: 10.1038/sj.npp.1300947. [DOI] [PubMed] [Google Scholar]
- 6.Melendez RI, McGinty JF, Kalivas PW, Becker HC. Brain region-specific gene expression changes after chronic intermittent ethanol exposure and early with-drawal in C57BL/6 J mice. Addict Biol. 2012;17:351–364. doi: 10.1111/j.1369-1600.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, Harris RA. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. J Neurochem. 2002;81:802–813. doi: 10.1046/j.1471-4159.2002.00860.x. [DOI] [PubMed] [Google Scholar]
- 8.Kerns RT, Ravindranathan A, Hassan S, Cage MP, York T, Sikela JM, et al. Ethanol-responsive brain region expression networks: implications for behavioral responses to acute ethanol in DBA/2 J versus C57BL/6 J mice. J Neurosci. 2005;25:2255–2266. doi: 10.1523/JNEUROSCI.4372-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansson AC, Rimondini R, Neznanova O, Sommer WH, Heilig M. Neuroplasticity in brain reward circuitry following a history of ethanol dependence. Eur J Neurosci. 2008;27:1912–1922. doi: 10.1111/j.1460-9568.2008.06159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 11.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 12.Kertes DA, Kalsi G, Prescott CA, Kuo PH, Patterson DG, Walsh D, et al. Neuro-transmitter and neuromodulator genes associated with a history of depressive symptoms in individuals with alcohol dependence. Alcohol Clin Exp Res. 2011;35:496–505. doi: 10.1111/j.1530-0277.2010.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang DO, Kim SM, Zhao Y, Hwang H, Miura SK, Sossin WS, et al. Synapse- and stimulus-specific local translation during long-term neuronal plasticity. Science. 2009;324:1536–1540. doi: 10.1126/science.1173205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Wang Z, Xu Y, Joo SH, Kim SK, Xue Z, et al. OsGSR1 is involved in crosstalk between gibberellins and brassinosteroids in rice. Plant J. 2009;57:498–510. doi: 10.1111/j.1365-313X.2008.03707.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang XQ, Kravchuk O, Liu PY, Kempf M, Boogaard CV, Lau P, et al. The evaluation of a clinical scar scale for porcine burn scars. Burns. 2009;35:538–546. doi: 10.1016/j.burns.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Raab-Graham KF, Haddick PC, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science. 2006;314:144–148. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- 17.Barak S, Liu F, Ben Hamida S, Yowell QV, Neasta J, Kharazia V, et al. Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nat Neurosci. 2013;16:1111–1117. doi: 10.1038/nn.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollingsworth EB, McNeal ET, Burton JL, Williams RJ, Daly JW, Creveling CR. Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex: cyclic adenosine 3':5'-monophosphate-generating systems, receptors, and enzymes. J Neurosci. 1985;5:2240–2253. doi: 10.1523/JNEUROSCI.05-08-02240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Kim S, Ryu WS. DDX3 DEAD-Box RNA helicase inhibits hepatitis B virus reverse transcription by incorporation into nucleocapsids. J Virol. 2009;83:5815–5824. doi: 10.1128/JVI.00011-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osterndorff-Kahanek E, Ponomarev I, Blednov YA, Harris RA. Gene expression in brain and liver produced by three different regimens of alcohol consumption in mice: comparison with immune activation. PLoS One. 2013;8:e59870. doi: 10.1371/journal.pone.0059870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang CY, Kim HH, Hiroi Y, Sawada N, Salomone S, Benjamin LE, et al. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal. 2009;2:ra11. doi: 10.1126/scisignal.2000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J Neurosci. 2012;32:1884–1897. doi: 10.1523/JNEUROSCI.3136-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Adachi Y, Imsumran A, Yamamoto H, Piao W, Li H, et al. Targeting for insulin-like growth factor-I receptor with short hairpin RNA for human digestive/gastrointestinal cancers. J Gastroenterol. 2010;45:159–170. doi: 10.1007/s00535-009-0151-6. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Brahmakshatriya V, Zhu H, Lupiani B, Reddy SM, Yoon BJ, et al. Identification of differentially expressed miRNAs in chicken lung and trachea with avian influenza virus infection by a deep sequencing approach. BMC Genomics. 2009;10:512. doi: 10.1186/1471-2164-10-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunning MJ, Ritchie ME, Barbosa-Morais NL, Tavare S, Lynch AG. Spike-in validation of an Illumina-specific variance-stabilizing transformation. BMC Res Notes. 2008;1:18. doi: 10.1186/1756-0500-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin SM, Du P, Huber W, Kibbe WA. Model-based variance-stabilizing transformation for Illumina microarray data. Nucleic Acids Res. 2008;36:e11. doi: 10.1093/nar/gkm1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kauffmann A, Gentleman R, Huber W. arrayQualityMetrics--a bioconductor package for quality assessment of microarray data. Bioinformatics. 2009;25:415–416. doi: 10.1093/bioinformatics/btn647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kauffmann A, Huber W. Microarray data quality control improves the detection of differentially expressed genes. Genomics. 2010;95:138–142. doi: 10.1016/j.ygeno.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Knaub LA, Jensen DR, Young Jung D, Hong EG, Ko HJ, et al. Skeletal muscle-specific deletion of lipoprotein lipase enhances insulin signaling in skeletal muscle but causes insulin resistance in liver and other tissues. Diabetes. 2009;58:116–124. doi: 10.2337/db07-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang K, Zhang H, Kugathasan S, Annese V, Bradfield JP, Russell RK, et al. Diverse genome-wide association studies associate the IL12/IL23 pathway with Crohn Disease. Am J Hum Genet. 2009;84:399–405. doi: 10.1016/j.ajhg.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang PC, Wester BA, Rajaraman S, Paik SJ, Kim SH, Allen MG. Hollow polymer microneedle array fabricated by photolithography process combined with micromolding technique. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:7026–7029. doi: 10.1109/IEMBS.2009.5333317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang K, Tang Z, Yang CJ, Kim Y, Fang X, Li W, et al. Molecular engineering of DNA: molecular beacons. Angew Chem Int Ed Engl. 2009;48:856–870. doi: 10.1002/anie.200800370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, O'Connell JR, McArdle PF, Wade JB, Dorff SE, Shah SJ, et al. From the Cover: Whole-genome association study identifies STK39 as a hypertension susceptibility gene. Proc Natl Acad Sci USA. 2009;106:226–231. doi: 10.1073/pnas.0808358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cajigas IJ, Tushev G, Will TJ, tom Dieck S, Fuerst N, Schuman EM. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron. 2012;74:453–466. doi: 10.1016/j.neuron.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, et al. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci USA. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature. 2009;459:528–533. doi: 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, et al. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- 41.Mayfield J, Ferguson L, Harris RA. Neuroimmune signaling: a key component of alcohol abuse. Curr Opin Neurobiol. 2013;23:513–520. doi: 10.1016/j.conb.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling ptby lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25(Suppl 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian QB, Nakayama K, Okano A, Suzuki T. Identification of mRNAs localizing in the postsynaptic region. Brain Res Mol Brain Res. 1999;72:147–157. doi: 10.1016/s0169-328x(99)00214-4. [DOI] [PubMed] [Google Scholar]
- 44.Eberwine J, Belt B, Kacharmina JE, Miyashiro K. Analysis of subcellularly localized mRNAs using in situ hybridization, mRNA amplification, and expression profiling. Neurochem Res. 2002;27:1065–1077. doi: 10.1023/a:1020956805307. [DOI] [PubMed] [Google Scholar]
- 45.Sung YJ, Weiler IJ, Greenough WT, Denman RB. Selectively enriched mRNAs in rat synaptoneurosomes. Brain Res Mol Brain Res. 2004;126:81–87. doi: 10.1016/j.molbrainres.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 46.Poon MM, Choi SH, Jamieson CA, Geschwind DH, Martin KC. Identification of process-localized mRNAs from cultured rodent hippocampal neurons. J Neurosci. 2006;26:13390–13399. doi: 10.1523/JNEUROSCI.3432-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang XQ, Liu PY, Kempf M, Cuttle L, Mill J, Kimble RM. Burn wound infection in a porcine burn model. J Burn Care Res. 2009;30:369–370. doi: 10.1097/BCR.0b013e318198a757. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Shi J, Kim YS, Zhai S, Jia B, Zhao H, et al. Improving tumor-targeting capability and pharmacokinetics of (99m)Tc-labeled cyclic RGD dimers with PEG (4) linkers. Mol Pharm. 2009;6:231–245. doi: 10.1021/mp800150r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smalheiser NR, Lugli G, Zhang H, Rizavi H, Cook EH, Dwivedi Y. Expression of microRNAs and other small RNAs in prefrontal cortex in schizophrenia, bipolar disorder and depressed subjects. PLoS One. 2014;9:e86469. doi: 10.1371/journal.pone.0086469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eipper-Mains JE, Kiraly DD, Palakodeti D, Mains RE, Eipper BA, Graveley BR. microRNA-Seq reveals cocaine-regulated expression of striatal microRNAs. RNA. 2011;17:1529–1543. doi: 10.1261/rna.2775511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Middaugh LD, Szumlinski KK, Van Patten Y, Marlowe AL, Kalivas PW. Chronic ethanol consumption by C57BL/6 mice promotes tolerance to its interoceptive cues and increases extracellular dopamine, an effect blocked by naltrexone. Alcohol Clin Exp Res. 2003;27:1892–1900. doi: 10.1097/01.ALC.0000099264.36220.48. [DOI] [PubMed] [Google Scholar]
- 52.Rajasethupathy P, Fiumara F, Sheridan R, Betel D, Puthanveettil SV, Russo JJ, et al. Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron. 2009;63:803–817. doi: 10.1016/j.neuron.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lugli G, Larson J, Martone ME, Jones Y, Smalheiser NR. Dicer and eIF2c are enriched at postsynaptic densities in adult mouse brain and are modified by neuronal activity in a calpain-dependent manner. J Neurochem. 2005;94:896–905. doi: 10.1111/j.1471-4159.2005.03224.x. [DOI] [PubMed] [Google Scholar]
- 54.Lugli G, Torvik VI, Larson J, Smalheiser NR. Expression of microRNAs and their precursors in synaptic fractions of adult mouse forebrain. J Neurochem. 2008;106:650–661. doi: 10.1111/j.1471-4159.2008.05413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang YW, Ruiz CR, Eyler EC, Lin K, Meffert MK. Dual regulation of miRNA biogenesis generates target specificity in neurotrophin-induced protein synthesis. Cell. 2012;148:933–946. doi: 10.1016/j.cell.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewohl JM, Nunez YO, Dodd PR, Tiwari GR, Harris RA, Mayfield RD. Up-regulation of microRNAs in brain of human alcoholics. Alcohol Clin Exp Res. 2011;35:1928–1937. doi: 10.1111/j.1530-0277.2011.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Booth CJ, Kim H, Qiu M, Constable RT. Evaluation of hepatic fibrosis with portal pressure gradient in rats. Magn Reson Med. 2009;61:1185–1192. doi: 10.1002/mrm.21964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao L, Lin EJ, Cahill MC, Wang C, Liu X, During MJ. Molecular therapy of obesity and diabetes by a physiological autoregulatory approach. Nat Med. 2009;15:447–454. doi: 10.1038/nm.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Roche O, Yan MS, Finak G, Evans AJ, Metcalf JL, et al. Regulation of endocytosis via the oxygen-sensing pathway. Nat Med. 2009;15:319–324. doi: 10.1038/nm.1922. [DOI] [PubMed] [Google Scholar]
- 62.Wang P, Bang JK, Kim HJ, Kim JK, Kim Y, Shin SY. Antimicrobial specificity and mechanism of action of disulfide-removed linear analogs of the plant-derived Cys-rich antimicrobial peptide Ib-AMP1. Peptides. 2009;30:2144–2149. doi: 10.1016/j.peptides.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 63.Wang J, Liu HB, Wang W, Kim I, Ha CS. A thiazoline-containing cobalt(II) complex based colorimetric fluorescent probe: ‘turn-on’ detection of fluoride. Dalton Trans. 2009;47:10422–10425. doi: 10.1039/b918887h. [DOI] [PubMed] [Google Scholar]
- 64.Wang JH, Bae JH, Lim HC, Shon WY, Kim CW, Cho JW. Medial open wedge high tibial osteotomy: the effect of the cortical hinge on posterior tibial slope. Am J Sports Med. 2009;37:2411–2418. doi: 10.1177/0363546509341174. [DOI] [PubMed] [Google Scholar]
- 65.Liu J, Lewohl JM, Dodd PR, Randall PK, Harris RA, Mayfield RD. Gene expression profiling of individual cases reveals consistent transcriptional changes in alcoholic human brain. J Neurochem. 2004;90:1050–1058. doi: 10.1111/j.1471-4159.2004.02570.x. [DOI] [PubMed] [Google Scholar]
- 66.Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. 2009;2:173–183. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gerstner JR, Vanderheyden WM, LaVaute T, Westmark CJ, Rouhana L, Pack AI, et al. Time of day regulates subcellular trafficking, tripartite synaptic localization, and polyadenylation of the astrocytic Fabp7 mRNA. J Neurosci. 2012;32:1383–1394. doi: 10.1523/JNEUROSCI.3228-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Glanzer J, Miyashiro KY, Sul JY, Barrett L, Belt B, Haydon P, et al. RNA splicing capability of live neuronal dendrites. Proc Natl Acad Sci USA. 2005;102:16859–16864. doi: 10.1073/pnas.0503783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sosanya NM, Huang PP, Cacheaux LP, Chen CJ, Nguyen K, Perrone-Bizzozero NI, et al. Degradation of high affinity HuD targets releases Kv1.1 mRNA from miR-129 repression by mTORC1. J Cell Biol. 2013;202:53–69. doi: 10.1083/jcb.201212089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zalfa F, Eleuteri B, Dickson KS, Mercaldo V, De Rubeis S, di Penta A, et al. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci. 2007;10:578–587. doi: 10.1038/nn1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akins MR, Berk-Rauch HE, Fallon JR. Presynaptic translation: stepping out of the postsynaptic shadow. Front Neural Circuits. 2009;3:17. doi: 10.3389/neuro.04.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang S, Kim S, Chawla S, Wolf RL, Zhang WG, O'Rourke DM, et al. Differentiation between glioblastomas and solitary brain metastases using diffusion tensor imaging. Neuroimage. 2009;44:653–660. doi: 10.1016/j.neuroimage.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.