Abstract
Adult stem cell deficiency has been implicated in the pathogenic mechanism for various diseases. Renal medullary dysfunction is one of the major mechanisms for the development of hypertension in Dahl salt-sensitive (S) rats. The present study first detected a stem cell deficiency in the renal medulla in Dahl S rats and then tested the hypothesis that transplantation of mesenchymal stem cells (MSCs) into the renal medulla improves salt-sensitive hypertension in Dahl S rats. Immunohistochemistry and flowcytometry analyses showed a significantly reduced number of stem cell marker CD133+ cells in the renal medulla from Dahl S rats compared with controls, suggesting a stem cell deficiency. Rat MSCs or control cells were transplanted into the renal medulla in uninephrectomized Dahl S rats, which were then treated with a low or high salt diet for 20 days. High salt-induced sodium retention and hypertension was significantly attenuated in MSC-treated rats compared with control cell-treated rats. Meanwhile, high salt-induced increases of pro-inflammatory factors, monocyte chemoattractant protein-1 and interleukin-1β, in the renal medulla were blocked by MSC treatment. Furthermore, immunostaining showed that high salt-induced immune cell infiltration into the renal medulla was substantially inhibited by MSC treatment. These results suggested that stem cell defect in the renal medulla may contribute to the hypertension in Dahl S rats and that correction of this stem cell defect by MSCs attenuated hypertension in Dahl S rats through anti-inflammation.
Keywords: CD133, inflammation, immune cell
Recent studies have suggested that many and perhaps all adult organs harbor stem cells [1], including the kidneys [2–4]. Organ-specific stem cells are necessary for organ repair during routine maintenance [5–6]. Rapid accumulating evidence shows that defects in organ-specific adult stem cells may be a pathogenic mechanism for diseases. The involvement of stem cell defects has been reported in various diseases, for example, hematologic disease [7], skin degeneration [8], aganglionic megacolon [9], keratopathy [10], muscular dystrophies [11], etc. The present study explored the possible involvement of renal medullary stem cell deficiency in the development of salt-sensitive hypertension and determined the effect of stem cell therapy on salt-sensitive hypertension in Dahl S rats.
Salt-sensitive hypertension accounts for 50% of hypertensive population [12]. Importantly, the salt sensitivity of blood pressure is closely associated with a much greater propensity to develop organ injuries in hypertension [13–14]. Mechanism for salt-sensitive hypertension remains unclear. It is well known that the renal medulla plays an important role in the regulation of sodium excretion and long-term blood pressure regulation [15–16]. Dahl S rat is a widely used genetic model of human salt-sensitive hypertension. Renal medullary dysfunction has long been recognized as one of the major mechanisms for the development of hypertension in Dahl S rats [16–17]. Interestingly, the renal medulla has been recently identified as a niche for stem cells [3]. Given the important role of stem cell in maintaining normal organ function, it is possible that the stem cells in the renal medulla may contribute to the maintenance of normal renal medullary function and thereby to the long-term regulation of arterial blood pressure. We therefore wondered whether there was stem cell deficiency in the renal medulla in Dahl S rats, and if so, whether stem cell defects in the renal medulla contributed to the development of salt-sensitive hypertension in Dahl S rats. The present study first examined the stem cell population and revealed a stem cell deficiency in the renal medulla, and then determined the effect of renal medullary transplantation of mesanchymal stem cells (MSCs) on salt-sensitive hypertension in Dahl S rats. Our results demonstrated that transplantation of MSCs into the renal medulla inhibited high salt-induced inflammation in the renal medulla and improved salt-sensitive hypertension in Dahl S rats
Materials and Methods
The detailed methods are available in online supplement.
Animals
Experiments used male Dahl S (Charles River), SS-13BN (Charles River) and Sprague-Dawley (Harlan) rats, weighing 250 to 350 g. Animal procedures were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University. SS-13BN rat was used in the present study because it is considered as one of the best normotensive control strains for Dahl S rat [18]. SS13-BN is a consomic subcolony of Dahl S rat with substitution of chromosome 13 from Brown Norway rat. The differences in genotype between SS-13BN rat and Dahl S rat is 1.95%, which is much smaller than the differences between Dahl S rat and other commonly used "control" strains: Dahl R 30%, Sprague-Dawley 52%, ACI 57%, and BN 77% [18].
Detection of CD133+, Oct-4+, CD90+ and CD43+ cells in the renal medulla
Immunohistochemistry was used to detect these cells in the medulla. Using an alternative method, single cell suspension from renal medullary tissues was prepared by collagenase digestion and cell strainer sieving and subjected to flow cytometric analysis of CD133+ cells. The appropriate isotype controls were included to assist in gating in flow cytometric analysis, and the forward scatter threshold was set to eliminate cell debris.
Ex-vivo expansion of rat MSCs and renal medullary interstitial cells (RMICs)
Rat MSCs were obtained from Texas A&M Health Science and cultured according to the instruction. RMICs were isolated from Sprague Dawley rats and cultured the same as MSCs. Totally 5×106 cells in 600 µl of 0.9% saline were used as described below.
Transplantation of MSCs or RMICs into renal medulla
Dahl S rats were uninephrectomized 1 week before. Cell suspensions prepared above were infused into the renal medulla of the remaining left kidney. A second cell infusion was performed 2 weeks later. RMICs were used in control animals. Animal groups included RMICs + low salt diet (Ctrl+LS), RMICs + high salt diet (Ctrl+HS), Saline+HS and MSC+HS.
Measurement of sodium balance
Additional groups of Dahl S rats were treated with MSCs or RMICs as described above, and then housed in metabolic cages 5 days after cell transplantation. Daily indexes of sodium balance were computed by subtracting urinary sodium excretion from total sodium intake and cumulative sodium balance was calculated. After a control day measurement, the animals were switched from tap water to 2% NaCl water and sodium balance measurements were continued for additional 3 days [19].
Monitoring of mean arterial pressure (MAP)
MAP was measured using a telemetry system for 3 days while the rats remained on a low salt diet, and then 20 more days when rats were on either a low salt diet (0.4%) or a high salt diet (8%). After the MAP measurement, the kidneys were saved for immunostaining or protein and RNA isolation.
Molecular biology assays
Protein levels of monocyte chemoattractant protein (MCP)-1 in renal medullary tissue were detected by Western blot. Interleukin (IL)-1β levels in the renal medullary tissues were measured using an enzyme-linked immunosorbent assay (ELISA) kit. CD90 mRNA levels were measured by quantitative RT-PCR
Statistics
Data are presented as means ± SE. The significance of differences in mean values within and between multiple groups was evaluated using an ANOVA followed by a Duncan’s multiple range test. Student’s t-test was used to evaluate statistical significance of differences between two groups. P<0.05 was considered statistically significant.
Results
Comparison of stem cell marker CD133+ cells in the renal medulla in different rats
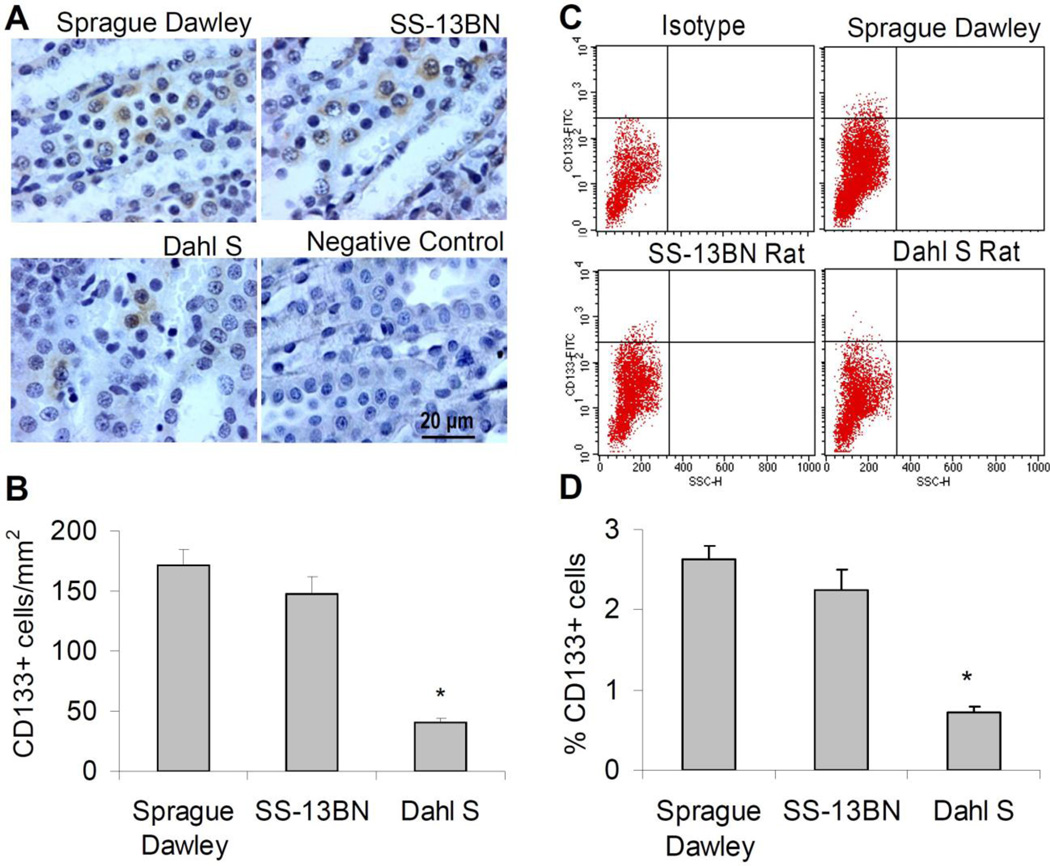
CD133 as a stem cell marker has been widely used to detect stem cells in a variety of organs such as brain [20], liver [21], muscle [22] as well as kidney [4, 23]. We thus chose CD133 as a marker to detect renal stem cells in the renal medulla. Double staining of CD133 with another stem cell marker Oct-4 [21] showed that both CD133 and Oct-4 co-expressed in the same cells in the renal medulla (supplement figure 1), further suggesting the stem cell identity of the CD133+ cells in the present study. Immunostaining showed a significantly reduced number of stem cell marker CD133+ cells in the renal medulla in Dahl S rats compared with normotensive Sprague-Dawley and SS-13BN rats (figure 1A&B). An alternative assay by flow cytometric analysis using single cell suspension prepared from the renal medulla also revealed that the percentage of CD133+ cells in Dahl S rats was significantly smaller than that in control rats (figure 1C&D). These data indicate a stem cell defect in the renal medulla in Dahl S rats.
Figure 1. CD133+ cells in the renal medulla.
A: Representative photomicrographs showing immunostaining of CD133+ cells. B: Quantitation of CD133+ cell counts in immunostaining. C: 2-D plots from flow cytometry analysis of CD133+ cell using single cell suspension, y axis, FITC signal;×axis, side scatter (SSC). D: Percentage of CD133+ cells in flow cytometry analysis. Animals were all on a low salt diet. * P<0.05 vs. other groups (n=6).
Effect of MSC transplantation on the level of CD90 in the renal medulla in Dahl S rats
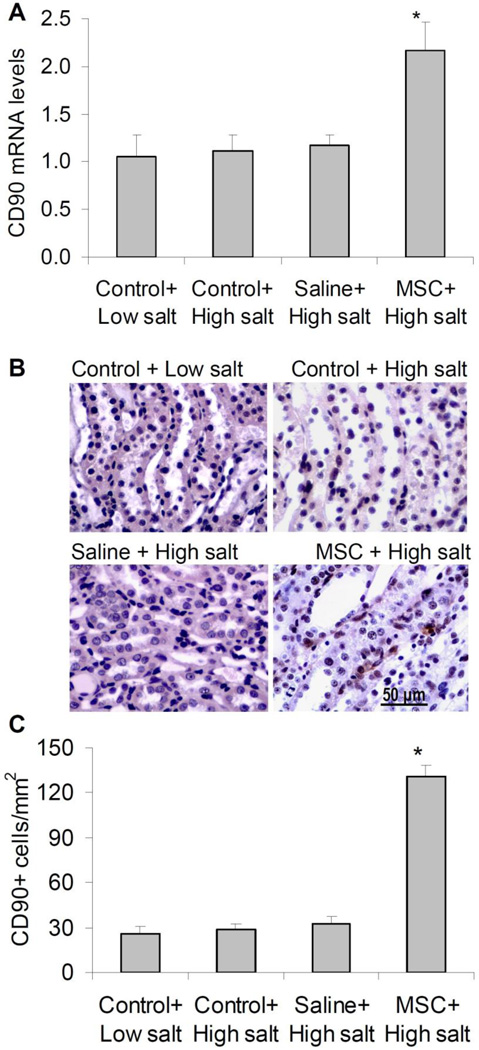
CD90 is a marker of MSCs suggested in the instruction from cell provider. We then detect the CD90+ cells to ensure a successful transplantation of MSCs at the endpoint of experiments after 20 day high salt challenge and MAP measurement. The number of CD90+ cells and the mRNA levels of CD90 in the renal medulla were greatly increased in MSC-treated rats compared with control rats (figure 2), suggesting a successful transplantation of MSCs into the renal medulla.
Figure 2. CD90 mRNA levels and immunohistochemistry analysis of CD90+ cells in the renal medulla in Dahl S rats.
A: CD90 mRNA levels by Real-Time RT-PCR analysis. B: Representative photomicrographs showing immunostaining of CD90+ cells in outer medulla. C: Quantitation of positive cell counts in immunostaining. Control = renal medullary interstitial cells, Saline = no cell, MSC = mesenchymal stem cells. * P<0.05 vs. other groups (n=5–6).
Effect of MSC transplantation into the renal medulla on sodium balance in Dahl S rats
High salt intake produced a positive sodium balance. The high salt-induced cumulative salt balance was significantly lower in MSC-treated rats than in control cell-treated rats (Figure 3A).
Figure 3. Effects of renal medullary transplantation of MSCs on salt balance and mean arterial pressure (MAP) in Dahl S rats.
A: sodium balance. B: MAP. Control = renal medullary interstitial cells, Saline = no cell, MSC = mesenchymal stem cells. * P < 0.05 vs. others.
Effect of MSC transplantation into the renal medulla on high salt induced increase in blood pressure in Dahl S rats
High salt intake remarkably increased mean arterial pressure (MAP) in control cell-treated rats. However this high salt-induced increase in MAP was significantly reduced in MSC-treated rats (figure 3), demonstrating that transplantation of MSCs into renal medulla attenuated salt sensitive hypertension in Dahl S rats. In an additional group of rats, we infused vehicle (0.9% saline only without cell suspension) into the renal medulla as a blank control. There was no significant difference in high salt-induced increase of MAP between RMIC- and saline-treated rats after high salt diet, suggesting that control cells did not produce damage compared with vehicle alone.
Effect of MSC transplantation on the levels of interleukin (IL)-1β and monocyte chemoattractant protein (MCP)-1, and CD43+ cell infiltration in the renal medulla
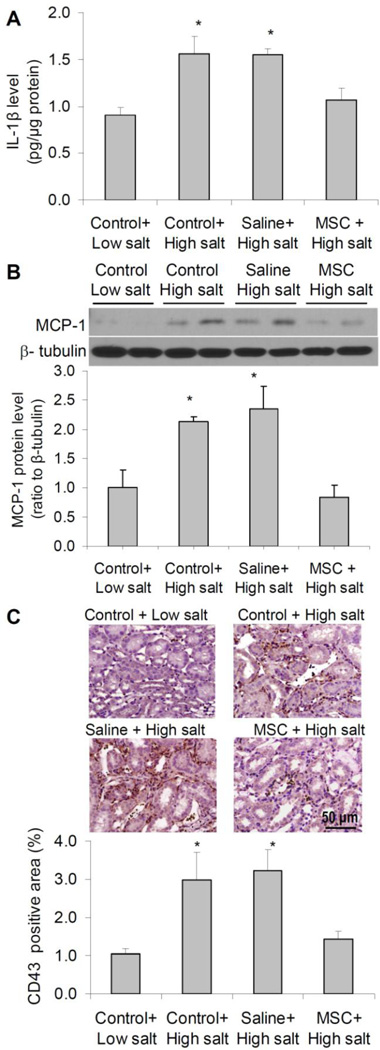
To determine the effect of MSC on inflammatory response to high salt challenge in the renal medulla, we detected the pro-inflammatory factors and immune cell infiltration in the renal medulla. IL-1β is closely associated with renal inflammation [24]. MCP-1 is one of the key chemokines that increases monocyte infiltration into inflamed tissues and an important inflammatory mediator [25]. Increased MCP-1 levels in the kidneys have been associated with renal inflammation and hypertension [26–28]. We then used IL-1β and MCP-1 to represent proinflammatory factors in the renal medulla. Our results showed that both IL-1β and MCP-1 levels were significantly increased after high salt challenge in control rats, which was blocked in MSC-treated rats (figure 4A&B). CD43 is a marker for identifying T cells and a subset of B cells and used as an indicator for immune cell infiltration and inflammation in the renal medulla in the present study. Our results showed a remarkable increase in CD43+ cells in the renal medulla after high salt challenge in control animals, which was blocked in MSC-treated rats (figure 4C). These results demonstrated that MSC treatment inhibited the inflammatory response to high salt challenge in the renal medulla in Dahl S rats.
Figure 4. Effect of MSCs on inflammatory factors in the renal medulla in Dahl S rats.
A: IL-1β levels by ELISA. B: MCP-1 levels by Western blot. C: Immunostaining of CD43+ cells in outer medulla. Control = renal medullary interstitial cells, Saline = no cell, MSC = mesenchymal stem cells. * P < 0.05 vs. Control + Low salt and MSC + High salt (n=5–6).
Discussion
The present study demonstrated that the number of stem cell marker CD133+ cells was decreased in the renal medulla and that transplantation of MSCs into the renal medulla significantly attenuated high salt-induced increase in blood pressure in Dahl S rats; the beneficial effect of MSCs was associated with reduction in high salt-stimulated increases in pro-inflammatory factors IL-β and MCP-1 as well as infiltration of immune cells in the renal medulla. These data suggested that there is a defect in stem cells in the renal medulla and that correction of the stem cell deficiency inhibits inflammatory response to high salt challenge in the renal medulla and attenuates salt-sensitive hypertension in Dahl S rats.
CD133 as a stem cell marker has been widely used to detect stem cells in a variety of organs including kidney [4]. Our data showed a significantly reduced number of CD133+ cells, which suggested a stem cell deficiency in the renal medulla in Dahl S rats. Given the fact that stem cell deficiency may play a role in the pathogenesis of diseases and that renal medullary dysfunction is one of the major mechanisms for hypertension in Dahl S rats, this finding may suggest that the stem cell deficiency participates in the mechanisms mediating renal medullary dysfunction and contributes to the pathogenesis of salt-sensitive hypertension in this animal model. If so, it would be expected that correction of this stem cell deficiency by transplanting stem cells into the renal medulla attenuated the salt-sensitive hypertension in Dahl S rats.
Stem cell therapies have been widely used for the regeneration and repair after acute ischemic and toxic organ damages. In addition, stem cell therapy has also been used in the treatment of different forms of diseases [29] and emerged as a new exciting therapeutic option for a variety of pathological conditions such as diabetes, multiple sclerosis, inflammatory bowel disease, liver disease, heart disease, and renal disease, etc. [29]. It is believed that stem cell therapy may be applied in more and more different diseases. The finding in the present study provided clue for using stem cell treatment in renal medullary dysfunction and salt-sensitive hypertension. Notably, among the cell sources for stem cell therapy MSCs may be the most widely used and currently preferred stem cell model and have been used in many clinical trials because of easy preparation, immunologic privilege and biosafety [30–31]. We then chose MSCs in our study and found that transplantation of MSCs into the renal medulla remarkably improved the capability of the kidneys to remove extra sodium load, which reduced sodium retention, and significantly attenuated hypertension in Dahl S rats. These data further support the view that stem cell deficiency in the renal medulla may be a novel mechanism contributing to the development of hypertension in Dahl S rats and that MSCs could be a potential therapeutic strategy for salt-sensitive hypertension.
There might be a concern that cell transplantation into the renal medulla would produce damage as well as renal medullary dysfunction, which would cause more increase of blood pressure after high salt challenge. If this was the case, there might be possibilities that control cells RMICs produced more damage than MSCs and that a reduced increase of blood pressure in MSC-treated rats after high salt challenge was due to less damage in the renal medulla in MSC-treated rats than that in control cell-treated rats. To rule out this possibility, we compared high salt-induced increase in blood pressure between control cell- and saline (vehicle)-treated rats and found no significant difference between these two groups, demonstrating that cell transplantation did not produce damage and dysfunction in the renal medulla compared with vehicle alone and that attenuation of salt sensitive hypertension in MSC-treated rats was indeed due to the beneficial effect of MSCs, not due to less damage produced by MSCs than RMICs.
We next wondered by what mechanism MSCs exerted the anti-hypertensive action in the renal medulla. It has been recognized that the beneficial effects of stem cell therapy are predominantly mediated by indirect paracrine mechanisms [30, 32]. In particular, it has been well documented that stem cells including MSCs possess immunomodulatory and anti-inflammatory functions [31–32]. Renal inflammation plays a pivotal role in salt-sensitive hypertension including that in Dahl S rats [33–34]. We therefore examined the pro-inflammatory factors and immune cell infiltration to determine whether anti-hypertensive action of MSC treatment was associated with anti-inflammatory effects in the renal medulla in Dahl S rats.
Our results demonstrated that high salt intake significantly increased pro-inflammatory factors and produced substantial immune cell infiltration in the renal medulla. However, MSC treatment blocked high salt-induced increases in pro-inflammatory factors and inhibited the inflammation in the renal medulla in Dahl S rats. These data suggested that normal stem cell behavior may preserve renal medullary function in response to high salt intake via well-maintained anti-inflammatory mechanisms, and that a deficient stem cell function in the renal medulla may result in an impairment of stem cell-mediated anti-inflammatory mechanisms and consequently incapacity to counterbalance inflammatory response to high salt challenge in Dahl S rat. MCS treatment corrected the impaired anti-inflammatory mechanisms associated with stem cell deficiency in the renal medulla and attenuated hypertension after high salt challenge in Dahl S rats. The mechanisms causing stem cell defect in Dahl S rats and how stem cells execute anti-inflammatory function require further investigation. It should be noted that although the causal role of renal inflammation in hypertension of Dahl S rats has been identified and that anti-inflammatory function of MSC has been defined, which suggests that MSC may attenuate hypertension via anti-inflammatory function, it however can not be totally ruled out that there might be other unknown mechanism of lowering blood pressure and that less hypertension led to less inflammation in MSC-treated rats.
Interestingly, in consistent with the findings in the present study, a most recent study showed that IV-injected MSCs localized into the kidneys and blocked the increase of pro-inflammatory factors IL-1β and TNF-α in the renal medulla [35], which was accompanied with normalization of the increased expression of pro-hypertensive renin-angiotensin system and the reduced expression of anti-hypertensive gene (type 2 angiotensin II receptor) in the renal medulla in two kidney-one clip rat, a renovascular hypertension model. Therefore, it is possible that there may also be interaction and regulation of pro-/anti-hypertensive genes by stem cells in the kidneys of Dahl S rats, a salt sensitive hypertension model, which is worth further evaluation.
In summary, the present study revealed a stem cell deficiency in the renal medulla in the Dahl S rats and that transplantation of MSCs into the renal medulla attenuated the salt-sensitive hypertension, which was associated with inhibition of high salt-induced increase of pro-inflammatory factors and immune cell infiltration in the renal medulla. It is concluded that stem cell deficiency in the renal medulla may be a novel mechanism contributing to the pathogenesis of salt-sensitive hypertension and that MSC therapy may serve as a new therapeutic strategy for salt-sensitive hypertension.
Supplementary Material
Stem cell defect in the renal medulla may contribute to salt-sensitive hypertension
Stem cell therapy is a potential therapeutic strategy for salt-sensitive hypertension
Normal stem cell inhibits the inflammatory response to high salt in the renal medulla
Acknowledgments
Sources of Funding
This work was supported by National Institute of Health grants HL-89563, HL-106042 and DK-54927; National Nature Science Foundation of China grant 81328006
Footnotes
Disclosures
None
Reference
- 1.Meirelles LdS, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 2.Lin F, Igarashi P. Searching for stem/progenitor cells in the adult mouse kidney. J Am Soc Nephrol. 2003;14:3290–3292. doi: 10.1097/01.asn.0000098682.51956.06. [DOI] [PubMed] [Google Scholar]
- 3.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of Renal Progenitor Cells from Adult Human Kidney. Am J Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haller H, de Groot K, Bahlmann F, Elger M, Fliser D. Stem cells and progenitor cells in renal disease. 2005;68:1932–1936. doi: 10.1111/j.1523-1755.2005.00622.x. [DOI] [PubMed] [Google Scholar]
- 6.Oliver JA. Adult renal stem cells and renal repair. Curr Opin Nephrol Hypertens. 2004;13:17–22. doi: 10.1097/00041552-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Raaijmakers MH, Scadden DT. Evolving concepts on the microenvironmental niche for hematopoietic stem cells. Curr Opin Hematol. 2008;15:301–306. doi: 10.1097/MOH.0b013e328303e14c. 00062752-200807000-00005 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Stout GJ, Blasco MA. Genetic dissection of the mechanisms underlying telomere-associated diseases: impact of the TRF2 telomeric protein on mouse epidermal stem cells. Dis Model Mech. 2009;2:139–156. doi: 10.1242/dmm.002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwashita T, Kruger GM, Pardal R, Kiel MJ, Morrison SJ. Hirschsprung disease is linked to defects in neural crest stem cell function. Science. 2003;301:972–976. doi: 10.1126/science.1085649. 301/5635/972 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagali N, Eden U, Utheim TP, Chen X, Riise R, Dellby A, Fagerholm P. In vivo morphology of the limbal palisades of vogt correlates with progressive stem cell deficiency in aniridia-related keratopathy. Invest Ophthalmol Vis Sci. 2013;54:5333–5342. doi: 10.1167/iovs.13-11780. DOI iovs.13-11780 [pii] 10.1167/iovs.13-11780. [DOI] [PubMed] [Google Scholar]
- 11.Wilschut KJ, Ling VB, Bernstein HS. Concise review: stem cell therapy for muscular dystrophies. Stem Cells Transl Med. 2012;1:833–842. doi: 10.5966/sctm.2012-0071. DOI sctm.2012-0071 [pii] 10.5966/sctm.2012-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II127–II134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 13.Campese V. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension. 1994;23:531–550. doi: 10.1161/01.hyp.23.4.531. [clinical conference]. [DOI] [PubMed] [Google Scholar]
- 14.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt Sensitivity, Pulse Pressure, and Death in Normal and Hypertensive Humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 15.Cowley AW, Jr MD, Lu S, Roman RJ. The renal medulla and hypertension. Hypertension. 1995;25:663–673. doi: 10.1161/01.hyp.25.4.663. [DOI] [PubMed] [Google Scholar]
- 16.Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2003;284:R13–R27. doi: 10.1152/ajpregu.00321.2002. [DOI] [PubMed] [Google Scholar]
- 17.Bergstrom G, Evans RG. Mechanisms underlying the antihypertensive functions of the renal medulla. Acta Physiologica Scandinavica. 2004;181:475–486. doi: 10.1111/j.1365-201X.2004.01321.x. [DOI] [PubMed] [Google Scholar]
- 18.Cowley AW., Jr Genomics and homeostasis. Am J Physiol Regul Integr Comp Physiol. 2003;284:R611–R627. doi: 10.1152/ajpregu.00567.2002. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Zhu Q, Xia M, Li PL, Hinton SJ, Li N. Hypoxia-inducible factor prolyl-hydroxylase 2 senses high-salt intake to increase hypoxia inducible factor 1alpha levels in the renal medulla. Hypertension. 2010;55:1129–1136. doi: 10.1161/HYPERTENSIONAHA.109.145896. DOI HYPERTENSIONAHA.109.145896 [pii] 10.1161/HYPERTENSIONAHA.109.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. PNAS. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kordes C, Sawitza I, Muller-Marbach A, Ale-Agha N, Keitel V, Klonowski-Stumpe H, Haussinger D. CD133+ hepatic stellate cells are progenitor cells. Biochem Biophys Res Commun. 2007;352:410–417. doi: 10.1016/j.bbrc.2006.11.029. DOI S0006-291X(06)02496-X [pii] 10.1016/j.bbrc.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia M. AC133 expression in human stem cells. Leukemia. 2001;15:1685–1688. doi: 10.1038/sj.leu.2402255. [DOI] [PubMed] [Google Scholar]
- 23.Bao J, Tu Z, Sun H, Luo G, Yang L, Song J, Qin M, Shi Y, Bu H, Li Y. R2: identification of renal potential progenitor/stem cells that participate in the renal regeneration processes of kidney allograft fibrosis. Nephrology (Carlton) 2008;13:500–507. doi: 10.1111/j.1440-1797.2008.00939.x. DOI NEP939 [pii] 10.1111/j.1440-1797.2008.00939.x. [DOI] [PubMed] [Google Scholar]
- 24.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. DOI kid929 [pii] 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 25.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein 1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takai S, Jin D, Sakonjo H, Miyazaki M. Combination therapy with irbesartan and efonidipine for attenuation of proteinuria in Dahl salt-sensitive rats. Hypertens Res. 2010;33:953–959. doi: 10.1038/hr.2010.90. DOI hr201090 [pii] 10.1038/hr.2010.90. [DOI] [PubMed] [Google Scholar]
- 27.Crowley SD, Song YS, Sprung G, Griffiths R, Sparks M, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux PL, Ruiz P. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension. 2010;55:99–108. doi: 10.1161/HYPERTENSIONAHA.109.144964. DOI HYPERTENSIONAHA.109.144964 [pii] 10.1161/HYPERTENSIONAHA.109.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujii S, Zhang L, Kosaka H. Albuminuria, expression of nicotinamide adenine dinucleotide phosphate oxidase and monocyte chemoattractant protein-1 in the renal tubules of hypertensive Dahl salt-sensitive rats. Hypertens Res. 2007;30:991–998. doi: 10.1291/hypres.30.991. DOI JST.JSTAGE/hypres/30.991 [pii] 10.1291/hypres.30.991. [DOI] [PubMed] [Google Scholar]
- 29.Larijani B, Esfahani EN, Amini P, Nikbin B, Alimoghaddam K, Amiri S, Malekzadeh R, Yazdi NM, Ghodsi M, Dowlati Y, Sahraian MA, Ghavamzadeh A. Stem cell therapy in treatment of different diseases. Acta Med Iran. 2012;50:79–96. DOI 20202 [pii] [PubMed] [Google Scholar]
- 30.Malliaras K, Marban E. Cardiac cell therapy: where we've been, where we are, where we should be headed. Br Med Bull. 2011;98:161–185. doi: 10.1093/bmb/ldr018. DOI ldr018 [pii] 10.1093/bmb/ldr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariani E, Facchini A. Clinical applications and biosafety of human adult mesenchymal stem cells. Curr Pharm Des. 2012;18:1821–1845. doi: 10.2174/138161212799859666. DOI CPD-EPUB-20120119-003 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Souidi N, Stolk M, Seifert M. Ischemia-reperfusion injury: beneficial effects of mesenchymal stromal cells. Curr Opin Organ Transplant. 2013;18:34–43. doi: 10.1097/MOT.0b013e32835c2a05. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Iturbe B, Quiroz Y, Herrera-Acosta J, Johnson RJ, Pons HA. The role of immune cells infiltrating the kidney in the pathogenesis of salt-sensitive hypertension. J Hypertens. 2002;(Suppl 20):S9–S14. [PubMed] [Google Scholar]
- 34.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2006;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. DOI 01.HYP.0000228320.23697.29 [pii] 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira-Sales EB, Maquigussa E, Semedo P, Pereira LG, Ferreira VM, Camara NO, Bergamaschi CT, Campos RR, Boim MA. Mesenchymal stem cells (MSC) prevented the progression of renovascular hypertension, improved renal function and architecture. PLoS One. 2013;8:e78464. doi: 10.1371/journal.pone.0078464. PONE-D-13-07146 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.